Abstract
Reward processing is often considered to be a monolithic construct, with different incentive types eliciting equivalent neural and behavioural responses. The majority of the literature on reward processing has used monetary incentives to elicit reward-related activity; yet social incentives may be particularly important due to their powerful ability to shape behaviour. Findings from studies comparing social and monetary rewards have identified both overlapping and distinct responses. In order to explore whether reward processing is domain-general or category-specific (i.e., the same or different across reward types), the present study recorded event-related potentials (ERPs) from early adolescents (ages 12-13) and emerging adults (ages 18-25) while they completed social and monetary reward tasks. Temporospatial principal components analysis revealed morphologically-similar reward positivities (RewPs) in the social and monetary reward tasks in each age group. In early adolescents, no significant difference was found between the magnitude of the RewP to social and monetary rewards. In emerging adults, however, the RewP to monetary rewards was significantly larger than the RewP to social rewards. Additionally, responses to feedback between the two tasks were not significantly correlated in either age group. These results suggest that both domain-general and category-specific processes underlie neural responses to rewards and that the relative incentive value of different types of rewards may change across development. Findings from this study have important implications for understanding the role that neural response to rewards plays in the development of psychopathology during adolescence.
Keywords: reward processing, reward positivity (RewP), social reward, monetary reward, early adolescence, emerging adulthood
The ability to detect and respond to rewarding outcomes of our actions is critical to adaptive functioning in a changing environment. Understanding whether our behaviours have resulted in positive or negative outcomes allows us to modify those behaviours in ways that increase the likelihood of receiving future rewards and of avoiding future punishments (Thorndike, 2000). A large body of cross-species evidence suggests that reward responding relies on neural circuitry responsible for the production and regulation of dopamine (DA; e.g., Delgado, 2007; Knutson & Wimmer, 2007). In humans, this network includes neurons in the ventral tegmental region of the midbrain projecting to the striatum and medial prefrontal cortex (Schultz, 2006). Activation of this system increases during the anticipation and receipt of many types of incentives (O’Doherty, 2004), such as winning money (Delgado, Nystrom, Fissell, Noll, & Fiez, 2000) or candy (Luking & Barch, 2013).
There are many types of potentially rewarding stimuli that can be used in experimental studies, but reward is often assumed to be a unitary construct, with different types of incentives assumed to generate equivalent behavioural and neural responses. In fact, the majority of this literature has used monetary incentives, which are easy to manipulate and robustly recruit DA activity, to elicit reward-related behaviour and neural activity (Foti, Weinberg, Bernat, & Proudfit, 2015; Izuma, Saito, & Sadato, 2008; Knutson, Bhanji, Cooney, Atlas, & Gotlib, 2008; Leotti & Delgado, 2014; Weinberg, Riesel, & Proudfit, 2014). Evidence from monetary incentive studies is then generalized across theories of reward processing, yet it is unclear whether all reward types are processed or shape behaviour in similar ways (Izuma et al., 2008; Kohls, Peltzer, Herpertz-Dahlmann, & Konrad, 2009; Rademacher et al., 2010; Spreckelmeyer et al., 2009). It is possible that a generic neural response identifies a stimulus or behaviour as rewarding but carries no meaning about the type of reward to be expected. Conversely, incentive-specific neural responses may allow for comparisons between the relative values of different kinds of rewards (Valentin & O’Doherty, 2009). Additional research is needed to establish whether reward processing is domain-general (i.e., the same across all incentive types) or category-specific (i.e., distinct across incentive types).
In particular, social incentives are of interest due to their powerful ability to shape behaviour (Fehr & Camerer, 2007). Consistent with this, recent studies have increasingly focused on the importance of social reward (Anderson, 2016; Forbes & Dahl, 2012; Guyer, Choate, Pine, & Nelson, 2012; Olino, Silk, Osterritter, & Forbes, 2015; Trezza, Damsteegt, Achterberg, & Vanderschuren, 2011). While studies examining neural response to social incentives have found similar patterns of activation as have been observed in other studies concerned with response to monetary incentives (Guyer et al., 2012; Olino et al., 2015), only a few studies to date have directly compared neural responses to social and monetary rewards. These have found evidence for both distinct and overlapping response patterns across incentive types. Initial evidence suggests that both social and monetary rewards recruit the striatum and medial prefrontal cortex (Izuma et al., 2008; Lin, Adolphs, & Rangel, 2012; Saxe & Haushofer, 2008; Zink et al., 2008), supporting the notion that different types of rewards have a “common currency” in terms of neural representations (Izuma et al., 2008; Saxe & Haushofer, 2008). Other work suggests, however, that the complete network of neural structures involved in processing different types of rewards may not be identical and may be sensitive to individual differences (Chan et al., 2016; Rademacher et al., 2010; Spreckelmeyer et al., 2009). However, these studies have also not matched the stimuli signaling reward on perceptual properties. It is possible that different perceptual properties of these stimuli, rather than the inherent incentive value of the rewards, drove the apparent differences in neural responses across the two reward types.
In addition, neural reward circuitry undergoes significant maturation from early adolescence to adulthood. Some evidence suggests that earlier development of the ventral striatum relative to the medial prefrontal cortex underlies heightened reward sensitivity during this period (Casey, Jones, & Hare, 2008; Galvan, 2010; Van Leijenhorst et al., 2010). The majority of studies investigating reward processing in adolescence have focused exclusively on monetary or other non-social rewards (Cohen et al., 2010; Lukie, Montazer-Hojat, & Holroyd, 2014; Luking, Luby, & Barch, 2014), yet adolescence marks the beginning of a shift in which social interactions and feedback from peers become paramount (Parker, Rubin, Erath, Wojslawowicz, & Buskirk, 2015; Vaillancourt, Brittain, McDougall, & Duku, 2013). This suggests that establishing reliable measures of neural response to social incentives will be critical to understanding developmental shifts in reward sensitivity. The present study aimed to characterize neural responses to social and monetary rewards in early adolescence (ages 12-13) and emerging adulthood (ages 18-25), in order to identify how responses to the two reward types converge and differ at each of these stages of development.
The index of neural response to reward used in the present study was an event-related potential (ERP) typically known as the feedback negativity (FN). The FN is maximal at frontocentral recording sites, occurs approximately 250-350ms following feedback, and has traditionally been conceptualized as a negative ERP deflection that is enhanced for nonreward compared to reward (Foti & Hajcak, 2009; Holroyd & Coles, 2002). However, recent work suggests that this apparent negativity is better described as a positivity that is enhanced for reward and absent for nonreward, which we, and others, have described as a reward positivity (RewP; Foti, Weinberg, Dien, & Hajcak, 2011; Holroyd, Pakzad-Vaezi, & Krigolson, 2008; Proudfit, 2015; Weinberg, Liu, Hajcak, & Shankman, 2015; Whitton et al., 2016). Multiple lines of evidence demonstrate the efficacy of the RewP as an index of reward-related neural activity. For instance, the magnitude of the RewP is associated with increased activation in reward-related brain structures (Becker, Nitsch, Miltner, & Straube, 2014; Carlson, Foti, Mujica-Parodi, Harmon-Jones, & Hajcak, 2011). Specifically, the magnitude of the RewP is correlated with midbrain volumes (Carlson, Foti, Harmon-Jones, & Proudfit, 2015), as well as with hemodynamic response in the ventral striatum, midcingulate, and midfrontal cortices following positive but not negative feedback (Becker et al., 2014; Carlson et al., 2011). The RewP is also moderated by genes regulating DA systems (Foti & Hajcak, 2012) and has been shown to track individual differences in reward sensitivity as measured by self-report and behaviour (Bress & Hajcak, 2013). Finally, the RewP can be reliably measured across multiple age groups (Bress, Meyer, & Proudfit, 2015; Kujawa, Proudfit, & Klein, 2014; Lukie et al., 2014; Weinberg et al., 2015), making it an ideal measure for comparing sensitivity to social and monetary rewards in both youth and adults.
Although there is a great deal of research examining the RewP in response to monetary rewards, only a few other studies to date have examined the RewP/FN in response to social reward and/or rejection (Crowley, Wu, Molfese, & Mayes, 2010; Kujawa, Arfer, Klein, & Proudfit, 2014; Sun & Yu, 2014; van der Veen, van der Molen, Sahibdin, & Franken, 2013). These studies have demonstrated that social feedback elicits ERP responses that are similar, though not identical in timing or morphology in every study, to those previously observed in response to monetary reinforcement. While Crowley and colleagues (2010) observed that social exclusion modulated a component similar to the FN, albeit non-significantly, they did not include an acceptance condition for comparison. In studies that have included both acceptance and rejection feedback, the results have been more mixed. For instance, Sun and Yu (2014) observed both a negative deflection in the ERP waveform in response to negative social feedback and a positive-going deflection in response to positive social feedback, while van der Veen and colleagues (2013) only observed a positive deflection in the ERP waveform in response to expected acceptance feedback. Kujawa, Arfer, and colleagues (2014), however, observed a negative-going deflection that was enhanced for rejection and reduced for acceptance feedback. Notably, these three studies were conducted in different age groups, and each used a time-window scoring method that can make it difficult to isolate reward-related activity from other neuroelectric contributions to the observed waveforms. Temporospatial principal components analysis (PCA), a data reduction technique that decomposes an observed ERP waveform into its underlying components in temporal and spatial dimensions (Foti et al., 2015; Foti et al., 2011; Proudfit, 2015) may be helpful in better characterizing the neural response to social rewards.
The primary aims of this study were first to test whether social and monetary reward elicit recognizable RewPs in both early adolescents and emerging adults, and also to explore whether the RewP is equally sensitive to both types of reinforcement at each developmental stage. In order to directly compare the RewP to social and monetary rewards, participants in the present study completed both a computerized social interaction task (the Island Getaway task (IG); Kujawa, Arfer, et al., 2014) and a forced-choice guessing task in which they could win money (the Doors task; Proudfit, 2015) while an electroencephalogram was recorded. We then decomposed the structure of the reward response using PCA in order to characterize the RewP elicited by social and monetary reward in early adolescents and emerging adults.
As processing social and monetary reward has been shown to recruit overlapping brain regions (Saxe & Haushofer, 2008) and there is some evidence to suggest the RewP elicited by social reward is similar to that elicited by monetary reward (Kujawa, Arfer, et al., 2014), we predicted that the RewP to the two reward types would be morphologically similar, with similar time courses and topographies. Additionally, we predicted that this RewP would be observed following both types of reward in both age groups. To our knowledge, however, no other studies have examined the association between the RewPs elicited by different incentive types within subjects. Moreover, the present study used comparable stimuli to represent social and monetary reward in two very different tasks, in order to better evaluate responses to the incentive types themselves, rather than perceptual properties of the stimuli. The novel design of the present study allowed for exploratory analyses assessing the RewP to social and monetary rewards across development, and evaluation of the evidence for category-specific or domain-general reward responses in early adolescence and emerging adulthood.
Method
Participants
Previous research has identified sex differences in reward-related neural responses (Spreckelmeyer et al., 2009). Moreover, girls tend to be more invested in relationships (Rose & Rudolph, 2006) and are more sensitive to interpersonal slights (MacEvoy & Asher, 2012) than boys. This suggests that it is important to examine reward processing generally, and social reward processing in particular, separately in males and females. In this preliminary study, therefore, only females were included in the samples in order to increase power to detect effects.
Early adolescents
The early adolescent sample was drawn from a large longitudinal study conducted at Stony Brook University (see e.g., Kujawa, Proudfit, & Klein, 2014; Olino, Klein, Dyson, Rose, & Durbin, 2010 for details). The present analyses were conducted on 39 individuals (M age = 12.38. SD) = 0.59), all of whom identified as Caucasian (15.4% also identified as Hispanic). After a description of the study was given, written informed consent was obtained from a parent or guardian and verbal assent was obtained from the adolescent. The Stony Brook University institutional review board approved all procedures conducted with this sample.
Emerging adults
Fifty-three undergraduates from McGill University were recruited to participate in the study through the McGill University undergraduate student research pool and flyers posted around the university campus; two were excluded due to excessive noise in the EEG data, and three because of equipment failure. Analyses were conducted on the remaining 48 participants (M age = 20.29, SD = 1.54). Fifty-two percent of the sample identified as Caucasian, 17% identified as Chinese, 10% identified as Korean, 6% identified as Arab/West Asian, 1% identified as Hispanic, and 13% identified as “Other.” Written informed consent was obtained from every participant prior to participation. The McGill University research ethics board approved all procedures conducted with this sample.
Procedure
For both samples, after providing assent and/or consent, EEG sensors were attached and participants completed two computer tasks involving social (IG) and monetary (Doors) reward. All stimuli were presented on a Pentium class computer using Python (version 2.7.10, Python Software Foundation) to present the IG task and Presentation (version 18.1, Neurobehavioural Systems, Inc.) to present the Doors task.
Tasks
Island Getaway
In the IG task, participants were told that they would be playing a “Survivor”-style game against eleven other people (co-players); in fact, the co-players were a part of the computer program (task modified from Kujawa, Arfer, et al., 2014). Co-player profiles were modified to depict peers in a similar age range to participants in different versions of the task designed for each age group. Task instructions indicated that participants would be travelling along a chain of six Hawaiian Islands and at each island they would have to vote whether they wanted each co-player to continue on with them to the next island or to be kicked out of the game. Each time participants voted to accept (“Keep”) or reject (“Kick out”) a co-player, they saw feedback indicating whether that co-player had voted to accept or reject them. Acceptance feedback was indicated by an image of a green “thumbs up” and rejection feedback was indicated by an image of a red “thumbs down.” Acceptance and rejection feedback were interpreted as social reward and nonreward, respectively. Each voting trial consisted of the following sequence: a co-player profile presented until vote; a fixation “+” presented for 1000 ms; feedback displayed for 2000 ms; and a blank screen presented for 1500 ms. In order to simulate variation in co-player response speed, a message saying, “Waiting for [co-player’s name] to vote…” was shown before the first fixation cross if participants voted faster than a simulated voting time selected for that co-player. In the emerging adult version of the task, visual analog scales (1 = not at all, 9 = extremely) were presented before the blank screen, on which participants indicated how much they liked and how much they thought others would like the previous co-player; results from these ratings are not presented here as they were not collected from both samples. There were 51 feedback trials split evenly between acceptance and rejection, with one trial type determined randomly. After each of the first five rounds of voting, participants were told that one of the co-players had been sent home, and after completing the sixth, participants were informed that they had made it to the “Big Island.”
Doors
The Doors task is a forced-choice guessing task commonly used to study responses to monetary reward in youth and adults (Proudfit, 2015). Prior to beginning the task, participants were informed that they would have the opportunity to win money during the task. This task consisted of three blocks of twenty trials. On each trial, participants saw two doors and were instructed to click the left or right mouse button to select which door they thought hid a prize. Following each choice, participants received feedback informing them of whether they won or lost money on that trial. A green arrow pointing up indicated that the participant had won $0.50, and a red arrow pointing down indicated that the participant had lost $0.25. Reward (i.e., win) and nonreward (i.e., loss) feedback were each presented on 50% of trials in random order. Each trial consisted of the following sequence: an image of two doors presented until mouse-click; a fixation “+” presented for 1000 ms; feedback arrow presented for 2000 ms; a fixation “+” presented for 1500 ms; and an image with “click for next round” presented until mouse-click. Prior to starting the task early adolescent participants were informed that they could win up to $5.00 and emerging adult participants were informed that they could win up to $10.00; following the task, all participants were given $5.00 (Stony Brook University participants were compensated in USD and McGill University participants were compensated in CAD).
Importantly, the feedback representing reward and nonreward in the two tasks was perceptually very similar. This allowed for an effective comparison of neural processing of these two types of reward based on their conceptual differences, rather than perceptual differences that might impact neural response.
EEG Data Acquisition and Processing
Early adolescents
Continuous EEG was recorded using a 34-electrode cap, based on the standard 10/20 layout, and a BioSemi system (BioSemi, Amsterdam, Netherlands). The electrooculogram (EOG) generated from eye movements and blinks was recorded using facial electrodes placed approximately 1cm above and below the left eye and 1cm from the outer corners of the eyes. Electrodes were also placed on the left and right mastoids. Recordings were digitized at a 24-bit resolution with a sampling rate of 1024 Hz using a low-pass fifth-order sync filter with a half power cutoff of 204 Hz. Each active electrode was measured online with respect to a common mode sense (CMS) active electrode, located between PO3 and POz, producing a monopolar (non-differential) channel. CMS forms a feedback loop with a paired driven right leg (DRL) electrode.
Emerging adults
Continuous EEG was recorded using a 32-electrode cap, based on the standard 10/20 layout, and a BrainVision actiCHamp system (Brain Products, Munich, Germany). The electrooculogram (EOG) generated from eye movements and blinks was recorded using facial electrodes placed approximately 1cm above and below the left eye, forming a bipolar channel, and referenced to an electrode on the back of the neck. Electrodes TP9 and TP10 were used as mastoid references. All electrode impedances were kept below 5 kΩ. Recordings were digitized at a 24-bit resolution with a sampling rate of 1000 Hz using an online 60 Hz low-pass filter.
For both samples, offline analysis was conducted using BrainVision Analyzer software (Brain Products, Munich, Germany). Data were re-referenced offline to include an average of the recordings from left and right mastoids and band-pass filtered with cutoffs of 0.01 and 30 Hz, and segmented for each trial either 200 ms (both tasks in early adolescents, Doors in emerging adults) or 500 ms (IG in emerging adults) before and 1000ms after feedback onset. Eye-blink correction (Gratton, Coles, & Donchin, 1983) and semi-automatic artifact rejection procedures were conducted. A voltage step of no more than 50.0μV between sample points, a maximum voltage difference of 175.0μV within a trial, and a minimum voltage difference of 0.50μV within 100ms intervals were the criteria used to automatically detect artifacts. Visual inspection of the data was then conducted to detect and reject remaining artifacts. Using this procedure, an average of 1.90 and 0.27 trials were rejected per person in the social task and an average of 1.18 and 0.25 trials were rejected per person in the monetary task for early adolescents and emerging adults, respectively. Additionally, PCA requires data from all channels at all time-points, and so channel averages that were based on fewer than five trials after artifact rejection were interpolated using 3-4 surrounding channels. ERPs were averaged across trials separately for reward (acceptance/gain) and nonreward (rejection/loss), and the activity in the 200ms window before feedback onset served as the baseline.
Analysis
Four separate temporospatial PCAs were conducted, one for each task and age group, using the ERP PCA Toolkit (Dien, 2010a). In each PCA, two averages (i.e., reward/nonreward) for each subject were entered into the data matrix. In each instance, a temporal PCA was performed first, followed by a spatial PCA (Dien, 2010b; Dien, Beal, & Berg, 2005; Dien, Khoe, & Mangun, 2007). The temporal PCA used all time points from each participant’s averaged data as variables, and it considered participants, trial types, and recording sites as observations. A Promax rotation was used to rotate to simple structure in the temporal domain (Dien, 2010b; Dien, Khoe, & Mangun, 2007). Following the first rotation, a parallel test (Horn, 1965) was conducted on the resulting Scree plot (Cattell, 1966), in which the Scree of the actual dataset is compared to a Scree plot derived from a fully random dataset. The number of factors retained is based on the largest number of factors that account for a greater proportion of variance than the fully random dataset (see Dien, 2010a for more information). Based on this criterion, 16 temporal factors in the social and monetary reward tasks in the early adolescent sample were extracted for rotation; in the emerging adult sample, 24 temporal factors in the social reward task and 21 temporal factors in the monetary reward task were extracted for rotation. The covariance matrix and Kaiser normalization were used (Dien, Beal, & Berg, 2005). For each factor, scores were derived for every combination of electrode, participant, and trial type. Each factor score represents the percentage of activity in the original data captured by that particular factor.
A spatial PCA was then conducted on each temporal factor in order to identify the spatial distribution of these factor scores. Variables consisted of all recording sites, and observations consisted of all participants, trial types, and temporal factor scores. Infomax was used to rotate the spatial factors to independence (Dien, 2010b; Dien et al., 2007). Based on the results of the parallel test (Horn, 1965), 4 spatial factors were extracted from each temporal factor for Infomax rotation in the early adolescent sample and the emerging adults sample in social reward, and 3 spatial factors were extracted in the emerging adult sample in monetary reward. The temporospatial PCA in the early adolescent sample resulted in factor combinations that accounted for 70% and 78% (64 factor combinations in each) of total variance in the data in the social and monetary reward tasks, respectively. Temporospatial PCA in the emerging adult sample resulted in factor combinations that accounted for 84% (96 factor combinations) and 82% (63 factor combinations) of total variance in the data in the social and monetary tasks, respectively.
Data exported for each participant then represent the loadings of that participant’s data onto the factor combination at the peak channel and time point. In order to directly assess timing and spatial voltage distributions, these factor loadings are translated back into voltages (see, e.g., Dien, 2012, or Foti, Weinberg, Dien, & Hajcak, 2011, for more detailed accounts of the methods). A robust analysis of variance (ANOVA; Keselman, Wilcox, & Lix, 2003) was then conducted on every temporospatial PCA factor combination that accounted for greater than 0.5% of variance in the data, to identify the factors that significantly differentiated between reward and nonreward. In each task, multiple factor combinations accounted for more than 1% of the variance (early adolescents: 15 in social reward, 13 in monetary reward; emerging adults: 13 in both reward types). Of these, there was one factor combination in each task that resembled the RewP, both in terms of timing and scalp distribution, and significantly differentiated reward from nonreward (described below for each task). As is evident from Figure 1, the social reward task appeared to elicit a more sustained response than the monetary reward task; the analyses that follow were limited to the RewP component derived from the PCA that was elicited in both tasks and age groups1.
Figure 1.
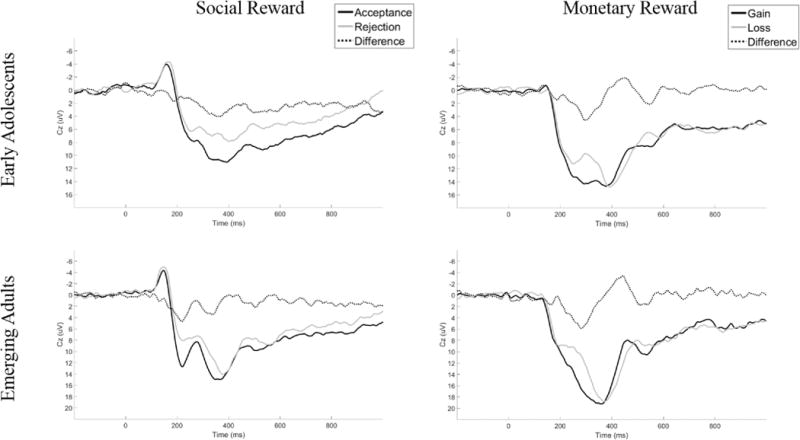
Waveforms depicting neural response to social and monetary reward tasks for each age group.
Subsequent analyses were conducted using SPSS (21.0; SPSS, Inc.). We did not conduct any between-group analyses because of differences in tasks and data acquisition between the samples. A two (feedback: reward, nonreward) by two (task: IG, Doors) repeated-measures ANOVA was conducted for the temporospatial PCA factor corresponding to the RewP in each sample (described further below); effect sizes are expressed as partial η2, calculated using the following formula: SSeffect / (SSeffect + SSerror). Within-subjects Pearson’s correlations were computed to assess the association between responses to social and monetary reward within each age group. All correlations involving PCA factors used a difference score of reward minus nonreward, in order to isolate activity specific to reward processing (Δ RewP).
Results
Figure 1 displays ERP waveforms for each task and each age group. As can be observed in this figure, the waveforms vary across age groups and tasks. Figure 2 displays the PCA-derived grand average response-locked ERPs at Cz for each task and each age group. Topographic maps are also shown, depicting voltage differences (in μV) across the scalp for nonreward minus reward feedback in the time window of the RewP.
Figure 2.
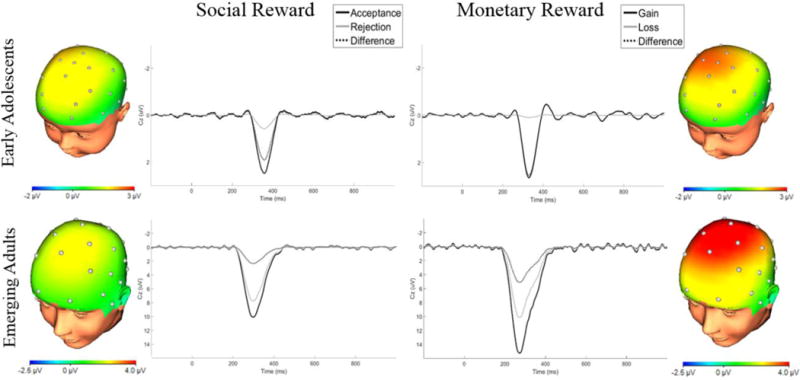
Waveforms and scalp topographies depicting temporospatial factor combinations corresponding to the Reward Positivity in social and monetary reward tasks for each age group. Scalp topographies show Δ RewP.
Early Adolescents
As indicated in Figure 2 and Table 1, the PCA factor combination corresponding to the RewP (temporal factor 5/spatial factor 1; TF5/SF1) in each of the two tasks represented a relative positivity maximal at frontocentral sites enhanced for reward trials and reduced for nonreward trials. However, the RewP elicited by social rewards became maximal approximately 25 ms later than the RewP elicited by monetary rewards. Results revealed a significant main effect of feedback, F(1, 38) = 14.47, p = .001; ηp2 = .28, such that the RewP was enhanced following reward (M = 3.05 μV, standard error of the mean (SEM) = 0.76) relative to nonreward (M = 0.39, SEM = 0.60). No significant main effect of task was found, F(1, 38) = 0.03, p = .88; ηp2 = .001, nor did task significantly interact with feedback type to predict neural response, F(1, 38) = 0.70, p = .41; ηp2 = .02. The magnitude of the Δ RewPs elicited in the social and monetary reward tasks were moderately but not significantly correlated with one another, r(37) = .28, p = .09; 90% CI [.01, .51]. Thus, although the two types of reward elicited morphologically-similar ERPs, we only found evidence for a modest and non-significant association between neural responses to social and monetary reward in early adolescence.
Table 1.
Temporospatial factor combinations corresponding to the reward positivity in social and monetary reward tasks for each age group
| Age Group | Task | Temporospatial factor combination | Variance explained (%) | Temporal loading peak (ms) | Spatial distribution | Nonreward vs. reward |
|---|---|---|---|---|---|---|
| early adolescents t(36) |
social | TF5/SF1 | 1.63 | 343 | frontocentral positivity | 8.37** |
| monetary | TF5/SF1 | 1.42 | 317 | frontocentral positivity | 10.26** | |
| emerging adults t(43) |
social | TF3/SF1 | 7.98 | 297 | frontocentral positivity | 9.45** |
| monetary | TF3/SF1 | 7.81 | 272 | frontocentral positivity | 37.30*** |
Note.
p < .01,
p < .001.
t-values were calculated using a robust ANOVA.
Emerging Adults
The PCA factor combination corresponding to the RewP (TF3/SF1) in each of the two tasks represented a relative positivity maximal at frontocentral sites enhanced for reward trials and reduced for nonreward trials (Table 1, Figure 2). As in the early adolescent sample, the RewP elicited by social rewards appeared to reach its peak 25 ms later than the RewP elicited by monetary rewards. Results identified significant main effects of feedback, (1, 47) = 47.54, p < .001; ηp2 = .50, and task, F(1, 47) = 19.44, p < .001; ηp2 = .29. These main effects were qualified by a significant feedback by task interaction, F(1, 47) = 9.10, p = .004; ηp2 = .16, such that the magnitude of the difference between reward and nonreward was larger for monetary (M = 5.10, SD = 5.22) than social reward (M = 2.33, SD = 4.56). In emerging adults, the magnitude of the Δ RewP in the social reward task was not significantly correlated with the magnitude of the Δ RewP in the monetary reward task, r(46) = .16, p = .27; 90% CI [−.08, .39]2.
Discussion
The primary aims of this study were to demonstrate that a recognizable RewP can be observed in response to distinct reward types and that it can be observed at different developmental stages. Using PCA, we empirically isolated a positivity that was enhanced for both social acceptance and winning money, and reduced for both social rejection and losing money. This RewP was evident in both early adolescence and emerging adulthood. Previous evidence suggests that the RewP is an effective index of activity in reward-related brain structures, specifically, the ventral striatum (Carlson et al., 2011; Foti et al., 2011). Whereas these studies have shown that the RewP is a useful marker of monetary reward processing (Becker et al., 2014; Bress, Smith, Foti, Klein, & Hajcak, 2012; Lukie et al., 2014; Nelson, Perlman, Klein, Kotov, & Hajcak, 2016), the data reported here suggest that the RewP may also be a useful marker of social reward sensitivity that can be observed in both early adolescence (e.g., Kujawa, Arfer, et al., 2014) and emerging adulthood. This social RewP, therefore, may be a useful marker of ventral striatum activation in response to social feedback. Studies recording EEG and fMRI in social reward tasks in the same participants will be necessary to test this possibility, but once validated, ERP paradigms can be more easily deployed to large and diverse samples to better understand neural processing of social incentives.
The results of this study are therefore likely to be useful to researchers interested in neural responses to social incentives, as well as the role that abnormal responses to social rewards and social functioning play in the pathogenesis of mental illness (Forbes & Dahl, 2012; Morgan, Olino, McMakin, Ryan, & Forbes, 2013). Although abnormal reward processing has been implicated in multiple forms of psychopathology (Baskin-Sommers & Foti, 2015; Chau, Roth, & Green, 2004; Dichter, Damiano, & Allen, 2012), including affective (Benson, Guyer, Nelson, Pine, & Ernst, 2015), substance use (Koob & Le Moal, 2001), and psychotic disorders (Arrondo et al., 2015), most research linking abnormal reward processing to mental illness has been conducted with monetary incentives (Casement et al., 2014; Foti & Hajcak, 2009). This literature often assumes findings from monetary incentives will generalize to other incentive types, but very few studies have compared neural responses to multiple types of reward within subjects (Izuma et al., 2008; Lin et al., 2012), and none have examined the category specificity of reward processing abnormalities in these populations. Yet social feedback powerfully shapes our behaviour (Fehr & Camerer, 2007), and there are robust associations between maladaptive social behaviour (e.g., aggression; Dirks, Treat, & Weersing, 2014) and mental illness. Future studies assessing neural response to multiple reward modalities might be useful in determining whether deficits are general, and might be driven by broad dysfunction of neural circuits mediating reward response, or if instead deficits are specific, suggesting abnormalities in higher-order evaluative processes.
Additionally, the neural systems supporting reward responding undergo significant changes from childhood to adulthood (Casey et al., 2008; Casey, Jones, & Somerville, 2011), and there is evidence that what is perceived as rewarding changes in meaningful ways across development (Jones et al., 2014; Somerville et al., 2013), suggesting that investigations of multiple reward modalities will be particularly important across adolescence. In the present study, the early adolescent sample displayed an equally large RewP to both reward types, and the effect of the feedback/task interaction was small (Cohen, 1992). This is consistent with evidence that children and early adolescents (ages 8-12) do not report subjective differences in how “rewarding” social and monetary feedback is (Kohls et al., 2009), suggesting the possibility of less differentiation of reward types—i.e., more domain-general reward processing—in this age group. However, there is also evidence that monetary rewards can lead to greater improvements in task performance than social rewards in children and adolescents (Kohls et al., 2009), suggesting it will be important for future studies to combine neural, behavioral, and subjective measures of reward sensitivity.
On the other hand, in the emerging adult sample, the RewP elicited by monetary rewards was significantly larger than that elicited by social rewards, an effect that was medium-to-large (Cohen, 1992), suggesting the possibility of greater category-specificity in this age group. The older sample may have been past the developmental period of heightened sensitivity to social feedback (Jones et al., 2014; Somerville et al., 2013), or the older sample might be more sensitive to other types of feedback, such as romantic (Aron et al., 2005; Collins, Welsh, & Furman, 2009) or achievement (Stipek & Mac Iver, 1989), rather than feedback from peers. Combined, the results of this study suggest there may be normative age-related changes in associations between, and relative weightings of, different incentive types. Given that adolescence is not only characterized by peak reward sensitivity (Casey et al., 2008; Casey et al., 2011), but also peak vulnerability to psychopathology (Kessler et al., 2007), and increasing sensitivity to social interactions and social feedback (Parker et al., 2015; Vaillancourt et al., 2013), future studies should assess neural response to multiple reinforcement types across this developmental period.
In our study, despite the use of perceptually similar stimuli to represent social and monetary reward feedback, RewP magnitudes to social and monetary rewards were not significantly correlated with one another in either sample, and the magnitudes of the associations were small in the emerging adult sample and medium in the early adolescent sample. While these correlations might reach significance with a larger sample size, these data demonstrate that the neural responses elicited in the two tasks are by no means redundant with one another. This suggests that identifying idiosyncratic patterns of sensitivity to distinct incentive types might be important in understanding the role that reward-processing abnormalities play in maladaptive behaviour. For instance, some people might exhibit a blunted response to monetary reward but a heightened response to social reward, while others might exhibit a blunted response to all reward types. These different patterns of reward responding might be associated with different outcomes; for example, the former pattern of responding may be more strongly associated with externalizing symptoms and behaviors (Kohls, Herpertz-Dahlmann, & Konrad, 2009), while the latter pattern of responding may be more strongly associated with symptoms of anhedonia (Meehl, 1975; Olino et al., 2014). Thus, identifying stable profiles of neural responses to different incentive types may be a fruitful avenue of future research.
Notwithstanding the methodological strengths and innovations of the present study, several potential limitations are apparent. First, participants included in this study were all female. Evidence suggests that positive social interactions may be particularly salient for females (Rose & Rudolph, 2006; Stroud, Salovey, & Epel, 2002), and there is some evidence that females demonstrate increased sensitivity to interpersonal conflict (Gillespie & Eisler, 1992; Rose & Rudolph, 2006; Stroud et al., 2002), which may play an important role in the development of internalizing problems among women and girls (Rudolph & Conley, 2005; Shih, Eberhart, Hammen, & Brennan, 2006). However, there may also be gender differences in neural responses to rewards in general (Kujawa, Proudfit, et al., 2014; Spreckelmeyer et al., 2009), and in response to social stimuli in particular (Guyer, McClure-Tone, Shiffrin, Pine, & Nelson, 2009; Spreckelmeyer et al., 2009). For these reasons, we chose to focus first on female participants; future studies should examine the generalizability of the patterns reported here to male participants.
Other areas of future study relate to developmental processes. For instance, we did not evaluate effects of pubertal development in the present study, but pubertal stage may impact the magnitude of neural responses to rewarding feedback (Forbes et al., 2010; LeMoult, Colich, Sherdell, Hamilton, & Gotlib, 2015; Op de Macks et al., 2011; Op de Macks et al., 2016). Future work should therefore include measures of puberty to further elucidate the influence of different aspects of adolescent development on processing of different incentive types. If domain-general increases in reward sensitivity follow the same adolescent-specific peak as has been observed in monetary reward studies (Casey et al., 2008; Casey et al., 2011), then it is possible that changes in reward sensitivity from childhood to adulthood are linked to puberty-driven neurochemical changes occurring in the adolescent brain (e.g., rising gonadal hormones). There may also be age-related changes in participants’ susceptibility to the deception involved in the Island Getaway task and belief that they were interacting with real peers in real time. Although the present study did not demonstrate a significant association between task engagement and the RewP2, it will be important for future studies to assess age-related and individual differences in belief in the task and its association with the RewP. Additionally, different EEG systems at different sites were used to collect data in each age group, precluding statistical comparisons between the two groups. Future studies might seek to replicate the results of the present study between the ages of 13 and 18, as evidence suggests that important changes in reward sensitivity occur during this time (Casey et al., 2008; Casey et al., 2011; Van Leijenhorst et al., 2010). Similarly, this cross-sectional study would be fruitfully followed by longitudinal studies looking at age- and puberty-related changes in neural response to social and monetary reward, as well as the relative weighting of each type of reward.
Importantly, real-life social situations are enormously more complex than can typically be modeled in a computer program. However, due to the nature of the two-way interactions between participants and co-players, the Island Getaway task used here may tap into processes that are particularly relevant for female participants in this age range – that is, social evaluation and approval (La Greca & Lopez, 1998; Parker et al., 2015; Vaillancourt et al., 2013). Moreover, as evidenced by the sustained nature of the neural responses to social feedback (Figure 1), it appears that the social task is tapping more elaborate neural processes than the monetary reward task and additional research is needed to further evaluate these processes.
In addition, despite being explicitly instructed that they and the co-players were voting simultaneously, and though the experimental design attempted to limit the impact of previous co-player voting on the RewP by separating co-players’ votes from behavior of participants, it is possible that co-players’ voting behaviour on previous rounds may have influenced participants’ behaviours and expectations in the task. It is also important to consider that neural responses to co-player feedback (acceptance or rejection) may have been influenced by participants’ recent voting behaviour (keep or kick out). In other words, receiving acceptance feedback from a co-player after voting to keep that player, and receiving acceptance feedback after voting to kick that player out may represent distinct psychological processes. The limited number of trials in the present study, however, did not allow for sufficient data to evaluate a stable RewP in each of these conditions (Levinson, Speed, Infantolino, & Hajcak, 2017).
Furthermore, while the feedback stimuli used in the present tasks were perceptually similar, they were not identical; thumbs up and down stimuli are inherently social (Morris, 1994), whereas the meaning of the arrows was learned before task administration. The onset of feedback following response selection was also not identical between the two tasks, which may have impacted response peak and magnitude of the RewP (Weinberg, Luhmann, Bress, & Hajcak, 2012). These task disparities may have introduced differences in strategizing or feedback processing between the two tasks, possibly accounting for weak associations between neural responses to the two feedback types. However, given the complexity of modeling real-world social interactions, methodological trade-offs were made in designing the task to enhance the perceived authenticity of the computerized social interactions. Future studies may benefit from further assessment of the complex component structure of neural responses to social feedback, the influence of participant behaviour on neural responses, and the impact of stimulus type and onset on the findings presented here. Nonetheless, this study adds to the growing body of literature suggesting that assessing sensitivity to social reward, in particular, is critical in understanding adolescent behaviour and the development of psychopathology in this population (Rudolph & Conley, 2005; Shih et al., 2006).
In sum, the present study was the first to assess ERPs to multiple types of reward in multiple age groups. Importantly, temporospatial PCA empirically identified a morphologically similar RewP across incentive types and groups. These findings, which support both domain-general and category-specific reward processes, have significant implications for future work assessing developmental changes in reward processing, as well as the role that sensitivity to specific reward types plays in the development of psychopathology. This work serves as a stepping stone to identifying idiosyncratic patterns of sensitivity to different reward types that may indicate risk for mental illness, and may help to inform prevention efforts throughout the high-risk period of adolescence and emerging adulthood.
Acknowledgments
Funding sources: Canada Research Chair grant to AW; National Institute of Mental Health Grant RO1 MH069942 awarded to DNK
Footnotes
Results of the full PCA are available upon request.
Self-reported task engagement in the social reward task was not significantly associated with the Δ RewP from the Island Getaway task in either sample (early adolescents: r = −.009, p = .96; emerging adults: r = −.18, p = .24), suggesting that the reduced Δ RewP to social feedback in the older sample was not fully explained by a lack of investment in the task.
Conflict of interest statement: The authors report no conflicts of interest.
References
- Anderson BA. Social reward shapes attentional biases. Cognitive Neuroscience. 2016;7(1–4):30–36. doi: 10.1080/17588928.2015.1047823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrondo G, Segarra N, Metastasio A, Ziauddeen H, Spencer J, Reinders NR, Murray GK. Reduction in ventral striatal activity when anticipating a reward in depression and schizophrenia: A replicated cross-diagnostic finding. Frontiers in Psychology. 2015;6:1280. doi: 10.3389/fpsyg.2015.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of Neurophysiology. 2005;94(1):327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Baskin-Sommers AR, Foti D. Abnormal reward functioning across substance use disorders and major depressive disorder: Considering reward as a transdiagnostic mechanism. International Journal of Psychophysiology. 2015;98(2):227–239. doi: 10.1016/j.ijpsycho.2015.01.011. [DOI] [PubMed] [Google Scholar]
- Becker MP, Nitsch AM, Miltner WH, Straube T. A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. The Journal of Neuroscience. 2014;34(8):3005–3012. doi: 10.1523/JNEUROSCI.3684-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson BE, Guyer AE, Nelson EE, Pine DS, Ernst M. Role of contingency in striatal response to incentive in adolescents with anxiety. Cognitive, Affective, & Behavioral Neuroscience. 2015;15(1):155–168. doi: 10.3758/s13415-014-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bress JN, Hajcak G. Self-report and behavioral measures of reward sensitivity predict the feedback negativity. Psychophysiology. 2013;50(7):610–616. doi: 10.1111/psyp.12053.. [DOI] [PubMed] [Google Scholar]
- Bress JN, Meyer A, Proudfit GH. The stability of the feedback negativity and its relationship with depression during childhood and adolescence. Development and Psychopathology. 2015;27(4pt1):1285–1294. doi: 10.1017/S0954579414001400. [DOI] [PubMed] [Google Scholar]
- Bress JN, Smith E, Foti D, Klein DN, Hajcak G. Neural response to reward and depressive symptoms in late childhood to early adolescence. Biological Psychology. 2012;89(1):156–162. doi: 10.1017/S0954579414001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Harmon-Jones E, Proudfit GH. Midbrain volume predicts fMRI and ERP measures of reward reactivity. Brain Structure and Function. 2015;220(3):1861–1866. doi: 10.1007/s00429-014-0725-9. [DOI] [PubMed] [Google Scholar]
- Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G. Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: A combined ERP and fMRI study. Neuroimage. 2011;57(4):1608–1616. doi: 10.1016/j.neuroimage.2011.05.037. [DOI] [PubMed] [Google Scholar]
- Casement MD, Guyer AE, Hipwell AE, McAloon RL, Hoffmann AM, Keenan KE, Forbes EE. Girls’ challenging social experiences in early adolescence predict neural response to rewards and depressive symptoms. Developmental Cognitive Neuroscience. 2014;8:18–27. doi: 10.1016/j.dcn.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124(1):111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Somerville LH. Breaking and accelerating of the adolescent brain. Journal of Research on Adolescence. 2011;21(1):21–33. doi: 10.1111/j.1532-7795.2010.00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RC, Li Z, Li K, Zeng Y-w, Xie W-z, Yan C, Jin Z. Distinct processing of social and monetary rewards in late adolescents with trait anhedonia. Neuropsychology. 2016;30(3):274–280. doi: 10.1037/neu0000233. [DOI] [PubMed] [Google Scholar]
- Chau DT, Roth RM, Green AI. The neural circuitry of reward and its relevance to psychiatric disorders. Current Psychiatry Reports. 2004;6(5):391–399. doi: 10.1007/s11920-004-0026-8. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nature Neuroscience. 2010;13(6):669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. A power primer. Psychological bulletin. 1992;112(1):155. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Collins WA, Welsh DP, Furman W. Adolescent romantic relationships. Annual Review of Psychology. 2009;60:631–652. doi: 10.1146/annurev.psych.60.110707.163459. [DOI] [PubMed] [Google Scholar]
- Crowley MJ, Wu J, Molfese PJ, Mayes LC. Social exclusion in middle childhood: Rejection events, slow-wave neural activity, and ostracism distress. Social Neuroscience. 2010;5(5–6):483–495. doi: 10.1080/17470919.2010.500169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104(1):70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nystrom LE, Fissell C, Noll D, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84(6):3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Damiano CA, Allen JA. Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: Animal models and clinical findings. Journal of Neurodevelopmental Disorders. 2012;4(1):1. doi: 10.1186/1866-1955-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dien J. The ERP PCA Toolkit: An open source program for advanced statistical analysis of event-related potential data. Journal of Neuroscience Methods. 2010a;187(1):138–145. doi: 10.1016/j.jneumeth.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Dien J. Evaluating two-step PCA of ERP data with geomin, infomax, oblimin, promax, and varimax rotations. Psychophysiology. 2010b;47(1):170–183. doi: 10.1111/j.1469-8986.2009.00885.x. [DOI] [PubMed] [Google Scholar]
- Dien J. Applying principal components analysis to event related potentials: A tutorial. Developmental Neuropsychology. 2012;37(6):497–517. doi: 10.1080/87565641.2012.697503. [DOI] [PubMed] [Google Scholar]
- Dien J, Beal DJ, Berg P. Optimizing principal components analysis of event-related potentials: matrix type, factor loading weighting, extraction, and rotations. Clinical Neurophysiology. 2005;116(8):1808–1825. doi: 10.1016/j.clinph.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Dien J, Khoe W, Mangun GR. Evaluation of PCA and ICA of simulated ERPs: Promax vs. Infomax rotations. Human Brain Mapping. 2007;28(8):742–763. doi: 10.1002/hbm.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks MA, Treat TA, Weersing VR. Youth’s Responses to Peer Provocation: Links to Symptoms of Anxiety and Depression. Journal of Psychopathology and Behavioral Assessment. 2014;36(3):339–349. doi: 10.1007/s10862-014-9406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends in Cognitive Sciences. 2007;11(10):419–427. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Research Review: Altered reward function in adolescent depression: what, when and how? Journal of Child Psychology and Psychiatry. 2012;53(1):3–15. doi: 10.1111/j.1469-7610.2011.02477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Ryan ND, Phillips ML, Manuck SB, Worthman CM, Moyles DL, Dahl RE. Healthy adolescents’ neural response to reward: Associations with puberty, positive affect, and depressive symptoms. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(2):162–172.e165. doi: 10.1097/00004583-201002000-00010. doi: http://dx.doi.org/10.1016/j.jaac.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Depression and reduced sensitivity to nonrewards versus rewards: Evidence from event-related potentials. Biological Psychology. 2009;81(1):1–8. doi: 10.1016/j.biopsycho.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Foti D, Hajcak G. Genetic variation in dopamine moderates neural response during reward anticipation and delivery: Evidence from event-related potentials. Psychophysiology. 2012;49(5):617–626. doi: 10.1111/j.1469-8986.2011.01343.x. [DOI] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Bernat EM, Proudfit GH. Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clinical Neurophysiology. 2015;126(7):1338–1347. doi: 10.1016/j.clinph.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti D, Weinberg A, Dien J, Hajcak G. Event-related potential activity in the basal ganglia differentiates rewards from nonrewards: Temporospatial principal components analysis and source localization of the feedback negativity. Human Brain Mapping. 2011;32(12):2207–2216. doi: 10.1002/hbm.21182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4(6):116–124. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie BL, Eisler RM. Development of the feminine gender role stress scale: A cognitive-behavioral measure of stress, appraisal, and coping for women. Behavior Modification. 1992;16(3):426–438. doi: 10.1177/01454455920163008. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7(1):81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80(4):1000–1015. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109(4):679. doi: 10.1037//0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, Pakzad-Vaezi KL, Krigolson OE. The feedback correct-related positivity: Sensitivity of the event-related brain potential to unexpected positive feedback. Psychophysiology. 2008;45(5):688–697. doi: 10.1111/j.1469-8986.2008.00668.x. [DOI] [PubMed] [Google Scholar]
- Horn JL. A rationale and test for the number of factors in factor analysis. Psychometrika. 1965;30(2):179–185. doi: 10.1007/BF02289447. [DOI] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58(2):284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Jones RM, Somerville LH, Li J, Ruberry EJ, Powers A, Mehta N, Casey BJ. Adolescent-specific patterns of behavior and neural activity during social reinforcement learning. Cognitive, Affective, & Behavioral Neuroscience. 2014;14(2):683–697. doi: 10.3758/s13415-014-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keselman H, Wilcox RR, Lix LM. A generally robust approach to hypothesis testing in independent and correlated groups designs. Psychophysiology. 2003;40(4):586–596. doi: 10.1111/1469-8986.00060. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Amminger GP, Aguilar-Gaxiola S, Alonso J, Lee S, Ustun TB. Age of onset of mental disorders: A review of recent literature. Current Opinion in Psychiatry. 2007;20(4):359. doi: 10.1097/YCO.0b013e32816ebc8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63(7):686–692. doi: 10.1016/j.biopsych.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Wimmer GE. Reward: Neural Circuitry for Social Valuation. In: Harmon-Jones E, Winkielman P, editors. Social neuroscience: Integrating biological and psychological explanations of social behavior. New York, NY: Guilford Associated Press; 2007. pp. 157–175. [Google Scholar]
- Kohls G, Herpertz-Dahlmann B, Konrad K. Hyperresponsiveness to social rewards in chilren and adolescents with attention-deficit/hyperactivity disorder (ADHD) Behavioral and Brain Functions. 2009;5(1):20. doi: 10.1186/1744-9081-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Herpertz-Dahlmann B, Konrad K. Differential effects of social and non-social reward on response inhibition in children and adolescents. Developmental Science. 2009;12(4):614–625. doi: 10.1111/j.1467-7687.2009.00816.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24(2)(00):97–129. 00195–0. doi: 10.1016/S0893-133X. [DOI] [PubMed] [Google Scholar]
- Kujawa A, Arfer KB, Klein DN, Proudfit GH. Electrocortical reactivity to social feedback in youth: A pilot study of the Island Getaway task. Developmental Cognitive Neuroscience. 2014;10:140–147. doi: 10.1016/j.dcn.2014.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa A, Proudfit GH, Klein DN. Neural reactivity to rewards and losses in offspring of mothers and fathers with histories of depressive and anxiety disorders. Journal of Abnormal Psychology. 2014;123(2):287. doi: 10.1037/a0036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Greca AM, Lopez N. Social anxiety among adolescents: Linkages with peer relations and friendships. Journal of Abnormal Child Psychology. 1998;26(2):83–94. doi: 10.1023/A:1022684520514. [DOI] [PubMed] [Google Scholar]
- LeMoult J, Colich NL, Sherdell L, Hamilton JP, Gotlib IH. Influence of menarche on the relation between diurnal cortisol production and ventral striatum activity during reward anticipation. Social Cognitive and Affective Neuroscience. 2015 doi: 10.1093/scan/nsv016. nsv016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti LA, Delgado MR. The value of exercising control over monetary gains and losses. Psychological Science. 2014;25(2):596–604. doi: 10.1177/0956797613514589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson AR, Speed BC, Infantolino ZP, Hajcak G. Reliability of the electrocortical response to gains and losses in the doors task. Psychophysiology. 2017 doi: 10.1111/psyp.12813. [DOI] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience. 2012;7(3):274–281. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukie CN, Montazer-Hojat S, Holroyd CB. Developmental changes in the reward positivity: An electrophysiological trajectory of reward processing. Developmental Cognitive Neuroscience. 2014;9:191–199. doi: 10.1016/j.dcn.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Barch DM. Candy and the brain: Neural response to candy gains and losses. Cognitive, Affective, & Behavioral Neuroscience. 2013;13(3):437–451. doi: 10.3758/s13415-013-0156-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luking KR, Luby JL, Barch DM. Kids, candy, brain and behavior: Age differences in responses to candy gains and losses. Developmental Cognitive Neuroscience. 2014;9:82–92. doi: 10.1016/j.dcn.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEvoy JP, Asher SR. When friends disappoint: Boys’ and girls’ responses to transgressions of friendship expectations. Child Development. 2012;83(1):104–119. doi: 10.1111/j.1467-8624.2011.01685.x. [DOI] [PubMed] [Google Scholar]
- Meehl PE. Hedonic capacity: Some conjectures. Bulletin of the Menninger Clinic. 1975;39(4):295–307. [PubMed] [Google Scholar]
- Morgan JK, Olino TM, McMakin DL, Ryan ND, Forbes EE. Neural response to reward as a predictor of increases in depressive symptoms in adolescence. Neurobiology of Disease. 2013;52:66–74. doi: 10.1016/j.nbd.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. Bodytalk: The meaning of human gestures. Crown Publishers; 1994. [Google Scholar]
- Nelson BD, Perlman G, Klein DN, Kotov R, Hajcak G. Blunted neural response to rewards as a prospective predictor of the development of depression in adolescent girls. American Journal of Psychiatry. 2016 doi: 10.1176/appi.ajp.2016.15121524. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: Insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Dyson MW, Rose SA, Durbin CE. Temperamental emotionality in preschool-aged children and depressive disorders in parents: Associations in a large community sample. Journal of Abnormal Psychology. 2010;119(3):468. doi: 10.1037/a0020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, McMakin DL, Morgan JK, Silk JS, Birmaher B, Axelson DA, Forbes EE. Reduced reward anticipation in youth at high-risk for unipolar depression: a perliminary study. Developmental Cognitive Neuroscience. 2014;8:55–64. doi: 10.1016/j.dcn.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Silk JS, Osterritter C, Forbes EE. Social reward in youth at risk for depression: A preliminary investigation of subjective and neural differences. Journal of Child and Adolescent Psychopharmacology. 2015;25(9):711–721. doi: 10.1089/cap.2014.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks ZA, Moor BG, Overgaauw S, Güroğlu B, Dahl RE, Crone EA. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Developmental Cognitive Neuroscience. 2011;1(4):506–516. doi: 10.1016/j.dcn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op de Macks ZA, Bunge SA, Bell ON, Kriegsfeld LJ, Kayser AS, Dahl RE. The effect of social rank feedback on risk taking and associated reward processes in adolescent girls. Social Cognitive and Affective Neuroscience. 2016 doi: 10.1093/scan/nsw125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JG, Rubin KH, Erath SA, Wojslawowicz JC, Buskirk AA. Peer relationships, child development, and adjustment: A developmental psychopathology perspective. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Theory and Method. Hoboken, NJ: John Wiley & Sons Inc; 2015. pp. 419–493. [DOI] [Google Scholar]
- Proudfit GH. The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology. 2015;52(4):449–459. doi: 10.1111/psyp.12370. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Grunder G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. Neuroimage. 2010;49(4):3276–3285. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Rudolph KD. A review of sex differences in peer relationship processes: potential trade-offs for the emotional and behavioral development of girls and boys. Psychological Bulletin. 2006;132(1):98. doi: 10.1037/0033-2909.132.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Conley CS. The socioemotional costs and benefits of social-evaluative concerns: Do girls care too much? Journal of Personality. 2005;73(1):115–138. doi: 10.1111/j.1467-6494.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, Hammen C. Age and gender as determinants of stress exposure, generation, and reactions in youngsters: A transactional perspective. Child Development. 1999;70(3):660–677. doi: 10.1111/1467-8624.00048. [DOI] [PubMed] [Google Scholar]
- Saxe R, Haushofer J. For love or money: A common neural currency for social and monetary reward. Neuron. 2008;58(2):164–165. doi: 10.1016/j.neuron.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annual Review of Psychology. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Shih JH, Eberhart NK, Hammen CL, Brennan PA. Differential exposure and reactivity to interpersonal stress predict sex differences in adolescent depression. Journal of Clinical Child and Adolescent Psychology. 2006;35(1):103–115. doi: 10.1207/s15374424jccp3501_9. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Ruberry EJ, Dyke JP, Glover G, Casey BJ. The medial prefrontal cortex and the emergence of self-conscious emotion in adolescence. Psychological Science. 2013;24(8):1554–1562. doi: 10.1177/0956797613475633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, Rademacher L, Irmak A, Konrad K, Grunder G. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009;4(2):158–165. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stipek D, Mac Iver D. Developmental change in children’s assessment of intellectual comptence. Child Development. 1989;60(3):521–538. doi: http://www.jstor.org/stable/1130719. [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biological Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. doi: http://dx.doi.org/10.1016/S0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Sun S, Yu R. The feedback related negativity encodes both social rejection and explicit social expectancy violation. Frontiers in Human Neuroscience. 2014;8 doi: 10.3389/fnhum.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike EL. Animal intelligence: Experimental studies. New Brunswick, NJ: Transaction Publishers; 2000. [Google Scholar]
- Trezza V, Damsteegt R, Achterberg EM, Vanderschuren LJ. Nucleus accumbens μ-opioid receptors mediate social reward. The Journal of Neuroscience. 2011;31(17):6362–6370. doi: 10.1523/JNEUROSCI.5492-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt T, Brittain HL, McDougall P, Duku E. Longitudinal links between childhood peer victimization, internalizing and externalizing problems, and academic functioning: Developmental cascades. Journal of Abnormal Child Psychology. 2013;41(8):1203–1215. doi: 10.1007/s10802-013-9781-5. [DOI] [PubMed] [Google Scholar]
- Valentin VV, O’Doherty JP. Overlapping prediction errors in dorsal striatum during instrumental learning with juice and money reward in the human brain. Journal of Neurophysiology. 2009;102(6):3384–3391. doi: 10.1152/jn.91195.2008. [DOI] [PubMed] [Google Scholar]
- van der Veen FM, van der Molen MW, Sahibdin PP, Franken IHA. The heart-break of social rejection versus the brain wave of social acceptance. Social Cognitive and Affective Neuroscience. 2013 doi: 10.1093/scan/nst120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Liu H, Hajcak G, Shankman SA. Blunted neural response to rewards as a vulnerability factor for depression: Results from a family study. Journal of Abnormal Psychology. 2015;124(4):878. doi: 10.1037/abn0000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Luhmann CC, Bress JN, Hajcak G. Better late than never? The effect of feedback delay on ERP indices of reward procsseing. Cognitive, Affective, and Behavioural Neuroscience. 2012;12:671–678. doi: 10.3758/s13415-012-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Riesel A, Proudfit GH. Show me the money: The impact of actual rewards and losses on the feedback negativity. Brain and Cognition. 2014;87:134–139. doi: 10.1016/j.bandc.2014.03.01. [DOI] [PubMed] [Google Scholar]
- Whitton AE, Kakani P, Foti D, Veer AV, Haile A, Crowley DJ, Pizzagalli DA. Blunted neural responses to reward in remitted major depression: A high-density event-related potential study. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2016;1(1):87–95. doi: 10.1016/j.bpsc.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink CF, Tong Y, Chen Q, Bassett DS, Stein JL, Meyer-Lindenberg A. Know your place: neural processing of social hierarchy in humans. Neuron. 2008;58(2):273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]


