Abstract
Background:
The squid ink extract is well known for its biomedical properties.
Objective:
In this study, squid Loligo vulgaris was collected from Tuticorin costal water, Bay of Bengal, India.
Materials and Methods:
Proximate composition of the crude squid ink was studied and found to have protein as the major component over lipid and carbohydrates. Further, bioactive fractions of squid ink were extracted with ethanol, and therapeutic applications such as hemolytic, antioxidant, antimicrobial, and in vitro anti-inflammatory properties were analyzed using standard methods.
Results:
In hemolytic assay, the squid ink extract exhibited a maximum hemolytic activity of 128 hemolytic unit against tested erythrocytes. In DPPH assay, the ethanolic extract of squid ink has exhibited an antioxidant activity of 83.5%. The squid ink was found to be potent antibacterial agent against the pathogens tested. 200 μL of L. vulgaris ink extract showed remarkable antibacterial activity as zone of inhibition against Escherichia coli (28 mm), Klebsiella pneumoniae (22 mm), Pseudomonas aeruginosa (21 mm), and Staphylococcus aureus (24 mm). The 68.9% inhibition of protein denaturation by the squid ink extract indicated that it has very good in vitro anti-inflammatory properties. The Fourier transform infrared spectroscopy analysis of the ethanolic extracts of the squid ink indicated the presence of functional groups such as 1° and 2° amines, amides, alkynes (terminal), alkenes, aldehydes, nitriles, alkanes, aliphatic amines, carboxylic acids, and alkyl halides, which complements the biochemical background of therapeutic applications.
Conclusion:
Hence, results of this study concluded that the ethanolic extract of L. vulgaris has many therapeutic applications such as antimicrobial, antioxidant, and anti-inflammatory activities.
SUMMARY
Squid ink is very high in a number of important nutrients. It’s particularly high in antioxidants for instance, which as well all know help to protect the cells and the heart against damage from free radicals. In the present study, the squid ink have antioxidant, anti-inflammatory and cytotoxic properties and can be considered as promising the developing the drugs.
Abbreviations Used: DPPH: 2,2-diphenyl-1-picrylhydrazyl, FTIR: Fourier-transform infrared spectroscopy, BSA: Bovine Serum Albumin
Keywords: Anti-inflammatory, antimicrobial, DPPH, hemolytic activity, ink extracts, Loligo vulgaris
INTRODUCTION
The marine organisms have had a major impact on the development of medical science. More recent studies on marine organisms have focused mainly on their application for the treatment of human diseases. Many marine chemicals often possess quite novel structures which lead to pronounced biological activity and novel pharmacological properties.[1] A number of discovery efforts have yielded several bioactive metabolites which have been successfully developed by the pharmaceutical industry.[2] The marine invertebrates rely solely on innate immune mechanisms for host defense which is a spectacular resource for the development of new bioactive compounds and have been recognized to offer a source of potential antimicrobial, anti-inflammatory, and antitumor drugs.[3]
Squids form the major constituent of the class Cephalopoda. The ink sacs form the waste material in squid. Squids are important seafood resource with pharmacological importance. In India, the sea squid ink has traditional applications in food and pharmacological products.[4] It was also reported that the squid ink has potential antiretroviral activity.[5] The peptidoglycan and acetone delipidated ink of squid showed antitumour activity.[6] The ink and tissue extracts of cuttlefish and squid showed antibacterial effect.[7] The squid ink is a multifunctional marine bioactive material and is also reported to exhibit antimicrobial activity. Mochizuki[8] studied the antibacterial activity of purified extract of the cuttlefish, Sepioteuthis lessoniana against Staphylococcus aureus. Takai et al.[9] reported the bioactivity of squid ink. Patterson and Murugan[10] observed broad spectrum of antibacterial activity for aqueous ink extract of the Loligo duvauceli and Sepia pharaonis against nine human pathogens. Rajaganapathi et al.[11] studied the squid ink for anti-retroviral activity. Antibacterial effect of the Indian squid L. duvauceli was reported by Nirmale et al.[12] Chacko and Patterson[13] described the antibacterial activity of pharaoh’s cuttlefish, S. pharaonis. Though several attempts are currently being made to isolate bioactive substances from these waste materials for biomedical research, comprehensive studies on testing multiple biological activities from the squid ink are yet to be done. Hence, the present study was designed to investigate the in vitro anti-inflammatory, antimicrobial, and antioxidants properties of ethanolic extracts Indian squid, Loligo vulgaris.
MATERIALS AND METHODS
Collection and identification
The specimens of L. vulgaris were collected from the Tuticorin fish landing center, India, during June of 2016. The specimens were thoroughly washed with treated sea water at the site of collection and transported with ice to laboratory, and specimens were identified by the standard literature of Fernando and Fernando.[14] The collected animals were held ventrally and the posterior end of the animal was squeezed to eject ink. The ink was collected and stored at −4°C.
Determination of proteins
In the present study, total protein content of the whole squid ink was determined by the method of Bradford.[15] The samples were mixed with 5 mL of Bradford reagent. Protein standard (bovine serum albumin [BSA]) solution was also prepared and absorbance was read at 595 nm using Shimadzu 160 ultraviolet-visible (UV-VIS) double-beam spectrophotometer.
Determination of lipids
The total lipids content of the whole squid ink was estimated by sulfo-phospho-vanillin method as described by Barnes and Blackstock.[16] The standard oil was taken in different concentrations and the ink samples were heated for 10 min in a boiling water bath and cooled, and an aliquot of each sample was placed in a dry tube. The blank contained concentrated sulfuric acid. In each of the test aliquots 6 ml of phospho-vanillin reagent was added and kept at room temperature for 15 min. The absorbance was read at 546 nm in UV VIS spectrophotometer.
Determination of total carbohydrate
The total carbohydrate of the whole ink extract was quantified by Anthrone method as described by Roe et al.[17] The weighed ink samples were taken into a boiling tube and were hydrolyzed by keeping in water bath for 3 h with 2.5 N HCl and cooled. After centrifugation, the supernatant was collected and aliquots were taken for analysis. Glucose solution was taken as standard and 2.5 N HCl with distilled water served as blank. Then, Anthrone reagent was added, heated, and then cooled and absorbance was read at 630 nm.
Determination of antioxidant activity
DPPH radical scavenging activity of the ethanolic extract of squid ink was determined by the method of Binsan et al.[18] The ink samples were prepared in concentrations of 50–200 μg/ml and 0.1 mM of DPPH in 95% (v/v) Methanol was added to the sample. Ascorbic acid was taken as the reference standard and in the blank deionized water was used instead of the sample. The mixture was allowed to stand at room temperature in the dark for 30 min. The absorbance of the resulting solution was measured at 517 nm using a spectrophotometer.
Hemolytic activity
The hemolytic assay was carried out using ethanolic extracts of squid ink according to Paniprasad and Venkateshvaran[19] in “U”- shaped microtiter plates. The blood was centrifuged thrice at 5000 rpm for 10 min. Five percent erythrocyte suspension was prepared for hemolytic study. The concentration of the extract tested was 10 mg/mL. Simultaneously, distilled water was used as negative control whereas saline water was used as positive control. The plates were incubated for 3 h at room temperature and the results were observed. Formation of a fine “button cell” with regular margin indicates the negative reaction. A uniform red-colored suspension of the lysed RBC indicates the positive result. Hemolytic activity was expressed as hemolytic unit (HU).
Antibacterial activity by agar well diffusion method
Antibacterial activity of ethanolic extracts of squid ink was investigated against Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and S. aureus These bacterial cultures (clinical isolates) were obtained from Meenakshi Hospital, Thanjavur. The antibacterial activity was carried out by agar well diffusion method[20] using different concentration, i.e., 20, 50, 100, and 200 μg/mL. Four wells of 5 mm size each made into the agar plates with the help of sterile cork borer. The wells were loaded with appropriate test concentrations of squid ink extract. All the plates were incubated at 37°C for 24 h. After incubation, the plates were observed for clear inhibition zone around the well, indicated the presence of antibacterial activity of tested samples.
Inhibition of protein denaturation
Inhibition of protein denaturation by the ethanolic extracts of squid ink was evaluated by the method of Mizushima and Kobayashi[21] with slight modification. 500 μL of 1% BSA was added to 100 μL of squid ink extract. This mixture was kept at room temperature for 10 min, followed by heating at 51°C for 20 min. The resulting solution was cooled down to room temperature and absorbance was recorded at 660 nm. Acetyl salicylic acid was taken as a positive control. The experiment was carried out in triplicates and percent inhibition for protein denaturation was calculated using:
%Inhibition of protein denaturation = 100 − ([A1 − A2]/A0 × 100)
where A1 is the absorbance of the sample, A2 is the absorbance of the product control, and A0 is the absorbance of the positive control.
Protease inhibition assay
Inhibition of trypsin by the ethanolic extracts of squid ink was evaluated by the method of Sakat et al.[22] 100 mL of BSA was added to 100 μL of squid ink extract. This was incubated at room temperature for 5 min. Reaction was inhibited by the addition of 250 μL of trypsin followed by centrifugation. The supernatant was collected, and absorbance was observed at 210 nm. Acetyl salicylic acid was used as a positive control. The experiment was carried out in triplicates and percent inhibition of protease inhibition was calculated.
%Protease inhibition = 100 − ([A1-A2]/A0 × 100)
where A1 is the absorbance of the sample, A2 is the absorbance of the product control, and A0 is the absorbance of the positive control.
Fourier transform infrared spectroscopy
The ethanolic extracts of the squid ink were freeze dried, and Fourier transform infrared (IR) spectra were recorded in Shimadzu IR Affinity-1S.
RESULTS AND DISCUSSION
Marine natural products continue to be a structurally diverse and pharmacologically most interesting source of bioactive metabolites. Some of them hold great potential for the development of new and much needed drugs primarily in the treatment of diabetic, inflammatory, cancer, etc. The traditional knowledge regarding the medicinal value of the marine fishes is prevalent among the local communities from the immemorial. In the absence of such traditional knowledge, the categorizing of the fishes according to their medicinal value is Herculean task as the varieties of fishes are more.
Squid ink is very rich in important nutrients. The proximate analysis of squid ink extracts for major biochemical contents were estimated. The carbohydrate content was found to be 4.81%, protein level 14.52%, and lipid level 0.82%. The percentage of carbohydrate and lipid content is very less and protein level is similar according to Nutrition Information of Squid Ink (http://www.fitbit.com/foods/Squid+Ink+Fettuccine+Pasta+Noodles/63658).
Squid ink has acquired unique space in the biomedical application, particularly high in antioxidants for instance, which as well all know help protect the cells and the heart against damage from free radicals. This means that squid ink might be useful in combating the visible signs of aging, heart disease and various threats to the immune system.[23] DPPH-free radical scavenging assay is an easy, rapid, and sensitive method for the antioxidant screening of animal extracts.[24] The DPPH radical scavenging activities are based on the ability of antioxidants to donate a hydrogen atom or an electron to stabilize radicals by converting them to the nonradical species.[25] DPPH is a radical having an odd electron and reacts with hydrogen donated from antioxidant. The DPPH radical obtain one more electron and the absorbance decreases.[26] In the present study, the L. vulgaris extracts has high DPPH scavenging capacity, which increased with increasing concentration [Figure 1] The DPPH assay was carried out at different concentrations of L. vulgaris extracts, such as 50, 100, 150, and 200 μg/mL. DPPH assay did not show any significant difference at 50 and 100 μg/mL concentrations in L. vulgaris sample; however, it was significant for 150 and 200 μg/mL for the extracts DPPH is a relatively stable-free radical. DPPH radical react with suitable reducing agents, the electrons become paired off, and the solution losses color stoichiometrically depending on the number of electrons taken up. Hence, this assay provides information on reactivity of test samples with a stable-free radical. The decrease in the absorbance of the DPPH radical caused by test samples was due to the scavenging of radical by electron donation.
Figure 1.
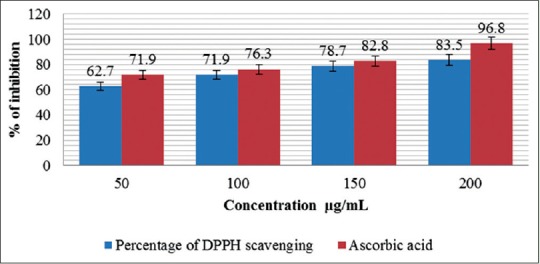
DPPH radical scavenging assay of Loligo vulgaris
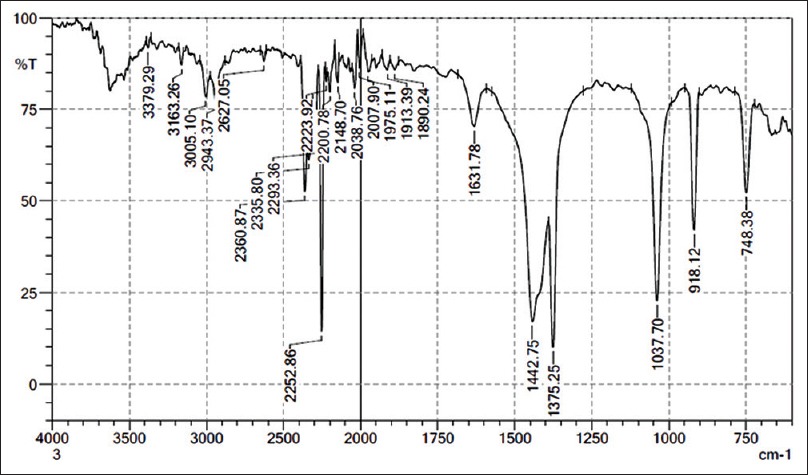
IR spectroscopy is among the most powerful spectroscopic techniques for food analysis since it covers the details on the functional group as well as chemical composition that is contained in the IR spectrum of specific substance.[27] IR spectroscopy is a powerful method for studying molecular structure and intramolecular interaction in biological tissues and cells.[28] Several authors have studied IR spectroscopy on biological substances such as muscle and liver. The IR spectroscopy is a promising technique both to define the biochemical basis of cell viability more clearly with quantitative information about chemical functional groups in cells and to identify those characteristics specific to viable cells.[29] Fourier transform IR spectroscopy (FTIR) allows measurement of the entire spectrum simultaneously, providing a means to collect spectral information accurately and rapidly.[30] The present study was carried out to analyze the functional group of ethanolic extract L. vulgaris ink using FTIR [Table 1].
Table 1.
Fourier transform infra-red spectroscopy analysis of ethanolic extraction of Loligo vulgaris ink
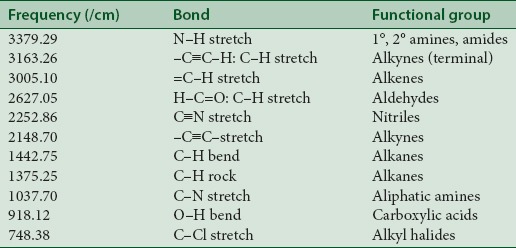
Figure 2 indicates that strong absorbance were observed at peak 3379.29 cm–1, 3163.26 cm–1, 3005.10 cm–1, 2627.05 cm–1, 2252.86 cm–1, 2148.70 cm–1, 1442.75 cm–1, 1375.25 cm–1, 1037.70 cm–1, 918.12 cm–1, 748.38 cm–1, which are characteristic of N–H stretch, –C≡C–H: C–H stretch, =C–H stretch, H–C=O: C–H stretch, C≡N stretch, –C≡C– stretch, C–H bend, C–H rock, C–N stretch, O–H bend, C–Cl stretch and the presence of functional groups such as 1° and 2° amines, amides, alkynes (terminal), alkenes, aldehydes, nitriles, alkanes, aliphatic amines, carboxylic acids, and alkyl halides. In overall, spectral profile is similar except for the variation in intensities of the bands. The most widely used modes in protein structural studies are 1° and 2° amines and amides. The broad band at 3297 cm–1 has been assigned that in the present study to O–H stretching, the bands of proteins have made a small contribution to it. The bands observed at 2923 cm–1 and at 3250 cm–1 are due to the asymmetric and symmetric starching modes of the methylene chain in the membrane lipids.[31] The sharp bands observed at 1653 cm–1 are assigned to the in plane C=O stretching vibration (amide) and to the aliphatic amines and carboxylic acid.[32]
Figure 2.
Fourier transform infrared spectroscopy analysis of Loligo vulgaris ink
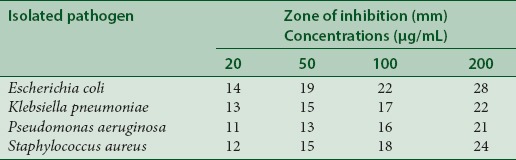
The squid ink of contains a rich array of chemical secretions to escape from predators; it contains many constituents of bioactive compounds and antimicrobial properties.[33] There are previous reports on the antibiotic effects of the fluid from the ink sac of cephalopods and the antibacterial activity in the extracts of gill and ink sac of cephalopods,[34] reported the ink of cephalopods exhibit antimicrobial activity. Purified extract of the cuttlefish, S. lessoniana ink showed antibacterial activity against S. aureus.[8] Smiline et al.[35] studied that the methanol extracts from the ink of L. duvaucelii exhibited activity against several bacteria. The methanol extract from ink of L. duvaucelii showed 18 mm zone of inhibition against the bacteria tested except E. coli and K. pneumoniae. These findings corroborate the results of the present study. In the present investigation, crude ink of L. duvauceli at 200 μl showed highest activity against E. coli (28 mm), K. pneumoniae (22 mm), P. aeruginosa (21 mm), and S. aureus (24 mm) [Table 2]. This results very similar to the Patterson and Murugan.[10]
Table 2.
Antimicrobial activity of Loligo vulgaris ink against tested pathogens
Hemolysis may be said to exist when the normal rate of red blood cell destruction is increased. Many of the biologically active compounds were found be responsible for hemolysis of the cell membrane. It has been proved that squid ink samples showed a better result which acted on the RBC’s and destructed them when compared to other test samples. Hemolytic activity was performed earlier[36] and was used as a reference work where the bioactive compounds from marine source have been studied. The breakdown of hemoglobin begins with oxidative ring rupture at the α-methene group of the porphyrin fraction of the molecule. In this study the hemolytic assay has been performed with human blood samples, B + ve group blood showed maximum 128 HU. A similar result was reported in the mucus of two other fish species, Channa punctatus and Cirrhinus mrigala.[37] Further, Uthayakumar et al.[38] observed that the mucus secretion of Mastacembelus armatus possesses a potent hemolytic activity against sheep and cow blood cells.
There are certain problems in using animals in experimental pharmacological research, such as ethical issues and the lack of rationale for their use when other suitable methods are available or could be investigated. Hence, in the present study, the protein denaturation and anti-proteinase action bioassay were selected for in vitro assessment of anti-inflammatory property of ethanolic extraction of L. vulgaris ink. The acetyl salicylic acid was used as a standard drug for inflammation which dose-dependent ability to thermally induced protein denaturation.[21] As a part of the investigation the anti-inflammatory activity of L. vulgaris was studied. It was effective in protein denaturation at different concentrations 50, 100, 150, 200 μg/mL, respectively. The L. vulgaris were observed as [Figure 3] squid ink of 68.9 μg/mL and acetyl salicylic acid of 77.9 μg/mL, respectively.
Figure 3.
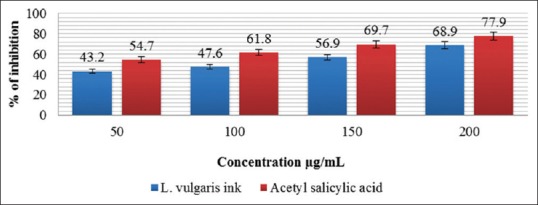
Inhibition of protein denaturation of Loligo vulgaris ink
Proteinases have been implicated in arthritic reactions. Neutrophils are known to be a rich source of proteinase which carries in their lysosomal granules many serine proteinases. It was previously reported that leukocytes proteinase play an important role in the development of tissue damage during inflammatory reactions and significant level of protection was provided by proteinase inhibitors.[39] In the present study, the antiproteinase ability of ethanolic extraction of L. vulgaris was investigated at different concentration. The ethanolic extracts of L. vulgaris have shown squid ink of 67.4 μg/mL and acetyl salicylic acid of 67.4 μg/mL [Figure 4].
Figure 4.
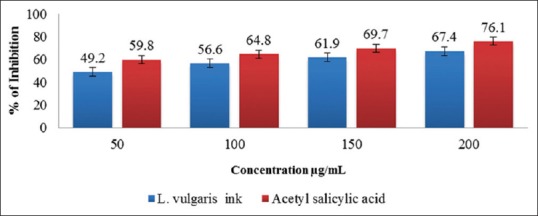
Inhibition Antiproteniase activity of L. vulgaris ink
CONCLUSION
Squid Ink has a diversity of benefits to offer us through industrial and medical applications. They are major potential benefits are identifying antimicrobials to treat products used in food, cosmetics and healthcare and developing drugs for use as antimicrobials, anti-inflammatory and anti-oxidants properties. It offers promise for identifying new, prospective drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We are thankful to the AMET University management for facilities provided.
REFERENCES
- 1.Lei J, Zhou J. A marine natural product database. J Chem Inf Comput Sci. 2002;42:742–8. doi: 10.1021/ci010111x. [DOI] [PubMed] [Google Scholar]
- 2.Kong F, Andersen RJ, Allen TM. Madangamine A, a novel cytotoxic alkaloid from the marine sponge Xestospongia ingens. J Am Chem Soc. 1994;116:6007. [Google Scholar]
- 3.Bansemir A, Blume M, Schröder S, Lindequist U. Screening of cultivated seaweeds for antibacterial activity against fish pathogenic bacteria. Aquaculture. 2006;252:79–84. [Google Scholar]
- 4.Nishimoto J, Motohiro T, Oomori H, Kuizumi C, Tamoto H, Kakehashi K, Shimuzu H, Kobatake A. Shiokara (salted and fermented seafoods). Shin Suisangaku Zenshu 25, Suisan Kakou Gijutsu Kouseisha - Kouseisha, Tokyo. 1980:240–4. [Google Scholar]
- 5.Suji S. Various uses of cephalopods. Fish Chim. 2009;29:24–6. [Google Scholar]
- 6.Sasaki J, Ishita K, Takaya Y, Uchisawa H, Matsue H. Anti-tumor activity of squid ink. J Nutr Sci Vitaminol (Tokyo) 1997;43:455–61. doi: 10.3177/jnsv.43.455. [DOI] [PubMed] [Google Scholar]
- 7.Zheng GL, Zhang XY, Zhou YG, Meng QC, Gong WG. Comparison between partial components and trace elements in sepia and squid ink. Chin J Mar Drugs. 2002;21:12–4. [Google Scholar]
- 8.Mochizuki A. An antiseptic effect of cuttlefish ink. Bull Jpn Soc Sci Fish. 1979;45:1401–3. [Google Scholar]
- 9.Takai M, Yamazaki K, Kawai Y, Inove N, Shinano H. Effect of squid liver skin and ink on microbiological characteristics of ‘Ika-Shiokara’during ripening process (part 2) Bull Jpn Soc Sci Fish. 1993;59:1617–23. [Google Scholar]
- 10.Patterson EJ, Murugan A. Screening of cephalopods for bioactivity. Phuket Mar Biol Cent Spl Publ. 2000;21:253–9. [Google Scholar]
- 11.Rajaganapathi J, Thyagarajan SP, Edward JK. Study on cephalopod's ink for anti-retroviral activity. Indian J Exp Biol. 2000;38:519–20. [PubMed] [Google Scholar]
- 12.Nirmale V, Nayak BB, Kannappan S, Basu S. Antibacterial effect of the Indian squid Loligo duvauceli (d’Orbigny) ink. J Indian Fish Assoc. 2002;29:65–9. [Google Scholar]
- 13.Chacko D, Patterson J. Effect of pharaoh's cuttlefish Sepia pharaonis ink against bacterial pathogens. Indian J Microbiol. 2005;45:223–6. [Google Scholar]
- 14.Machado P, Brown CP, Butts J, Eastoe E, Padron Hernandez FLdA, de Oliveira RJ. Chem Commun. 2002;49:2765–7. doi: 10.1039/c3cc00103b. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Barnes H, Blackstock J. Estimation of lipids in marine animals and tissues: Detailed investigation of the sulpho-phosphovanilun method for ‘total’lipids. J Exp Mar Biol Ecol. 1973;12:103–18. [Google Scholar]
- 17.Roe JH, Epstein JH, Goldstein NP. A photometric method for the determination of insulin in plasma and urine. J Biol Chem. 1949;178:839–45. [PubMed] [Google Scholar]
- 18.Binsan W, Benjakul S, Visessanguan W, Roytrakul S, Tanaka M, Kishimura H. Antioxidative activity of Mungoong, an extract paste, from the cephalothorax of white shrimp (Litopenaeus vannamei) Food Chem. 2008;106:185–93. [Google Scholar]
- 19.Paniprasad K, Venkateshvaran K. Microhaemolytic assay. In: Venkateshvaran K, Prasad KP, editors. Training Mannual on Advance Techniques Marine Biotoxinology. India: Central Institute Fisheries Education; 1997. p. 41. [Google Scholar]
- 20.Vairamani S, Subha Pradha N, Ramasamy P, Barwin Vino A, Raveendran S, Shanmugam S. Antibacterial activity of extract of whole body tissue and Ethylenediamine tetra acetate extract of cuttlebone of Sepiella inermis (Orbigny.1848) Res J Microbiol. 2012;7:263–72. [Google Scholar]
- 21.Mizushima Y, Kobayashi M. Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol. 1968;20:169–73. doi: 10.1111/j.2042-7158.1968.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 22.Sakat S, Juvekar AR, Gambhire MN. In vitro antioxidant and antiinflammatory activity of methanol extract of Oxalis corniculata Linn. Int J Pharm Pharm Sci. 2010;2:146–56. [Google Scholar]
- 23.Vate NK, Benjakul S. Antioxidative activity of melanin-free ink from splendid squid (Loligo formosana) Int Aquat Res. 2013;5:9. [Google Scholar]
- 24.Hagerman AE, Riedl KM, Jones GA, Sovik KN, Ritchard NT, Hartzfeld PW, et al. High molecular weight plant polyphenolics (tannins) as biological antioxidants. J Agric Food Chem. 1998;46:1887–92. doi: 10.1021/jf970975b. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekara A, Shahidi F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J Agric Food Chem. 2010;58:6706–14. doi: 10.1021/jf100868b. [DOI] [PubMed] [Google Scholar]
- 26.Brand-Williams W, Cuvelier ME, Berse C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995;28:25–30. [Google Scholar]
- 27.Kumosinski TF, Farell HM. Determination of the global secondary structure of proteins by Fourier Transform Infrared (FTIR) spectroscopy. Trends Food Sci Technol. 1993;4:169–75. [Google Scholar]
- 28.Patrick TT, Lacelle WS, Hossien MY. Malignant human colonic tissue investigation by pressure tunning FTIR spectroscopy. Appl Spectrosc. 1993;47:1830–6. [Google Scholar]
- 29.Palaniappan PL, Vijayasundaram V. FTIR study of the arsenic induced biochemical changes on the liver tissues of freshwater fingerlings Labeo rohitha. Rom J Biophys. 2008;18:135–44. [Google Scholar]
- 30.Mondon JA, Duda S, Nowak BF. Histological, growth and 7-ethoxyresorufin O-deethylase (EROD) activity responses of greenback flounder rhombosolea tapirina to contaminated marine sediment and diet. Aquat Toxicol. 2001;54:231–47. doi: 10.1016/s0166-445x(01)00146-1. [DOI] [PubMed] [Google Scholar]
- 31.Patrick TT, Lacelle WS, Hossien MY. Malignant human colonic tissue investigation by pressure tunning FTIR spectroscopy. Appl Spectrosc. 1993;47:1830–6. [Google Scholar]
- 32.Parveez I, Servercan HF. FTIR spectroscopic characterization of protein structure in aqueous and non aqueous media. J Mol Catal B Enzym. 1999;7:207–21. [Google Scholar]
- 33.Zhong JP, Wang G, Shang JH, Pan JQ, Li K, Huang Y, et al. Protective effects of squid ink extract towards hemopoietic injuries induced by cyclophosphamine. Mar Drugs. 2009;7:9–18. doi: 10.3390/md7010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sheu TY, Chou CC. Antimicrobial activity of squid ink. J Chin Agric Chem Soc. 1990;28:59–68. [Google Scholar]
- 35.Smiline Girija AS, Hariprasad G, Vijayashree Priyadharsini J, Pandi Suba K, Raghumaran R, Cnanavendhan SG. Antimicrobial potential of Loligo duvauceli ink against the common clinical bacterial &yeast isolates. Biomedicine. 2008;28:213–5. [Google Scholar]
- 36.Malcok HK, Aktas E, Ayyildiz A, Yigit N, Yazgi H. Hemolytic activities of the Candida species in liquid medium. Eurasian J Med. 2009;41:95–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Kuppulakshmi C, Prakash M, Gunasekaran G, Manimegalai G, Sarojini S. Antibacterial properties of fish mucus from Channa punctatus and Cirrhinus mrigala. Eur Rev Med Pharmacol Sci. 2008;12:149–53. [PubMed] [Google Scholar]
- 38.Uthayakumar V, Ramasubramanian V, Senthilkumar D, Priyadarisini VB, Harikrishnan R. Biochemical characterization, antimicrobial and hemolytic studies on skin mucus of fresh water spiny eel Mastacembelus armatus. Asian Pacific Journal of Tropical Biomedicine. 2012:S863–9. [Google Scholar]
- 39.Oyedepo OO, Femurewa AJ. Anti-protease and membrane stabilizing activities of extracts of Fagra zanthoxiloides, Olax subscorpioides and Tetrapleura tetraptera. Int J Pharmacogn. 1995;33:65–9. [Google Scholar]


