Summary
The insect wing is a key evolutionary innovation that was essential for insect diversification. Yet despite their importance, there is still debate about their evolutionary origins. Two main hypotheses have been proposed: the paranotal hypothesis suggests that wings evolved as an extension of the dorsal thorax, while the gill-exite hypothesis proposes that wings were derived from a modification of a pre-existing branch at the dorsal base (subcoxa) of the leg. Here we address this question by studying how wing fates are initially specified during Drosophila embryogenesis, by characterizing a cis-regulatory module (CRM) from the snail (sna) gene, sna-DP (for dorsal primordia). sna-DP specifically marks the early primordia for both the wing and haltere, collectively referred to as the dorsal primordia. We found that the inputs that activate sna-DP are distinct from those that activate Distalless, a marker for leg fates. Further, in genetic backgrounds in which the leg primordia are absent, the dorsal primordia are still partially specified. However, lineage-tracing experiments demonstrate that cells from the early leg primordia contribute to both ventral and dorsal appendage fates. Together, these results suggest that the wings of Drosophila have a dual developmental origin: two groups of cells, one ventral and one more dorsal, give rise to the mature wing. We suggest that the dual developmental origins of the wing may be a molecular remnant of the evolutionary history of this appendage, in which cells of the subcoxa of the leg coalesced with dorsal outgrowths to evolve a dorsal appendage with motor control.
Keywords: Insect wing evolution, appendage development, wing primordia, haltere primordia, Drosophila development, leg primordia, embryogenesis, Wingless, Decapentaplegic
eTOC blurb
By studying a cis-regulatory module that is specifically active in the embryonic dorsal (wing and haltere) primordia of Drosophila, Requena et al demonstrate that dorsal fates are derived from two separate groups of cells, one that shares a lineage with the ventral primordia. These data are consistent with a dual evolutionary origin of the wing.
Introduction
It is estimated that nearly three quarters of the species currently living on Earth are insects. Although there have been several hypotheses to explain this vast diversity, one likely contributing factor was the acquisition of flight due to the development of wings approximately 350 million years ago, long before any vertebrate acquired the ability to fly [1, 2]. Yet despite their importance to life on Earth, there is still debate about the evolutionary origins of insect wings [3]. One set of ideas, collectively termed the paranotal hypothesis, suggests that wings evolved as an extension from a part of the dorsal thorax called the thoracic tergum, or paranotal lobe [4, 5]. According to this hypothesis, this anatomical outgrowth initially gave insects the ability to glide, creating the opportunity for the evolution of wing anatomy and function. However, the paranotal lobe was unlikely to have any musculature or neural innervation, raising significant questions about how motor control of these dorsal outgrowths would have evolved. An alternative hypothesis proposes that wings were derived from a modification of a pre-existing branch at the dorsal base of the leg, a region referred to as the subcoxa [6–10]. In aquatic arthropods this structure may have initially evolved as a gill, specializing in gas exchange, and was subsequently modified to become a wing following terrestrialization of some crustacean lineages.
Two principal approaches have informed our current view of insect wing evolution. One approach, which depends on careful examination of the fossil record to trace the origins of the wing, has provided support for both a paranotal lobe and subcoxa origin [5–7, 11]. One limitation of this approach is that there is a large gap in the fossil record that spans the period of time when wings first appeared. An alternative approach relies on comparing the expression patterns of molecular markers of appendages in extant insects and crustaceans that may represent early steps of wing evolution [3]. Using this approach, for example, genes that are expressed in the developing wing of Drosophila melanogaster were also found to be expressed in the subcoxal gills of branched appendages in two different crustaceans, consistent with a subcoxal origin for the insect wing [8, 12]. Although compelling, these types of studies also have their limitations. For one, the presence of similar markers in the fly wing and non-wing structures such as the crustacean gill could represent examples of convergent, instead of divergent, evolution. Second, many of the markers used in these studies are expressed in both leg and wing precursors, or at late stages of development, making them less definitive [13]. More recently, in two insects wing markers were found to be expressed in two separate domains, corresponding to the positions of dorsal outgrowths and subcoxal branches, providing evidence for the idea that wings evolved from a fusion of these two initially distinct structures [3, 14, 15]. This dual origin hypothesis has also been supported by functional studies and recent fossil analysis [11, 16–18].
A complementary approach that may help inform the origins of insect appendages are experiments that characterize how the wing and leg primordia are initially specified during development using cis-regulatory modules (CRMs) that are active in the appendage primordia. These CRMs are not only useful as markers, but they can be used to genetically label and trace the progeny of these primordia. For example, the characterization of an early CRM from the Distalless (Dll) gene in Drosophila called Dll304 has already provided evidence for a subcoxal origin of the wing [19, 20]. Although Dll function is not required for the establishment of wing fates, Dll304 is active in a group of ~30 ventral cells early in embryogenesis, and the progeny of these cells contributes to both the ventral (leg) and dorsal (wing and haltere) appendages [20, 21]. Slightly later in embryogenesis, a different Dll CRM called DllLT is active in a subset of the cells that previously expressed Dll304 [19–21]. Unlike Dll304, DllLT-expressing cells only give rise to part of the leg and do not contribute to dorsal appendages [21, 22]. In sum, these experiments reveal that Dll-expressing ventral cells in the early embryo contribute to both leg and wing fates, but soon thereafter the only adult structure that Dll-expressing cells give rise to is legs (reviewed in [23]).
To the extent that developmental studies in extant organisms can be used to inform evolution, these findings are consistent with a shared evolutionary origin of legs and wings. However, an important but currently missing test of this idea is to characterize CRMs that are specifically active in the dorsal (wing and haltere) primordia (DP). Although several genes, including vestigial (vg), snail (sna), and escargot (esg), are well known embryonic markers of the DP in Drosophila, no CRMs have yet been described that specifically label these cells [24–29]. Here we describe the first such CRM that is specifically active in both the wing and haltere primordia during Drosophila embryogenesis. We use this CRM, derived from the sna gene, to analyze the signals and transcription factors that are required for the specification of the DP. We find that the inputs that activate this CRM are distinct from those that activate Dll, suggesting that the DP are, at least in part, specified independently of the ventral (leg) primordia (VP). Moreover, in genetic backgrounds in which the VP are absent or ablated, DP fates are still partially specified. Together, these results demonstrate that the dorsal appendages of Drosophila have a dual developmental origin: although some DP cells share a lineage with the VP, much of the DP is independently derived from non-VP cells. Based on these developmental data in Drosophila, we discuss the idea of a dual evolutionary origin of wings, in which cells of the subcoxa migrated and coalesced with dorsal outgrowths to evolve a dorsal appendage with motor control.
Results
Identification of the sna-DP CRM
The formation of the wing and haltere primordia requires the function of sna and esg [27]. Although sna expression is restricted to the DP, esg is also expressed in the VP [24–27] (Figure 1B and 2A). Therefore, we searched for CRMs of the sna gene specifically active in the DP.
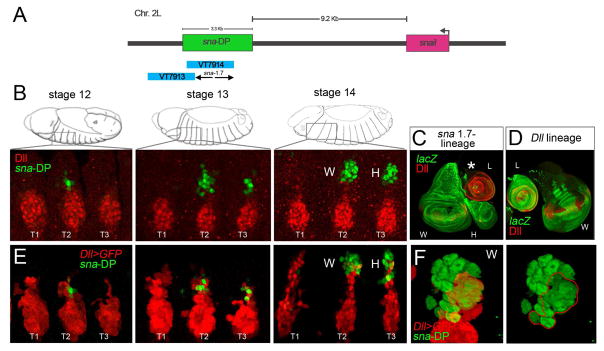
Figure 1. Overview of the sna-DP enhancer and its relationship to Dll expression.
(A) The sna genomic region. sna-DP CRM (green bar) is a 3.3 kb fragment 3′ to the sna transcription start site. VT7914, but not VT7913, (both from VDRC) is active in the DP, and sna-1.7 is defined by the non-overlapping region of these two fragments.
(B) Embryonic time course of sna-DP activity (green) compared to Dll protein (red).
(C, D) Lineage tracing results for sna-DP (C) and Dll (D). (C) The progeny of sna-DP cells (green) label the entire wing and haltere imaginal discs and a small number of cells in the leg (asterisk). (D) The progeny of Dll expressing cells (green) contribute to the entire leg and to parts of the wing disc (see also Figure S2).
(E, F) Time course of sna-DP activity (driving nuclear lacZ; green) compared to Dll>GFP (red). Note that some cells derived from the VP (due to perdurance of GFP from Dll>GFP; red) overlap with sna-DP expressing DP cells (green). (F) An enlargement of a stage 14 wing primordia. W, wing primordia. H, haltere primordia. L, leg primordia. See also Figures S1 and S2 and Movie S1.
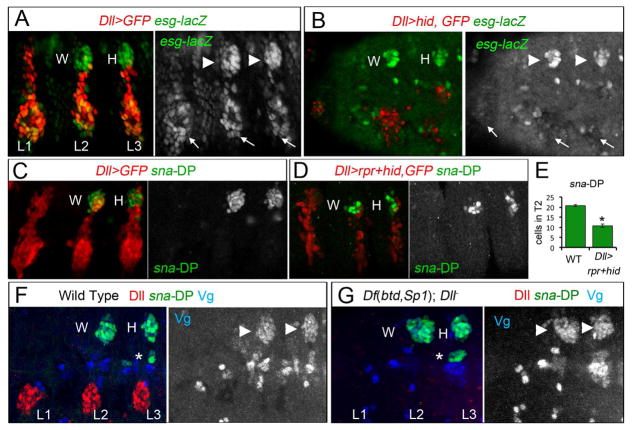
Figure 2. The DP is reduced when the VP is ablated.
(A) Thoracic view of esg-lacZ (green) and Dll>GFP (red) expression in wild type stage 14 embryos. The three leg and DP are labeled with arrows and arrowheads, respectively.
(B) Genetic ablation of the ventral primordia in Dll>hid embryos (red and arrows) reduced the size of the DP (arrowheads) and eliminates ventral expression of esg-lacZ, (arrows).
(C) Thoracic view of sna-DP (green) and Dll>GFP (red) in wild type stage 14 embryo.
(D) Ablation of the ventral primordia in Dll>rpr+hid embryos (red and arrows) reduced the size of the DP (green).
(E) Quantification of the number in T2 of sna-DP (green bars) in wild type and Dll>rpr+hid enbryos. * p<0.05 with Student’s t-test.
(F) Wild type stage 14 embryo stained for sna-DP-lacZ (green), Vg (blue) and Dll (red).
(G) A stage 14 Df(btd,Sp1); Dll− embryo. DP size is unaffected. An asterisk (*) in F and G label a band of muscle cells that express sna-DP and Vg. See also Figure S2.
Although multiple sna CRMs have been identified [30–32], none of them drive expression in the DP. An unbiased scan of the locus identified a single fragment that had such activity; notably, this fragment overlaps with VT7914 from the Vienna Drosophila Resource Center (VDRC) that also shows activity in the DP (Figure 1A). We named this CRM sna-DP and used it to drive lacZ reporter genes and compare its activity with Dll during embryonic development (Figure 1B). sna-DP was first detected at stage 12/13 in a few cells dorsal to Dll expressing cells in the second and third thoracic segment (T2 and T3) and, by stage 14, the number of sna-DP cells in T2 and T3 increased to an average number of 20 and 14 cells, respectively. The sna-DP reporter overlaps perfectly with Sna and Vg protein (Figure S1). Like sna, sna-DP is not active in third instar imaginal discs. However, cell-lineage tracing experiments using a minimized version of sna-DP (sna-1.7; see below) efficiently labeled the entire wing and haltere imaginal discs and a small number of proximal cells of all three leg discs in third instar larvae (Figure 1C and Figure S2). Thus, sna-DP marks DP cells that will give rise to the dorsal regions of the T2 and T3 segments of the adult, including the wing and haltere appendages, respectively.
Partially independent origins of the dorsal and ventral primordia
Because of the shared lineages for the ventral and dorsal appendage primordia [20, 21, 33], we investigated the relationship between these primordia using sna-DP as a marker. First, we compared the spatial and temporal expression pattern between cells that had expressed Dll, using DllMD23-Gal4; UAS-GFP (Dll>GFP), and the sna-DP reporter (Figure 1E). The perdurance of Gal4 and GFP allowed us to trace the cells that had activated, but no longer actively transcribe, Dll. The initial activation of sna-DP at stage 12/13 was observed primarily in Dll>GFP cells, while at stage 14 approximately half of the sna-DP-expressing cells were also labeled with Dll>GFP (Figure 1E, F and Movie S1), consistent with Dll lineage tracing experiments (Figure 1D and Figure S2). Although Dll-expressing cells can give rise to all regions of the third instar wing disc, individual wing discs are only partially labeled and there is a bias for these cells to populate the ventral portion of the disc (Figure 1D and Figure S2). The partial labeling of the wing discs by DllMD23-Gal4 contrasts with near 100% labeling of the leg imaginal discs, arguing that it is unlikely due to a low efficiency of the lineage tracing method. Curiously, lineage tracing with the early Dll304 CRM, which is only active early and transiently, reveals a bias for labeling the anterior compartment (Figure S2).
Although these lineage tracing experiments demonstrate that the progeny of Dll-expressing cells of the VP contribute to dorsal structures, they do not address if the DP requires a contribution from the VP. We first tested this by using Dll MD23-Gal4 to ablate the ventral progenitor cells by expressing the pro-apoptotic gene head involution defective (hid). The leg and wing primordia were visualized with esg-lacZ (Figure 2A, B and Figure S1C). As expected, no VP cells were observed in these embryos, as seen by the absence of ventral esg-lacZ expression. In contrast, DP cells were still present, although the size of dorsal primordia was reduced by approximately 50% (Figure 2B). Similar results were observed when the dose of the pro-apoptotic genes was increased (Figure 2C–E).
We further tested the independence of the DP by examining embryos mutant for Dll and the ventral selector genes buttonhead (btd) and Sp1 [23, 34–36]. Even in these triple mutant embryos sna-DP and vg were normally expressed (Figure 2G). However, although Dll protein is not detected we note that Dll MD23-Gal4 is still active in the triple mutant, suggesting that VP fates are still partially specified (Figure S2G).
Together, these results suggest that in the absence of the VP, or when the VP is severely compromised, DP fates are still specified, although its size is reduced.
Distinct regulation of the dorsal and ventral primordia
If the DP arise in part independently of the VP, we would expect them to have distinct genetic inputs. However, before carrying out a detailed analysis, we used both gain- and loss-of-function manipulations to demonstrate that sna-DP is not a Sna-dependent autoregulatory element, and that it is activated independently of vg, presumably by signals and other transcription factors present in these embryos at the correct time and position (Figure S3).
Dll expression in the VP is activated by Wingless (Wg) and restricted dorsally and ventrally by the Decapentaplegic (Dpp) and Epidermal Growth Factor Receptor (EGFR) pathways, respectively [20, 37–39]. We therefore compared how these three pathways influence the formation and size of both primordia, using sna-DP-lacZ and Dll expression as readouts. In addition to examining mutants, as described below we manipulated these pathways in various ways using prd-Gal4, which is expressed throughout the T2, but not the T3, segment, thus allowing a comparison of prd-Gal4 expressing and non-expressing segments in the same embryo (Figure S3).
Wg
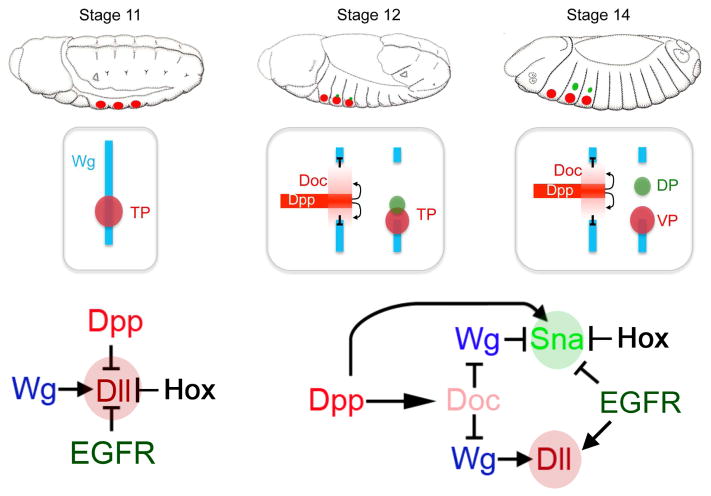
Initially, wg is expressed in dorso-ventral stripes in the anterior compartment of each thoracic segment that are later interrupted due to repression by the T-box transcription factors encoded by the three Dorsocross (Doc) genes [40]. Repression of wg by Doc creates a Wg free domain in the lateral ectoderm (Figure 3A–C). sna-DP activity is only observed after wg repression and within the Doc expression domain (Figure 3D–F). Consistent with these expression patterns, ectopic activation of the Wg pathway by expressing an activated form of the transcriptional co-activator Armadillo (Arm*) results in a smaller DP and an increase in the number of VP cells (Figure 4C and O). Conversely, downregulation of the Wg pathway using a dominant negative version of the Wg transcriptional effector TCF (TCFDN) had no effect on sna-DP, but completely abolished Dll expression (Figure 4B and O). Moreover, in embryos homozygous for a deficiency that removes all three Doc genes, Df(3L)DocA, the DP are absent and there is a dramatic expansion of the Dll-expressing VP (Figure 4D and O). Ectopic expression of one of the Doc genes, Doc-2, eliminates the VP, while the number of sna-DP positive cells remains unchanged (Figure 4E and O). These data are consistent with the idea that Doc-mediated repression of wg is necessary for the formation of the DP, and that wg is an essential activator of the VP [20, 39, 41].
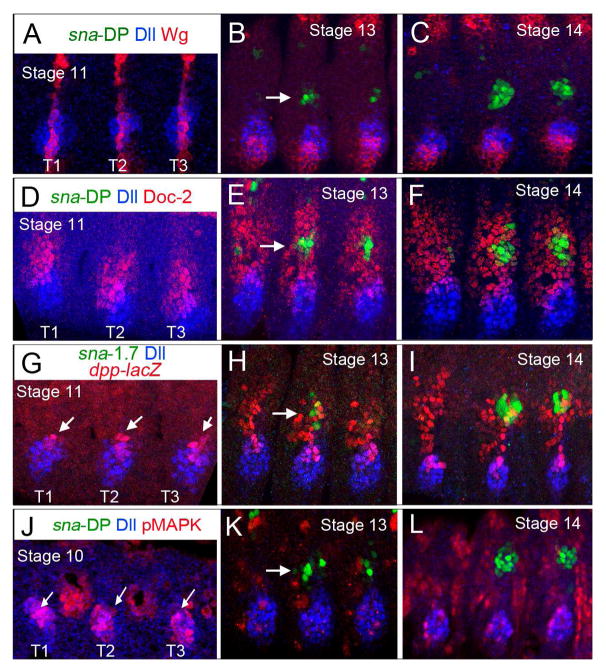
Figure 3. Relationship between sna-DP, Dll, Doc, and signaling pathways.
All panels show thoracic views of wild type embryos stained for the indicated markers.
(A–C) At stage 11 Wg is expressed in uninterrupted dorso-ventral stripes. Later sna-DP is activated in a lateral domain where the Wg stripe is interrupted (arrow).
(D–F) sna-DP is activated within the Doc-2 domain (arrow). (G–I) dpp is initially expressed as a dorsal spot within Dll expressing cells (arrows). At stage 13 and 14 sna-DP is activated in dpp-lacZ expressing cells.
(J–L) pMAPK is detected within Dll expressing cells at stage 10 (arrows in J). sna-DP is active in cells with low or no pMAPK staining. See also Figures S3.
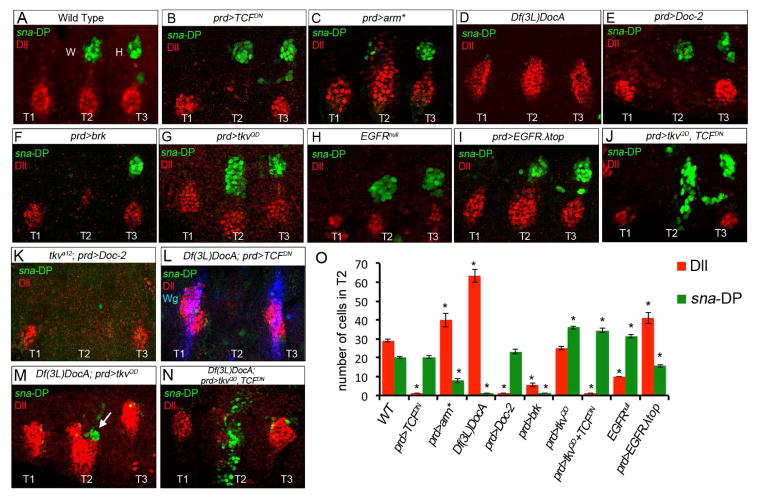
Figure 4. Distinct regulation of Dll and sna-DP.
Thoracic regions of stage 14 embryos stained for Dll (red) to mark the VP, sna-DP (green) to mark the DP, and Wg (L, blue).
(A) Wild type.
(B) In prd>TCFDN embryos the VP is absent and DP not affected in T2.
(C) In prd>arm* embryos VP size increases and DP size decreases in T2.
(D) In Df(3L)DocA embryos DP is absent and VP size is doubled.
(E) In prd>Doc-2 embyros, VP is absent and DP size is unchanged.
(F) In prd>brk embryos both the VP and DP are absent in T2.
(G) In prd>tkvQD embryos DP size increases, while VP size is unchanged in T2.
(H) In EGFRnull mutant embryos, VP size is reduced and DP size increases.
(I) In prd>EGFR. top VP size increases and DP size is reduced in T2.
(J) In prd>tkvQD, TCFDN embryos DP size increases and shifts them ventrally. VP are absent.
(K) A tkva12, prd>Doc-2 embryo. Without Dpp signaling, resupplying Doc-2 does not rescue DP formation.
(L) A Df(3L)DocA, prd>TCFDN embryo. Reducing Wg pathway activation in the absence of the Doc genes fails to rescue DP formation. Dll and wg expression are absent in T2.
(M) A Df(3L)DocA, prd>tkvQD embryo. Increasing Dpp pathway activity partially rescues DP formation (arrow).
(N) A Df(3L)DocA; prd>tkvQD, TCFDN embryo. Simultaneously activating the Dpp pathway and repressing the Wg pathway in the absence of the Doc genes further increases DP size (compare with M).
(O) Quantification of DP (green bars) and VP (red bars) size in the genetic backgrounds shown in (A–J). * p<0.05 with Student’s t-test indicates a significant difference from wild type T2.
Dpp
The dorsal and ventral appendage primordia also have different responses to dpp, which is initially expressed as a dorsal spot within Dll expressing cells and gradually expands dorsally along with sna-1.7 activity (minimized version of sna-DP) (Figure 3G–I). The expression of a constitutively active version of the Dpp receptor, thickveinsQD (tkvQD) almost doubles the size of the DP without affecting the size of the VP (Figure 4G and O). Inhibition of the Dpp pathway through the expression of the Dpp pathway repressor Brinker (Brk), abolishes both sna-DP and Dll expression (Figure 4F and O). The effects of Dpp manipulations on sna-DP are consistent with previous findings that dpp is required for Doc expression [40, 41].
EGFR
Activation of the EGFR pathway, as visualized using an antibody to phospho-MAP Kinase (pMAPK), is initially detected in Dll expressing cells at stage 10/11 and by stage 14 is restricted to a subset of the VP and is mostly absent from the DP (Figure 3J–L). Consistent with previous results [38], in EGFR mutant embryos the size of the DP increases while the size of the VP is reduced (Figure 4H and O). Conversely, expression of a constitutively active version of the EGFR receptor (EGFR.λtop) reduces the size of the DP and increases the size of the VP (Figure 4I and O).
Epistasis experiments
The above experiments suggest that the activation of the Doc genes by Dpp results in the repression of wg in the lateral ectoderm, thus generating a permissive domain where sna-DP can be activated. To further investigate the role of Dpp, Doc and Wg we carried out epistasis experiments to more precisely decipher the logic of sna-DP activation.
Initially, we tested if the activation of the Dpp pathway, which increases the size of the DP, would increase their size further when the Wg pathway was downregulated. In prd>tkvQD, TCFDN embryos, the size of the DP increased compared to WT (compare Figure 4J with 4A), but was similar to the size observed in prd>tkvQD embryos (compare Figure 4J with 4G and 4O).
We next asked if the Doc genes play a role in sna-DP activation besides its indirect role through repression of wg. First, we tried to rescue the lack of sna-DP activation in tkva12 null mutant embryos by expressing Doc-2 with prd-Gal4. Doc-2 was not sufficient to induce sna-DP in the absence of tkv, suggesting a Doc-independent role for the Dpp pathway in activating sna-DP (Figure 4K). Similarly, sna-DP expression was not rescued in Df(3L)DocA embryos in which the the Wg pathway was downregulated by the expression of TCFDN, suggesting that Doc plays a positive role in addition to repression of wg (Figure 4L). In a third experiment, we examined Df(3L)DocA embryos in which the Dpp pathway was also upregulated. In these embryos, we observed a small number of sna-DP positive cells dorsal to the Dll domain (Figure 4M). This limited rescue could be due to ectopic expression of wg typical of Df(3L)DocA mutant embryos [40]. To test for this, we also downregulated the Wg pathway in these embryos (Df(3L)DocA; prd>tkvQD, TCFDN). In these embryos activation of the Dpp pathway was sufficient to increase the number of sna-DP expressing cells (Figure 4N).
Together, these epistasis experiments indicate that sna-DP is activated by the Dpp pathway by two parallel mechanisms: one is via Dpp’s activation of Doc (which represses wg) and one that is independent of Doc (Figure 7). We further conclude that the primary role for Doc in the activation of sna-DP is to repress wg.
Figure 7. Origin and specification of the Drosophila wing.
At stage 11, wg is expressed in continuous dorso-ventral stripes in each segment of the embryo. As the initial group of Dll expressing cells (red circles) contributes to both the DP and VP we refer to them as thoracic primordia (TP). By stage 12, Doc is activated by Dpp and represses wg in the lateral ectoderm. The dorsal primordia (DP, green circle) originates from two populations of cells: one within the TP (cells that had previously expressed Dll) and a second group dorsal to the TP. As embryogenesis progresses the DP separates from the VP. The bottom panels show the known inputs into Dll and sna-DP when Dll is first activated (left) and when sna-DP is activated (right).
Hox regulation of sna-DP
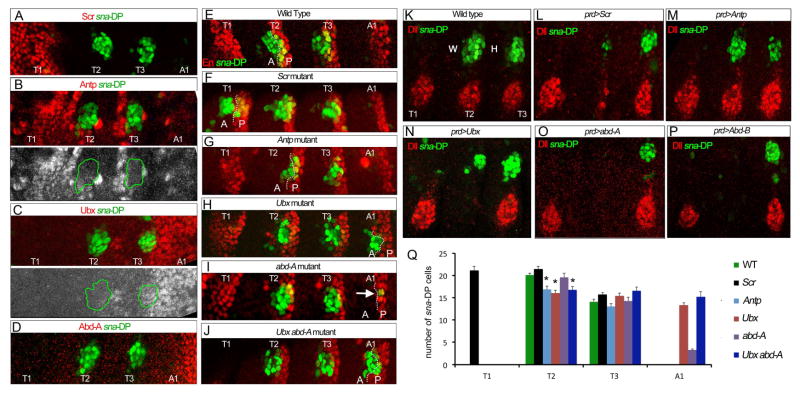
Although the Wg, Dpp, and EGFR pathways are deployed similarly in all thoracic and abdominal segments of the embryo, the DP are only formed in the T2 (wing) and T3 (haltere) segments (Figure 1B). Previous results suggest that the Hox proteins control the segmental expression of Dll and vg [19, 29] and, for Dll, direct Hox inputs have been defined that restrict its expression to the three thoracic segments [19, 42, 43]. To investigate the relationship between the Hox genes and the DP, we first compared the expression of the Hox genes Scr, Antp, Ubx and abd-A with sna-DP (Figure 5A–D). At the stage when sna-DP is fully activated in T2 and T3 (stage 14), Scr is restricted to the first thoracic segment, T1 (Figure 5A). Antp protein is observed in all three thoracic segments where it overlaps with sna-DP at stage 13 (Figure S4). However, by stage 14 Antp is not observed in sna-DP positive cells (Figure 5B). In contrast, Ubx overlaps with sna-DP in the haltere primordia in T3 but not with the wing primordia in T2 (Figure 5C). Abd-A is restricted to the abdominal segments, with higher levels in the posterior compartment (Figure 5D).
Figure 5. Hox regulation of sna-DP.
(A–D) Thoracic and first abdominal segments of stage 14 embryos stained for sna-DP (green) and Scr (red), Antp (red or white in B), Ubx (red or white in C) and Abd-A (red in D). The wing and haltere primordia are outlined in green.
(E–J) Thoracic and first abdominal segments of stage 14 embryos stained for sna-DP (green) and En (red) in wild type (E), Scr (F), Antp (G), Ubx (H), abd-A (I) and Ubx abd-A double (J) mutant embryos. The anterior (A) –posterior (P) compartment border is indicated with a dotted line. Arrow in (H) points to sna-DP cells in the P compartment of A1 in abdA mutant embryos.
(K–P) Thoracic segments of stage 14 embryos stained for sna-DP (green) and Dll (red) in wild type (K), prd>Scr (L), prd>Antp (M), prd>Ubx (N), prd>abd-A (O) and prd>Abd-B (P) embryos. Ectopic expression of Scr, abd-A or Abd-B in T2 represses Dll and sna-DP while prd>Antp does not change these readouts. prd>Ubx reduces the size of the DP domain and eliminates Dll.
(Q) Quantification of DP size in T2 in Hox mutant genotypes. * p<0.05 with Student’s t-test indicates a significant difference from wild type T2. See also Figures S4 and S5.
As the regulation of the VP gene Dll is compartment specific [43], we examined the relationship between sna-DP and the posterior compartment gene engrailed (en) in WT and Hox mutant embryos. In Scr mutant embryos ectopic DP is observed in the T1 segment, in both anterior and posterior compartments (Figure 5F). In Antp mutants sna-DP is expressed in both compartments, but the number of cells is reduced in T2 (Figure 5G and Q). In Ubx mutant embryos, sna-DP is derepressed in the anterior compartment of the first abdominal segment (A1) (Figure 5H and Q). Consistent with this observation, in abd-A mutant embryos sna-DP is derepressed in ~4 posterior compartment cells in abdominal segments (Figure 5I), and in Ubx abd-A double mutant embryos sna-DP is derepressed in both anterior and posterior compartment cells of the abdominal segments (Figure 5J). In Ubx and Ubx abdA mutant embryos we also noticed a reduction in the number of sna-DP cells in T2 compared to wild type embryos, which may be a consequence of partial derepression of Scr (Figure 5Q and Figure S4).
Ectopic expression experiments, using the prd-Gal4 driver, were also informative (Figure 5K–P). prd>Scr is able to nearly eliminate both sna-DP and Dll, while expressing abd-A or Abd-B completely eliminates both primordia. In contrast, although prd>Ubx fully eliminates the VP, it only reduces the size of the DP. prd>Antp did not have any noticeable effect on either Dll or sna-DP. Finally, because a portion of the DP is derived from the VP, we considered the possibility that Hox repression of the DP could in part be an indirect consequence of VP repression. However, by using a Gal4 driver that is active in dorsal, but not ventral, regions of each segment, to drive the expression of abdominal Hox proteins, we found that they can repress the DP independently of the VP (Figure S5).
Molecular dissection of sna-DP
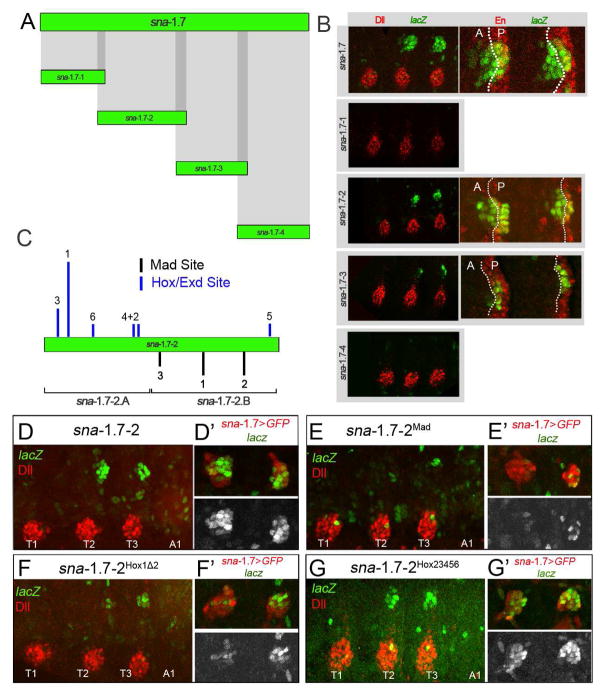
To address how the signals that regulate the expression of sna are integrated at a molecular level we further dissected the sna-DP CRM. We reduced the original sna-DP to a 1.7 Kb fragment (sna-1.7) by comparison to other reporter lines (Figure 1A and Methods). Next, we subdivided the sna-1.7 CRM into 4 overlapping fragments (Figure 6A and B). Although the background of lacZ increased after minimizing the sna-1.7 fragment, the activity in the DP stands out compared to the background. Only the sna-1.7-2 fragment reproduced the expression of sna in both the anterior and posterior compartments of the dorsal primordia (Figure 6B). sna-1.7-3 activity was mostly restricted to the posterior compartment (Figure 6B), but can eventually contribute to both compartments when tested in lineage tracing experiments (Figure S2). Further attempts to dissect sna-1.7-2 into smaller subfragments (sna-1.7-2A and sna-1.7-2.B) were unsuccessful, suggesting that both halves are required for activity (Figure S6). Notably, when in trans to an intact sna-1.7 reporter inserted into the same chromosomal location, the activity of these subfragments was rescued, most likely by a phenomenon known as transvection [44] (Figure S6).
Figure 6. Molecular dissection of sna-DP.
(A) Division of sna-1.7 into 4 overlapping fragments.
(B) Thoracic segments of stage 14 embryos stained for Dll or En (red) and βgal (green). sna-1.7 reproduces the activity of sna-DP in both the A and P compartments. sna-1.7-2 is active in both compartments, while sna-1.7-3 is mostly restricted to the P compartment. sna-1.7-1 and sna-1.7-4 do not drive any expression in the DP.
(C) sna-1.7-2 has predicted binding sites for Hox/Exd (blue lines) and Mad (black lines). The height of the bar indicates the relative score (JASPAR). Subfragments sna-1.7-2.A and sna-1.7-2.B are indicated.
(D–G) Thoracic and first abdominal segments of stage 14 embryos stained for Dll (red) and βgal (green). The right panels show the DP region where the activity of sna-1.7>GFP (red) is compared to the activity of mutant sna-1.7-2 elements (green or white). (D) sna-1.7-2.
(E) sna-1.7-2Mad with the three predicted Mad sites mutated.
(F) sna-1.7-2Hox1Δ2 with Hox site 1 mutated. (G) sna-1.7-2Hox23456 with Hox sites 2–6 mutated. See also Figures S6 and S7.
Because of Dpp’s positive role in sna activation we searched for binding sites of the transcription factor Mothers against Dpp (Mad) within the minimal sna-1.7-2. We found 3 predicted sites that when mutated (sna-1.7-2Mad) strongly reduced the levels of the reporter gene expression (Figure 6E and Figure S6). We also identified a site that binds Antp together with the Hox cofactors Extradenticle (Exd) and Homothorax (Hth) that, when mutated, reduced expression (sna-1.7-2Hox1Δ2) (Figure 6F and Figure S7). However, mutation of this or other putative Hox binding sites failed to result in derepression in the abdominal or T1 segments, leaving open the question of whether Hox repression of sna-DP in these segments is direct (Figure 6F and G).
Discussion
Here, we describe the first CRM in Drosophila, sna-DP, that is specifically active in the embryonic progenitors of the wing and haltere imaginal discs. Lineage tracing experiments using this element demonstrate that the embryonic cells marked by sna-DP are the progenitors for the entire adult thorax of segments T2 and T3, not just the dorsal appendages (wing and haltere). Thus, by comparing the regulation of sna-DP to that of VP-restricted genes and CRMs, we have been able to unambiguously compare the genetic inputs that specify these two primordia, as well as their lineages and spatial relationship to each other. Below we discuss these findings and how they help inform the evolutionary origins of the wing.
Different inputs for specifying the VP and DP
Using Dll and a sna-DP reporter gene as readouts, we extend previous findings to derive the regulatory inputs into the initial specification of these two primordia [19–21, 29, 37–39, 42, 43]. Our key findings, combined with previous observations, are summarized in Figure 7. In stage 11 embryos, Dll is first activated in a group of ~30 cells in each thoracic segment in a Wg-dependent manner. Because these cells can give rise to both ventral and dorsal structures, we refer to this group of cells as the Thoracic Primordia (TP), to highlight the fact that they have a broader developmental potential compared to the VP, which is defined by the set of Dll-expressing cells a few hours later. At this initial stage, the size of the TP is restricted by EGFR ventrally and Dpp dorsally [20, 37]. Also at this early stage, Wg is expressed in a continuous stripe along the dorsal-ventral axis. Soon thereafter, expression of the Doc genes is activated in a set of lateral cells in a Dpp-dependent manner [41] and is responsible for repressing wg, thus interrupting the Wg stripe [40]. Our experiments demonstrate that both conditions – an absence of Wg and presence of Dpp – are required for the initial expression of sna-DP, which is activated in Dpp- and Doc-expressing and Wg-non-expressing region of the embryo. The identification of essential Mad binding sites suggests that the activation of sna-DP by Dpp is direct. In contrast to Dpp-activation of the DP, the primary inducer of Dll in the VP is Wg [21, 22, 39]. The role of EGFR signaling is more complex: although it is initially required to restrict Dll expression from the ventral midline, this expansion of Dll is likely because of a transformation of cell fate [37]. Later in embryogenesis, EGFR signaling plays an activating role in specifying the VP and a negative role in specifying the DP, consistent with previous observations [38].
The TP and, subsequently, the VP are not present in abdominal segments due to repression by the abdominal Hox proteins Ubx and AbdA [19, 42, 43]. There are interesting similarities and differences in the Hox regulation of DP formation. Unlike the VP, which is present in all three thoracic segments, in WT embryos the DP only forms in T2 and T3. Consistently, in Scr null embryos sna-DP is derepressed in T1. Antp has a positive, but not essential role in forming the DP because there are fewer sna-DP-expressing cells in Antp null embryos. Interestingly, Antp has recently been shown to have a positive role in VP size [45]. Further, as observed for Dll [43], repression of sna-DP by the abdominal Hox proteins occurs in a compartment-specific manner. We also found that a subfragment of sna-DP (sna-1.7-3) is mostly expressed in the posterior compartment of T2 and T3, suggesting that the compartment-specific repression by Ubx and Abd-A is mediated by distinct inputs into sna-DP. However, unlike the regulation of Dll in the VP, we have been unable to separate the positive (by Antp) and negative (by Scr, Ubx and Abd-A) Hox inputs into sna-DP: when Hox binding sites in sna-DP were mutated, we only observed reduced expression, thereby leaving unresolved if Hox-mediated abdominal repression of sna-DP is direct. Further, we note that assessing Ubx’s role in T3 is not straightforward: while the size of the DP is smaller in T3 compared to T2, in Ubx null embryos DP size in T3 does not change. This may in part be because of derepression of Scr, which could limit our ability to observe the expected increase in DP size in Ubx null embryos. Nevertheless, ectopic expression of abd-A in T2 completely eliminates the DP, while ectopic expression of Ubx in T2 only reduces the size of DP, highlighting an interesting difference between the activities of these two abdominal Hox proteins.
Implications for the evolution of wings
Our lineage tracing data demonstrate that the wing and haltere imaginal discs are derived from two populations of cells: those that originate in the TP (marked by activity of the early Dll CRM, Dll304), and those that receive distinct cues (high Dpp, low Wg) in more dorsal positions of the thoracic segments. Although these lineage tracing experiments suggest that both populations of cells have the potential to give rise to any part of the wing and haltere imaginal discs, we highlight two differences. First, lineage tracing using TP drivers labels only a portion of each wing disc, and the labeled cells have a tendency to be in the ventral portion of the disc. The ventral bias may be a consequence of the ventral position of the TP relative to the DP, suggesting that the fate of these cells is due to their relative position in the embryo. In contrast, lineage tracing performed with sna-DP is 100% efficient, consistently labeling the entire wing and haltere imaginal discs. The complete labeling of these discs by sna-1.7 argues that the cells that express that CRM, which include the cells derived from the TP, are the precursors of the entire dorsal thorax.
We suggest that the dual developmental origins of the wing primordia may be a molecular remnant of the evolutionary history of this appendage and thus support a dual evolutionary origin of the wing. Interestingly, this idea has also been suggested based on recent expression studies of wing marker genes [3, 14, 15], functional approaches [16, 17] and fossil analysis [18]. In future work, it will be interesting to investigate whether similar CRMs with sna-DP-like activity are conserved in other organisms such as crustaceans. Interestingly, and consistent with this notion, it is noteworthy that a sna ortholog is expressed adjacent to the limb buds in the crustacean Parhyale hawaiensis [46].
In summary, by carrying out CRM-based lineage analyses and genetic studies, our observations complement comparative expression approaches and provide additional support for a dual origin model of the dorsal appendages. From an evolutionary point of view, there are two advantages of such a model. For one, the dorsal contribution to the wing could have provided an initial wing-like structure that allowed airborne insects to glide. Second, a ventral/coxa contribution could have provided an initial source of muscles innervated by motor neurons, allowing directed movements of this structure. It is particularly striking that the dual origins of the DP are still observable in a dipteran fly such as Drosophila, which unlike crustaceans undergoes holometabolan development where the adult structures develop from cells set aside early in embryogenesis. If the dual specification of the dorsal appendage occurs in both holo- and hemimetabolan insects, it would support the idea that it predates the origin of holometabolous metamorphosis.
STAR Methods
Contact for reagents and resource sharing
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Carlos Estella (cestella@cbm.csic.es).
Experimental model and subject details
Fly and embryo culture: Drosophila melanogaster were maintained at 25°C on standard cornmeal agar diet in a humidified incubator. Embryos were collected in apple juice agar plates for 12 hrs. Fly strains are provided in the key resources table.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-β-Gal | MP | 559761 |
| Mouse anti-β-Gal | Promega | Z378A |
| Rabbit anti-GFP | ThermoFisher | A6455 |
| Mouse anti-Wg | DSHB | 4D4 |
| Mouse anti-En | DSHB | 4D9 |
| Mouse anti-Scr | DSHB | 6H4.1 |
| Mouse anti-Ubx | DSHB | FP3.38 |
| Mouse anti-Antp | DSHB | 4C3 |
| Rabbit anti-Abd-A | Santa Cruz | d-130 |
| Guinea Pig-anti-Sna | gift from Yutaka Nibu, Cornell University, USA | |
| Rabbit anti-Vg | gift of Sean Carroll University of Wisconsin-Madison, USA | |
| Guinea Pig anti-Dll | [19] | |
| Guinea Pig anti-Hth | [19] | |
| Rabbit anti-Doc-2 | gift from Ingof Reim, Friedrich-Alexander University Erlangen-Nürnberg, Germany | |
| Rabbit anti-Phospho-p44/42 MAPK | Cell Signaling | 9101 |
| Experimental Models: Organisms/Strains | ||
| prd-Gal4 | Flybase | FBtp0000358 |
| esgNP5130-Gal4 | Flybase | FBal0098823 |
| DllMD23-Gal4 | Flybase | FBti0002783 |
| Doc-1-Gal4 (GMR 45H05) | Flybase | FBsf0000164776 |
| Dll304-Gal4 | Flybase | FBal0288749 |
| esg05730-lacZ | Flybase | FBti0008070 |
| dpp10638 -lacZ | Flybase | FBti0002737 |
| act5C>stop>lacZ; UAS-flp | [46] | |
| Scr4 | Flybase | FBal0015280 |
| Antp25 | Flybase | FBal0000566 |
| Ubx1 | Flybase | FBal0017338 |
| UbxMx12abd-AM1 | Gift from Ernesto Sanchez-Herrero | |
| Df(btd,Sp1) | Flybase | FBab0047246 |
| EGFRnull | Flybase | FBal0066102 |
| Df(3l)DocA | Flybase | FBab0037663 |
| tkva12 | Flybase | FBal0016821 |
| vgnull | Flybase | FBal0093753 |
| snaV2 | Flybase | FBal0015896 |
| UAS-rpr | Flybase | FBst0005823 |
| UAS-hid | Flybase | FBtp0012437 |
| UAS-GFP | Flybase | FBti0012493 |
| UAS-TCFDN | Flybase | FBtp0001721 |
| UAS-arm* (delta N) | Flybase | FBtp0001725 |
| UAS-brk | Flybase | FBtp0085350 |
| UAS-tkvQD | Flybase | FBtp0001199 |
| UAS-Doc-2 | Flybase | FBtp0017741 |
| UAS-EGFRλtop4.2 | Flybase | FBtp0008722 |
| UAS-Scr | Flybase | FBtp0000719 |
| UAS-Antp | Flybase | FBtp0014554 |
| UAS-Ubx | Flybase | FBal0039098 |
| UAS-abd-A | Flybase | FBtp0085557 |
| UAS-Abd-B | Flybase | FBal0038086 |
| UAS-vg | Flybase | FBtp0051400 |
| UAS-sna | Flybase | FBtp0009053 |
| Recombinant Proteins | ||
| His-tag HM (Hth) | [40] | |
| His-tag Exd | [40] | |
| His-tag Dfd | [40] | |
| His-tag Antp | [40] | |
| His-tag Ubx | [40] | |
| His-tag Abd-A | [40] | |
| Oligonucleotides | ||
| sna-DP sense: cagtAAGCTTgtggagcgcaccccaaagct | ||
| sna-DP asense: cagtAGATCTaagggatctgataaagaacgatctcc | ||
| sna-1.7 sense: cagtAAGCTTggttggggttaaagtagagcggc | ||
| sna-1.7 asense: cagtAGATCTtggcaaccgactaacaacgcatc | ||
| sna-1.7-1 sense: cagtAAGCTTggttggggttaaagtagagc | ||
| sna-1.7-1 asense: cagtAGATCTttgatcttgcgggtaagccc | ||
| sna-1.7-2 sense: cagtAAGCTTttcttatgggcttacccgca | ||
| sna-1.7-2 asense: cagtAGATCTcaaagctcagcagcggcagc | ||
| sna-1.7-3 sense: cagtAAGCTTgctgccgctgctgagctttg | ||
| sna-1.7-3 asense: cagtAGATCTatacgttaggcattgctatc | ||
| sna-1.7-4 sense: cagtAAGCTTtagcaatgcctaacgtatcg | ||
| sna-1.7-4 asense: cagtAGATCTtggcaaccgactaacaacgc | ||
| sna-1.7-2A sense: cagtAAGCTTttcttatgggcttacccgca | ||
| sna-1.7-2A asense: cagtAGATCTggtaggaataaaccggaggag | ||
| sna-1.7-2B sense: cagtAAGCTTgaatggcgcccgcctcgattt | ||
| sna-1.7-2B asense: cagtAGATCTcaaagctcagcagcggcagc | ||
| sna-1.7 sense pEntry: CACCggttggggttaaagtagagcggc | ||
| sna-1.7-2 sense pEntry: CACCttcttatgggcttacccgca | ||
| sna-1.7-3 sense pEntry: CACCgctgccgctgctgagctttg | ||
| Mad-1 sense: gtccgccattaaacgatATCATAAtgtTAATtatgtttacagatttgtcg | ||
| Mad-1 asense: cgacaaatctgtaaacataATTAacaTTATGATatcgtttaatggcggac | ||
| Mad-2 sense: ccggtttattcctaccgaatTTATAAATTTtcgattttattaccttc | ||
| Mad-2 asense: gaaggtaataaaatcgaAAATTTATAAattcggtaggaataaaccgg | ||
| Mad-3 sense: ccttatctatcggaccggtctTAAGTAAATAAtgtctgtctgtccccatatctttcagg | ||
| Mad-3 asense: cctgaaagatatggggacagacagacaTTATTTACTTAagaccggtccgatagataagg | ||
| Hox-1 Δ2 sense: agatttacgacagcatttcaCCCCttatgtcacattctaggg | ||
| Hox-1 Δ2 asense: ccctagaatgtgacataaGGGGtgaaatgctgtcgtaaatct | ||
| Hox-2 sense: gcgcatctccgccgtaaaccGTCGTCACGttttatgttaatgcaac | ||
| Hox-2 asense: gttgcattaacataaaaCGTGACGACggtttacggcggagatgcgc | ||
| Hox-3 sense: tgatctgcgatcgacccaagCGGGCATCAagcatttcataatttatgtc | ||
| Hox-3 asense: gacataaattatgaaatgctTGATGCCCGcttgggtcgatcgcagatca | ||
| Hox-4 sense: ggctaagcgcatctccTACTGCCCAAGGatgacatttttatgttaatgc | ||
| Hox-4 asense: gcattaacataaaaatgtcatCCTTGGGCAGTAggagatgcgcttagcc | ||
| Hox-5 sense: gggccatttaattgtctcgaCGTCCGGTAGTAcgctgctgagctttg | ||
| Hox-5 asense: caaagctcagcagcgTACTACCGGACGtcgagacaattaaatggccc | ||
| Hox-6 sense: cgaaccatttgaaaatacccccgccCGCCGGCGTTCgccccgctcctattcagttgcaaa | ||
| Hox-6 asense: tttgcaactgaataggagcggggcGAACGCCGGCGggcgggggtattttcaaatggttcg | ||
| Recombinant DNA | ||
| attB-hs43-nuc-lacZ | [19] | |
| pBPGUw (Gal4) | [47] | |
| Software and Algorithms | ||
| Image J | https://imagej.nih.gov/ij/ | |
| JASPAR | http://jaspar.genereg.net/) | |
| Target Explorer | http://te.cryst.bbk.ac.uk/ [48] | |
| Vienna Drosophila Resource Center | http://enhancers.starklab.org/ | |
| Other | ||
Fly stocks
prd-Gal4, esgNP5130-Gal4, DllMD23-Gal4, Dll304-Gal4, tubGal80ts, Doc-1-Gal4 (GMR 45H05), esg05730-lacZ and dpp10638-lacZ are all described in Flybase and key resource table. For lineage trace analyses the act5C>stop>lacZ; UAS-flp [47] stock was crossed with the corresponding Gal4 lines. For Dll lineage analysis, we restricted the activity of the DllMD23-Gal4 line to embryogenesis using the tubGal80ts. Briefly, embryos were collected at 25° for 12hrs, transferred to 29° for 24hrs and then to 17° until dissection to shutdown Gal4 activity.
Scr4, Antp25, UbxMx12abd-AM1, Df(btd,Sp1), Df(3l)DocA, EGFRnull, tkva12, DllSA1, vgnull and snaV2 are described in Flybase. UAS-hid, UAS-rpr, UAS-GFP, 20XUAS-6XGFP, UAS-TCFDN, UAS-arm* (delta N), UAS-brk, UAS-tkvQD, UAS-TCFDN, UAS-Doc-2, UAS-EGFRλtop4.2, UAS-Scr, UAS-Antp, UAS-Ubx, UAS-abd-A, UAS-Abd-B, UAS-vg, UAS-sna are described in Flybase and key resource table.
Method Details
Immunofluorescence
Imaginal discs were dissected in PBS and fixed with 4% paraformaldehyde in PBS for 25 minutes at room temperature. They were blocked in PBS, 1% BSA, 0.3% Triton for 1 hour, incubated with the primary antibody over night at 4°C, washed four times in blocking buffer, and incubated with the appropriate fluorescent secondary antibody for 1 hour at room temperature in the dark. They were then washed and mounted in Vectashield (Vector Laboratories). Embryos were collected every 12 hrs and dechorionated in 100% bleach for 3 minutes and fixed in 1X PBS, 4% formaldehyde and heptane solution for 25 minutes. Then embryos were devitellinized with methanol and wahsed in PBT (1X PBS and 0.1% tween 20). Embryos were blocked in 3% BSA PBT for 1 hour and incubated with primary antibodies over night, washed four times in PBT, and incubated with the appropriate fluorescent secondary antibody for 1 hour at room temperature in the dark. They were then washed and mounted in Vectashield.
Confocal images were obatined with a Zeiss LSM510 coupled to a vertical Axio Imager.Z1 M.
For visualization a Z-projection was generated using Image J (https://imagej.nih.gov/ij/) for representative embryos of each stage and genotype.
Generation of transgenes
sna-DP, sna-1.7, sna-1.7-1, sna-1.7-2, sna-1.7-3 and sna-1.7-4 sequences were cloned into the attB-hs43-nuc-lacZ plasmid vector [22]. sna-1.7 and sna-1.7-3 were also cloned in the pBPGUw (Gal4) vector [48]. The primers used for cloning each reporter line are described in the key resources table.
Putative Mad and Hox binding sites were identified on the basis of a bioinformatics analysis combining data from the JASPAR CORE Insecta database (http://jaspar.genereg.net/) and the Target Explorer tool [49]. Mutagenesis of the Mad and Hox putative binding sites was performed using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). The primers used for the mutagenesis are described in in the key resources table. All reporter constructs were inserted in the same site 3R (86Fb) to allow proper comparisons. In addition, sna-DP-lacZ and sna-1.7-Gal4 were also inserted in 2R (51D).
Electrophoretic mobility shift assays (EMSAs)
Binding experiments were performed as described previously [42]. Proteins were expressed as His-tag fusions and purified from BL21 cells. HM protein (HM refer to the full-length homeodomainless isoform of Hth) was purified in complex with Exd. Binding reactions for Dfd, Ubx, and AbdA were performed with 150nM Hox protein and 75nM Exd/HM. Antp protein was used at a concentration of 75nM with 30nM Exd/HM. DNA probes were radiolabeled with P32 and used at a concentration of 6nM in the binding reaction.
Quantification and Statistical Analysis
sna-DP and Dll positive cells were counted by taking multiple Z stacks to encompass the entire primordia using Image J (https://imagej.nih.gov/ij/). At least 10 embryos were counted per genotype. Statistical analysis, * p<0.05 with Student’s t-test. Error bars represent the standard error of the mean.
Supplementary Material
3D view of stage 14 wing primordia. Red (Dll>20XUAS-6XGFP) and green (sna-DP). Note that aprox. half of the sna-DP primordia is labeled by Dll>20XUAS-6XGFP.
Highlights.
An enhancer that marks the initial wing primordia in Drosophila is defined
The signals that initiate wing and leg development are distinct
Lineage experiments show the wing comes from ventral and dorsal primordia
Insect wings may have evolved from dual ventral and dorsal inputs
Acknowledgments
We thank Ingolf Reim, Ernesto Sánchez-Herrero and Fernando Diaz-Benjumea for fly stocks and reagents. We thank Richard Allan for his initial contributions to the project and Nuria Esteban for technical help. We also thank Ernesto Sánchez-Herrero, Molly Przeworski, and members of the Mann and Estella labs for comments on the manuscript. This study was supported by grants from the Secretaría de Estado de Investigación, Desarrollo e Innovación (Ministerio de Economía y Competitividad) [No. BFU2015-65728-P to C.E.] and NIH grants RO1GM058575 and R35GM118336 awarded to R.S.M.
Footnotes
Author Contributions:
D.R, J.A.A, H.G, R.L, and C.E performed the experiments. D.R, J.A.A, H.G, R.L, C.E and R.S.M. analyzed the data. C.E. and R.S.M. wrote the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Engel MS. Insect evolution. Current biology : CB. 2015;25:R868–872. doi: 10.1016/j.cub.2015.07.059. [DOI] [PubMed] [Google Scholar]
- 2.Grimaldi D, Engel M. Evolution of the Insects. Cambridge University Press; 2005. [Google Scholar]
- 3.Clark-Hachtel CM, Tomoyasu Y. Exploring the origin of insect wings from an evo-devo perspective. Curr Opin Insect Sci. 2016;13:77–85. doi: 10.1016/j.cois.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Snodgrass RE. Principles of insect morphology. New York: McGraw Hill; 1935. p. 667. [Google Scholar]
- 5.Crampton G. The phylogenetic origin and the nature of the wings of insects according to the paranotal theory. Journal of the New York Entomological Society. 1916;24:1–39. [Google Scholar]
- 6.Kukalova-Peck J. Origin of the insect wing and wing articulation from the arthropodan leg. Canadian Journal of Zoology. 1983;61:1618–1669. [Google Scholar]
- 7.Kukalova-Peck J. Origin and evolution of insect wings and their relation to metamorphosis, as documented by the fossil record. Journal of Morphology. 1978;156:53–125. doi: 10.1002/jmor.1051560104. [DOI] [PubMed] [Google Scholar]
- 8.Averof M, Cohen SM. Evolutionary origin of insect wings from ancestral gills. Nature. 1997;385:627–630. doi: 10.1038/385627a0. [DOI] [PubMed] [Google Scholar]
- 9.Wigglesworth V. Evolution of Insect Wings and Flight. Nature. 1973;246:127–129. [Google Scholar]
- 10.Coulcher JF, Edgecombe GD, Telford MJ. Molecular developmental evidence for a subcoxal origin of pleurites in insects and identity of the subcoxa in the gnathal appendages. Sci Rep. 2015;5:15757. doi: 10.1038/srep15757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rasnitsyn A. A modified paranotal theory of insect wing origin. Journal of morphology. 1981;168:331–338. doi: 10.1002/jmor.1051680309. [DOI] [PubMed] [Google Scholar]
- 12.Damen WG, Saridaki T, Averof M. Diverse adaptations of an ancestral gill: a common evolutionary origin for wings, breathing organs, and spinnerets. Current biology : CB. 2002;12:1711–1716. doi: 10.1016/s0960-9822(02)01126-0. [DOI] [PubMed] [Google Scholar]
- 13.Jockusch EL, Nagy LM. Insect evolution: how did insect wings originate? Current biology : CB. 1997;7:R358–361. doi: 10.1016/s0960-9822(06)00174-6. [DOI] [PubMed] [Google Scholar]
- 14.Clark-Hachtel CM, Linz DM, Tomoyasu Y. Insights into insect wing origin provided by functional analysis of vestigial in the red flour beetle, Tribolium castaneum. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16951–16956. doi: 10.1073/pnas.1304332110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niwa N, Akimoto-Kato A, Niimi T, Tojo K, Machida R, Hayashi S. Evolutionary origin of the insect wing via integration of two developmental modules. Evol Dev. 2010;12:168–176. doi: 10.1111/j.1525-142X.2010.00402.x. [DOI] [PubMed] [Google Scholar]
- 16.Elias-Neto M, Belles X. Tergal and pleural structures contribute to the formation of ectopic prothoracic wings in cockroaches. R Soc Open Sci. 2016;3:160347. doi: 10.1098/rsos.160347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Medved V, Marden JH, Fescemyer HW, Der JP, Liu J, Mahfooz N, Popadic A. Origin and diversification of wings: Insights from a neopteran insect. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:15946–15951. doi: 10.1073/pnas.1509517112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prokop J, Pecharova M, Nel A, Hornschemeyer T, Krzeminska E, Krzeminski W, Engel MS. Paleozoic Nymphal Wing Pads Support Dual Model of Insect Wing Origins. Current biology : CB. 2017;27:263–269. doi: 10.1016/j.cub.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Vachon G, Cohen B, Pfeifle C, McGuffin ME, Botas J, Cohen SM. Homeotic genes of the Bithorax complex repress limb development in the abdomen of the Drosophila embryo through the target gene Distal-less. Cell. 1992;71:437–450. doi: 10.1016/0092-8674(92)90513-c. [DOI] [PubMed] [Google Scholar]
- 20.Cohen B, Simcox AA, Cohen SM. Allocation of the thoracic imaginal primordia in the Drosophila embryo. Development. 1993;117:597–608. doi: 10.1242/dev.117.2.597. [DOI] [PubMed] [Google Scholar]
- 21.McKay DJ, Estella C, Mann RS. The origins of the Drosophila leg revealed by the cis-regulatory architecture of the Distalless gene. Development. 2009;136:61–71. doi: 10.1242/dev.029975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Estella C, McKay DJ, Mann RS. Molecular integration of wingless, decapentaplegic, and autoregulatory inputs into Distalless during Drosophila leg development. Developmental cell. 2008;14:86–96. doi: 10.1016/j.devcel.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Estella C, Voutev R, Mann RS. A dynamic network of morphogens and transcription factors patterns the fly leg. Current topics in developmental biology. 2012;98:173–198. doi: 10.1016/B978-0-12-386499-4.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whiteley M, Noguchi PD, Sensabaugh SM, Odenwald WF, Kassis JA. The Drosophila gene escargot encodes a zinc finger motif found in snail-related genes. Mechanisms of development. 1992;36:117–127. doi: 10.1016/0925-4773(92)90063-p. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi S, Hirose S, Metcalfe T, Shirras AD. Control of imaginal cell development by the escargot gene of Drosophila. Development. 1993;118:105–115. doi: 10.1242/dev.118.1.105. [DOI] [PubMed] [Google Scholar]
- 26.Alberga A, Boulay JL, Kempe E, Dennefeld C, Haenlin M. The snail gene required for mesoderm formation in Drosophila is expressed dynamically in derivatives of all three germ layers. Development. 1991;111:983–992. doi: 10.1242/dev.111.4.983. [DOI] [PubMed] [Google Scholar]
- 27.Fuse N, Hirose S, Hayashi S. Determination of wing cell fate by the escargot and snail genes in Drosophila. Development. 1996;122:1059–1067. doi: 10.1242/dev.122.4.1059. [DOI] [PubMed] [Google Scholar]
- 28.Williams JA, Bell JB, Carroll SB. Control of Drosophila wing and haltere development by the nuclear vestigial gene product. Genes & development. 1991;5:2481–2495. doi: 10.1101/gad.5.12b.2481. [DOI] [PubMed] [Google Scholar]
- 29.Carroll SB, Weatherbee SD, Langeland JA. Homeotic genes and the regulation and evolution of insect wing number. Nature. 1995;375:58–61. doi: 10.1038/375058a0. [DOI] [PubMed] [Google Scholar]
- 30.Ip YT, Park RE, Kosman D, Yazdanbakhsh K, Levine M. dorsal-twist interactions establish snail expression in the presumptive mesoderm of the Drosophila embryo. Genes & development. 1992;6:1518–1530. doi: 10.1101/gad.6.8.1518. [DOI] [PubMed] [Google Scholar]
- 31.Ip YT, Levine M, Bier E. Neurogenic expression of snail is controlled by separable CNS and PNS promoter elements. Development. 1994;120:199–207. doi: 10.1242/dev.120.1.199. [DOI] [PubMed] [Google Scholar]
- 32.Dunipace L, Ozdemir A, Stathopoulos A. Complex interactions between cis-regulatory modules in native conformation are critical for Drosophila snail expression. Development. 2011;138:4075–4084. doi: 10.1242/dev.069146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wieschaus E, Gehring W. Clonal analysis of primordial disc cells in the early embryo of Drosophila melanogaster. Developmental biology. 1976;50:249–263. doi: 10.1016/0012-1606(76)90150-0. [DOI] [PubMed] [Google Scholar]
- 34.Cordoba S, Requena D, Jory A, Saiz A, Estella C. The evolutionarily conserved transcription factor Sp1 controls appendage growth through Notch signaling. Development. 2016;143:3623–3631. doi: 10.1242/dev.138735. [DOI] [PubMed] [Google Scholar]
- 35.Estella C, Mann RS. Non-redundant selector and growth-promoting functions of two sister genes, buttonhead and Sp1, in Drosophila leg development. PLoS genetics. 2010;6:e1001001. doi: 10.1371/journal.pgen.1001001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Estella C, Rieckhof G, Calleja M, Morata G. The role of buttonhead and Sp1 in the development of the ventral imaginal discs of Drosophila. Development. 2003;130:5929–5941. doi: 10.1242/dev.00832. [DOI] [PubMed] [Google Scholar]
- 37.Goto S, Hayashi S. Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development. 1997;124:125–132. doi: 10.1242/dev.124.1.125. [DOI] [PubMed] [Google Scholar]
- 38.Kubota K, Goto S, Eto K, Hayashi S. EGF receptor attenuates Dpp signaling and helps to distinguish the wing and leg cell fates in Drosophila. Development. 2000;127:3769–3776. doi: 10.1242/dev.127.17.3769. [DOI] [PubMed] [Google Scholar]
- 39.Kubota K, Goto S, Hayashi S. The role of Wg signaling in the patterning of embryonic leg primordium in Drosophila. Developmental biology. 2003;257:117–126. doi: 10.1016/s0012-1606(03)00062-9. [DOI] [PubMed] [Google Scholar]
- 40.Reim I, Lee HH, Frasch M. The T-box-encoding Dorsocross genes function in amnioserosa development and the patterning of the dorsolateral germ band downstream of Dpp. Development. 2003;130:3187–3204. doi: 10.1242/dev.00548. [DOI] [PubMed] [Google Scholar]
- 41.Hamaguchi T, Yabe S, Uchiyama H, Murakami R. Drosophila Tbx6-related gene, Dorsocross, mediates high levels of Dpp and Scw signal required for the development of amnioserosa and wing disc primordium. Developmental biology. 2004;265:355–368. doi: 10.1016/j.ydbio.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 42.Gebelein B, Culi J, Ryoo HD, Zhang W, Mann RS. Specificity of Distalless repression and limb primordia development by abdominal Hox proteins. Developmental cell. 2002;3:487–498. doi: 10.1016/s1534-5807(02)00257-5. [DOI] [PubMed] [Google Scholar]
- 43.Gebelein B, McKay DJ, Mann RS. Direct integration of Hox and segmentation gene inputs during Drosophila development. Nature. 2004;431:653–659. doi: 10.1038/nature02946. [DOI] [PubMed] [Google Scholar]
- 44.Duncan IW. Transvection effects in Drosophila. Annual review of genetics. 2002;36:521–556. doi: 10.1146/annurev.genet.36.060402.100441. [DOI] [PubMed] [Google Scholar]
- 45.Uhl JD, Zandvakili A, Gebelein B. A Hox Transcription Factor Collective Binds a Highly Conserved Distal-less cis-Regulatory Module to Generate Robust Transcriptional Outcomes. PLoS genetics. 2016;12:e1005981. doi: 10.1371/journal.pgen.1005981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hannibal RL, Price AL, Parchem RJ, Patel NH. Analysis of snail genes in the crustacean Parhyale hawaiensis: insight into snail gene family evolution. Development genes and evolution. 2012;222:139–151. doi: 10.1007/s00427-012-0396-6. [DOI] [PubMed] [Google Scholar]
- 47.Struhl G, Basler K. Organizing activity of wingless protein in Drosophila. Cell. 1993;72:527–540. doi: 10.1016/0092-8674(93)90072-x. [DOI] [PubMed] [Google Scholar]
- 48.Pfeiffer BD, Jenett A, Hammonds AS, Ngo TT, Misra S, Murphy C, Scully A, Carlson JW, Wan KH, Laverty TR, et al. Tools for neuroanatomy and neurogenetics in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9715–9720. doi: 10.1073/pnas.0803697105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sosinsky A, Bonin CP, Mann RS, Honig B. Target Explorer: An automated tool for the identification of new target genes for a specified set of transcription factors. Nucleic acids research. 2003;31:3589–3592. doi: 10.1093/nar/gkg544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
3D view of stage 14 wing primordia. Red (Dll>20XUAS-6XGFP) and green (sna-DP). Note that aprox. half of the sna-DP primordia is labeled by Dll>20XUAS-6XGFP.