Abstract
Background:
Mortality rate is increasing due to cardiovascular problems throughout the world. These cardiac problems are directly associated with dyslipidemia.
Aim:
The aim of this study was to evaluate the antihyperlipidemic effect of aqueous extract and methanol extract of Trachyspermum ammi at 1 g/kg, 3 g/kg, and 5 g/kg dose levels in rats.
Materials and Methods:
For this purpose, 45 male albino rats were used and randomly divided into nine equal groups (n = 5). The lipid levels were increased after 24 h of single intraperitoneal injection of Triton X-100 (100 mg/kg) in rats. Aqueous and methanol extracts equivalent to 1 g/kg, 3 g/kg, and 5 g/kg were administered orally to the rats for 21 days. Atorvastatin (10 mg/kg) was used as standard drug. Blood samples were collected at 0, 2nd, 9th, 16th, and 23rd day by a direct cardiac puncture in Vacuette® heparin tubes. Serum was separated and then analyzed for lipid profile, liver function test (LFT), and renal function test (RFT) using standard diagnostic kits.
Results:
Results showed that extracts at 3 g/kg and 5 g/kg decreased the levels of total cholesterol, triglyceride, and low-density lipoprotein and increased high-density lipoprotein concentration in serum. T. ammi also decreased LFT and RFT parameters at the end of the study.
Conclusion:
T. ammi possessed antioxidant and antihyperlipidemic activities along with hepato- and nephro-protective effects.
SUMMARY
Aqueous and methanol extracts of T. ammi were administered orally at 1-, 3-, and 5 g/kg doses to hyperlipidemic rats (Triton X-100 induced hyperlipidemia) and atorvastatin (10 mg/kg, orally) was used as standard drug. Methanol extract at 5 g/kg showed antihyperlipidemic effect that is identical to that of standard drug.
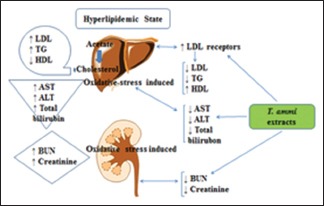
Abbreviations Used: LDL: Low-density lipoprotein; TC: Total cholesterol; VLDL: Very low-density lipoprotein; HDL: High-density lipoprotein; T. ammi: Trachyspermum ammi; WHO: World Health Organization; CAD: Coronary artery disease; BHT: Butylated hydroxytoluene; BUN: Blood urea nitrogen; AST: Aspartate transaminase; ALT: Alanine transaminase; IP: Intraperitoneal.
Keywords: Antihyperlipidemic effect, hepatoprotective, oxidative stress, Trachyspermum ammi
INTRODUCTION
In the world’s developing and under developing countries, mortality rate is increasing due to cardiovascular problems.[1,2] These cardiac problems are directly associated with dyslipidemia, which is the leading cause of atherosclerosis, arteriosclerosis, stroke, obesity, coronary artery diseases, and heart attack.[3,4,5]
The normal level of cholesterol in plasma can be maintained by biosynthesis of cholesterol, absorption of cholesterol from diet, cholesterol removal from the circulation and elimination of cholesterol through feces and bile.[6] Hyperlipidemia is majorly linked with increased level of low-density lipoprotein (LDL), total cholesterol (TC), very LDL (VLDL), and decreased the level of high-density lipoprotein (HDL) in serum.[7,8] Initially, such abnormal accumulation of lipid in liver causes steatosis or fatty liver. At the chronic stages, destruction of hepatocytes occurs.[9,10]
In general, statins as antihyperlipidemic drugs are most commonly used to decline the increased level of cholesterol and lipid in blood. However, these allopathic medicines have many side effects such as myopathy, hyperuricemia, flushing, hepatotoxicity, dry skin[11,12,13] and gastric disturbance, abdominal pain, and flatulence.[14,15]
Medicinal plants play an important role in preventing and treating a variety of diseases. Plant and herbal drugs are safer and well tolerated as compared to synthetic drugs.[16] The World Health Organization (WHO) has been estimated that almost eighty percent world’s population trusts on the usage of herbal methods for treatment.[17] Therefore, researchers are also interested on herbal therapies of cardiovascular complaints having less adverse effects.
Trachyspermum ammi is an annual ayurvedic herbaceous plant, belongs to the important medicinal family, Umbelliferae or Apiaceae.[18] It is well known by several common names such as ajwain/omum, carom seed, and bishop’s weed.[19,20] Al-Yunan or Kammun, agyptischer, Xi Ye Cao Guo Qin, ajowan, adiowan, hounastan, ajwan.[21,22] Ajwain is an indigenous plant of Egypt. It grows widely in soil having a great amount of salts such as Southwest Asia and Mediterranean Sea.[23] Ajwain is cultivated and widely distributed in Pakistan, India, Iraq, Afghanistan, and Iran.[24] It is reported that T. ammi seeds have anti-platelet,[25] analgesic,[26] antihypertensive,[27] antibacterial,[28] antifungal,[29] antioxidant,[30] insecticidal,[31] anthelmintic,[32] anti-inflammatory,[33] diuretic and anti-lithiasis,[34] detoxification,[35] antiviral,[36] spermicidal,[37] antitussive and bronchodilatory,[38] antiulcer,[39] antispasmodic,[40] digestive stimulant,[41] hepatoprotective,[42] estrogenic,[43] and nematicidal activities.[44] As compared to other effects, the antihyperlipidemic effect of T. ammi is still not well explored and well documented.
Latest studies have revealed that ingestion of vegetable oil containing polyunsaturated fatty acids (such as omega-3 and omega-6) decreases plasma level of triacylglycerol and cholesterol and inversely linked to the occurrence of heart diseases.[45]
Phytochemical studies revealed that T. ammi contains a significant amount of polyunsaturated fatty acids as well as a good source of dietary fibers. Hence, there is a great need to well explore the antihyperlipidemic activity of T. ammi.
To keep in view the medicinal value of T. ammi as described in literature review, the present study was carried out to evaluate the antihyperlipidemic effect of aqueous extract and methanol extract of T. ammi at 1 g/kg, 3 g/kg, and 5 g/kg dose levels in rats.
MATERIALS AND METHODS
Collection of plant material
T. ammi seeds are widely distributed in Pakistan. They have pale brown color and oval shape. They are bitter and pungent in taste. They are also used as a flavor, spice, and preservative. Seeds of T. ammi were purchased from local market. Identification and authentication were done by a Botanist from Department of Botany, Government College University Faisalabad. Seeds were washed and dried under shade. After drying, they were ground to fine powder.
Preparation of extracts
The extract was prepared by cold extraction method. The fine powder (2 kg) was soaked separately with distilled water (6 L) and 98% methanol (6 L) in a sealed close container for 2 weeks with occasional stirring and shaking. The extract was filtered first using muslin cloth and then with Whatman filter paper No. 1. Excess of solvent was evaporated by rotary evaporator at 40°C to get semisolid texture of extract.
Approval by Animal Ethical Committee
This experiment was conducted after the approval from Animal Ethical Committee of Faculty of Pharmaceutical Science, Government College University Faisalabad.
Qualitative phytochemical analysis
A qualitative phytochemical analysis of extracts was carried out to identify the presence of different phytochemicals such as tannin, saponin, alkaloid, steroids, flavonoid, carbohydrates, proteins, phenols, and terpenoids using standard biochemical techniques.[46,47]
Antioxidant activity by 1,1-diphenyl-2-picrylhydrazyl method
Liyana-Pathirana and Shahidi[48] was used to analyze the antioxidant activity of extracts. Briefly, it is described as 1,1-diphenyl-2-picrylhydrazyl (DPPH) solution (0.1 mM) and different concentrations of samples solutions (0.1–1.1 mg/mL) in methanol were prepared. Butylated hydroxytoluene (BHT) was dissolved in methanol using as a positive control or reference standard. Test samples (1 mL) and BHT (1 mL) were mixed with DPPH solution (1 mL) separately in test tubes, and these test tubes were kept at 28°C ± 2°C (room temperature) for 30 min. Similarly, blank was prepared, it contained 1 mL of methanol instead of reference standard or sample. After an incubation period at room temperature, absorbance was measured against blank at 517 nm. Each sample was tested in triplicate. Percentage scavenging effect was calculated with following formula:
Scavenging activity (%) = (Ablank − Asample/Ablank) × 100
Where a blank was optical density (OD) of control.
A sample was OD of test sample.
Experimental animals
Forty-five male albino rats of 80–130 g were used in the present study. They were purchased from the Institute of National Health – Islamabad, Pakistan and kept at the animal house of Faculty of Pharmaceutical Science, Government College University Faisalabad, at a temperature of 25°C ± 1°C and humidity (65%–70%) conditions with 12 h light-dark cycle. Animals were placed in cages 7 days before study for acclimatization and randomly divided into nine equal groups (n = 5). Water and chow were provided ad libitum.
Induction of hyperlipidemia
Rats were starved for 18 h, and then, hyperlipidemia was induced by single intraperitoneal (IP) injection of 100 mg/kg Triton X-100 prepared in normal saline. After 24 h, serum triglyceride and cholesterol levels were checked and rats with serum TC levels more than 130 mg/dL were considered hyperlipidemic. Treatment was started orally after 24 h of Triton X-100 injection (when hyperlipidemia was induced) for 21 days.[49]
Experimental design
Each group contained 5 rats. After inducing hyperlipidemia, Triton X-100 was injected IP (100 mg/kg) daily during study before administering the extracts/standard.
Group 1: Served as normal control while Group 2: Served as disease control, which received 100 mg/kg Triton X-100 IP injection daily during the study. Group 3: Standard group receiving Triton X-100 + 10 mg/kg atorvastatin daily and Groups 4–9 were treatment groups; Group 4 was given Triton X-100 + aqueous extract at 1 g/kg daily; Group 5 was administered Triton X-100 + 3 g/kg aqueous extract daily; Group 6 receiving Triton X-100 + aqueous extract (5 g/kg) daily; Group 7 was administered Triton X-100 + 1 g/kg methanol extract daily; Group 8 was given Triton X-100 + methanol extract at 3 g/kg daily; and Group 9 received Triton X-100 + methanol extract (5 g/kg) daily.
T. ammi extracts declined the high level of cholesterol caused by Triton X-100.
Collection of blood samples
Blood samples (2.5 mL/sample) were collected on the days 0, 2nd, 9th, 16th, 23rd in Vacuette® heparin tubes by direct cardiac puncture under mild chloroform anesthesia. These collected samples were centrifuged for 10 min at 4000 rpm to get the serum. Then serum was examined for the assessment of biochemical parameters such as triglycerides, TC, LDL, VLDL and HDL, aspartate transaminase (AST), alanine transaminase (ALT), total bilirubin, creatinine, albumin, and blood urea nitrogen (BUN).
Statistical analysis
Data were expressed as mean ± standard deviation. One way analysis of variance followed by post hoc “Tukey” test was applied using IBM SPSS software, version 20.0 to calculate the significance level. P < 0.05 was considered statistically significant.
RESULTS
Qualitative phytochemical analysis
A qualitative phytochemical analysis of aqueous and methanol extracts showed that tannins, terpenoids, alkaloids flavonoids, steroids, phenols, and carbohydrates were present in both extracts. Saponins were absent in aqueous extract, but they were present in methanol extract, whereas amino acids were absent in both aqueous and methanol extracts [Table 1].
Table 1.
Phytochemical analysis of aqueous and methanol extracts of Trachyspermum ammi

Antioxidant activity
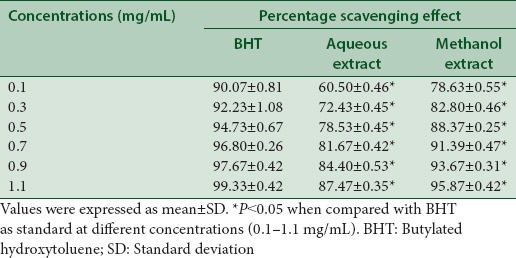
Aqueous and methanol extracts showed significant (P < 0.05) dose-dependent % scavenging effect as compared to standard (BHT). At maximum concentration (1.1 mg/mL) BHT, aqueous and methanol extracts exhibited % scavenging effect of 99.33%, 87.47%, and 95.87% respectively as presented in Table 2.
Table 2.
Antioxidant activity of aqueous and methanol extracts of Trachyspermum ammi by 1,1-diphenyl-2-picrylhydrazyl method
Antihyperlipidemic effect
Antihyperlipidemic effect of aqueous and methanol extracts at a dose of 1 g/kg, 3 g/kg, and 5 g/kg is given in Tables 3-7, respectively. It is manifested from these tables that both extracts at a dose of 1 g/kg did not show the antihyperlipidemic activity.
Table 3.
Effect of aqueous and methanol extracts of Trachyspermum ammi on triglyceride levels (mg/dL)
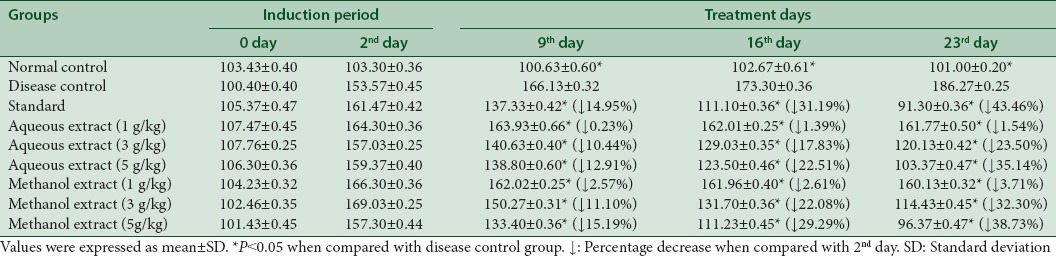
Table 7.
Effect of aqueous and methanol extracts of Trachyspermum ammi on very low-density lipoprotein levels (mg/dL)
Effect on triglyceride levels (mg/dL)
Aqueous extract reduced the serum levels of triglyceride up to 1.54%, 23.50%, and 35.14% at dose of 1 g/kg, 3 g/kg, and 5 g/kg, respectively. Similarly, methanol extract lowered the triglyceride levels to 3.71%, 32.30%, and 38.73% at dose of 1 g/kg, 3 g/kg, and 5 g/kg respectively at 23rd day as shown in Table 3.
Effect on total cholesterol levels (mg/dL)
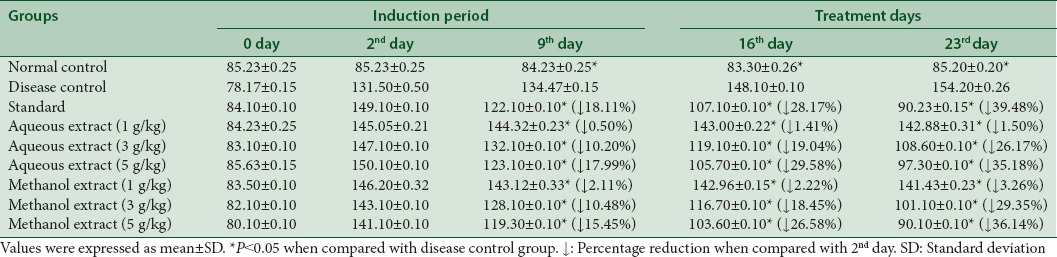
Aqueous extract declined the serum levels of TC to 1.50%, 26.17%, and 35.18% at dose of 1 g/kg, 3 g/kg, and 5 g/kg, respectively. Similarly, methanol extract dropped the TC levels up to 3.26%, 29.35%, and 36.14% at dose of 1 g/kg, 3 g/kg, and 5 g/kg, respectively at 23rd day [Table 5].
Table 5.
Effect of aqueous and methanol extracts of Trachyspermum ammi on total cholesterol levels (mg/dL)
Effect on low-density lipoprotein levels (mg/dL)
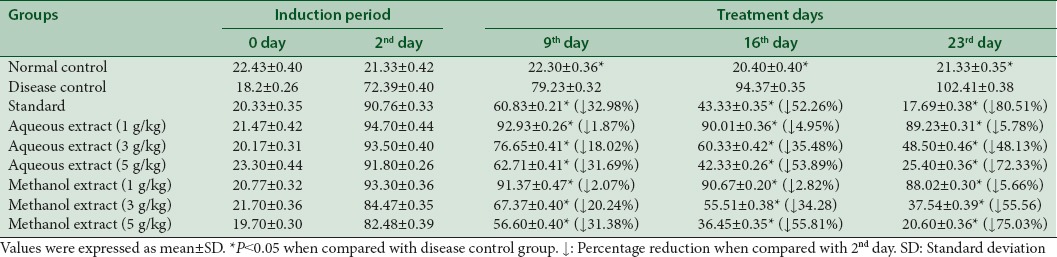
Table 6 shows that aqueous extract decreased the serum levels of LDL up to 5.78%, 48.13%, and 72.33% at dose of 1 g/kg, 3 g/kg, and 5 g/kg, respectively. Similarly, methanol extract reduced the LDL levels to 5.66%, 55.56%, and 75.03% at dose of 1 g/kg, 3 g/kg, and 5 g/kg, respectively at 23rd day.
Table 6.
Effect of aqueous and methanol extracts of Trachyspermum ammi on low-density lipoprotein levels (mg/dL)
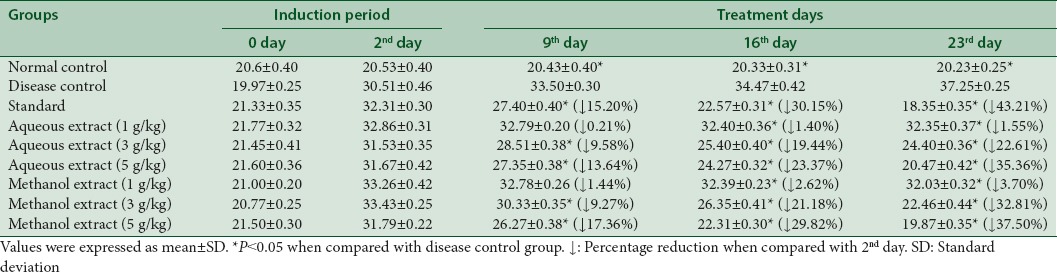
Effect on very low-density lipoprotein levels (mg/dL)
Aqueous extract lowered the serum levels of VLDL to 1.55%, 22.61%, and 35.36% at dose of 1 g/kg, 3 g/kg, and 5 g/kg, respectively. Similarly, methanol extract declined the VLDL up to 3.70%, 32.81%, and 37.50% at dose of 1 g/kg, 3 g/kg, and 5 g/kg respectively at 23rd day as given in Table 7.
Effect on high-density lipoprotein levels (mg/dL)
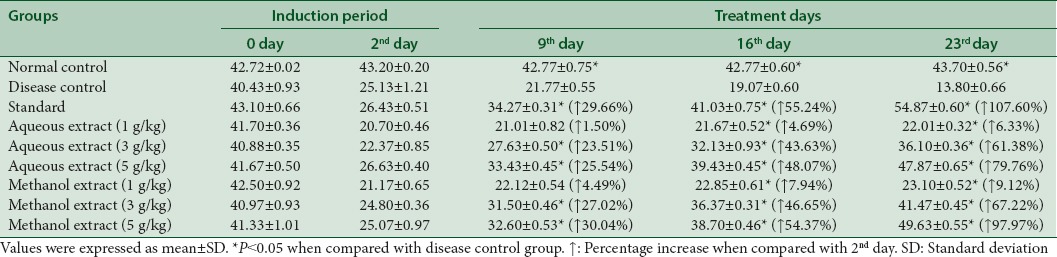
Table 4 exhibits that aqueous extract raised the serum levels of HDL up to 6.33%, 61.38%, and 79.76% at dose of 1 g/kg, 3 g/kg, and 5 g/kg, respectively. Similarly, methanol extract increased the HDL levels to 9.12%, 67.22%, and 97.97% at dose of 1 g/kg, 3 g/kg, and 5 g/kg respectively at 23rd day.
Table 4.
Effect of aqueous and methanol extracts of Trachyspermum ammi on high-density lipoprotein levels ( mg/dL)
Methanol extract at 5 g/kg dose showed promising antihyperlipidemic effect that is identical to that of standard atorvastatin therapy.
Figure 1.
Experimental design
Effect on liver function test
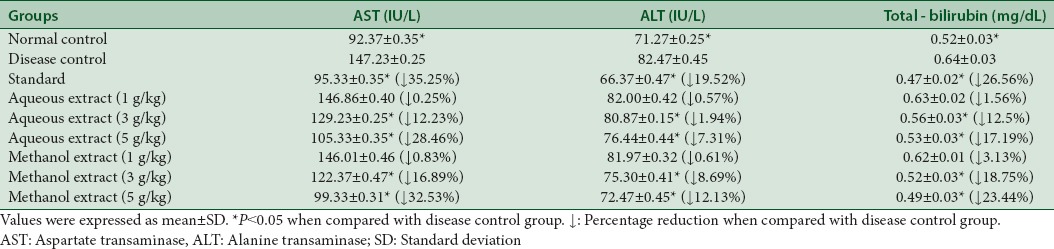
AST, ALT, and total bilirubin levels got raised in hyperlipidemic state as indicated in disease control group. Aqueous and methanol extracts showed a dose-dependent decrease in liver enzymes (AST, ALT, and total bilirubin) and exhibited liver protected effect as given in Table 8.
Table 8.
Effect of aqueous and methanol extracts of Trachyspermum ammi on liver function tests
Effect on renal function test
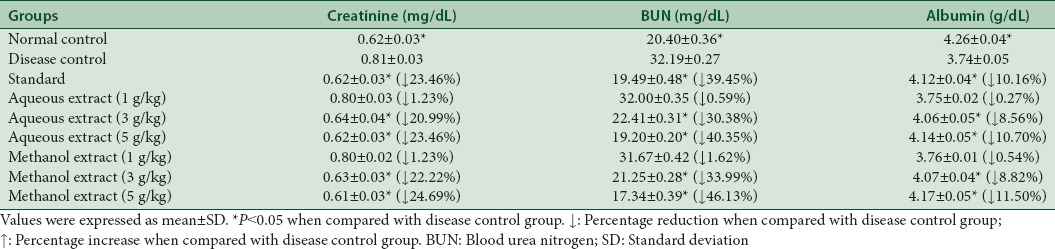
Table 9 exhibits the nephroprotective effect of extracts. Both extracts caused a dose-dependent reduction in creatinine, BUN and increase in albumin. There is increase in serum creatinine, BUN and decrease in albumin in hyperlipidemic condition due to oxidative stress which is confirmed by elevated levels of renal function parameters in disease control group as compared to normal control group values [Table 9].
Table 9.
Effect of aqueous and methanol extracts of Trachyspermum ammi on renal function tests
DISCUSSION
Hyperlipidemia is a well-considered threat factor for cardiovascular disorders, especially CAD (coronary artery disease). Numerous studies have exhibited a clear-cut relationship between cardiovascular events and high cholesterol levels.[50] Cardiovascular ailments have been thought globally as chief cause of death, almost 16.7 million deaths per year according to WHO. One out of each four middle-aged personnel in Pakistan is suffering from cardiovascular complaints.[51] Various studies exposed that food plays an essential role in the development of atherosclerosis and hyperlipidemia. Several studies on the animals and human have assessed that the saturated fatty acids and cholesterol cause hypercholesterolemia by changing the lipoprotein mechanisms and pattern and elevating TC. In several animal models, cholesterol-rich food has been often used to induce hypercholesterolemia, raise the cholesterol levels, and correlated metabolic changes.[52]
Triton X-100 has been commonly used to induce the acute hyperlipidemia by blocking the removal of triglyceride and cholesterol in various animal models[53,54] particularly the rat models, for the screening of synthetic or natural antihyperlipidemic drugs.[55] The present study indicated that IP injection of Triton X-100 (100 mg/kg) prepared in normal saline was appropriate to induce hyperlipidemia in rats. Similar findings were reported by Jahromi and Ray,[56] Sudha et al.,[57] and Gundamaraju et al.[58] that Triton X-100 (100 mg/kg) in rats elevated their serum lipid profile parameters. It is well identified that HDL has a defending role in cardiovascular diseases, particularly coronary artery disease.[59] Similarly, high levels of LDL in serum correlate with an increased risk for atherosclerosis development.[60] There was a significant elevation in TC, triglyceride, LDL, and VLDL levels, whereas the level of HDL in all groups was reduced after 24 h of IP injection of Triton X-100 [Tables 3 and 4]. When aqueous and methanol extracts of T. ammi were co-supplemented with Triton X-100, the increased levels of triglycerides, TC, VLDL, and LDL declined considerably. These results correlated with the findings of Dhulasavant et al.[61] and Iqbal et al.[62] who described that aqueous and ethanolic extracts of Cinnamomum tamala considerably reduced the levels of triglyceride, TC, LDL, and VLDL whereas both extracts increased the HDL levels significantly. Martinez et al.[63] also stated similar findings that correlate with our results in the present study.
In our study, extracts at different doses dropped the triglyceride, TC, LDL, and VLDL levels in the serum of rats and a significant increase was also observed in HDL levels, and it can be suggested that extracts have a direct role in lipids metabolism. These findings correlate with the results of Khan et al.[64] who evaluated that cinnamon bark at different doses reduced triglyceride and TC levels and controlled free fatty acids levels in type 2 diabetic patients. Hence, it indicates the efficacy of T. ammi in preventing the increased levels of lipid profile in hyperlipidemia.
Biochemical studies disclosed that triglycerides are independently linked to cardiovascular diseases.[65] Most of the antihypercholesterolemic drugs do not diminish the triglycerides levels but the aqueous and methanol extracts of T. ammi equivalent to 5 g/kg significantly reduced it by 35.14% and 38.73% respectively at 3 weeks treatment in the current study and a substantial lessening in triglycerides and LDL levels after supplementation of extracts at dose of 3 g/kg and 5 g/kg demonstrated its importance in prevention and management of cardiovascular diseases. These results also correlate with the findings of Zari and Allogmani[66] who stated that Cinnamomum zeylanicum oil caused a considerable reduction in triglycerides, TC, LDL, and VLDL levels with a significant increase in HDL level in streptozocin-induced diabetic rats. Hence, it may be considered that the T. ammi may be helpful in controlling the metabolism of certain lipoproteins that demonstrating the antihyperlipidemic effect of the T. ammi. Similar results also described by Wiesenfeld et al.[67] who assessed that flaxseed plays a main role in declining free and ester cholesterol in humans or animal models.
Reactive oxygen species and free radicals are most important causative agents for the development of several diseases such as cardiovascular diseases, cancer and neurodegenerative diseases.[68] Extracts showed a powerful antioxidant activity at 1.1 mg/mL when assessed against DPPH free radical [Table 2]. In this study, the effectiveness of extracts was compared with atorvastatin-a standard lipid-lowering drug. Khanna et al.[69] also stated a similar comparison between Phyllanthus niruri and standard drug in albino rats. In the present study, treatment with either extracts or atorvastatin improved the lipid profile. Methanolic extract at a dose of 5 g/kg significantly reduced the TC (36.14%), triglyceride (38.73%) and LDL concentration (75.03%) and markedly increased the HDL concentration (97.97%) in serum at treatment day 23rd. T ammi extracts treated groups at dose of 3 g/kg and 5 g/kg revealed a considerable antihyperlipidemic effect when compared with untreated control group. This may be occurred due to the existence of some antihyperlipidemic compounds which may act like inhibitor of some enzymes as HMG CoA reductase that inhibits the production of cholesterol in liver or declined the absorption of cholesterol from intestine.[70] Similar results were observed by Farnier and Davignon[71] and Mozaffarian et al.[72] who stated that administration of statins such as atorvastatin, lovastatin, and pravastatin decreased the production of cholesterol in liver by inhibiting the HMG-CoA reductase enzyme.
On the basis of these findings, it may safely be said that methanolic extract (5 g/kg) and atorvastatin (10 mg/kg) were equally efficacious in the management of hyperlipidemia. These results recommended that T. ammi produced an alteration in hepatic fat content, serum lipids, and showing a protective role on hyperlipidemia with good antioxidant effect. Therefore, T. ammi may be one of the potential sources for preventing and management of hyperlipidemia and its complications with less side effects and well tolerance. This data can be supportive in future for the treatment of hyperlipidemia in patients.
CONCLUSION
It was concluded that T. ammi possessed good antioxidant potential and significantly lowered the levels of triglyceride, TC, LDL, VLDL, ALT, AST, total bilirubin and increased the levels of HDL, albumin along with decreasing BUN and creatinine. It is suggested that T. ammi can act as antihyperlipidemic agent by sharing the mechanism of action of statins along with ameliorating the oxidative stress in vital organs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Koh JH, Kim JM, Chang UJ, Suh HJ. Hypocholesterolemic effect of hot-water extract from mycelia of Cordyceps sinensis. Biol Pharm Bull. 2003;26:84–7. doi: 10.1248/bpb.26.84. [DOI] [PubMed] [Google Scholar]
- 2.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141:122–31. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Mazumder A, Saravanan V. Antihyperlipidemic activity of Camellia Sinensis leaves in triton wr-1339 induced albino rats. Pharmacogn Mag. 2008;4:60. [Google Scholar]
- 4.Rizvi F, Iftikhar M, George JP. Beneficial effects of fish liver preparations of sea bass (Lates calcarifer) versus gemfibrozil in high fat diet-induced lipid-intolerant rats. J Med Food. 2003;6:123–8. doi: 10.1089/109662003322233521. [DOI] [PubMed] [Google Scholar]
- 5.Gao W, He HW, Wang ZM, Zhao H, Lian XQ, Wang YS, et al. Plasma levels of lipometabolism-related miR-122 and miR-370 are increased in patients with hyperlipidemia and associated with coronary artery disease. Lipids Health Dis. 2012;11:55. doi: 10.1186/1476-511X-11-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adekunle AS, Adedeji AL, Oyewo EO, Adedosu OT, Omotoso AT. Hyperlipidemia induced by atherogenic diet enhanced oxidative stress in the kidney and inflammatory responses: An in-vivo study. Asian J Nat Appl Sci. 2013;2:82–93. [Google Scholar]
- 7.Vogel H, editor. Drug Discovery and Evaluation: Pharmacological Assays. Springer Science & Business Media. 2007 [Google Scholar]
- 8.Saravanan R, Rajendra Prasad N, Pugalendi KV. Effect of Piper betle leaf extract on alcoholic toxicity in the rat brain. J Med Food. 2003;6:261–5. doi: 10.1089/10966200360716689. [DOI] [PubMed] [Google Scholar]
- 9.El-Demerdash FM, Yousef MI, El-Naga NI. Biochemical study on the hypoglycemic effects of onion and garlic in alloxan-induced diabetic rats. Food Chem Toxicol. 2005;43:57–63. doi: 10.1016/j.fct.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Jain KS, Kathiravan MK, Somani RS, Shishoo CJ. The biology and chemistry of hyperlipidemia. Bioorg Med Chem. 2007;15:4674–99. doi: 10.1016/j.bmc.2007.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Simons LA. Additive effect of plant sterol-ester margarine and cerivastatin in lowering low-density lipoprotein cholesterol in primary hypercholesterolemia. Am J Cardiol. 2002;90:737–40. doi: 10.1016/s0002-9149(02)02600-0. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho AA, Lima UW, Valiente RA. Statin and fibrate associated myopathy: Study of eight patients. Arq Neuropsiquiatr. 2004;62:257–61. doi: 10.1590/s0004-282x2004000200013. [DOI] [PubMed] [Google Scholar]
- 13.Rao SK, Prasad T, Mohanta GP, Manna PK. An overview of statins as hypolipidemic drugs. Int J Pharm Sci Drug Res. 2011;3:178–83. [Google Scholar]
- 14.Ballantyne CM, Blazing MA, Hunninghake DB, Davidson MH, Yuan Z, DeLucca P, et al. Effect on high-density lipoprotein cholesterol of maximum dose simvastatin and atorvastatin in patients with hypercholesterolemia: Results of the Comparative HDL Efficacy and Safety Study (CHESS) Am Heart J. 2003;146:862–9. doi: 10.1016/S0002-8703(03)00440-X. [DOI] [PubMed] [Google Scholar]
- 15.Newman CB, Palmer G, Silbershatz H, Szarek M. Safety of atorvastatin derived from analysis of 44 completed trials in 9,416 patients. Am J Cardiol. 2003;92:670–6. doi: 10.1016/s0002-9149(03)00820-8. [DOI] [PubMed] [Google Scholar]
- 16.Javed I, Zia-Ur-Rahman N, Khan MZ, Muhammad F, Aslam B, Iqbal Z, et al. Antihyperlipidaemic efficacy of Trachyspermum ammi in albino rabbits. Acta Vet Brno. 2009;78:229–36. [Google Scholar]
- 17.Mueller MS, Mechler E. Medicinal Plants in Tropical Countries: Traditional Use Experience Facts. Georg Thieme Verlag. 2005 [Google Scholar]
- 18.Gersbach PV, Reddy N. Non-invasive localization of thymol accumulation in Carum copticum (Apiaceae) fruits by chemical shift selective magnetic resonance imaging. Ann Bot. 2002;90:253–7. doi: 10.1093/aob/mcf179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anilakumar KR, Saritha V, Khanum F, Bawa AS. Ameliorative effect of ajwain extract on hexachlorocyclohexane-induced lipid peroxidation in rat liver. Food Chem Toxicol. 2009;47:279–82. doi: 10.1016/j.fct.2008.09.061. [DOI] [PubMed] [Google Scholar]
- 20.Pathak AK, Nainwal N, Goyal BM, Singh R, Mishra V, Nayak S, et al. Pharmacological activity of Trachyspermum ammi: A review. J Pharm Res. 2010;3:895–9. [Google Scholar]
- 21.Lim TK. Edible Medicinal and Non-Medicinal Plants. Netherlands: Springer; 2013. Blighiasapida; pp. 4–14. [Google Scholar]
- 22.Ashraf M, Orooj A. Salt stress effects on growth, ion accumulation and seed oil concentration in an arid zone traditional medicinal plant ajwain (Trachyspermum ammi [L.] Sprague) J Arid Environ. 2006;64:209–20. [Google Scholar]
- 23.Jeet K, Devi N, Narender T, Sunil T, Lalit S, Raneev T. Trachyspermum ammi (ajwain): A comprehensive review. Int Res J Pharm. 2012;3:133–8. [Google Scholar]
- 24.Shojaaddini M, Moharramipour S, Sahaf B. Fumigant toxicity of essential oil from Carum copticum against Indian meal moth Plodia interpunctella. J Plant Prot Res. 2008;48:411–9. [Google Scholar]
- 25.Zarshenas MM, Moein M, Samani SM, Petramfar P. An overview on ajwain (Trachyspermum ammi) pharmacological effects;modern and traditional. J Nat Remedies. 2013;14:98–105. [Google Scholar]
- 26.Dashti-Rahmatabadi MH, Hejazian SH, Morshedi A, Rafati A. The analgesic effect of Carum copticum extract and morphine on phasic pain in mice. J Ethnopharmacol. 2007;109:226–8. doi: 10.1016/j.jep.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 27.Bairwa R, Sodha RS, Rajawat BS. Trachyspermum ammi. Pharmacogn Rev. 2012;6:56–60. doi: 10.4103/0973-7847.95871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaur GJ, Arora DS. Bioactive potential of Anethum graveolens Foeniculum vulgare and Trachyspermum ammi belonging to the family Umbelliferae-current status. J Med Plants Res. 2010;4:87–94. [Google Scholar]
- 29.Wadhwa S. Antimicrobial activity of essential oils of Trachyspermum ammi. Int J Pharm Biol Arch. 2010;1 [Google Scholar]
- 30.Umar S, Asif M, Sajad M, Ansari MM, Hussain U, Ahmad W, et al. Anti inflammatory and antioxidant activity of Trachyspermum ammi seeds in collagen induced arthritis in rats. Int J Drug Dev Res. 2012;4:210–19. [Google Scholar]
- 31.Chaubey MK. Fumigant toxicity of essential oils from some common spices against pulse beetle Callosobruchus chinensis (Coleoptera Bruchidae) J Oleo Sci. 2008;57:171–9. doi: 10.5650/jos.57.171. [DOI] [PubMed] [Google Scholar]
- 32.Tamura T, Iwamoto H. Thymol: A classical small-molecule compound that has a dual effect (potentiating and inhibitory) on myosin. Biochem Biophys Res Commun. 2004;318:786–91. doi: 10.1016/j.bbrc.2004.04.085. [DOI] [PubMed] [Google Scholar]
- 33.Thangam C, Dhananjayan R. Antiinflammatory potential of the seeds of Carum copticum Linn. Indian J Pharmacol. 2003;35:388–91. [Google Scholar]
- 34.Sabar AG. Lithotripsy of different urinary tract stones by using seeds of Carum copticum. italic>Iraqi J Pharm Sci. 2010;19:38–42. [Google Scholar]
- 35.Velazhahan R, Vijayanandraj S, Vijayasamundeeswari A, Paranidharan V, Samiyappan R, Iwamoto T, et al. Detoxification of aflatoxins by seed extracts of the medicinal plant Trachyspermum ammi (L.) Sprague ex Turrill-structural analysis and biological toxicity of degradation product of aflatoxin G1. Food Control. 2010;21:719–25. [Google Scholar]
- 36.Hussein G, Miyashiro H, Nakamura N, Hattori M, Kakiuchi N, Shimotohno K. Inhibitory effects of sudanese medicinal plant extracts on hepatitis C virus (HCV) protease. Phytother Res. 2000;14:510–6. doi: 10.1002/1099-1573(200011)14:7<510::aid-ptr646>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 37.Paul S, Kang SC. In vitro determination of the contraceptive spermicidal activity of essential oil of Trachyspermum ammi (L.) Sprague ex Turrill fruits. N Biotechnol. 2011;28:684–90. doi: 10.1016/j.nbt.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 38.Boskabady MH, Alizadeh M, Jahanbin B. Bronchodilatory effect of Carum copticum in airways of asthmatic patients. Therapie. 2007;62:23–9. doi: 10.2515/therapie:2007007. [DOI] [PubMed] [Google Scholar]
- 39.Ramaswamy S, Sengottuvelu S, Sherief SH, Jaikumar S, Saravanan R, Prasadkumar C, et al. Gastroprotective activity of ethanolic extract of Trachyspermum ammi fruit. Int J Pharm Bio Sci. 2010;1:1–5. [Google Scholar]
- 40.Mahboubi M, Kazempour N. Chemical composition and antimicrobial activity of Satureja hortensis and Trachyspermum copticum essential oil. Iran J Microbiol. 2011;3:194–200. [PMC free article] [PubMed] [Google Scholar]
- 41.Platel K, Srinivasan K. Studies on the influence of dietary spices on food transit time in experimental rats. Nutr Res. 2001;21:1309–14. [Google Scholar]
- 42.Gilani AH, Jabeen Q, Ghayur MN, Janbaz KH, Akhtar MS. Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum copticum seed extract. J Ethnopharmacol. 2005;98:127–35. doi: 10.1016/j.jep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Kaur H. Estrogenic activity of some herbal galactogogue constituents. Indian J Anim Nutr. 1998;15:232–4. [Google Scholar]
- 44.Jabbar A, Iqbal Z, Khan MN. In vitro anthelmintic activity of Trachyspermum ammi seeds. Pharmacogn Mag. 2006;2:126. [Google Scholar]
- 45.Hasani-Ranjbar S, Nayebi N, Moradi L, Mehri A, Larijani B, Abdollahi M. The efficacy and safety of herbal medicines used in the treatment of hyperlipidemia;a systematic review. Curr Pharm Des. 2010;16:2935–47. doi: 10.2174/138161210793176464. [DOI] [PubMed] [Google Scholar]
- 46.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005;4:685–8. [Google Scholar]
- 47.Patra JK, Panigrahi TK, Rath SK, Dhal NK, Thatoi HN. Phytochemical screening and antimicrobial assessment of leaf extracts of Excoecaria agallocha L. A mangal species of Bhitarkanika, Orissa, India. Adv Nat Appl Sci. 2009;3:241–6. [Google Scholar]
- 48.Liyana-Pathirana CM, Shahidi F. Antioxidant activity of commercial soft and hard wheat (Triticum aestivum L.) as affected by gastric pH conditions. J Agric Food Chem. 2005;53:2433–40. doi: 10.1021/jf049320i. [DOI] [PubMed] [Google Scholar]
- 49.Keshetty V, Srinivas Pabba RG, Kandukuri JM, Allenki V. Antihyperlipidemic activity of methanolic extract of Garlic (Allium sativum L.) in Triton X 100 induced hyperlipidemic rats. J Pharm Res. 2009;2:777–80. [Google Scholar]
- 50.Bays HE, Moore PB, Drehobl MA, Rosenblatt S, Toth PD, Dujovne CA, et al. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: Pooled analysis of two phase II studies. Clin Ther. 2001;23:1209–30. doi: 10.1016/s0149-2918(01)80102-8. [DOI] [PubMed] [Google Scholar]
- 51.Raza JA, Babb JD, Movahed A. Optimal management of hyperlipidemia in primary prevention of cardiovascular disease. Int J Cardiol. 2004;97:355–66. doi: 10.1016/j.ijcard.2003.07.039. [DOI] [PubMed] [Google Scholar]
- 52.Zulet MA, Barber A, Garcin H, Higueret P, Martínez JA. Alterations in carbohydrate and lipid metabolism induced by a diet rich in coconut oil and cholesterol in a rat model. J Am Coll Nutr. 1999;18:36–42. doi: 10.1080/07315724.1999.10718825. [DOI] [PubMed] [Google Scholar]
- 53.Kellner A, Correll JW, Ladd AT. Sustained hyperlipemia induced in rabbits by means of intravenously injected surface-active agents. J Exp Med. 1951;93:373–84. doi: 10.1084/jem.93.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiser RH, Jr, Denniston JC, Rindsig RB, Beisel WR. Triglyceride Secretion Rates: Use of Triton WR 1339 in the Rhesus Moneky. Army Medical Research Institute of Infectious Diseases, Frederick MD. 1973 doi: 10.1093/jn/104.2.223. [DOI] [PubMed] [Google Scholar]
- 55.Schurr PE, Schultz JR, Parkinson TM. Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids. 1972;7:68–74. doi: 10.1007/BF02531272. [DOI] [PubMed] [Google Scholar]
- 56.Jahromi MA, Ray AB. Antihyperlipidemic effect of flavonoids from Pterocarpus marsupium. J Nat Prod. 1993;56:989–94. doi: 10.1021/np50097a001. [DOI] [PubMed] [Google Scholar]
- 57.Sudha SS, Karthic R, Naveen JR. Anti hyperlipidemic activity of Spirulina platensis in Triton X-100 induced hyperlipidemic rats. Hygeia. 2011;3:32–7. [Google Scholar]
- 58.Gundamaraju R, Hwi KK, Singla RK, Vemuri RC, Mulapalli SB. Antihyperlipidemic potential of Albizia amara (Roxb) Boiv. bark against Triton X-100 induced hyperlipidemic condition in rats. Pharmacognosy Res. 2014;6:267–73. doi: 10.4103/0974-8490.138237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilson PW, Abbott RD, Castelli WP. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 1988;8:737–41. doi: 10.1161/01.atv.8.6.737. [DOI] [PubMed] [Google Scholar]
- 60.Warnholtz A, Mollnau H, Oelze M, Wendt M, Münzel T. Antioxidants and endothelial dysfunction in hyperlipidemia. Curr Hypertens Rep. 2001;3:53–60. doi: 10.1007/s11906-001-0081-z. [DOI] [PubMed] [Google Scholar]
- 61.Dhulasavant V, Shinde S, Pawar M, Naikwade NS. Antihyperlipidemic activity of Cinnamomum tamala Nees, on high cholesterol diet induced hyperlipidemia. Int J PharmTech Res. 2010;2:2517–21. [Google Scholar]
- 62.Iqbal Z, Iqbal K, Mudassar M. Hepatoprotective effect of Cinnamon on cholesterol induced fatty changes in albino rats. Isra Med J. 2015;7:225–7. [Google Scholar]
- 63.Martinez LO, Jacquet S, Tercé F, Collet X, Perret B, Barbaras R. New insight on the molecular mechanisms of high-density lipoprotein cellular interactions. Cell Mol Life Sci. 2004;61:2343–60. doi: 10.1007/s00018-004-4087-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Khan A, Safdar M, Ali Khan MM, Khattak KN, Anderson RA. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care. 2003;26:3215–8. doi: 10.2337/diacare.26.12.3215. [DOI] [PubMed] [Google Scholar]
- 65.Bainton D, Miller NE, Bolton CH, Yarnell JW, Sweetnam PM, Baker IA, et al. Plasma triglyceride and high density lipoprotein cholesterol as predictors of ischaemic heart disease in British men. The Caerphilly and Speedwell Collaborative Heart Disease Studies. Br Heart J. 1992;68:60–6. doi: 10.1136/hrt.68.7.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zari TA, Allogmani AS. Long-term effects of Cinnamomum zeylanicum Blume oil on some physiological parameters in streptozotocin-diabetic and non-diabetic rats. Bol Latinoam Caribe. 2009;8:266–74. [Google Scholar]
- 67.Wiesenfeld PW, Babu US, Collins TF, Sprando R, O’Donnell MW, Flynn TJ, et al. Flaxseed increased alpha-linolenic and eicosapentaenoic acid and decreased arachidonic acid in serum and tissues of rat dams and offspring. Food Chem Toxicol. 2003;41:841–55. doi: 10.1016/s0278-6915(03)00035-8. [DOI] [PubMed] [Google Scholar]
- 68.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41(12 Pt 2):1819–28. [PubMed] [Google Scholar]
- 69.Khanna AK, Rizvi F, Chander R. Lipid lowering activity of Phyllanthus niruri in hyperlipemic rats. J Ethnopharmacol. 2002;82:19–22. doi: 10.1016/s0378-8741(02)00136-8. [DOI] [PubMed] [Google Scholar]
- 70.Sharma SB, Nasir A, Prabhu KM, Murthy PS, Dev G. Hypoglycaemic and hypolipidemic effect of ethanolic extract of seeds of Eugenia jambolana in alloxan-induced diabetic rabbits. J Ethnopharmacol. 2003;85:201–6. doi: 10.1016/s0378-8741(02)00366-5. [DOI] [PubMed] [Google Scholar]
- 71.Farnier M, Davignon J. Current and future treatment of hyperlipidemia: The role of statins. Am J Cardiol. 1998;82:3J–10J. doi: 10.1016/s0002-9149(98)00423-8. [DOI] [PubMed] [Google Scholar]
- 72.Mozaffarian D, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354:1601–13. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]


