The number of phosphorylated sites on transcription factor SOG1 affects the strength of various DNA damage responses including DNA repair, replication arrest, cell death, and cell differentiation.
Abstract
The Arabidopsis thaliana transcription factor SUPPRESSOR OF GAMMA RESPONSE1 (SOG1) regulates hundreds of genes in response to DNA damage, and this results in the activation of cell cycle arrest, DNA repair, endoreduplication, and programmed cell death. However, it is not clear how this single transcription factor regulates each of these pathways. We previously reported that phosphorylation of five Ser-Gln (SQ) motifs in the C-terminal region of SOG1 are required to activate downstream pathways. In this study, we introduced Ser-to-Ala (AQ) substitutions in these five SQ motifs to progressively eliminate them and then we examined the effects on DNA damage responses. We found that all SQs are required for the full activation of SOG1 and that the expression level of most downstream genes changed incrementally depending on the number of phosphorylated SQ sites. Genes involved in DNA repair and cell cycle progression underwent stepwise activation and inhibition respectively as the number of phosphorylated SQ sites increased. Also, inhibition of DNA synthesis, programmed cell death, and cell differentiation were incrementally induced as the number of phosphorylated SQ sites increased. These results show that the extent of SQ phosphorylation in SOG1 regulates gene expression levels and determines the strength of DNA damage responses.
INTRODUCTION
Because of a sessile lifestyle, plants cannot escape from environmental stresses such as drought, temperature extremes, and high light intensity. These stresses induce production of reactive oxygen species (ROS), which cause DNA damage (Roldán-Arjona and Ariza, 2009). Furthermore, although sunlight is required by plants to convert solar energy to chemical energy in chloroplasts via photosynthesis, UV light from sunlight constantly damages the genome of plant cells. In addition, ROS are generated during photosynthesis itself (Foyer and Shigeoka, 2011). During photosynthesis, energy from sunlight is transferred to photosystems, but it is not uncommon that leakage of electron to O2 results in the generation of ROS (Cruz de Carvalho, 2008). Other DNA-damaging agents in plants include aluminum and boron from soil (Sakamoto et al., 2011; Nezames et al., 2012; Sjogren et al., 2015). Because of these stressors and the inability of plants to avoid them, their genomic DNA is constantly exposed to greater DNA damage compared with animals. To cope with this, plants have developed an efficient system to counteract various kinds of DNA damage. When genomic DNA of plants suffers DNA damage, it is detected by sensor proteins and the signal is transduced to the key kinases ATM (ATAXIA-TELANGIECTASIA) and ATR (ATM- and RAD3-RELATED). These kinases phosphorylate downstream factors that determine and trigger the final response pathways whether it is cell cycle arrest, DNA repair, endoreduplication, or programmed cell death. This highly coordinated system is called the DNA damage response (DDR) (Garcia et al., 2003; Culligan et al., 2004; Fulcher and Sablowski, 2009; Adachi et al., 2011).
When genomic DNA in Arabidopsis thaliana is threatened by an agent that induces DNA double-strand breaks (DSBs), such as gamma irradiation or exposure to zeocin, the most remarkable response is a rapid and robust transcriptional regulation of numerous genes (Culligan et al., 2006; Yoshiyama et al., 2009; Adachi et al., 2011). The expression of hundreds of genes is changed, and this transcriptional response is primarily governed by a single transcription factor known as SUPPRESSOR OF GAMMA RESPONSE1 (SOG1) (Yoshiyama et al., 2009). Because several other transcription factors are downstream targets of SOG1, SOG1 both directly and indirectly regulates hundreds of genes and induces a broad cascade of transcriptional responses. In a SOG1 mutant line, cell cycle arrest, DNA repair, endoreduplication, and programmed cell death are all compromised, indicating that SOG1 has an essential role in regulating these response pathways (Yoshiyama et al., 2009; Furukawa et al., 2010; Adachi et al., 2011). Numerous response pathways are regulated by SOG1, but how this single transcription factor regulates each pathway is unclear.
Posttranslational modifications of transcription factors have crucial roles in the regulation of downstream gene expression. These modifications include phosphorylation, methylation, acetylation, ubiquitination, SUMOylation, and glycosylation (Meek and Anderson, 2009). Protein phosphorylation as a crucial regulatory mechanism has been extensively studied (Hunter and Karin, 1992; Whitmarsh and Davis, 2000). Previously, we found that, although the level of SOG1 transcription and amount of protein is unchanged in response to DNA damage (Yoshiyama et al., 2013), SOG1 activity is regulated by phosphorylation (Yoshiyama et al., 2013). SOG1 has five Ser-Gln (SQ) motifs in its C-terminal transcription regulatory domain, and the Ser residues in these SQ motifs are phosphorylated by an ATM kinase in response to DNA damage. Furthermore, SOG1 and ATR are required for response to aluminum toxicity, and SOG1 is phosphorylated by recombinant ATR in vitro (Sjogren et al., 2015).
These results indicate that SOG1 is activated by both ATM and ATR via phosphorylation. SQ phosphorylation is indispensable for SOG1 function, and the extent of phosphorylation is dependent on the severity of the DSB inducer (Yoshiyama et al., 2013). However, it is not known whether all five SQ motifs are required for activation of SOG1 function. The finding that a SOG1-like protein found in a gymnosperm has only three of these five SQ motifs at conserved sites (Yoshiyama et al., 2014; Yoshiyama, 2016) suggests that three SQ motifs are important to exert full SOG1 functions in Arabidopsis. Furthermore, we hypothesized that the specific pattern of SQ phosphorylation determines which DDR pathway is activated. To investigate the relationship between SQ phosphorylation and DDR activation, we introduced mutations at SQ motifs (SQ to AQ) and generated SOG1 mutant lines with a differing number of SQ phosphorylation sites. We found that increasing the number of SQ phosphorylation sites in SOG1 changes the expression level of downstream genes and strengthens DNA damage responses in Arabidopsis.
RESULTS
Phosphorylation Sites of SOG1 Are Determined in Response to DNA Damage
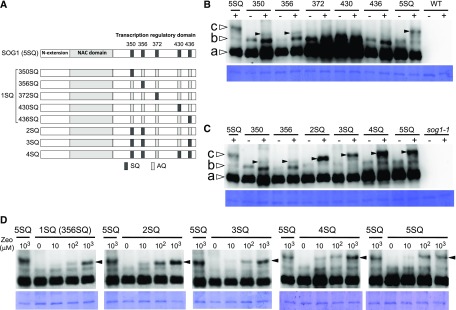
Arabidopsis SOG1 contains three functional domains: the N-terminal extension, the central NAC (NAM, ATAF1/2, and CUC2) domain, and the C-terminal transcription regulatory domain (Figure 1A) (Yoshiyama, 2016). The central NAC domain is required for recognition and DNA binding to the promoters of SOG1 target genes (Jensen et al., 2010; Sjogren et al., 2015). SOG1 has five SQ motifs in its C-terminal region, and their phosphorylation is essential to activate SOG1 function (Yoshiyama et al., 2013). To determine which SQ phosphorylations are required for SOG1 function, we generated five Arabidopsis sog1-1 transgenic plants expressing ProSOG1:SOG1(1SQ)-Myc (Figure 1A), in which the promoter and coding regions of SOG1 (Ser-to-Ala substitutions [AQ] at four of the five SQ motifs) are fused in-frame to the 10xMyc tag. The 10xMyc tag does not affect SOG1 function; we previously confirmed that the SOG1-10xMyc protein is functional (Yoshiyama et al., 2013). Because sog1-1 was originally isolated in the Landsberg erecta (Ler) background and the sog1-1 mutant used in this study was backcrossed at least six times to Columbia (Col), the Col/Ler hybrid effect is minimally suppressed in this sog1-1 line.
Figure 1.
Structural Features and Phosphorylation Pattern of SOG1 Mutants.
(A) Structural features of SOG1. The N-terminal extension, NAC domain, and transcription regulatory domain are shown. The five Ser-Gln (SQ) motifs are represented by dark-gray boxes, and the mutated (to Ala-Gln) AQ motifs are represented by light-gray boxes.
(B) and (C) Detection of phosphorylated forms of SOG1. sog1-1 lines harboring ProSOG1:SOG1[1SQs (350SQ, 356SQ, 372SQ, 430SQ, or 436SQ)]-Myc (B) and sog1-1 lines harboring ProSOG1:SOG1[2SQ, 3SQ, 4SQ, and 5SQ]-Myc (C) were used. Five-day-old seedlings grown on MS plates were transferred to liquid medium with (+) or without (−) 1 mM zeocin, and total protein was extracted 1 h later. Phosphorylated forms of SOG1 were detected using an SDS-PAGE gel containing Phos-tag. Coomassie blue staining is shown below. Nonphosphorylated, phosphorylated, and hyperphosphorylated SOG1-Myc (bands a, b, and c, respectively) observed in wild-type SOG1 are indicated by white arrowheads. Hyperphosphorylated SOG1-Myc observed in phosphorylated SOG1 mutants are indicated by black arrowheads. As negative controls, wild-type (B) or sog1-1 plants (C) with no SOG1-Myc were loaded on the right side of the gel.
(D) Effect of zeocin concentration on phosphorylation of SQ sites in SOG1. This experiment was conducted similarly to the ones shown in (B) and (C), using a variety of zeocin concentrations (0, 10, 100, and 1000 μM). Black arrowheads indicate hyperphosphorylated SOG1-Myc.
Zeocin is a radiomimetic reagent that causes DSBs similar to those caused by radiation (Huang et al., 1981). We previously reported that the amount of SOG1 phosphorylation is dependent on the concentration of zeocin, but the phosphorylation pattern was not changed by the zeocin concentration. When wild-type 5-d-old seedlings treated with 100 or 1000 μM zeocin for 1 h were allowed to recover by transferring the seedlings to Murashige and Skoog (MS) plates, we found that root growth partially recovered after exposure to 100 μM zeocin but not after 1000 μM zeocin treatment (Supplemental Figure 1A). Furthermore, using a comet assay, we found that the number of DSBs increased as the zeocin concentration increased (Supplemental Figure 1B).
Because these results showed that 1000 μM zeocin confers a high level of DNA damage, we used 1 mM zeocin to clearly observe SOG1 phosphorylation. 1SQ seedlings [sog1-1/SOG1(1SQ)-Myc] were treated for 1 h with 1000 μM zeocin and total protein was extracted from root tips and immunoblotted with an anti-Myc antibody. As we previously reported, in addition to the SOG1 band (Figure 1B, band a, white arrowhead), an anti-Myc antibody detected a more slowly migrating band (Figure 1B, band b, white arrowhead) in untreated plants, representing phosphorylated SOG1 in a DNA damage-independent manner (Yoshiyama et al., 2013). We previously reported that this DNA damage-independent phosphorylation of SOG1 (appearing as band b in Figure 1B) does not involve the SQ motifs because band b was also detected in SOG1(5AQ)-Myc plants, which have Ser-to-Ala substitutions at all five SQ motifs (Yoshiyama et al., 2013). In plants treated with zeocin, sog1-1/SOG1(350SQ) and sog1-1/SOG1(356SQ), we observed a third band, which was an even more slowly migrating band (Figure 1B, band c, black arrowhead), suggesting that SOG1 was phosphorylated in a DNA damage-dependent manner. However, the band position was different from that observed in wild-type SOG1 (Figure 1B, band c, white arrowhead). A third band was not found in the other 1SQ lines (372SQ, 430SQ, and 436SQ). These immunoblotting results showed that the SOG1 phosphorylation sites that are important in the response to DNA damage were 350SQ and 356SQ.
Next, we transformed the sog1-1 mutant with SOG1 (2SQ), which included both 350SQ and 356SQ (Figure 1A), to determine whether 350SQ and 356SQ are sufficient to show the same phosphorylation pattern observed in wild-type SOG1(5SQ). In the 2SQ line, the DNA damage-dependent band c (Figure 1C, band c, black arrowhead) migrated more slowly compared with those observed in the 350SQ or 356SQ lines, but still migrated more rapidly than that found in wild-type SOG1(5SQ). This finding indicates that phosphorylation of 2SQ (350SQ and 356SQ) was less than the phosphorylation with wild-type SOG1(5SQ). We previously reported that 350SQ, 356SQ, and 436SQ are well conserved among SOG1-like proteins found from angiosperms to gymnosperms, but 372SQ and 430SQ are not conserved in gymnosperms (Yoshiyama et al., 2014). Based on these findings, we hypothesized that 350SQ, 356SQ, and 436SQ are the most important SQ sites to exert full SOG1 function. Therefore, we constructed a sog1-1 mutant with SOG1 (3SQ), which included 350SQ, 356SQ, and 436SQ, and another mutant SOG1 (4SQ), which included 350SQ, 356SQ, 430SQ, and 436SQ, transgenic lines (Figure 1A). In zeocin-treated 3SQ and 4SQ lines, new phosphorylated bands appeared (Figure 1C, black arrowhead). Because phosphorylation of 372SQ, 430SQ, and 436SQ was not observed in 1SQ lines (Figure 1B), these results indicated that phosphorylation of these sites was not occurring independently. Furthermore, the phosphorylation of 372SQ, 430SQ, and 436SQ appears to require the presence of 350SQ and 356SQ. Taken together, we conclude from these findings that 350SQ and 356SQ are phosphorylated first and that their phosphorylation triggers the phosphorylation of other SQ sites.
We then speculated that the pattern of phosphorylation sites of SOG1 was affected by the amount of DNA damage. For instance, if there were minimal DNA damage, we posit that few SOG1 SQ sites would be phosphorylated, but if there was significant DNA damage, more SQ sites would be phosphorylated. To examine the possibility, the phosphorylation pattern of each mutant was evaluated at different zeocin concentrations. We found that band c (Figure 1D, black arrowhead), which is DNA damage dependent, appeared following 10 μM zeocin treatment in each mutant and that the band intensity incrementally increased in a dose-dependent manner with zeocin concentrations from 10 to 1000 μM. However, we found no differences in the band pattern among phosphorylation mutants (Figure 1D). These results suggested that although increases in the amount of phosphorylated SOG1 were dependent on the extent of DNA damage in any of the mutant lines, there was no relationship between the amount of DNA damage and the number of SOG1 phosphorylation sites.
The Expression Level of DNA Repair Genes Is Dependent on the Number of Phosphorylated SQ Motifs in SOG1
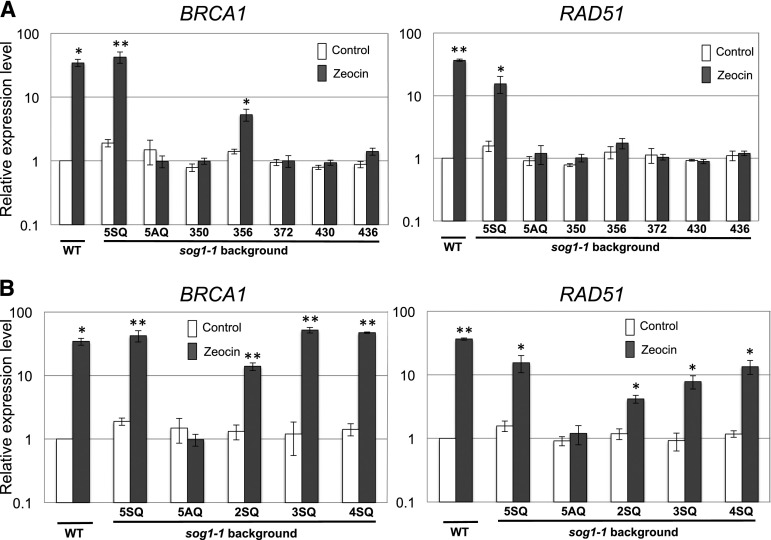
To clarify the relationship between the regulation of DNA repair genes and SOG1 phosphorylation, the expression of DNA repair genes was examined in various SOG1 SQ mutants using qRT-PCR. We used both BRCA1 (BREAST CANCER SUSCEPTIBILITY GENE1), which is involved in DSB repair, and RAD51 (RADIATION SENSITIVE 51), which is an essential component of homologous recombination, because both of these genes are induced in response to DNA damage and their expression is dependent on SOG1 (Culligan et al., 2006; Yoshiyama et al., 2009). BRCA1 is a direct target of SOG1 (Sjogren et al., 2015). However, it is not known whether RAD51 is a direct or indirect target of SOG1. Each SOG1 mutant seedling was treated with 100 μM zeocin for 2 h, and total RNA was extracted from root tips. As previously reported, transcriptional induction of BRCA1 and RAD51 due to zeocin treatment was restored in sog1-1 carrying SOG1(5SQ)-Myc but not SOG1(5AQ)-Myc (Figure 2A) (Yoshiyama et al., 2013). With respect to the five 1SQ SOG1 mutants, only the 356SQ line showed a slight induction of BRCA1 by zeocin. However, the extent of induction was not as high as that found in wild-type SOG1 (5SQ) (Figure 2A). No other 1SQ lines (350SQ, 372SQ, 430SQ, or 436SQ) showed induction of BRCA1 and RAD51 by zeocin treatment. Although 350SQ lines showed no induction of BRCA1 and RAD51, we posit that 350SQ and 356SQ are required to promote phosphorylation of other SQ sites to fully induce expression of DNA repair genes because not only 356SQ but also 350SQ was phosphorylated in response to DNA damage (Figure 1B). In the case of the 2SQ, 3SQ, and 4SQ lines, we found that the expression of these two genes increased as the number of phosphorylated SQ sites increased (Figure 2B). These findings indicate that the expression level of DNA repair genes is controlled by the number of phosphorylation sites of SOG1 and that phosphorylation of 350SQ and 356SQ triggers subsequent phosphorylation of other SQ sites in SOG1.
Figure 2.
qRT-PCR Quantification of BRCA1 and RAD51 Expression in Roots of Phosphorylated SOG1 Mutants.
Expression of BRCA1 and RAD51 in the wild type and sog1-1 harboring various transgene SOG1s. Seedlings (5 d old) were either treated with 100 μM zeocin or left untreated and then harvested for RNA preparation 2 h later. All values are normalized to the expression level of EIF4A-1. Results are reported as the mean relative to the expression level in the untreated wild-type sample. Error bars indicate se for biological triplicates. Asterisks indicate significance at *P < 0.05 or **P < 0.01 versus control (untreated wild type) using a one-tailed Student’s t test.
Expression of Most Target Genes Incrementally Increased or Decreased as the Number of SQ Sites Increased
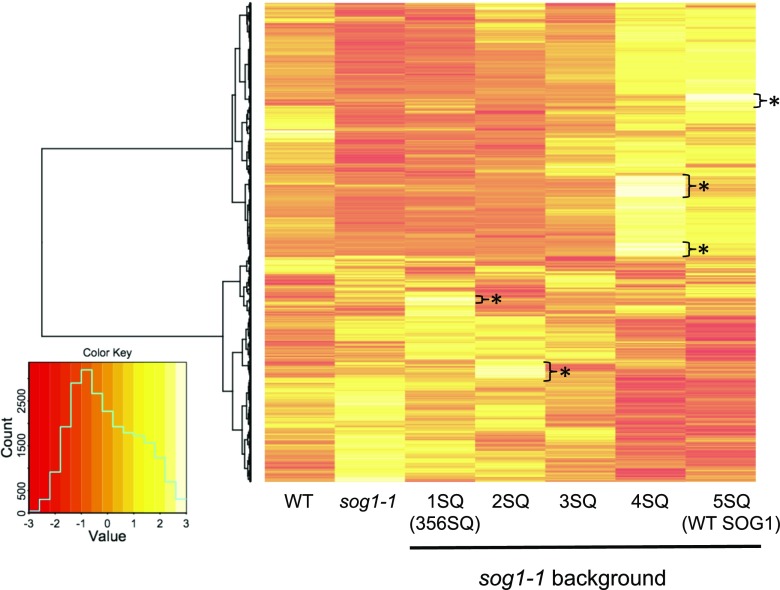
Based on our qRT-PCR results that the DNA repair genes BRCA1 and RAD51 are incrementally induced as the number of SOG1 phosphorylation sites increase, and because SOG1 regulates hundreds of genes in response to DNA damage, we next investigated how the number of phosphorylated SQ motifs affects the overall transcriptional regulation. RNA-seq analysis was performed with seven lines [the wild type, sog1-1, sog1-1 with 1SQ (356SQ), 2SQ, 3SQ, 4SQ, and 5SQ]. RNA was extracted from root tips of plants either treated with 100 μM zeocin for 2 h or left untreated. To analyze expression changes triggered by DNA damage, a set of differentially expressed genes (DEGs) derived from each line was selected according to their significance in fold change expression (false discovery rate [FDR] < 0.01) relative to the respective control. Heat map analysis showed that there was a large overlap between the DEGs of phosphorylated mutants and that expression of most genes incrementally increased or decreased as the number of SQ sites increased (Figure 3). Hierarchical clustering analysis also strongly supported the above conclusion; the expression data of seven samples with a similar number of SQ phosphorylation sites were closely grouped (Supplemental Figure 2A). Furthermore, this cluster analysis showed that the expression pattern of the wild type (no SOG1 transgene) was not similar to that of sog1-1/SOG1 (5SQ), but rather was similar to the expression pattern of sog1-1/SOG1 (3SQ). Because the SOG1 transcript level in sog1-1/SOG1 (5SQ) was 7 times higher than in the wild type (Supplemental Figure 2B), it is possible that the expression level of downstream targets in 5SQ lines is higher than in the wild type. Also, there are genes whose expression was induced in a specific phosphorylated mutant (1SQ, 2SQ, 4SQ, and 5SQ) (Figure 3, single asterisk). These observations indicate that certain SOG1 phosphorylation patterns induce this group of genes.
Figure 3.
Changes in Expression of Genes in the SOG1 Phosphorylation Mutants in Response to DNA Damage.
Heat map showing the DEGs between control and zeocin treatment. Columns and rows in the heat map represent samples and genes, respectively. A color key and density plot (green line) are provided in the graphic shown at the bottom left. The gradient color scale indicates the relative expression level. Results of hierarchal clustering are shown in the right part of the heat map. Asterisks in the map show genes whose expression is induced only in 1SQ, 2SQ, 4SQ, and 5SQ.
Genes Involved in DNA Repair and Cell Cycle Inhibition Are Induced as the Number of SQs Increases
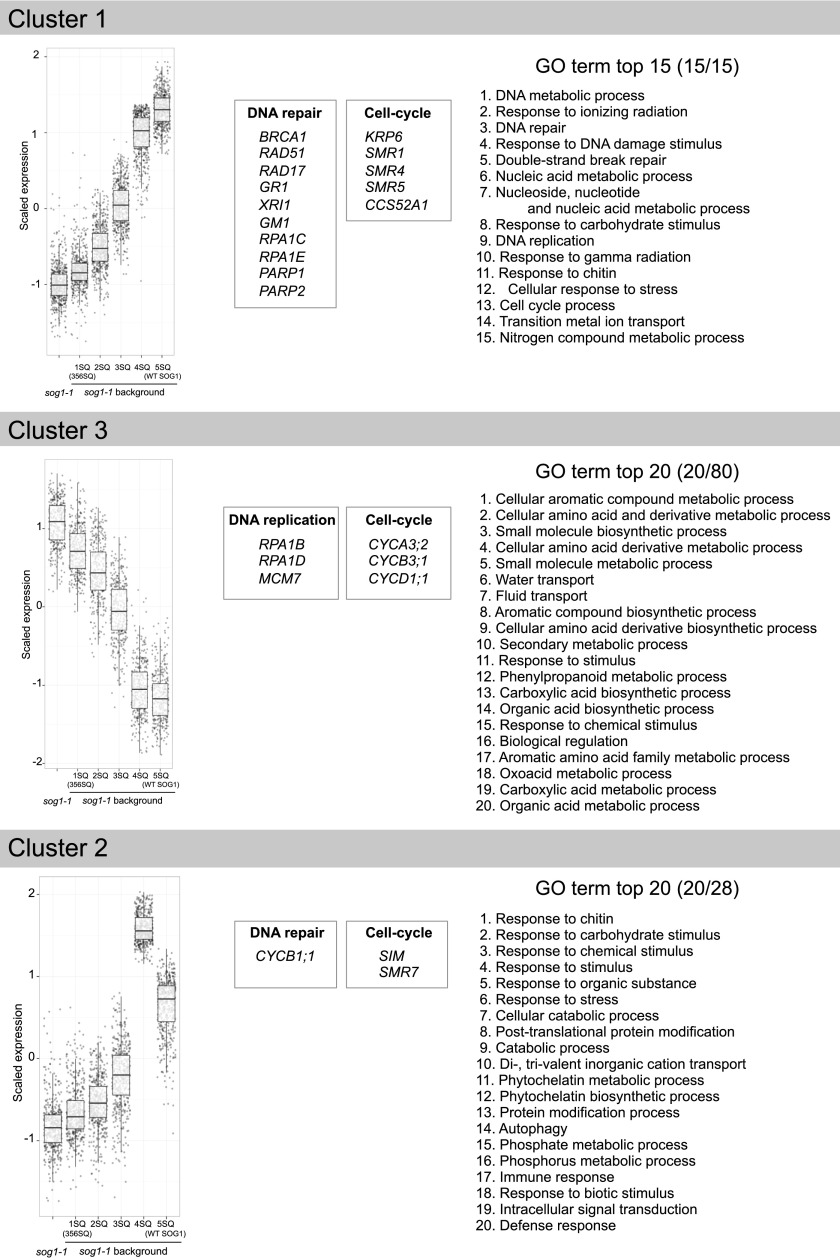
To facilitate cluster analysis of gene expression, the expression profiles of DEGs were determined by self-organizing maps (SOM) cluster analysis. In these experiments, we used sog1-1 and transgenic plants [sog1-1 with 1SQ (356SQ), 2SQ, 3SQ, 4SQ, or 5SQ] to examine the effects of the number of phosphorylated SQ sites on transcriptional response. We found that the expression of 2759 DEGs was significantly changed by zeocin treatment in at least one of the seven lines (Supplemental Data Set 1) and that all DEGs were divided into 12 groups based on their expression pattern (Supplemental Figures 3 and 4 and Supplemental Data Set 1). The most abundant Clusters 1, 2, and 3 contained 674, 441, and 356 genes, respectively. Cluster 1 contained genes whose expression increased from 1SQ to 5SQ (Figure 4, Cluster 1). Most DNA repair genes known to be induced by genotoxic agents or gamma irradiation were found in this cluster, including BRCA1, RAD51, RAD17, GMI1 (GAMMA-IRRADIATION and MITOMYCIN C INDUCED1; involved in homologous recombination), XRI1 (X-RAY INDUCED TRANSCRIPT1; involved in DNA repair), and PARP1 and PARP2 [POLY (ADP-ribose) polymerase 1 and 2; repair of DNA damage].
Figure 4.
Box Plot and Common GO Terms of the Cluster 1, Cluster 2, and Cluster 3 DEGs.
The expression profiles of DEGs were determined by SOM cluster analysis and all DEGs were divided into 12 clusters based on their expression patterns. Box plots and the most common GO terms of the most abundant group Cluster 1, Cluster 2, and Cluster 3 genes are shown. Representative genes in each cluster are also shown. The boundaries of the boxes indicate the 25th and 75th percentiles. The median is marked by a black line within the box. The lower whisker extends from the box bottom to the lowest value within 1.5 * IQR (interquartile range; defined as the distance between the first and third quartiles) of the first quartile. The upper whisker extends from the box top to the highest value that is within 1.5 * IQR of the third quartile. 1SQ (356SQ); 2SQ, 3SQ, 4SQ as in Figure 1A; 5SQ is wild-type SOG1 in a sog1-1 background.
RPA (REPLICATION PROTEIN A) is a single-stranded DNA binding protein required for eukaryotic DNA replication, repair, and recombination. There are five RPA1 paralogs (RPA1A to RPA1E) in Arabidopsis, of which RPA1C and RPA1E, which are primarily responsible for DNA damage repair (Aklilu and Culligan, 2016), were found in Cluster 1. Because RPAs are bound by ATR interacting protein (Cimprich and Cortez, 2008), SOG1 may induce RPAs via the ATR pathway. Furthermore, cell cycle inhibitors also are found in this cluster, including the cyclin-dependent kinase inhibitors SMR1 (SIAMESE-RELATED1), SMR4, SMR5, and KRP6 (KIP-related protein 6).
To further our understanding of the biological processes that are regulated by SOG1 phosphorylation, we next tested for enrichment of DEGs with a Gene Ontology (GO) enrichment analysis using BiNGO (Maere et al., 2005). With GO annotations, we examined biological processes and pathways, and we identified the distribution of genes in different GO categories (Supplemental Figure 5 and Supplemental Data Set 2). The major GO biological processes associated with DEGs in Cluster 1 were DNA metabolic process, response to ionizing radiation, DNA repair, response to various stimuli, and DSB break repair (Figure 4, Cluster 1, right panel; Supplemental Figure 5A). Furthermore, we also found that genes induced by chitin were enriched in Cluster 1 (Figure 4, right panel). Because chitin is a component of fungal cell walls, it is recognized as a general elicitor of plant defense responses, indicating that part of the defense system is activated in response to DNA damage. This observation is supported by findings from a previous study that the DDR and immune responses are closely related (Yan et al., 2013).
Genes Involved in DNA Replication and Cell Cycle Progression Are Suppressed as the Number of SQs Increases
Cluster 3 contained genes whose expression decreased from 1SQ to 5SQ (Figure 4, Cluster 3). RPA1B and RPA1D, which are responsible for normal DNA replication (Aklilu and Culligan, 2016), were found in this cluster. In addition, CYCA3;2 (CYCLIN-DEPENDENT PROTEIN KINASE3;2), CYCB3;1 (CYCLIN B3;1), and CYCD1;1 were assigned to this cluster. CYCA3;2 is highly expressed at the G1/S phase and has a role in actively dividing tissues (Takahashi et al., 2010), whereas CYCB3;1 has an expression peak at the G2/M transition phase (Menges et al., 2005). The suppression of these cyclins may be required for cell cycle arrest. In addition, although they are not in the top 20, we found in Cluster 3 that DNA replication and regulation of cellular processes were enriched by GO analysis (Supplemental Figure 5B and Supplemental Data Set 2), suggesting that DNA replication and cell cycle progression are inhibited as the number of phosphorylation sites of SOG1 increases.
Genes with the Highest Expression in 4SQ but Are Suppressed in 5SQ
Cluster 2 included genes whose expression incrementally increased as the number of SQ motifs increased, but showed the highest expression in the 4SQ lines with slightly repressed induction found in the 5SQ lines (Figure 4, Cluster 2). This cluster included CYCB1;1, which is induced by gamma irradiation and is a major regulator of homologous recombination (Weimer et al., 2016). SIM (SIAMESE) and SMR7, which are cyclin-dependent kinase inhibitors, are also found in this cluster. Because it was reported that SMR7 regulates the cell cycle checkpoint of the DDR in response to ROS (Yi et al., 2014), this kinase inhibitor may play a role in regulating the DDR in response to DSBs. In our GO analysis, we found that genes involved in response to chitin, carbohydrate, and stimulus were enriched in Cluster 2 (Figure 4, right panel; Supplemental Figure 5C). We noted that the phosphorylation difference between 5SQ and 4SQ was due to the phosphorylation status of 372SQ. Therefore, the nonfunctioning SQ motif in 4SQ (372AQ) contributes to the strong induction of genes in Cluster 2, and the phosphorylation of 372SQ suppresses the expression of these genes to a certain extent. Because Cluster 2 has the second-largest number of genes in our SOM analysis, it demonstrates that the phosphorylation of 372SQ has an important role in suppressing many genes in response to DNA damage.
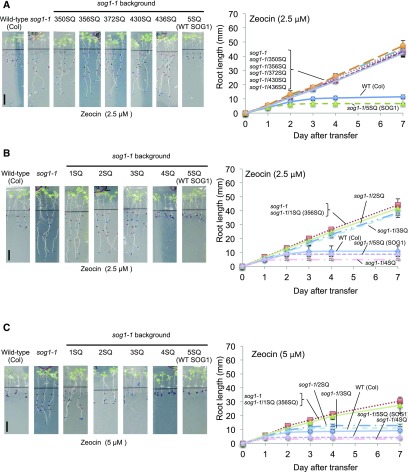
Root Growth Inhibition by Zeocin Treatment in SOG1 Phosphorylation Mutants
Previous studies reported that root growth was inhibited after transfer of wild-type seedlings to zeocin-containing medium, but it was not inhibited in sog1-1 seedlings (Adachi et al., 2011; Yoshiyama et al., 2014). These findings demonstrated that SOG1 is required for DNA damage-induced root growth arrest. Because our RNA-seq results showed that the expression of genes involved in cell cycle arrest and DNA replication were incrementally induced and repressed as the number of SQ sites increased, respectively, we hypothesized that the root growth of each mutant would also be incrementally inhibited. To test this hypothesis, 5-d-old seedlings were transferred to plates with either MS medium or MS medium containing 2.5 μM zeocin, and root growth was measured. We found that not only the sog1-1 mutants with SOG1 (1SQs) but also those with SOG1(2SQ and 3SQ) did not complement the sog1-1 phenotype (Figures 5A and 5B; Supplemental Figures 6A and 6B). On the other hand, the 4SQ and 5SQ lines did complement it (Figures 5A and 5B; Supplemental Figures 6A and 6B). Next, these experiments were performed with 5 μM zeocin, and we found that the root growth of 2SQ and 3SQ lines was intermediate between that of the resistant lines (sog1-1 and 1SQ) and the sensitive lines (the wild type, 4SQ, and 5SQ) (Figure 5C). These results indicated that root growth was incrementally inhibited as the number of SQ sites increased. We also observed that root growth inhibition was stronger in the 4SQ and 5SQ lines than in the wild type. This finding may be because expression of SOG1 is higher in phosphorylation mutant lines than in the wild type (Supplemental Figure 2B). As shown in Figure 3, increased expression of SOG1 strongly induces or suppresses downstream genes and therefore may account for why root growth inhibition on zeocin plates may be greater in 4SQ and 5SQ lines compared with that found in the wild type.
Figure 5.
Zeocin Sensitivity of the Roots of SOG1 Phosphorylated Mutants.
(A) and (B) Root growth of phosphorylated mutants on 2.5 μM zeocin plates. Four-day-old seedlings of the wild type, sog1-1, and sog1-1 harboring SOG1 [1SQs, 2SQ, 3SQ, 4SQ, and 5SQ(SOG1)] were transferred onto plates containing 2.5 μM zeocin, and root growth was measured.
(C) Root growth of phosphorylated mutants on 5 μM zeocin plates. Four-day-old seedlings of the wild type, sog1-1, and sog1-1 harboring SOG1 [1SQ (356SQ), 2SQ, 3SQ, 4SQ, and 5SQ(SOG1)] were transferred onto plates containing 5 μM zeocin, and root growth was measured. Bars = 10 mm. Data are presented as mean values ± sd from three independent experiments.
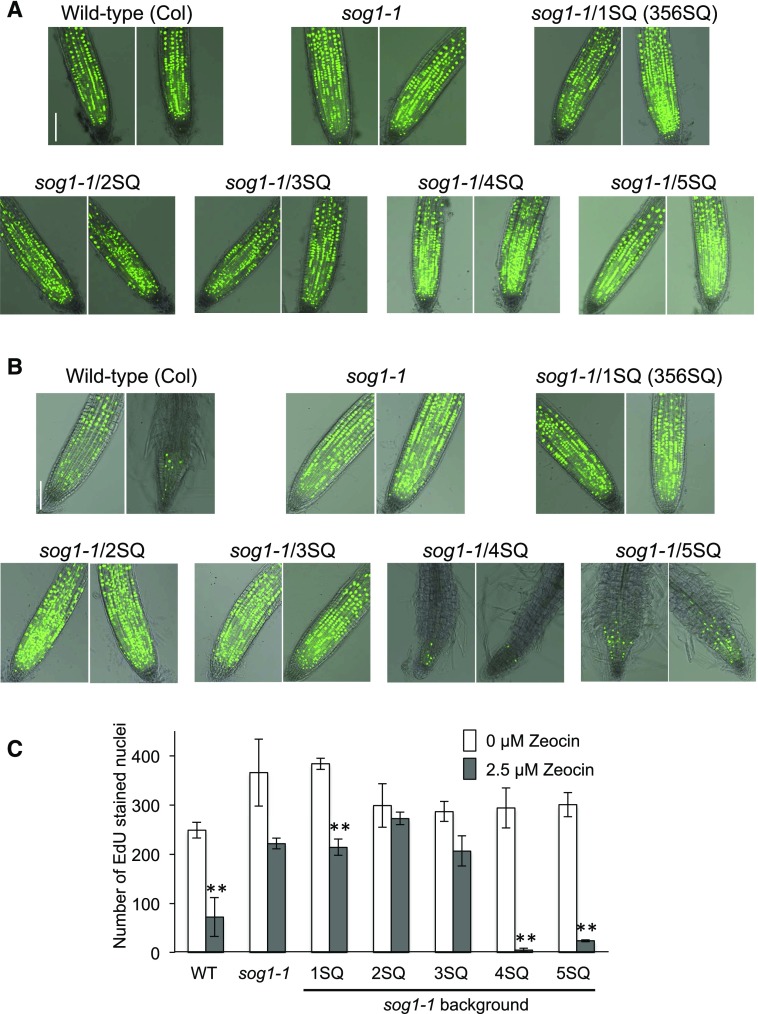
To determine whether DNA replication is arrested in these SQ mutant lines, we measured 5-ethynyl-2-deoxyuridine (EdU) incorporation 3 d after 2.5 μM zeocin treatment, which is when root growth arrest starts in the wild type. In wild-type seedlings, EdU signals were weak in the meristematic zone after zeocin treatment compared with untreated controls, showing that DNA synthesis was downregulated by the induction of DNA damage (Figures 6A to 6C). These results were consistent with the results of root growth on zeocin plates (Figure 5A); wild-type root growth inhibition started on the third day after transfer to zeocin plates. Therefore, these observations suggest that inhibition of DNA synthesis caused the root growth inhibition. In contrast, replication in meristematic cells in the sog1-1 mutant was slightly downregulated, but was still active after zeocin treatment (Figures 6A to 6C). 1SQ, 2SQ, and 3SQ lines showed active EdU incorporation in the meristematic zone similar to that found in sog1-1 (Figures 6A to 6C). In contrast, the EdU signals in 4SQ and 5SQ seedlings were diminished and their root morphology was also changed because of cell differentiation. These EdU results are consistent with our findings on root growth of 1SQ to 5SQ lines on zeocin plates (Figure 5B), which showed that root growth of 1SQ, 2SQ, and 3SQ lines is zeocin resistant, while that of 4SQ and 5SQ is zeocin sensitive.
Figure 6.
Roots Stained with EdU and the Number of EdU-Stained Nuclei.
(A) and (B) Roots stained with EdU. Four-day-old seedlings were transferred to MS plates containing either 0 μM (A) or 2.5 μM (B) zeocin. After 3 d, roots were labeled with EdU (green) to evaluate DNA synthesis. Bars = 0.1 mm.
(C) Number of EdU-labeled nuclei in the meristematic zone. Nuclei were counted using Image J with the ITCN plug-in. Bars represent the mean of the number of EdU-stained nuclei in 8 to 15 seedlings of control samples (no zeocin, white) or samples treated with 2.5 μM zeocin (gray). Error bars show se (n = 3 or 4). Asterisks indicate significance at *P < 0.05 or **P < 0.01 versus controls (no zeocin) using a two-tailed Student’s t test.
Induction of Programmed Cell Death and Cell Differentiation in SOG1 Phosphorylation Mutants
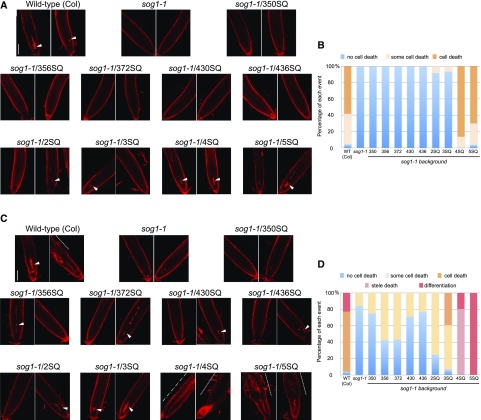
Programmed cell death is an important response to DNA damage in Arabidopsis. Zeocin treatment leads to SOG1-dependent cell death in root stem cells above the quiescent center (Yoshiyama et al., 2013). To elucidate the relationship between cell death and SOG1 phosphorylation, we examined the ability of SOG1 phosphorylation mutants to induce cell death, which was visualized using propidium iodide (PI) staining. In wild-type (Col) plants, stem cell death was induced by 5 μM zeocin treatment for 24 h, but it was not induced in sog1-1 plants (Figures 7A and 7B; Supplemental Figure 7 and Supplemental Table 1) (Yoshiyama et al., 2013). We did not observe induction of stem cell death after zeocin treatment in the 1SQ lines, including 350SQ and 356SQ (Figures 7A and 7B; Supplemental Figure 7 and Supplemental Table 1). On the other hand, some cell death was observed in 2SQ and 3SQ lines after zeocin treatment, whereas zeocin treatment in the 4SQ and 5SQ (wild-type SOG1) lines caused strong cell death (Figures 7A and 7B; Supplemental Figure 7 and Supplemental Table 1). Furthermore, 48 h after zeocin treatment, we found that the proportion of the cell death observed in 2SQ and 3SQ lines increased as the number of SQ sites increased (Figures 7C and 7D; Supplemental Figure 7B and Supplemental Table 2), which indicates that cell death was gradually induced as the number of SQ sites increased.
Figure 7.
Cell Death and Cell Differentiation in Zeocin-Treated Root Tips.
(A) to (D) Cell death and cell differentiation induced by zeocin treatment. Five-day-old seedlings were transferred to MS plates containing 5 μM zeocin, and roots were stained with PI (red) 24 h later ([A] and [B]) or 48 h later ([C] and [D]). Arrowheads indicate cell death, and the broken line, single-dot dashed line, and arrow show cell differentiation, stele death, and root constriction, respectively. Bars = 0.1 mm.
(B) The proportion of roots with no cell death, some cell death, and cell death in each SOG1 phosphorylated mutant 24 h following zeocin treatment.
(D) The proportion of roots with no cell death, some cell death, cell death, stele death, and cell differentiation in each SOG1 phosphorylated mutant 48 h following zeocin treatment.
Cell differentiation is another important response to DNA damage in Arabidopsis. Zeocin treatment causes an early transition from cell division to cell differentiation in the root meristem (Adachi et al., 2011). In this study, we also observed that cells in the upper part of the root meristem expanded 48 h after zeocin treatment in the wild type (Col) but not in sog1-1 mutant plants (Figures 7C and 7D). Because cell death and cell differentiation in the wild type was observed 24 and 48 h, respectively, after zeocin treatment, we know that DNA damage related to zeocin treatment first induces stem cell death followed by cell differentiation. Cell differentiation was not observed in any of the 1SQs, 2SQ, and 3SQ lines, but was observed in the 4SQ and 5SQ lines (Figures 7C and 7D; Supplemental Table 2), indicating that cell differentiation was induced as the number of SQ sites increased. In addition, it should be noted that the 4SQ line exhibited a strange phenotype, showing stele death with no cell differentiation and constriction of root tips (Figure 7C, 4SQ, single-dot dashed line and arrow). Because 372SQ was not phosphorylated in 4SQ lines, unphosphorylated 372SQ seems to have caused the unexpected phenotypes observed in 4SQ plants.
DISCUSSION
Effects of SOG1 Phosphorylation Deficiency on Transcriptional Responses
SOG1 is a transcription factor that regulates hundreds of genes in response to DNA damage, resulting in the activation of cell cycle arrest, DNA repair, cell differentiation, and programmed cell death (Yoshiyama et al., 2009; Furukawa et al., 2010). A critical unresolved issue regarding the DDR is how a single transcription factor SOG1 regulates these various pathways. To resolve this issue, we constructed several SOG1 phosphorylation mutants in Arabidopsis and examined their phosphorylation states in response to DNA damage. We showed that the five SQ motifs in the SOG1 C-terminal region are all phosphorylated and that each SQ motif is indispensable for full activation of SOG1 functions in the regulation of the various cell processes of the DDR. We found that 350SQ and 356SQ of SOG1 are the first motifs phosphorylated in response to DNA damage and that this phosphorylation is a priming event for the subsequent phosphorylation of other SQs (372SQ, 430SQ, and 436SQ). It should be noted that not all of the SOG1 molecule is phosphorylated, but rather part of SOG1 is phosphorylated in response to DNA damage (Figures 1B to 1D). One reason may be that SOG1 phosphorylation is dependent on tissue type. In this study, protein extraction was prepared from root tips, which inherently includes various tissues; therefore, SOG1 may be phosphorylated in some tissues in response to DNA damage. Another consideration is that SOG1 binds to DNA, and when DNA is damaged, SOG1 near the damage site is only phosphorylated by ATM or ATR, which are rapidly recruited to damaged sites. In any case, why only a part of SOG1 is phosphorylated by DNA damage is an important question to solve and warrants further investigation. We also noted that the intensity of band b in zeocin-treated 350SQ was higher than in untreated 350SQ. Perhaps the band position of phosphorylated 350SQ is overlapping band b. In this case, the upper phosphorylated band (band c) in 350SQ is SOG1, which is phosphorylated at two sites (a DNA damage-independent site and 350SQ).
Surprisingly, the expression of most target genes was either incrementally activated or repressed as the number of SOG1 phosphorylation sites increased. In particular, we found that genes involved in DNA repair and cell cycle arrest underwent gradual activation and genes involved in DNA replication and cell cycle progression underwent gradual repression. We have demonstrated that the number of phosphorylation sites in a transcription factor can incrementally affect the expression level of global downstream target genes in plants.
Using GO analysis, we found that many genes involved in biological processes were enriched in Cluster 3, which contained genes whose expression decreased from 1SQ to 5SQ (Figure 4, Cluster 3). Specifically, metabolic processes of aromatic compounds and amino acids, and biosynthetic processes of small molecules, carboxylic acids, and organic acids were enriched (Figure 4, Cluster 3, right panel; Supplemental Figure 5B). Because some genes involved in the metabolism of aromatic compounds are related to the accumulation of salicylic acid, which plays an important role in the induction of plant defenses against various stresses (Zheng et al., 2009), we propose that at least some aspect of the defense pathway is suppressed by DNA damage. Although this observation is inconsistent with our previously described results that some genes identified in Cluster 1 were induced by chitin (Figure 4, Cluster 1), considering the complexity of the defense pathway in plants, we posit there are different responses to DNA damage in which one part of the defense system may be suppressed while another is activated.
Effects of SOG1 Phosphorylation Deficiency on the DDR
Root growth is inhibited after transfer of wild-type seedlings to zeocin-containing medium. However, sog1-1 seedlings continue to grow (Figure 5A). Our results showed that root growth is incrementally inhibited in response to DNA damage as the number of SQ sites increased (Figure 5). The EdU signals of plants whose root growth was inhibited on 2.5 μM zeocin plates (the wild type, 4SQ, and 5SQ) were diminished, while the EdU signals of plants whose root growth was not inhibited (sog1-1, 1SQ, 2SQ, and 3SQ) were not diminished (Figure 6). These observations revealed that inhibition of DNA synthesis is very likely the cause of root growth inhibition. Cell death and cell differentiation were also rapidly induced in the wild type, 4SQ, and 5SQ compared with other lines (Figure 7). Furthermore, cell death and differentiation may be incrementally induced as the number of SQ sites increased. Data for 5SQ in Figure 1D showed that an intermediate phosphorylation state was not observed in the 5SQ line even if the amount of DNA damage changed. Although an intermediate phosphorylation state may not have a biological roles, there remains the possibility that intermediate phosphorylation exists at an undetectable level.
Genes that induce stem cell death and cell differentiation that are activated by DNA damage have not been identified. Because induction of cell death and cell differentiation is dependent on the number of SQ phosphorylation sites, there is a possibility that such genes may belong to Cluster 1, in which the gene expression profiles increased from 1SQ to 5SQ. It should be noted that the phenotype of 4SQ was different from that of 5SQ (Figures 7C and 7D). Zeocin treatment of 4SQ lines induced stele death with no cell differentiation and constriction of root tips (Figure 7C, single-dot dashed line and arrow), which are effects that were not found in 5SQ lines, but this may be because the phosphorylation pattern of 4SQ evokes an uncoordinated DDR. In fact, the expression pattern of genes in Cluster 2 identified using SOM analysis was highest in 4SQ but was suppressed in 5SQ (Figures 3, single asterisk for 4SQ, and 4, Cluster 2). Thus, genes from Cluster 2 may contribute to the abnormal phenotypes of the 4SQ lines and perhaps nonfunctional 372SQ (372AQ) is required to suppress these genes. Further investigation to identify these genes that confer such harmful effects and the continued analysis of genes that differ between 4SQ and 5SQ are of particular interest.
To summarize our findings, the efficiency of DNA damage responses (DNA repair gene induction, DNA synthesis arrest, cell death, and cell differentiation) is consistent with our RNA-seq results in which the expression of most genes is incrementally repressed and induced in proportion to the number of phosphorylated SOG1 SQ sites. These results indicate that the efficiency of the DDR is dictated by the number of SQ sites phosphorylated in SOG1 and that the lack of a single SQ phosphorylation can lead to an aberrant DDR.
Functional Consequences of SOG1 Phosphorylation in the Regulation of the DDR Pathways
Changes in protein phosphorylation represent a mechanism that is frequently used by cells to regulate transcription factor activity. Protein phosphorylation directly regulates distinct aspects of transcription factor functions including protein stability, cellular localization, protein-protein interactions, and DNA binding. In this broad context, we asked how phosphorylation affects the function of SOG1. We previously showed that the amount and localization of SOG1 protein was not affected by DNA damage (Yoshiyama et al., 2013). However, in this study, we report the contrasting finding that the expression of target genes is incrementally regulated by the number of phosphorylated SOG1 SQ motifs. Based on these findings, we proposed the following model to account for the effects of phosphorylation on SOG1 function. The surface of the DNA binding site in the NAC domain may be progressively exposed by SQ phosphorylation, which causes a gradual increase in DNA binding affinity. For such a model, we asked how 4SQ elicits an uncoordinated DDR. If 372 SQ is required for interaction with other factors, which work with SOG1 to suppress some genes (i.e., genes found in Cluster 2), unphosphorylated 372SQ causes the aberrant phenotype of 4SQ. If the phosphorylation pattern of SOG1 determines the affinity of interacting factors, which may be other transcription factors or coregulators, it would be of interest to identify SOG1-interacting factors using a coimmunoprecipitation assay. Another possibility is that the phosphorylation pattern of SOG1 directly dictates the promoter preference of target genes. In other words, the binding of SOG1 to different promoter sites can be regulated by the degree of protein phosphorylation. The SOG1 NAC domain, a DNA binding domain, is distinct from the five SQ sites that are in the C-terminal region. However, it is possible that the DNA binding activity of SOG1 is regulated indirectly by phosphorylation at residues that are remote from the DNA binding domain. A chromatin immunoprecipitation experiment can be used to analyze for differences in target genes among SOG1 phosphorylation mutants.
Predicting Functions of SOG1 Orthologs in Other Plants
SOG1-like proteins are found in other eudicots (Glycine max, Medicago truncatula, Vitis vinifera, and Populus trichocarpa), monocots (Oryza sativa, Zea mays, and Sorghum bicolor), an ancestor of the angiosperms (Amborella trichopoda), and in a gymnosperm (Picea glauca) (Yoshiyama et al., 2014). These SOG1-like proteins have SQ motifs at conserved positions in their C-terminal regions. The SOG1-like proteins found in eudicots and monocots have all five SQ motifs. The SOG1-like protein found in A. trichopoda has four of the five conserved SQ motifs (lacking 372SQ), whereas the SOG1-like protein found in the gymnosperm P. glauca has three of the five motifs (lacking 372SQ and 430SQ). These observations indicate that 350SQ, 356SQ, and 436SQ are evolutionally conserved among these plants and that these SQ motifs may therefore be crucial for the DDR via SOG1 activation. It is consistent with our findings that 350SQ and 356SQ are the first sites that are phosphorylated and that they are important as priming sites for additional phosphorylation. Because A. trichopoda has four of the five and P. glauca has three of the five conserved SQ motifs of SOG1, it is possible that the expression level of DNA repair genes in response to DNA damage in these plants is not as high as that found in Arabidopsis. However, both plants have other SQ motifs positioned at nonconserved sites in their transcription regulatory domain, which may induce maximal gene induction. In this study, we showed that loss of the 372SQ site results in perturbed DDR responses. However, A. trichopoda has 4SQ (350SQ, 356SQ, 430SQ, and 436SQ), which is the same as our 4SQ (350SQ, 356SQ, 430SQ, and 436SQ). Further study is warranted to answer the very interesting question of how 4SQ confers a negative impact in Arabidopsis. Furthermore, it would also be interesting to analyze the evolutionary conservation of SOG1-like proteins and SQ motifs in future studies because it will provide an improved understanding of DDR evolution in other plant species.
METHODS
Plant Materials and Growth Conditions
The Arabidopsis thaliana plants used in this study were either grown on soil or on MS media (1× MS salts including vitamins, 2% [w/v] sucrose, pH 6.0, and 0.8% [w/v] gellan gum agar for solid medium) under continuous light with a light intensity of ∼50 μmol photons m−2 s−1 at 22°C. The accession Col-0 was used as the wild-type strain, and the sog1-1 line has been described previously (Yoshiyama et al., 2009). For analysis of zeocin sensitivity of root growth, 5-d-old seedlings were transferred to plates containing either MS media or MS media plus 2.5 or 5 μM zeocin (Invitrogen), and root growth was measured. Error bars indicate sd from biological triplicates.
Generation of Transgenic Plants Carrying SOG1 Phosphorylation Mutation
To generate various SOG1 phosphorylated mutants, we performed multiple site-directed mutagenesis using a pENTR plasmid containing native promoter-driven SOG1 and native promoter-driven SOG1 (5AQ), which were previously described (Yoshiyama et al., 2013), and changes were confirmed by sequencing. iProof polymerase (Bio-Rad) was used for the site-directed mutagenesis reaction using the following primers: 350ASF1 and 350ASR1 for SOG1 350SQ, 356ASF1 and 356ASR1 for 356SQ, 372ASF1 and 372ASR1 for 372SQ, g430ASF1 and 430ASR1 for 430SQ, and 436ASF1 and g436ASR1 for 436SQ. To generate 2SQ, 3SQ, and 4SQ, we performed multiple site-directed mutagenesis using a pENTR plasmid containing native promoter-driven SOG1 (350SQ). The following primers were used for the mutagenesis: 356ASF1 and 356ASR1 for 2SQ, 356ASF1, 436ASF1, and 356ASR1 for 3SQ, and 356ASF1, 430ASF1, 436ASF1, and 356ASR1 for 4SQ. Primer sequences are listed in Supplemental Table 5. To add the Myc tag to the constructs, we used pGWB19 (no promoter, C-10xMyc) binary plasmids (Nakagawa et al., 2007) with LR clonase (Invitrogen), which were introduced into Arabidopsis via Agrobacterium tumefaciens (GV3101)-mediated floral dip transformation (Clough and Bent, 1998). Transformants with a single insertion were selected based on kanamycin resistance. T3 homozygous mutants were used in these experiments.
Immunoblotting
Five-day-old seedlings were transferred to MS liquid medium containing 0 or 1 mM zeocin. After a 1-h incubation, a pool of root tips (from ∼100 seedlings) was excised and ground in the following buffer: 10 mM Tris (pH 7.6), 150 mM NaCl, 2 mM EDTA, 0.5% (v/v) Nonidet P-40 (Nacalai Tesque), 1 mM DTT, protease inhibitor cocktail (Sigma-Aldrich), and a phosphatase inhibitor cocktail (0.1 mM Na3VO4, 1 mM NaF, 60 mM β-glycerophosphatase, and 20 mM p-nitrophenylphosphate). The slurry was centrifuged (20,000g) twice to remove debris, and the supernatant was recovered and used for subsequent analysis. Proteins (1 μg) were separated on an 8% SDS-PAGE gel containing 40 μM MnCl2 and 20 μM Phos-tag (NARD Institute), which was used for identification of phosphorylated SOG1 protein. Phosphorylated SOG1 is visualized as bands that migrate more slowly than those of nonphosphorylated proteins. After electrophoresis, the proteins were electroblotted to a PVDF membrane (Merck Millipore) in the following buffer: 6.3 mM NaHCO3, 4.3 mM Na2CO3, pH 9.5, and 20% methanol. Because SOG1-Myc can be detected using an anti-Myc antibody, the membrane was incubated for 2 h at room temperature in the anti-Myc primary antibody A-14 (1:2000 dilution, lot no. F0810; Santa Cruz Biotechnology), rinsed three times with 1× TBST (0.01 M Tris-HCl, 0.15 M NaCl, and 0.05% Triton X-100), and incubated with an anti-rabbit immunoglobulin horseradish peroxidase-conjugated secondary antibody (1:4000 dilution, lot no. 187662; Promega) to detect SOG1-Myc. Next, the membrane was washed three times with 1× TBST and processed with a LAS-4000 luminescent image analyzer (Fujifilm) after incubation with the ECL Prime enhanced chemiluminescence kit (GE Healthcare).
qRT-PCR
Five-day-old seedlings were transferred to MS liquid medium containing 0 or 100 μM zeocin. After a 2-h incubation, total RNA was extracted from a pool of root tips containing ∼100 seedlings using an RNeasy Plant Mini Kit (Qiagen) according to the manufacturer’s instructions. All samples were treated with DNase I using the Qiagen RNase-Free DNase set and quantified. To produce cDNA for qRT-PCR, 0.3 μg of total RNA was reverse transcribed using random hexamer primers and the Transciptor Universal cDNA Master (Roche) according to the manufacturer’s protocol. qRT-PCR reactions were performed using iQ SYBR Green Supermix (Bio-Rad) and the MyiQ single-color real-time PCR detection system (Bio-Rad) with the following reaction conditions: an initial 3-min denaturation at 95°C, followed by 50 cycles of 30 s at 95°C, 30 s at 60°C, and 45 s at 72°C, followed by a melting curve generation with 80 consecutive 0.5°C increase from 60 to 100°C. Images were captured during the 72°C extension step. The expression level relative to the level in the 0-h (untreated) wild-type sample was calculated using the value of the relative RNA levels normalized to the internal control ELF4A-1 (eukaryotic translation initiation factor 4A-1; At3g13920). The following primer sets were used: ELF4A1 control primers (elf4A1 and elf4A5); BRCA1 BREAST CANCER SUSCEPTIBILITY GENE1 (At4g21070) primers (brca1F2 and brca1rtR2); and RAD51 (At5g20850) primers (rad51AF1 and rad51ArtR1), which are found in Supplemental Table 5. Three biological replicates and three technical replicates were performed. Significance was tested using a one-tailed t test with equal variance.
Visualization of Cell Death and Cell Swelling
Five-day-old seedlings were transferred to MS plates containing 0 or 2.5 μM zeocin. Seedlings were stained 1 d and 2 d later with PI (10 μg/mL, 100× dilution; Nacalai Tesque) on a slide for 5 min. Stained seedlings were observed by confocal laser scanning microscopy using a TCS SP8 microscope (Leica Camera). The fluorescence excitation maximum of PI was 535 nm and the emission maximum was 617 nm. The Z-score test for two populations (wild type versus SQ mutant) was used to determine whether the two populations differed significantly on a single characteristic. Using Excel, the probability (P value) associated with the Z-score was assessed (1-norm.s.dist(z,true)).
EdU Incorporation
Five-day-old seedlings were transferred to MS plates containing 0 or 2.5 μM zeocin. Three days after transfer, the seedlings were measured for entry into S-phase using the Click-iT EdU Alexa Fluor 488 imaging kit (Invitrogen) according to the manufacturer’s instructions. Seedlings were incubated with 2.5 μM EdU for 4 h, and EdU-stained seedlings were observed by confocal laser scanning microscopy using a TCS SP8 microscope (Leica Camera). Nuclei were counted in the mitotic zone using Image J (https://imagej.nih.gov/ij/), with the ITCN plug-in using a width of 15, minimal distance of 7.5, and a threshold of 0.2.
RNA-Seq Analysis
Five-day-old seedlings were transferred to MS liquid medium containing 0 or 100 μM zeocin. After 2-h incubation, total RNA was extracted from a pool of root tips containing ∼100 seedlings using an RNeasy Plant Mini Kit (Qiagen). All samples were treated with DNase I using the Qiagen RNase-Free DNase Set (Qiagen) and quantified. Three independent RNA samples were used for the following analyses. RNA quality was checked by determining the RNA integrity number with an RNA 6000 bioanalyzer and RNA Nano Chip (Agilent Technologies). For library preparation, 0.5 µg of total RNA from samples with a RNA integrity number value ≥ 8 was used. All libraries were prepared using a TruSeq Stranded mRNA LT Sample kit (Illumina) according to the manufacturer’s protocol. Pooled libraries were sequenced on a NextSeq 500 (Illumina), and single-end reads of 75 bp length were obtained. The obtained reads were mapped to the reference Arabidopsis genome (TAIR10) by TopHat2 (Kim et al., 2013) and the htseq-counts script in the HTSeq library was used to count the reads (Anders et al., 2015). Count data were subjected to trimmed mean of M-values normalization in EdgeR (Robinson et al., 2010; McCarthy et al., 2012). Transcript expression and DEGs were defined with the EdgeR GLM approach (Robinson et al., 2010), and genes with FDRs <0.01 were classified as DEGs. Scaled expression values (fold change) were used for clustering based on SOM (Kohonen, 1984; Wehrens and Buydens, 2007). To identify enrichment of GO terms in the different sets of DEGs, GO enrichment analysis was performed using Cytoscape with the BiNGO add-on (http://apps.cytoscape.org/apps/bingo) (Maere et al., 2005). The GO term finder tests for overrepresentation of GO categories using a hypergeometric test and FDR correction for multiple testing (P ≤ 0.05).
Comet Assay
To detect DNA damage, comet assays were performed using the Comet assay kit (Trevigen) according to the manufacturer’s protocol. Briefly, root tips of 7-d-old seedlings in PBS buffer were chopped with a fresh razor blade on ice. Nuclei suspensions were filtered through a 40-μm mesh EASY strainer (Greiner Bio-One) and mixed with low-melting-point agarose at 37°C at a ratio of 1:10 (v/v). Samples (50 μL) were loaded on to a comet slide and then incubated at 4°C for 60 min. Next, the slides were incubated for 20 min in alkaline solution (200 mM NaOH and 1 mM EDTA) at room temperature and washed with 1× TBE buffer (90 mM Tris-borate and 2 mM EDTA) or 5 min. Electrophoresis was performed at 4°C in 1× TBE buffer for 10 min at 18 V (1 V/cm). The slides were then dehydrated in 70% (v/v) ethanol for 5 min and air dried. Next, 50 μL of SYBR Green (Thermo Fisher Scientific) was added and images were captured using ECLIPSE 80i (Nikon). Pictures were taken in black and white to clearly visualize the comet tails.
Accession Numbers
RNA-seq data reported here are available in the DDBJ Sequenced Read Archive under the following accession numbers: DRX083737 to DRX083772. Accession numbers for genes are as follows: BRCA1 (At4g21070), EIF4A-1 (At3g13920), RAD51 (At5g20850), and SOG1 (At1g25580).
Supplemental Data
Supplemental Figure 1. The Effect of 100 and 1000 μM Zeocin on Plants.
Supplemental Figure 2. Expression Changes of Genes among the SOG1 Phosphorylation Mutants in Response to DNA Damage.
Supplemental Figure 3. SOM Cluster Analysis of Gene Expression in SOG1 Phosphorylated Mutants.
Supplemental Figure 4. SOM Cluster Analysis of Gene Expression in SOG1 Phosphorylated Mutants.
Supplemental Figure 5. GO Enrichment Maps with DEGs.
Supplemental Figure 6. Root Growth of SOG1 Phosphorylated Mutants.
Supplemental Figure 7. Cell Death and Cell Differentiation in Control Root Tips.
Supplemental Table 1. The Proportion of Cell Death Observed 24 h Following Zeocin Treatment in Various SOG1 Phosphorylation Mutants.
Supplemental Table 2. The Proportion of Cell Death, Stele Death, and Cell Differentiation Observed 48 h Following Zeocin Treatment in Various SOG1 Phosphorylation Mutants.
Supplemental Table 3. Statistical Comparisons of Cell Death between Wild Type and SQ Mutants.
Supplemental Table 4. Statistical Comparisons of Cell Death, Stele Death, and Cell Differentiation between Wild Type and SQ Mutants.
Supplemental Table 5. Sequences of Primers Used in This Study.
Supplemental Data Set 1. SOM Cluster Analysis.
Supplemental Data Set 2. GO Biological Process Terms Significantly Overrepresented in the 12 Clusters Generated by SOM Analysis of SOG1 Phosphorylation Mutants.
Acknowledgments
We thank Hokuto Nakayama and Akiko Nakamasu for helpful discussions. This work was supported by JSPS KAKENHI (13J40017 and 17K07455 to K.O.Y., and JP16H01472 and JP16K07408 to S.K.) and by MEXT Supported Program for the Strategic Research Foundation at Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (Grant S1511023 to S.K.).
AUTHOR CONTRIBUTIONS
K.O.Y. planned the experiments and performed most of the experiments. K.K. and T.S. performed the RNA-seq experiment. S.K. performed data RNA-seq data analysis. K.O.Y. and S.K. conceived the project and wrote the manuscript.
References
- Adachi S., et al. (2011). Programmed induction of endoreduplication by DNA double-strand breaks in Arabidopsis. Proc. Natl. Acad. Sci. USA 108: 10004–10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aklilu B.B., Culligan K.M. (2016). Molecular evolution and functional diversification of Replication Protein A1 in plants. Front. Plant Sci. 7: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S., Pyl P.T., Huber W. (2015). HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31: 166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich K.A., Cortez D. (2008). ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9: 616–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743. [DOI] [PubMed] [Google Scholar]
- Cruz de Carvalho M.H. (2008). Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 3: 156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan K., Tissier A., Britt A. (2004). ATR regulates a G2-phase cell-cycle checkpoint in Arabidopsis thaliana. Plant Cell 16: 1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culligan K.M., Robertson C.E., Foreman J., Doerner P., Britt A.B. (2006). ATR and ATM play both distinct and additive roles in response to ionizing radiation. Plant J. 48: 947–961. [DOI] [PubMed] [Google Scholar]
- Foyer C.H., Shigeoka S. (2011). Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 155: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulcher N., Sablowski R. (2009). Hypersensitivity to DNA damage in plant stem cell niches. Proc. Natl. Acad. Sci. USA 106: 20984–20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T., Curtis M.J., Tominey C.M., Duong Y.H., Wilcox B.W., Aggoune D., Hays J.B., Britt A.B. (2010). A shared DNA-damage-response pathway for induction of stem-cell death by UVB and by gamma irradiation. DNA Repair (Amst.) 9: 940–948. [DOI] [PubMed] [Google Scholar]
- Garcia V., Bruchet H., Camescasse D., Granier F., Bouchez D., Tissier A. (2003). AtATM is essential for meiosis and the somatic response to DNA damage in plants. Plant Cell 15: 119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C.H., Mirabelli C.K., Jan Y., Crooke S.T. (1981). Single-strand and double-strand deoxyribonucleic acid breaks produced by several bleomycin analogues. Biochemistry 20: 233–238. [DOI] [PubMed] [Google Scholar]
- Hunter T., Karin M. (1992). The regulation of transcription by phosphorylation. Cell 70: 375–387. [DOI] [PubMed] [Google Scholar]
- Jensen M.K., Kjaersgaard T., Nielsen M.M., Galberg P., Petersen K., O’Shea C., Skriver K. (2010). The Arabidopsis thaliana NAC transcription factor family: structure-function relationships and determinants of ANAC019 stress signalling. Biochem. J. 426: 183–196. [DOI] [PubMed] [Google Scholar]
- Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. (2013). TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14: R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohonen T. (1984). Self-organized formation of topologically correct feature maps. Biol. Cybern. 43: 59–69. [Google Scholar]
- Maere S., Heymans K., Kuiper M. (2005). BiNGO: a Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21: 3448–3449. [DOI] [PubMed] [Google Scholar]
- McCarthy D.J., Chen Y., Smyth G.K. (2012). Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40: 4288–4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meek D.W., Anderson C.W. (2009). Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harb. Perspect. Biol. 1: a000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menges M., de Jager S.M., Gruissem W., Murray J.A. (2005). Global analysis of the core cell cycle regulators of Arabidopsis identifies novel genes, reveals multiple and highly specific profiles of expression and provides a coherent model for plant cell cycle control. Plant J. 41: 546–566. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Kurose T., Hino T., Tanaka K., Kawamukai M., Niwa Y., Toyooka K., Matsuoka K., Jinbo T., Kimura T. (2007). Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 104: 34–41. [DOI] [PubMed] [Google Scholar]
- Nezames C.D., Sjogren C.A., Barajas J.F., Larsen P.B. (2012). The Arabidopsis cell cycle checkpoint regulators TANMEI/ALT2 and ATR mediate the active process of aluminum-dependent root growth inhibition. Plant Cell 24: 608–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M.D., McCarthy D.J., Smyth G.K. (2010). edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26: 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán-Arjona T., Ariza R.R. (2009). Repair and tolerance of oxidative DNA damage in plants. Mutat. Res. 681: 169–179. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Inui Y.T., Uraguchi S., Yoshizumi T., Matsunaga S., Mastui M., Umeda M., Fukui K., Fujiwara T. (2011). Condensin II alleviates DNA damage and is essential for tolerance of boron overload stress in Arabidopsis. Plant Cell 23: 3533–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjogren C.A., Bolaris S.C., Larsen P.B. (2015). Aluminum-dependent terminal differentiation of the Arabidopsis root tip is mediated through an ATR-, ALT2-, and SOG1-regulated transcriptional response. Plant Cell 27: 2501–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Kojima S., Sakaguchi N., Umeda-Hara C., Umeda M. (2010). Two Arabidopsis cyclin A3s possess G1 cyclin-like features. Plant Cell Rep. 29: 307–315. [DOI] [PubMed] [Google Scholar]
- Wehrens R., Buydens L.M. (2007). Self- and super-organizing maps in R: The kohonen package. J. Stat. Softw. 21: 1–19. [Google Scholar]
- Weimer A.K., et al. (2016). The plant-specific CDKB1-CYCB1 complex mediates homologous recombination repair in Arabidopsis. EMBO J. 35: 2068–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh A.J., Davis R.J. (2000). Regulation of transcription factor function by phosphorylation. Cell. Mol. Life Sci. 57: 1172–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S., Wang W., Marqués J., Mohan R., Saleh A., Durrant W.E., Song J., Dong X. (2013). Salicylic acid activates DNA damage responses to potentiate plant immunity. Mol. Cell 52: 602–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi D., et al. (2014). The Arabidopsis SIAMESE-RELATED cyclin-dependent kinase inhibitors SMR5 and SMR7 regulate the DNA damage checkpoint in response to reactive oxygen species. Plant Cell 26: 296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama K., Conklin P.A., Huefner N.D., Britt A.B. (2009). Suppressor of gamma response 1 (SOG1) encodes a putative transcription factor governing multiple responses to DNA damage. Proc. Natl. Acad. Sci. USA 106: 12843–12848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama K.O. (2016). SOG1: a master regulator of the DNA damage response in plants. Genes Genet. Syst. 90: 209–216. [DOI] [PubMed] [Google Scholar]
- Yoshiyama K.O., Kimura S., Maki H., Britt A.B., Umeda M. (2014). The role of SOG1, a plant-specific transcriptional regulator, in the DNA damage response. Plant Signal. Behav. 9: e28889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama K.O., Kobayashi J., Ogita N., Ueda M., Kimura S., Maki H., Umeda M. (2013). ATM-mediated phosphorylation of SOG1 is essential for the DNA damage response in Arabidopsis. EMBO Rep. 14: 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Qualley A., Fan B., Dudareva N., Chen Z. (2009). An important role of a BAHD acyl transferase-like protein in plant innate immunity. Plant J. 57: 1040–1053. [DOI] [PubMed] [Google Scholar]