Abstract
Objective
One of the common findings in autism spectrum disorder (ASD) is limited selective attention toward social objects such as faces. Evidence from both human and nonhuman primate studies suggests that selection of objects for processing is guided by the appraisal of object values. We hypothesized that impairments in selective attention in ASD may reflect a disruption of a system supporting learning about object values in social domain.
Method
We examined value learning in social (faces) and nonsocial (fractals) domains in preschoolers with ASD (n = 25) and typically developing (TD) controls (n = 28) using a novel value learning task implemented on a gaze-contingent eye-tracking platform consisting of value learning and selective attention choice test.
Results
Children with ASD performed more poorly than TD controls in the social value learning task, but both groups performed similarly on the nonsocial task. Within-group comparisons indicated that value learning in TD children was enhanced on the social compared to nonsocial task, but no such enhancement was seen in children with ASD. Performance in the social and nonsocial conditions was correlated in the ASD but not in the TD group.
Conclusion
The study provides support for a domain-specific impairment in value learning for faces in ASD and suggest that in ASD value learning in social and nonsocial domains may rely on a shared mechanism. These findings have implications for both models of selective social attention deficits in autism and identification of novel treatment targets.
Keywords: autism, value learning, face, eye-tracking, reward system
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by marked social deficits and the presence of stereotyped interests and behaviors.1 A core feature of ASD is atypical selective attention, i.e., the ability to prioritize for processing those social objects that hold the greatest informational value and to ignore those that are irrelevant.2–4 In naturalistic and laboratory settings, young children with ASD often fail to select faces for processing.5–9, 10 Moreover, when they do attend to faces, children with ASD have difficulty selecting the parts of faces that are most relevant in a given context (e.g., eyes versus mouth).5,11,12 Signs of atypical selective attention begin to emerge during the prodromal stages of ASD13–17 and are prevalent in toddlers with ASD.18,19 Limited ability to select most relevant faces for processing may hinder the development of drawing inferences about others’ intentions, mental states, and goals,20–22 coordinating attention and actions with others in order to reach common goals,23 and observational learning.24 Relatedly, atypical social attention in ASD is strongly related to increased autism symptom severity and poor adaptive functioning.25–27 Early individual differences in selective social attention contribute to the variability in social, adaptive, and cognitive outcomes observed in preschoolers with ASD.25–27 While impairments in selective attention to faces in young children with ASD are well documented, the evidence regarding selective attention of non-animate objects is more limited, especially to objects that are task-relevant rather than distractors.6 The extant free-viewing studies suggest that toddlers with ASD typically show limited attention to faces compared to developmentally delayed and typically developing (TD) peers, but there is no strong evidence for enhanced attention to objects in general,5,28 unless the objects map onto special-interests children with ASD.29 Despite the prominence of atypical selective attention in ASD, the mechanisms that give rise to such differences are not well understood.
One of the critical questions in the field of attention research is how an individual “knows” which objects (social and nonsocial) should be prioritized for processing within a given context. Evidence from research on both human and nonhuman primates suggests that attentional selection is guided by the appraisal of object values.2,30–35 In visual attention, object value is defined primarily by the usefulness or relevance of the information the object provides to the observer.2,3 As such, eye movements harvest information that then guides behavior in service of maximizing adaptation and survival. This principle is reflected in the finding that monkeys are willing to give up food to obtain visual information (i.e., “pay-per-view”) about members of their social group (i.e., their availability for mating).36,37 Numerous studies illustrate faster response times to or increased detection of high-value stimuli,38 as well as robust effects of value information on visual attentional selection,39 including enhanced attention to high-value objects and suppressed attention to low-value objects.40
The effects of value learning can operate without conscious awareness of associations between value and specific stimulus features41 and may endure days past the training stage (long-term or “stable” values).40 However, in everyday situations, object values often need to be learned rapidly and flexibly updated as task demands change (short-term values).30 For example, the informational value of faces of interactive partners is likely to vary depending on context (e.g., mother versus stranger, or teacher versus peer), and as a result, their values may need to be updated as interactions unfold. Value learning is subserved by the reward systems of the brain. In primates, flexible (short-term) and stable (long-term) values are encoded in the head and the tail of the caudate nucleus (CN), respectively,4,30,31 and value information is sent to superior colliculi (SC) biasing gaze to high-value objects.42 Information about object values can also be transmitted to cortical brain regions including orbital and medial prefrontal cortices43,44 or to the anterior cingulate gyrus, which integrates and utilizes value information to modify behavior and response selection.45
Research on the reward system in ASD has focused primarily on examining functional brain activation patterns in response to various types of rewards, with a primary focus on dissociable46 anticipatory (perceived reward value) and motivational (drive to obtain the reward) components. Very little work has focused on how initially neutral objects acquire reward value for individuals with ASD47,48 and whether this process is comparable to that observed in unaffected individuals.48,49 The few studies that address value learning in ASD typically employ the probabilistic learning task and examine whether learning the values of nonsocial objects (e.g., abstract images) differs depending on reward type: social (e.g., verbal praise or a smiling face) or nonsocial (monetary).50,51 Extant, albeit limited, evidence suggests that TD adults and those with ASD learn to select high-value objects associated with both social (e.g., happy faces) and nonsocial (monetary) rewards.50 However, compared to controls, adults with ASD appear to perform worse at learning object values when the rewards are social, suggesting that social rewards may have a lower motivational value in ASD.50 To our best knowledge, there have been no studies examining either how individuals with ASD learn the values of social objects (here, faces) or whether this process is impaired relative to learning the values of nonsocial objects. Moreover, studies on value learning in ASD have thus far focused exclusively on high-functioning adolescents and adults. Therefore, neither the ontogeny of observed abnormalities nor their role in autistic psychopathology has been adequately investigated. Finally, no studies have examined the relationship between value learning and selective attention in ASD.
To begin to fill in these gaps, here we examined short-term flexible value learning for both social and nonsocial objects in young children with ASD and TD controls. To do so, we designed a novel implicit learning task inspired by a value learning task previously developed and validated in non-human primates.32,52 The task measures whether reinforcement for attending to a particular object during training trials biases visual attention toward the reinforced objects (whether social or nonsocial) during subsequent testing trials. With regard to the social version of the task, we examined if children can learn, as they would in real-world, which face carries more information or is ‘nice’, i.e., responds contingently with positive affect when looked upon. We intentionally used ecologically-valid pairings of social cues (neutral faces) with consequences (smiling faces) to examine if and how children with ASD learn about value of faces and whether this process is distinct from that observed in response to pairings of nonsocial events. The value learning task requires no verbal instructions and relies on eye movements as the response modality; thus, it can be administered to individuals with a wide age and/or IQ range.
We hypothesize that in the social condition, children with ASD will learn more poorly compared to TD controls. In the nonsocial condition, we hypothesize that children with ASD will exhibit value learning either commensurate with that observed in TD controls or their learning will be enhanced compared to TD controls, which would suggest that nonsocial objects have a preferential status in their reward learning system. With regard to the within-group patterns, we hypothesized that the TD group would show enhanced learning during the social compared to the nonsocial condition of the value learning task. Children with ASD, however, were expected to either perform better in the nonsocial compared to the social version of the task, which would support the notion that objects rather than faces have a preferential status in this group. We also examined the degree of correlation between social and nonsocial reward learning to gain insight into whether two forms of learning rely on shared underlying mechanisms. In an exploratory manner, we also examined frequency of positive affective responses (smiling) during the social and nonsocial value learning tasks. We reasoned that if one type of stimuli held greater hedonic value to one group versus the other, we would observe group-related differences in the frequency of positive affective response to the specific conditions and that the degree to which children find the tasks enjoyable, may be related to their value learning.
METHOD
The study was approved by the Human Investigations Committee of Yale School of Medicine, and informed written consent was obtained from all parents prior to testing. Diagnoses and assessments were conducted at the Toddler Developmental Disabilities Clinic.
Participants
The ASD group consisted of 25 children (100% male), with a mean age of 43.9 (SD = 18) months (21 to 78 months), and the TD group consisted of 27 children (37% male), with a mean chronological age of 38.6 (SD = 14) months (20 to 65 months). Children with ASD were recruited from the university-based clinic specializing in the diagnosis of autism, and TD children were recruited through advertisement. Exclusionary criteria were gestational age below 34 weeks, hearing or visual impairment, non-febrile seizure disorders, or a known genetic syndrome. The diagnostic status of each child with ASD was ascertained based on a review of his medical and developmental history, as well as assessments of autism symptom severity (using the Autism Diagnostic Observation Schedule-2, ADOS-2) and cognitive levels (using the Mullen Scales of Early Learning, MSEL, or Differential Ability Scales-II, DAS-II, based on age). Seven out of 25 children with ASD were diagnosed before the age of 36 months. Research on stability of diagnosis assigned before 3 years suggests that only a small fraction of children (~10%) are expected to change diagnosis over short- and long-term follow-up.53–57 Should any diagnostic shifts occur amongst the 7 children, they would be unlikely to affect the existing pattern of results. Developmental status in the TD group was confirmed using parent report and the MSEL. The groups did not differ with regard to chronological age (F[1, 51] = 1.45 p = .235), but, as expected, the group with ASD had significantly lower nonverbal developmental quotients (DQ) (p < .001; Table 1). Across the two groups, nonverbal mental age ranged from 13 to 78 months. Given the comorbidity between ASD and developmental delay/intellectual disability, unselected toddlers/preschoolers are likely to have DQ scores as those seen in the present study. Given the young age of the participants, the children were not evaluated for conditions often comorbid with ASD such as anxiety or attention-deficit/hyperactivity disorder (ADHD). The groups also differed in sex distribution (X2(1) = 23.39, p < .001); therefore, we examined sex effects on the primary dependent measure in the TD group before collapsing across sex for between-group comparisons. Please see the Preliminary Analyses section for details. Taking advantage of a relatively broad chronological age range, we directly evaluated the effects of age on performance by including it within the models as a covariate.
Table 1.
Sample Characterization
| ASD | TD | |
|---|---|---|
| n | 25 | 27 |
| # of Males | 25 | 10 |
| Age | 43.9 (17) | 38.6 (14) |
| MSEL Nonverbal DQ | 76.9 (24) | 111 (14) |
| ADOS-2 SA CSS | 5.75 (1.8) | — |
| ADOS-2 RRB CSS | 7.58 (1.9) | — |
Note: ADOS = Autism Diagnostic Observation Schedule; ASD = autism spectrum disorder; CSS = calibrated severity scores; DQ = developmental quotient; MSEL = Mullen Scales of Early Learning; RRB = restrictive and repetitive behaviors; SA = social affect; TD = typically developing.
Stimuli
Dynamic fractals were drawn from popular genetic algorithm-based fractal generation software,58 with opening frames of the videos selected as the static stimuli. Humans’ happy, dynamic facial expressions were drawn from the BU-4DFE 3D Dynamic Facial Database.59 To ensure the perceptual salience equivalency of the stimuli, 12 facial and 12 fractal stimuli were rated by 17 adults through crowdsourcing60 via Qualtrics on 10-point scales targeting the saliency of each image and, for the faces, of each emotional expression (neutral and happy). Each stimulus was ranked twice, first as a static still-frame (first frame of the video) and again as a dynamic display. Ratings were averaged, and the chosen stimuli were matched to within half a point for all ratings. Please see Figure 1 for illustration of the static versions of the stimuli. Moreover, the design involved value reversal on successive blocks of trials, therefore counterbalancing stimuli within each category with regard to their assigned value (high/low). Stimuli sets were standardized with regard to luminance, contrast density, texture similarity, size, color, intensity, and positioning.61 Two static and two dynamic versions of fractals and faces of comparable salience were chosen for the task.
Figure 1.
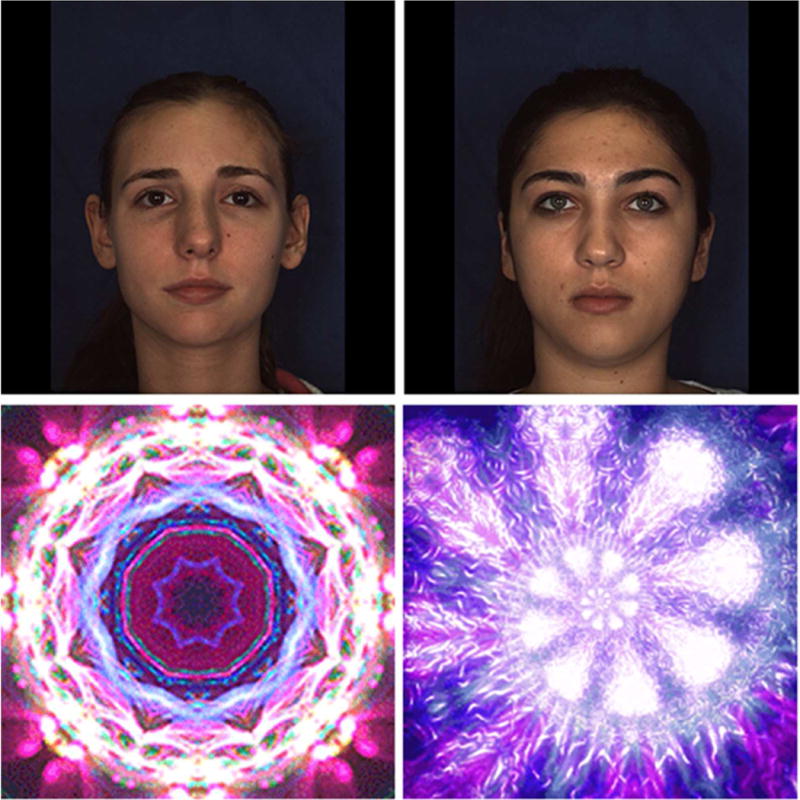
Stimuli used in the social and nonsocial version of the Flexible Value Learning Task
Apparatus
The gaze-contingent experimental design was implemented on an SR EyeLink 1000 Plus 500 Hz eye tracker configured for remote mode using a 5-point calibration procedure augmented with smooth pursuit calibration correction.62 Eye-tracking data were processed using custom software written in MATLAB. The software accommodated standard steps for processing eye-tracking data, including data quality check (QC) and data extraction (DE) steps. QC steps evaluate calibration and validation errors in visual angles and the overall attention to the scene based on the overall proportion of valid looking times. DE steps include blink detection, region of interest (ROI) identification, dwell time on specific ROIs, frequency and duration of fixations, and event-triggered saccadic reaction time.
Procedure
The design of the experimental flexible value learning task is based on a marker task developed by Hikosaka et al.31 The following adaptations to the original primate task were made: (a) Learning load: given that training on an extended number of stimuli sets would not be feasible with young and disabled children, we limited the number of stimuli to be learned to 2. (b) Domain specificity: to address the question of domain generality vs. specificity in value learning, we created two versions of the tasks: social, where high-value faces turn from neutral and static to dynamic and smiling, and nonsocial, where high-value fractals turn from static to dynamic displays. (b) Reward type: given that real-life visual exploration aims to harvest information rather than primary rewards, we replaced the drop of juice delivered contingently to primates upon fixation on the HV object with a contingent delivery of a dynamic novel display (evolving fractal or a smiling face). Although the dynamic display can be considered rewarding in a hedonic sense, delivering pleasure upon encounter, it also can be considered rewarding because it holds more information than the static display (e.g., “who is nice”), information that can be used to guide attention and behavior and to enhance adaptation.
The value learning task consisted of four blocks of trials, each containing 24 training trials, followed by 8 testing trials. Training: The same two objects (A and B), either two fractals or two faces, were presented in all blocks. Within each block, one object (e.g., A) was assigned a priori to be high-value (HV) and the other one (e.g., B) low-value (LV). If a child looked at an HV object (face or fractal), the object became dynamic, turning into a smiling face (Social) or a swirling fractal (Nonsocial), with a probability equal to 1 for 2 seconds. Fixation on the LV object did not result in a change to its display and the stimulus was presented on the screen for 2s. In Blocks 1 and 3, object A was assigned HV and object B was assigned LV; values reversed in Blocks 2 and 4. Each trial started with the appearance of a central fixation point for 600ms (Figure 2). Then, an object (A or B) appeared randomly in one of four locations for 2 seconds. The fixation point was extinguished 300ms after object onset. Out of 24 training trials, 12 displayed the HV object and 12 the LV object. Testing: The Choice Test consisted of 8 trials at the end of each block. During each trial, the two objects (HV and LV) from the training trials were presented simultaneously for 2 seconds in two of four locations, selected at random, and a saccade toward the HV but not the LV object activated its dynamic display. The proportion of test trials during which a child fixated on the HV object standardized over all valid test trials (#HV/(#HV + #LV)) across all testing blocks served as a dependent variable (%HV). Each child first viewed the nonsocial (fractal) condition and then the social (face) condition. The task lasted approximately 11 minutes.
Figure 2.
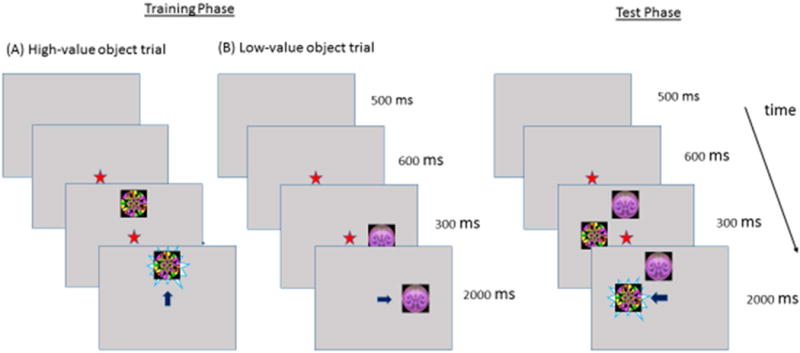
Short-term value learning task. Note: High-value (HV) object training trials (A): fixation (marked as an arrow) on the HV object results in activation of a dynamic display (revolving fractal, shown, or smiling face, not shown). Low-value (LV) object training trials (B): fixation on the LV object results in no change to its display. Choice test trials consist of a simultaneous display of HV and LV objects presented randomly in two of four locations. Four blocks of training and testing trials were shown, with object values alternating between the blocks. Only the nonsocial (fractal) version of the task is shown below.
Affective responses to value learning tasks
In addition to value learning, we also examined a positive affective component of the task performance: participants’ frequency of smiling during the social and nonsocial conditions. An instance of a smile was coded if the child’s lips moved from a neutral to an upturned position. A maximum of one smile could be coded per each trial. If a child started smiling during a trial and continued to do so during a subsequent trial, an instance of “smile” was coded for both trials. Two coders with previously established inter-rater reliability viewed the recorded sessions off-line and coded instances of smiling during the presentation of training and test trials in each condition. Subsequently, a proportion of trials during which the children exhibited positive affect (%Smile) during the task was computed. The raters independently coded affective responses for 20% of the total sample and were highly reliable (α = .95). Affective responses were coded for all but 2 participants (both with ASD) due to occlusion of the mouth by a pacifier or another object.
Data reduction
The visual scene was divided into six ROIs including the central fixation point, the four potential object locations, and the entire screen area. For a trial to be considered valid, a child was required to look at the central fixation stimulus and then to execute a saccade toward a single object presented on the screen (training trials) or toward one of the two objects presented simultaneously on the screen (test trials). All 52 children contributed valid data in the nonsocial condition. In the social condition, three children with ASD failed to contribute any valid data due to fatigue or a truncated visit, and two children in the TD group had to be excluded due to calibration errors. After these exclusions, in the nonsocial condition, 25 children with ASD and 27 TD controls contributed data, compared to 22 children with ASD and 25 TD controls in the social condition.
Statistical analyses
The hypotheses were tested using analysis of variance (ANOVA), with diagnostic group as a between-group factor, condition as a within-group factor, and chronological age (months) as a covariate. Significant group × condition interaction effects were followed by planned within- and between-group contrasts. All planned contrasts are reported with a Holm-Bonferroni correction for multiple comparisons. Associations between variables of interest were examined using the Pearson’s r correlation coefficient analysis. Data analysis was implemented in SAS 9.4.
RESULTS
Preliminary Analysis
Sex effects in the TD group
A within-group ANOVA on %HV in the TD group indicated no main effects of sex (F[1, 21] = .09, p = .768), a significant effect of condition (F[1,21] = 5.07, p = .035), and no sex × condition interaction (F[1, 21] = 1.03, p = .322). In the nonsocial condition, %HV was 73.9% (SD = 12) in females and 71.8% (SD = 13) in males (p = .701). In the social condition, %HV was 81.7% in females (SD = 11) and 86.4% (SD = 7) in males (p = .304). Thus, males and females in the TD group were collapsed for the subsequent analyses.
Value learning and nonverbal DQ
Considering that the two groups differed in nonverbal DQ scores, we first evaluated if performance on the tasks was correlated with DQ. Pearson’s r correlational analysis between %HV and nonverbal DQ in the ASD and TD groups yielded non-significant results. In the ASD group, the correlations were r(24) = .196, p = .36 and r(24) = .006, p = .77 in the nonsocial and social conditions, respectively, and in the TD group, analogous correlations were r(27) = −.10, p = .60, and r(24) = .313, p = .14. Thus, the task requirements did not appear to differentially affect children across the spectrum of cognitive ability.
Flexible Value Learning Task
Training Trials
On average, valid eye-tracking data were recorded from 57 (SD = 22) and 63 (SD = 19) training trials in the nonsocial condition and from 51 (SD = 20) and 68 (SD = 12) training trials in the social condition in the ASD and TD groups, respectively. A group × condition ANOVA, with age as a covariate, on the number of valid training trials indicated a significant effect of diagnosis (F[1,44] = 5.55, p = .023), no effect of condition (F[1,44] = .16, p = .694), and a significant diagnosis × condition interaction (F[1, 44] = 4.80, p = .034). The effect of age was not significant (p = .229). Planned contrasts showed that in TD group, the number of valid training trials was higher in the social than nonsocial condition (p = .014), but valid training trials were comparable across conditions in the ASD group (p = .150). The ASD group did not differ from TD controls concerning the number of valid trials completed in the nonsocial condition (p = .358, d = .32), but they had fewer valid trials in the social condition (p < .004, d = .88). Thus, attention to the social compared to nonsocial version of the task was enhanced in the TD but not in the ASD group. In addition, although there were significant correlations between the number of completed training trials and %HV in the TD group (r[24] = .49, p = .018 and r[24] = .63, p = .001 in the nonsocial and social conditions, respectively), the correlations were not significant in the ASD group (r[22] = .20, p = .38 and r[22] = .36, p = .10).
Value learning
During the Test trials, both groups looked at the HV compared to LV objects within each category significantly more often than expected by chance (all p-values < .001; Figure 3). A diagnosis × condition ANOVA on %HV revealed no significant effect of diagnosis (F[1, 43] = 2.21, p = .144), or condition (F[1, 43] = .59, p = .542) but a significant diagnosis × condition interaction (F[1, 43] = 8.88, p = .005). The effect of age was not significant (p = .900). Between-group comparisons showed no significant difference in the nonsocial condition (p = .577, d = .29) but a significant TD group advantage in the social condition (p = .007, d = .85). Within-group contrasts showed that, following training, the bias toward HV objects in the TD group in the social condition was stronger compared to the nonsocial condition (p = .007), but this bias was comparable between conditions in the ASD group (p = .164; Figure 3).
Figure 3.
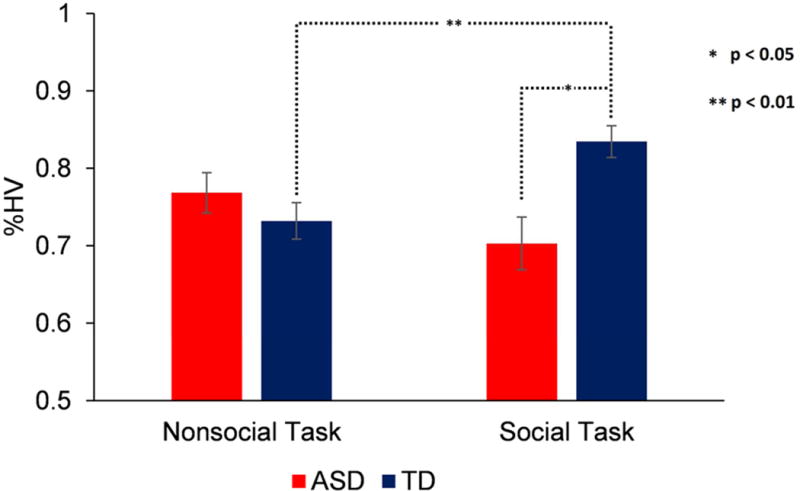
Average proportion of test trials during which the participants in the autism spectrum disorder (ASD) and typically developing (TD) groups selected the high-value objects (%HV) in the social and nonsocial conditions.
Inter-correlations between performance in social and nonsocial conditions
Within the ASD group, there was a significant correlation between performance on the social and nonsocial conditions (r[22] = .47, p = .025); the correlation was negligible in the TD group (r[24] = .086, p = .689; Figure 4). The pattern was unchanged when the correlation effects were partialled for effects of chronological age.
Figure 4.
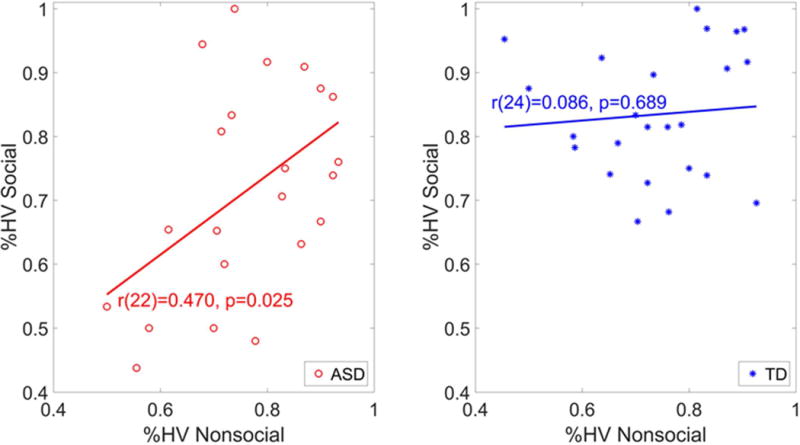
Correlation between learning in the social (faces) and nonsocial (fractals) conditions for the autism spectrum disorder (ASD) and typically developing (TD) groups.
Affective Responses
Subsequently, we evaluated whether the groups differed in their positive affective responses to when viewing faces (social condition) and fractals (nonsocial condition). On average, children with ASD exhibited positive affective responses on 8.1% (SD = 11) and 5.2% (SD = 11) of trials in the social and nonsocial conditions, respectively, compared to 8.3% (SD = 13) and 4.1% (SD = 5) in the TD group. A diagnosis × condition ANOVA on the %Smile variable indicated no effect of diagnosis (F[1, 45] = .13, p = .723), or condition (F[1, 45] = 2.16, p = .148), and no diagnosis × condition interaction (F[1, 45] = .120, p = .878), or effect of age (F[1, 45] = .24, p = .629). Although both groups exhibited somewhat higher rates of positive affective responses to the social than nonsocial condition, the differences were not statistically significant. There were also no significant correlations between %Smile and performance on the social and nonsocial conditions in either group. In the ASD group, the correlations were r(23) = −.083, p = .712 and r(23) = −.029, p =. 893, for the social and nonsocial conditions, respectively, and, in the TD group, they were r(24) = −.199, p = .349 and r(26) = −.267, p = .186. The pattern of results was unchanged when the correlation analysis was partialled for the effects of chronological age.
DISCUSSION
To the best of our knowledge, this is the first study to report on a selective impairment in flexible value learning for faces in preschoolers with ASD. As expected, children with ASD performed more poorly when learning values of faces compared to TD controls. The two groups did not differ on a task involving learning values of fractals, which suggests that children with ASD do not exhibit enhanced flexible value learning in nonsocial condition. Consistent with our hypothesis, value learning for faces compared to fractals was enhanced in TD children. In contrast, preschoolers with ASD performed comparably on face and fractal versions of the task. This study extends work by Lin et al.,50 indicating impaired value learning in children with ASD when both the objects and associated rewards are social and ecologically valid and intact value learning when both objects and rewards are drawn from the nonsocial domain. Importantly, social and nonsocial value learning were not correlated in the TD sample, suggesting that learning related to each domain may rely on at least partially distinct mechanisms. In the ASD group, however, performance in the two conditions was highly correlated, which suggests potential reliance on the shared mechanism for value learning of social and nonsocial objects in this group.
In the real-world environment, relative informational value of faces needs to be appraised rapidly, retained, and then updated as task requirements change. We propose that ASD may be characterized by an early disruption of the brain circuitry supporting value learning of social objects such as faces. Such disruption may prevent young children with ASD from learning the informational relevance of social stimuli. Consequently, looking at faces may offer less support with regard to guiding their attention and action and, ultimately, be less likely to drive behavior. The results also suggest that unlike in typical controls, in ASD, attention and learning are not augmented by the social nature of the stimuli. Specifically, it is not that children with ASD attend more poorly or learn less effectively in social than nonsocial conditions. Their performance on the fractal test was on par with TD controls, but they did not exhibit enhanced performance when faces are present the same way TD children did. At present, it is not clear what mechanism might be responsible for this pattern of results. We hypothesize that, when faces are concerned, in TD children, value learning typically subserved by the reward system of the brain may be augmented by the social brain circuitry. In children with ASD, the same pathway may not be fully functional, and thus social value learning may not be supported to the same extent as in unaffected individuals. Affected children may then rely primarily on nonsocial learning circuitry in all contexts, leading to diminished learning from social stimuli.
Importantly, despite showing less effective value learning for faces by young children with ASD, the frequency of positive affective responses to the face and fractal stimuli was comparable across groups, and there was no correlation between frequency of smiling during training and performance on test trials. The latter finding is consistent with evidence of behavioral, anatomical, and neurophysiological distinctions between hedonic (liking) and motivation (wanting) circuitry within the reward system in the brain.46,63 Although liking and wanting are typically tightly linked, they can be dissociated, as has been demonstrated in some disorders, including depression and schizophrenia.64 That is, affected individuals may be able to experience pleasure upon encountering social stimuli (liking) but may not be able to spontaneously pursue them (wanting).65 This interpretation aligns with findings suggesting relatively spared hedonic but impaired motivational components of the reward system in ASD.48,49 Further work is warranted to elucidate the relationships between affective, motivational, and learning components of reward system in ASD on a mechanistic level using behavioral, physiological, and neuroimaging measures.
During the face value learning task, attention of typically developing controls was enhanced compared to the fractal value learning task. No such enhancement was observed in preschoolers with ASD. Limited attention to tasks involving faces is consistent with numerous earlier findings,5,13,16 but this is the first report of less effective value learning within the social domain. Although one might conclude that limited attention to training leads to less effective value learning, the relationship between attention and learning in preschoolers with ASD appeared complex. Unlike in TD controls, the magnitude of the learning effect was not correlated with the number of completed training trials in children with ASD. That is, children with ASD who were more attentive during training did not necessarily learn more about the face-value associations. This is perhaps not surprising, for although attention gates learning, simply looking at faces does not guarantee that their essential features66 or associated value will be extracted, particularly if the mechanism underlying learning is compromised.
There were no associations between the ages of participants and value learning indices in either group. This finding suggests that deficits in value learning in ASD may emerge early in development. Indeed, limited attention toward faces has been reported within the first year of life, before most behavioral symptoms of ASD begin to emerge.13,16 Although it has been hypothesized that limited attention to faces in ASD may be due to impairments in the innate perceptual biases that prime TD newborns to detect and process faces faster and more effectively than other types of stimuli,67 support for this hypothesis has been limited.15,68,69 Instead, atypical attention to faces may result from an impaired ability to assign value to faces based on a history of interactions with the faces in real-life contexts. The discovery of the role of value learning in the emergence and maintenance of attentional atypicalities would be essential for elucidating the mechanisms driving social impairment in ASD and would be best addressed through examination of the developmental dynamics of the two systems through prospective studies of infants at risk for ASD.
Although no sex effects were found in the TD group, it will be necessary to examine if the results observed in males with ASD generalize to females affected by the disorder. There was also a significant difference between the ASD and TD groups in intellectual functioning. Though value learning was not correlated with nonverbal DQ in either group, the specificity of the observed effects in ASD versus other developmental disorders remains to be examined. Future larger studies should also evaluate potential contribution of comorbid attentional and affective factors to value learning in ASD. As described, all children first viewed the Fractal and then the Face version of the task. Of note, however, although both attention and performance on the Social version of the task was enhanced in TD controls, the ASD group performed at the same level in the social and nonsocial versions. Thus, it appears that performance differences were driven by facilitation of attention to faces in the TD group, rather than fatigue-driven waning of attention to faces in the ASD group. The hypothesis that children with ASD rely on the same mechanism for value learning in social and nonsocial domains will have to be verified through functional neuroimaging studies. Lastly, the present study examined flexible value learning in ASD, and is it not clear if the observed deficits extend to the ability to encode and retain long-term (stable) value information.
The current study tested a novel gaze-contingent value learning task modeled after a task previously developed and validated in primates.4,32 Results demonstrate for the first time domain-specific impairments in flexible value learning in young children with ASD. Such impairments may impact ability to select for processing the most relevant social stimuli in the context of rapidly changing contingencies and consequently, affect efficacy of social approaches and communicative bids. This important new lead should be investigated through prospective longitudinal studies of infants at risk for ASD, which would help determining if deficits in flexible value learning precede emergence of abnormal selective attention patterns and other symptoms in ASD. Moreover, future studies investigating the mechanistic role of value learning in social attention in ASD are warranted. The present data suggest that children with ASD may employ a shared mechanism for learning the values of social and nonsocial stimuli. This important new lead calls for investigation into neural networks supporting such learning in affected and unaffected individuals. The novel eye tracking paradigm employed in the present study also offers a new way for investigating learning in individuals across a range of chronological and mental ages, which is essential given concerns regarding generalizability of some of the findings in ASD literature across developmental epochs and cognitive phenotypes. Our work provides support for the social motivation hypothesis of autism,47 while highlighting the role of the value learning dysfunction in pathophysiology of ASD. Clinical implications of this work hinge on future discoveries of the role that value learning plays in emergence of autistic psychopathology. If the deficits in social value learning are seen in at-risk infants who later are diagnosed with ASD, social value learning may constitute an early biomarker for ASD-linked vulnerabilities. Should the mechanistic role of social value learning in development of social impairments be confirmed, value learning may constitute a new treatment target, with interventions to be implemented through, for example, implicit attention training protocols. Finally, a better understanding of the neural mechanisms underlying impaired social value learning in humans with ASD may open new avenues for reverse translational studies in genetic, molecular physiology, and drug discovery.
Clinical Guidance.
The study tests, for the first time, a novel task designed to investigate reward value learning of social (faces) and nonsocial (fractals) stimuli in young and disabled participants.
The study suggests that preschoolers with ASD may rely on a different neural mechanism to abstract information about reward value of social stimuli than typically developing controls and that their learning of reward value in the social but not in the non-social domains is impaired.
The findings shed light on mechanisms underlying impaired social attention in ASD and have important implications for identification of novel treatment targets.
Lay Summary.
The study documents, for the first time, that reward value learning in social domain is impaired in preschoolers with ASD. The study also suggests that preschoolers with ASD may rely on a different neural mechanism to abstract information about reward value of social stimuli than typically developing controls. The findings shed light on mechanisms underlying impaired social attention in ASD and have important implications for identification of novel treatment targets.
Acknowledgments
The study was supported by the National Institutes of Mental Health (R01MH100182) and the V & L Marx Foundation (to K.C.).
The authors wish to express our appreciation to the families and their children for their time and participation. They would also like to thank Kelly Powell, PhD, Scuddy Fontenelle, PhD, Megan Lyons, MS, Karyn Bailey, MSW, and Amy Giguere Carney, MSW, of Yale School of Medicine, for their contribution to sample characterization and Emily Hilton, BA, and Anna Milgramm, BA, of Yale School of Medicine, for their assistance in data collection and manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This article is discussed in an editorial by Dr. Paula Fitzpatrick on page xx.
Clinical guidance is available at the end of this article.
Drs. Wang and Chawarska served as the statistical experts for this research.
Disclosure
Drs. Wang, Hampson, Chawarska, and Mss. DiNicola and Heymann report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Drs. Quan Wang, Yale School of Medicine, Child Study Center, New Haven, CT.
Mss. Lauren DiNicola, Yale School of Medicine, Child Study Center, New Haven, CT.
Mss. Perrine Heymann, Yale School of Medicine, Child Study Center, New Haven, CT.
Drs. Michelle Hampson, Yale School of Medicine, Child Study Center, New Haven, CT.
Drs. Katarzyna Chawarska, Yale School of Medicine, Child Study Center, New Haven, CT.
References
- 1.Psychiatric Association A. Diagnostic and Statistical Manual of Mental Disorders: DSM 5. 5th. Washington, DC: Author; 2013. [Google Scholar]
- 2.Gottlieb J, Hayhoe M, Hikosaka O, Rangel A. Attention, Reward, and Information Seeking. The Journal of Neuroscience. 2014;34(46):15497–15504. doi: 10.1523/JNEUROSCI.3270-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tatler BW. Eye Movements from Laboratory to Life. In: Horsley M, Toon N, Knight BA, Reilly R, editors. Current Trends in Eye Tracking Research. Switzerland: Springer; 2014. pp. 17–35. [Google Scholar]
- 4.Hikosaka O, Nakamura K, Nakahara H. Basal ganglia orient eyes to reward. Journal of Neurophysiology. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- 5.Chawarska K, Macari S, Shic F. Context modulates attention to social scenes in toddlers with autism. Journal of Child Psychology and Psychiatry. 2012;53(8):903–913. doi: 10.1111/j.1469-7610.2012.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shic F, Bradshaw J, Klin A, Scassellati B, Chawarska K. Limited activity monitoring in toddlers with autism spectrum disorder. Brain Research. 2011;1380:246–254. doi: 10.1016/j.brainres.2010.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Hofsten C, Uhlig H, Adell M, Kochukhova O. How children with autism look at events. Research in Autism Spectrum Disorders. 2009;3(2):556–569. [Google Scholar]
- 8.Bedford R, Elsabbagh M, Gliga T, et al. Precursors to Social and Communication Difficulties in Infants At-Risk for Autism: Gaze Following and Attentional Engagement. Journal of Autism and Developmental Disorders. 2012:1–11. doi: 10.1007/s10803-012-1450-y. Online First. [DOI] [PubMed] [Google Scholar]
- 9.Pierce K, Conant D, Hazin R, Stoner R, Desmond J. Preference for geometric patterns early in life as a risk factor for autism. Archives of General Psychiatry. 2011;68(1):101–109. doi: 10.1001/archgenpsychiatry.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falck-Ytter T, Thorup E, Bölte S. Brief Report: Lack of Processing Bias for the Objects Other People Attend to in 3-Year-Olds with Autism. J Autism Devel Disord. 2014;45:1897–1904. doi: 10.1007/s10803-014-2278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawarska K, Ye S, Shic F, Chen L. Multilevel Differences in Spontaneous Social Attention in Toddlers With Autism Spectrum Disorder. Child Development. 2016;87:543–557. doi: 10.1111/cdev.12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones W, Carr K, Klin A. Absence of preferential looking to the eyes of approaching adults predicts level of social disability in 2-year-old toddlers with autism spectrum disorder. Archives of General Psychiatry. 2008;65:946–954. doi: 10.1001/archpsyc.65.8.946. [DOI] [PubMed] [Google Scholar]
- 13.Shic F, Macari S, Chawarska K. Speech disturbs face scanning in 6-month-old infants who develop autism spectrum disorder. Biological Psychiatry. 2014;75:231–237. doi: 10.1016/j.biopsych.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones E, Venema K, Earl R, et al. Reduced engagement with social stimuli in 6-month-old infants with later autism spectrum disorder: a longitudinal prospective study of infants at high familial risk. Journal of neurodevelopmental disorders. 2016;8(1):1. doi: 10.1186/s11689-016-9139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones W, Klin A. Attention to eyes is present but in decline in 2–6-month-old infants later diagnosed with autism. Nature. 2013;504(7480):427–431. doi: 10.1038/nature12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chawarska K, Macari S, Shic F. Decreased Spontaneous Attention to Social Scenes in 6-Month-Old Infants Later Diagnosed with Autism Spectrum Disorders. Biol Psychiatry. 2013;74:195–203. doi: 10.1016/j.biopsych.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baranek GT. Autism during infancy: A retrospective video analysis of sensory-motor and social behaviors at 9–12 months of age. J Autism Devel Disord. 1999;29:213–224. doi: 10.1023/a:1023080005650. [DOI] [PubMed] [Google Scholar]
- 18.Chawarska K, Macari SL, Volkmar FR, et al. ASD in Infants and Toddlers. In: Volkmar FR, Rogers SJ, Paul R, Pelphrey KA, editors. Handbook of Autism and Pervasive Developmental Disorders. Fourth. Hoboken, NJ: John Wiley and Sons, Inc.; 2014. [Google Scholar]
- 19.Pierce K, Marinero S, Hazin R, McKenna B, Barnes CC, Malige A. Eye tracking reveals abnormal visual preference for geometric images as an early biomarker of an autism spectrum disorder subtype associated with increased symptom severity. Biological psychiatry. 2016;79:657–666. doi: 10.1016/j.biopsych.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blakemore SJ, Decety J. From the perception of action to the understanding of intention. Nature Reviews Neuroscience. 2001;2(8):561–567. doi: 10.1038/35086023. [DOI] [PubMed] [Google Scholar]
- 21.Frischen A, Bayliss AP, Tipper SP. Gaze cueing of attention: Visual attention, social cognition, and individual differences. Psychological Bulletin. 2007;133:694–724. doi: 10.1037/0033-2909.133.4.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tomasello M. Culture and cognitive development. Curr Directions Psychol Sci. 2000;9:37–40. [Google Scholar]
- 23.Sebanz N, Bekkering H, Knoblich G. Joint action: Bodies and minds moving together. Trends in Cognitive Sciences. 2006;10:71–76. doi: 10.1016/j.tics.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 25.Campbell DJ, Shic F, Macari S, Chawarska K. Gaze response to dyadic bids at 2 years related to outcomes at 3 years in autism spectrum disorders: a subtyping analysis. Journal of Autism and Developmental Disorders. 2014;44:431–442. doi: 10.1007/s10803-013-1885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsabbagh M, Bedford R, Senju A, Charman T, Pickles A, Johnson MH. What you see is what you get: contextual modulation of face scanning in typical and atypical development. Social Cognitive and Affective Neuroscience. 2014;9(4):538–543. doi: 10.1093/scan/nst012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norbury CF. Sources of variation in developmental language disorders: evidence from eye-tracking studies of sentence production. Philosophical Transactions of the Royal Society B: Biological Sciences. 2014;369:20120393. doi: 10.1098/rstb.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klin A, Lin DJ, Gorrindo P, Ramsay G, Jones W. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasson NJ, Touchstone EW. Visual attention to competing social and object images by preschool children with autism spectrum disorder. J Autism Devel Disord. 2014;44:584–592. doi: 10.1007/s10803-013-1910-z. [DOI] [PubMed] [Google Scholar]
- 30.Hikosaka O, Kim HF, Yasuda M, Yamamoto S. Basal ganglia circuits for reward value-guided behavior. Annual review of neuroscience. 2014;37:289. doi: 10.1146/annurev-neuro-071013-013924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamamoto S, Kim HF, Hikosaka O. Reward value-contingent changes of visual responses in the primate caudate tail associated with a visuomotor skill. J Neurosci. 2013;33:11227–11238. doi: 10.1523/JNEUROSCI.0318-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim HF, Hikosaka O. Distinct basal ganglia circuits controlling behaviors guided by flexible and stable values. Neuron. 2013;79:1001–1010. doi: 10.1016/j.neuron.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Awh E, Belopolsky AV, Theeuwes J. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends in cognitive sciences. 2012;16:437–443. doi: 10.1016/j.tics.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 35.Bourgeois A, Chelazzi L, Vuilleumier P. How motivation and reward learning modulate selective attention. Progress in Brain Research. 2016;229:325–342. doi: 10.1016/bs.pbr.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Shepherd SV, Deaner RO, Platt ML. Social status gates social attention in monkeys. Current Biology. 2006;16(4):R119–R120. doi: 10.1016/j.cub.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Current Biology. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 38.Engelmann JB, Damaraju E, Padmala S, Pessoa L. Combined effects of attention and motivation on visual task performance: transient and sustained motivational effects. Front Hum Neurosci. 2009;3:4. doi: 10.3389/neuro.09.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson BA. The attention habit: how reward learning shapes attentional selection. Annals of the New York Academy of Sciences. 2016;1369:24–39. doi: 10.1111/nyas.12957. [DOI] [PubMed] [Google Scholar]
- 40.Della Libera C, Chelazzi L. Learning to attend and to ignore is a matter of gains and losses. Psychological Science. 2009;20:778–784. doi: 10.1111/j.1467-9280.2009.02360.x. [DOI] [PubMed] [Google Scholar]
- 41.Anderson BA, Laurent PA, Yantis S. Reward predictions bias attentional selection. Front Hum Neurosci. 2013;7:262. doi: 10.3389/fnhum.2013.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yasuda M, Hikosaka O. Functional territories in primate substantia nigra pars reticulata separately signaling stable and flexible values. J Neurophysiol. 2015;113:1681–1696. doi: 10.1152/jn.00674.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current opinion in neurobiology. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 44.Rudebeck PH, Saunders RC, Prescott AT, Chau LS, Murray EA. Prefrontal mechanisms of behavioral flexibility, emotion regulation and value updating. Nat Neurosci. 2013;16:1140–1145. doi: 10.1038/nn.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chudasama Y, Daniels TE, Gorrin DP, Rhodes SE, Rudebeck PH, Murray EA. The role of the anterior cingulate cortex in choices based on reward value and reward contingency. Cerebral Cortex. 2013;23:2884–2898. doi: 10.1093/cercor/bhs266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller EM, Shankar MU, Knutson B, McClure SM. Dissociating motivation from reward in human striatal activity. J Cogn Neurosci. 2014;26:1075–1084. doi: 10.1162/jocn_a_00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chevallier C, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in Cognitive Sciences. 2012;16:231–239. doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohls G, Schulte-Rüther M, Nehrkorn B, et al. Reward system dysfunction in autism spectrum disorders. Social Cognitive and Affective Neuroscience. 2012;8:565–572. doi: 10.1093/scan/nss033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW. Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci. 2012;7:160–172. doi: 10.1093/scan/nsq095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin A, Adolphs R, Rangel A. Impaired learning of social compared to monetary rewards in autism. Frontiers in neuroscience. 2012;6:143. doi: 10.3389/fnins.2012.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Solomon M, Frank MJ, Ragland JD, et al. Feedback-driven trial-by-trial learning in autism spectrum disorders. American Journal of Psychiatry. 2015;172:173–181. doi: 10.1176/appi.ajp.2014.14010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto S, Monosov IE, Yasuda M, Hikosaka O. What and where information in the caudate tail guides saccades to visual objects. J Neurosci. 2012;32:11005–11016. doi: 10.1523/JNEUROSCI.0828-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim SH, Macari S, Koller J, Chawarska K. Examining the phenotypic heterogeneity of early autism spectrum disorder: subtypes and short-term outcomes. J Child Psychol Psychiatry. 2016;57:93–102. doi: 10.1111/jcpp.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chawarska K, Klin A, Paul R, Macari S, Volkmar F. A prospective study of toddlers with ASD: short-term diagnostic and cognitive outcomes. J Child Psychol Psychiatry. 2009;50:1235–1245. doi: 10.1111/j.1469-7610.2009.02101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guthrie W, Swineford LB, Nottke C, Wetherby AM. Early diagnosis of autism spectrum disorder: stability and change in clinical diagnosis and symptom presentation. Journal of Child Psychology and Psychiatry. 2013;54:582–590. doi: 10.1111/jcpp.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozonoff S, Young GS, Landa RJ, et al. Diagnostic stability in young children at risk for autism spectrum disorder: a baby siblings research consortium study. J Child Psychol Psychiatry. 2015;56:988–998. doi: 10.1111/jcpp.12421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lord C, Risi S, DiLavore PS, Shulman C, Thurm A, Pickles A. Autism From 2 to 9 Years of Age. Archives of General Psychiatry. 2006;63:694–701. doi: 10.1001/archpsyc.63.6.694. [DOI] [PubMed] [Google Scholar]
- 58.Draves S. The electric sheep screen-saver: A case study in aesthetic evolution. Applications on Evolutionary Computing. 2005;3449:458–467. [Google Scholar]
- 59.Zhang X, Yin L, Cohn J, et al. BP4D-Spontaneous: A high resolution spontaneous 3D dynamic facial expression database. Image and Vision Computing. 2014;32:692–670. special issue of the Best of FG13. [Google Scholar]
- 60.Redi JA, Hoßfeld T, Korshunov P, Mazza F, Povoa I, Keimel C. Crowdsourcing-based multimedia subjective evaluations: a case study on image recognizability and aesthetic appeal. Proceedings of the 2nd ACM international workshop on Crowdsourcing for multimedia; Barcelona, Spain. 2013. pp. 29–34. [Google Scholar]
- 61.Itti L. Quantifying the contribution of low-level saliency to human eye movements in dynamic scenes. Visual Cognition. 2005;12:1093–1123. [Google Scholar]
- 62.Celebi FM, Kim ES, Wang Q, Wall CA, Shic FA. Smooth Pursuit Calibration Technique. Proceedings of the Symposium on Eye Tracking Research and Applications; Safety Harbor, FL. 2014. pp. 375–376. [Google Scholar]
- 63.Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- 64.Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends in neurosciences. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: the role of prefrontal–striatal interactions. Schizophrenia Bulletin. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chawarska K, Shic F. Looking but not seeing: Atypical visual scanning and recognition of faces in 2 and 4-year-old children with autism spectrum disorder. J Autism Devel Disord. 2009;39:1663. doi: 10.1007/s10803-009-0803-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnson MH. Subcortical face processing. Nat Rev Neurosci. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- 68.Johnson MH. Autism: demise of the innate social orienting hypothesis. Curr Biol. 2014;24:R30–1. doi: 10.1016/j.cub.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 69.Chawarska K, Klin A, Volkmar F. Automatic attention cueing through eye movement in 2-year-old children with Autism. Child development. 2003;74:1108–1122. doi: 10.1111/1467-8624.00595. [DOI] [PubMed] [Google Scholar]


