Abstract
Background:
Corbichonia decumbens (Forssk.) Exell (Molluginaceae), recently has moved to Lophiocarpaceae as per angiospermic plant group (APG) III system, is an annual or short-lived, dwarf, glabrous subshrub, prefers to grow on rocky places and on sand-stones in dry, hot areas of Rajasthan. This is the potential plant with medicinal properties. Vegetative organs under study show antioxidant, anti-inflammatory, antiulcer, antimicrobial, and antinociception activity.
Objective:
This study was carried out to identify the phytoconstituents present in the methanolic and ethyl-acetate extract of root and stem of C. decumbens by GC-MS analysis.
Materials and Methods:
Powdered test samples were sequentially extracted with methanol and ethyl-acetate. The compounds obtained as a result of GC-MS screening were identified on the basis of their retention time, peak area and compared with that of literature available and by interpretation of mass spectra.
Results:
GC-MS analysis of a methanolic extract of root detected mome-inositol (49.53%), guanosine (20.91%), and cis-vaccenic acid (9.25%). While ethyl-acetate extract of root analyzed pentadecanoic acid (17.91%), octadecanoic acid (15.01%) and cis-vaccenic acid (12.04%). Methanolic extract of stem detected mome-inositol (75.47%), pentadecanoic acid (6.04%), and 7-tetradecenal, (Z) (4.54%) while ethyl-acetate extract of stem revealed the presence of 1-heptacosanol (17.35%), hexadecanoic acid (17.17%), and octadecanal (12.64%).
Conclusion:
The results suggest that C. decumbens (Forssk.) Exell is a plant of potential medicinal value, yielding various bioactive compounds that confirm the application of this plant as a plant-based drug in pharmacy-industry.
SUMMARY
Extraction is the most important step in the analysis of bioactive compounds present in botanical preparations. The strength of solvent plays a key role in this process, methanol as well as ethyl-acetate showed better response as far as extraction potency is concerned. Gas chromatography mass spectrometry analysis is highly reliable, and the interpretations of the results are of high-quality. This tool is in particular useful for confirming of the presence of bioactive-substances. The results suggest that Corbichonia decumbens (Forssk.) Exell can be used for drug formulations against some major disorders, i.e., cancer, ulcer, tuberculosis, arthritis, etc.

Abbreviations Used: GC-MS: Gas Chromatography-Mass Spectrometry, kPa: Kilopascal, RT: Retention time, MF: Molecular formula, MW: Molecular weight
Keywords: Bioactive compounds, Corbichonia decumbens, gas chromatography mass spectrometry screening, lophiocarpaceae, phytochemical screening, retention time
INTRODUCTION
Plants have contributed a lot of medicinal compounds being used today to treat diseases such as cancer, hormonal imbalances, jaundice, diabetes, inflammation, etc. Plants are commonly available in abundance, especially in the tropics.[1] Most of the world’s population relies upon traditional medicine, particularly plant-based drug for the primary health care.[2] In developing countries, low-income people such as farmers, people of small isolated villages, and native communities use folk medicine for the treatment of common infections.[3] Researches into medicinal plants have shown that they contain secondary metabolites which possess a variety of structural arrangement and properties.[4]
The plant family, lophiocarpaceae is comprised of two genera with approximately 25 species.[5] These plants are commonly known as stone plants or carpet weeds. Corbichonia decumbens is a creeping, well-branched, diffuse-ascending, and prostrate to procumbent, semi-succulent, and annual herb of Rajasthan.[6]
The methanol extract of leaves of this plant has shown significant anti-inflammatory effects in various animal models and is used as an antiulcer.[7,8] Leaves of this plant are used as a herbal alternative for heal of various diseases.[9] The present investigation was carried out to detect phytochemicals from root and stem using gas chromatography coupled with mass spectrometry. The molecular weight and structure of the compounds of test materials were ascertained by interpretation on mass spectrum of gas chromatography-mass spectrometry (GC-MS) using the database of National Institute of Standard and Technology (NIST). The GC-MS analysis will provide a representative spectral output of all the compounds that get separated from the sample.[10] This tool facilitates the relationship between the compounds extracted from plants and their pharmacological efficacies.
MATERIALS AND METHODS
Fresh and healthy plant material was collected from rocky places of Jodhpur (Beri-Ganga, Machia-Safari, Bheem-Bhadak and Ossian) District of Rajasthan in the month of July–October 2016. The specimen authentication and taxonomic identification were done by Botanical Survey of India Jodhpur, Rajasthan.
The plant material was washed 2–3 times with running fresh water, shade-dried, and grinded to powder. The powdered plant material was kept in small and labeled plastic bags. Two gram powder was transferred to round bottom flask each containing 100 ml of solvent, i.e., methanol and ethyl-acetate, boiled at 65°–75°C for 6 h using Soxhlet extractor. Extract was filtered using Whatman filter no. 1 and evaporated to dryness. A volume of 2 μl of this solution was employed for GC-MS analysis.[11] The GC-MS analysis was performed at Advanced Instrumentation Research Facility (AIRF) JNU, Delhi. Syringe insertion and injection speed were high, and it was pumped for five times. Temperature of injection port was maintained at 260.0°C, column oven temperature was set at 80°C. For GC pressure was maintained at 81.9 kPa. Split ratio was 50.0, and ion source temperature was maintained at 230°C.
Standard analytical procedures were used for screening of preliminary phytochemicals, i.e., Wagner’s Test (for alkaloids), Braymer’s Test (for tannins), Salkowski’s Test (for steroids), Sodium Hydroxide Test (for flavonoids), Frothing Test (for saponins), and Molisch’s Test (for carbohydrates). The extract contained polar as well as nonpolar phytoconstituents. The spectrum of unknown components was compared with spectrum stored in the NIST library version. The eluted compounds were characterized on the basis of their molecular formula, structure, retention time, and peak % area.
RESULTS AND DISCUSSION
The preliminary phytochemical screening of root and stem extract in methanol and ethyl-acetate as solvent showed the presence of various metabolic compounds such as alkaloids, tannins, steroids, flavonoids, and carbohydrates. The compounds have shown an affirmative and strong response in both the solvents under the study of the stem as compared to root. Methanolic stem extract and ethyl-acetate root extract showed better response as compared to methanolic root and ethyl-acetate stem extract.
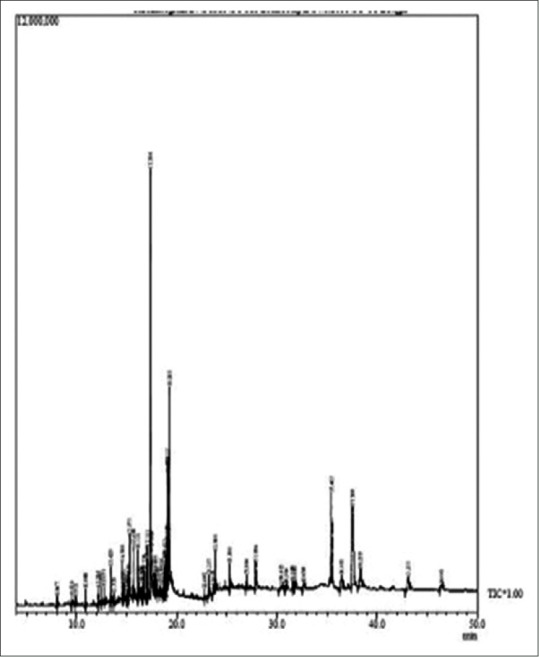
The more precise information in qualitative analysis can be obtained by GC-MS.[12] Root extract in methanol revealed 44 peaks [Figure 1] indicating the presence of 39 compounds and stem revealed 38 peaks [Figure 2] indicating 34 compound. 14 compounds showed biological activity in methanolic root and stem extract [Table 1]. Ethyl-acetate extract of root show the presence of 60 peaks [Figure 3] indicating 46 compounds and stem shows 56 peaks [Figure 4] indicating 44 compounds. Twenty compounds showed biological activity in ethyl-acetate root and stem extract [Table 2].
Figure 1.
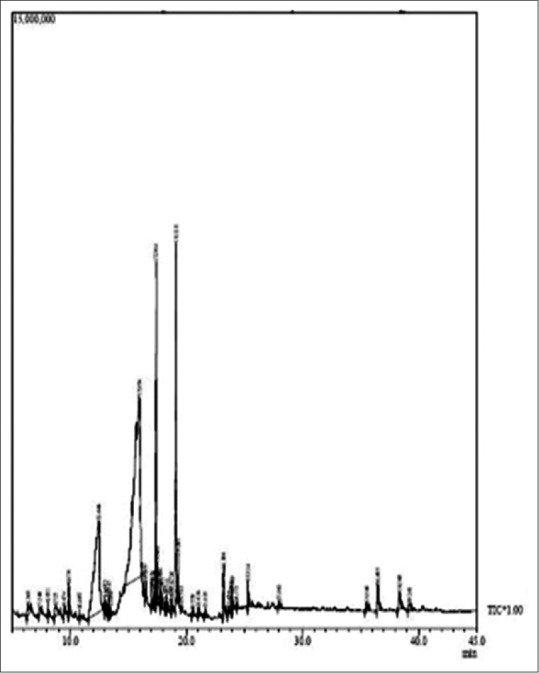
Gas chromatography-mass spectrometry chromatogram of methanolic root extract
Figure 2.
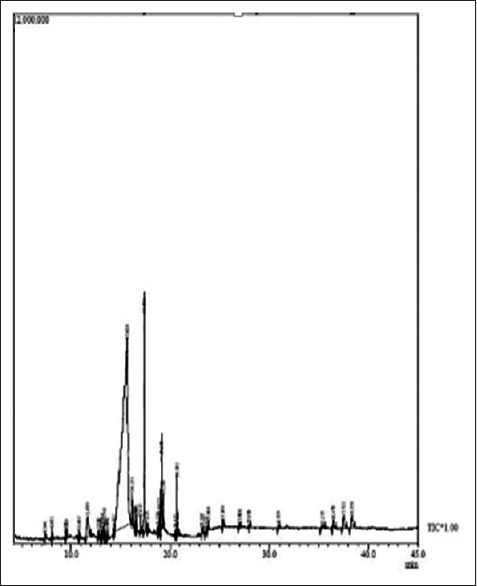
Gas chromatography-mass spectrometry chromatogram of methanolic stem extract
Table 1.
Bioactive compounds from vegetative parts (in methanol extract)
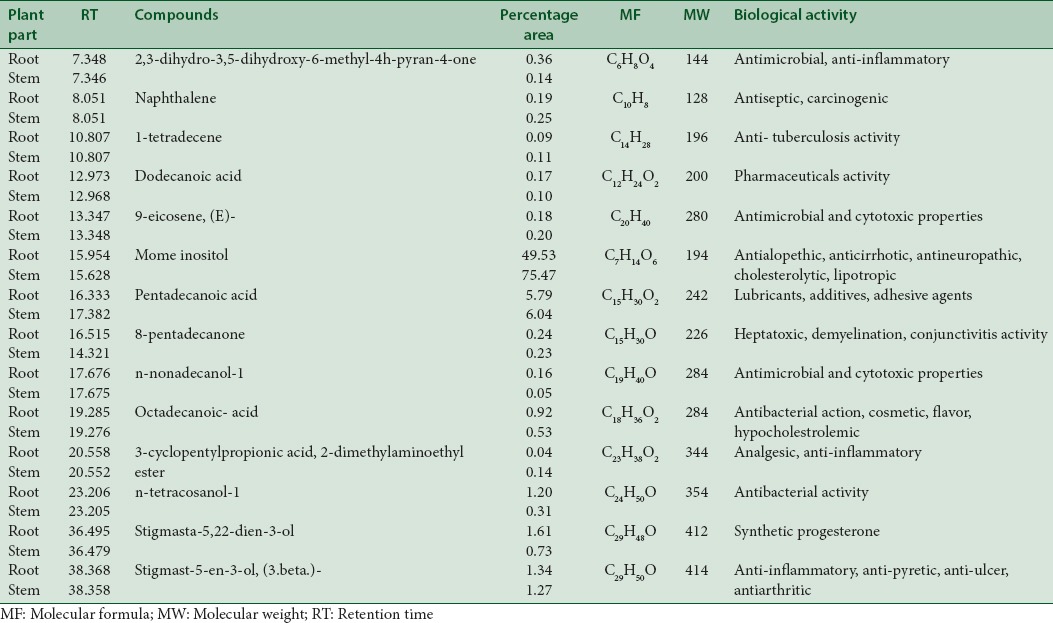
Figure 3.

Gas chromatography-mass spectrometry chromatogram of ethyl-acetate root extract
Figure 4.
Gas chromatography-mass spectrometry chromatogram of ethyl-acetate stem extract
Table 2.
Bioactive compounds from vegetative parts (in ethyl-acetate extract)
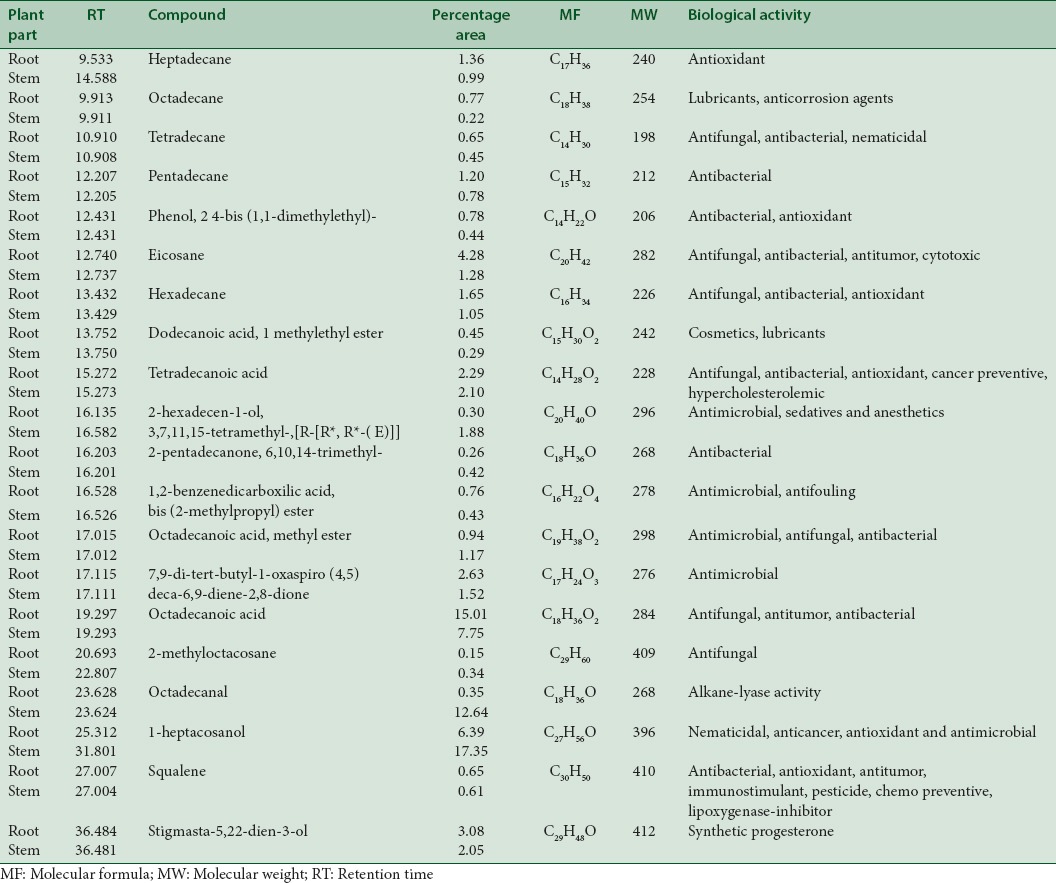
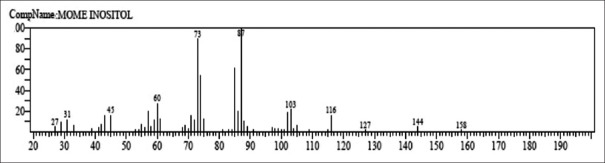
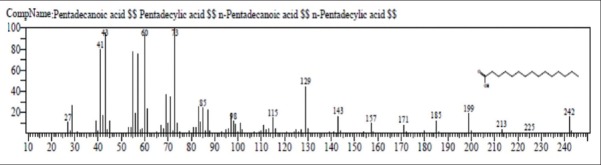
The first compound identified in methanolic root extract of C. decumbens with less retention time (RT = 6.365) was 1,3,5-triazine-2,4,6-triamine (0.88%), whereas 1-H-cyclopenta (A) pentalene, 2,3B,6,6A,7,7A-hexahydro-2, 2, 3B-trimethyl, (3B. Alpha., 6A. Alpha. 7A. Beta-(0.44%) was the last compound which take longest retention time (RT = 39.241). Mome-inositol [Figure 5] showed higest % area (49.53%) in methanolic root extract. In ethyl-acetate root extract, decane, 3-methyl showed less retention time (RT = 8.068) with 0.32% area whereas tetrakis (2,3-ditert-butylphenyl)-4,4’- biphenylene diphosphonate was the last compound which was retained for longest time (RT = 42.600) with 1.27% area. Pentadecanoic acid [Figure 6] showed highest % area (17.91%).
Figure 5.
Mass spectrum of mome inositol
Figure 6.
Mass spectrum of pentadecanoic acid
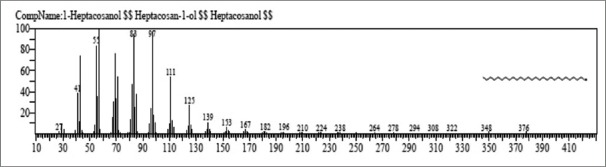
The first compound identified in methanolic stem extract of C. decumbens with less retention time (RT = 7.346) was 2, 3-dihydro-3, 5-dihydrdxy-6-methyl-4H-pyran-4-one with 0.14% area whereas stigmast-5-en-3-ol, (3 beta)-take longest retention time (RT = 38.358) with 1.27% area. Highest % area in methanolic stem extract is taken by mome-inositol (75.47%). In ethyl-acetate stem extract, dodecane showed less retention time (RT = 8.067) with 0.26% area, whereas 1-heptacosanol [Figure 7] was the last compound which take longest retention time (RT = 46.441) and highest % area (17.35%).
Figure 7.
Mass spectrum of 1-heptacosanol
These observations support stronger extraction capacity of methanol and ethyl-acetate extract that could have been produced a number of active constituents responsible for many biological activities. The compounds identified by preliminary qualitative analysis and GC-MS analysis are medicinally important as they possess unique structure with specific biological activities. Many plant parts are used in Ayurvedic, traditional, folk, and homoeopathic medicines to treat several ailments, including liver and spleen enlargement, hepatitis, nervous disorders, renal disorders, bronchitis and asthma, and whooping cough.[13,14] For extraction of these bioactive compounds effects on extraction rate of solvents should be investigated.[15] The accuracy of a method can be measured through comparing with the external standard method.[16] Before using herbal or plant-based drugs further toxicity studies using human cell lines are needed to determine the suitability of these preparations.[17]
CONCLUSION
These results may help in standardization, characterization and identification of bioactive compounds to carry out further research. The presence of various bioactive compounds justifies the use of root and stem to cure various ailments by conventional practitioners. Hence, it is recommended as a plant of potential pharmaceutical importance. Thus, this plant can be used as a potential source in the field of drug formulations. Further research is desired to resolve ethical and legal challenges.
Financial support and sponsorship
The authors are thankful to AIRF, JNU, Delhi and CAS department of Botany JNVU, Jodhpur (Rajasthan) for providing infrastructure and technical support.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Padmanabhan P, Jangle SN. Evaluation of in-vitro anti-inflammatory activity of herbal preparation, a combination of four medicinal plants. Int J Basic Appl Med Sci. 2012;2:109–16. [Google Scholar]
- 2.Dubey NK, Kumar R, Tripathi P. Global promotion of herbal medicine;India's opportunity. Curr Sci. 2004;86:37–41. [Google Scholar]
- 3.Balandrin MF, Klocke JA, Wurtele ES, Bollinger WH. Natural plant chemicals: Sources of industrial and medicinal materials. Science. 1985;228:1154–60. doi: 10.1126/science.3890182. [DOI] [PubMed] [Google Scholar]
- 4.Theresa IE, Stephen OO, Adeola OO, Franklin A. Quantitative determination of the saponin contents and GC-MS study of the medicinal plants Cassytha filiformis (Linn.) leaves. J Coastal Life Med. 2016;4:154–6. [Google Scholar]
- 5.Roskov Y, Kunze T, Orrell T, Abucay L, Paglinawan L, Culham A, et al. Didžiulis V, editor. ‘Species 2000 & ITIS Catalogue of Life: Annual Checklist’. Species. 2000 [Google Scholar]
- 6.Arora S, Saini M. Morphological studies on medicinally important plant of Gisekia pharnaceoides Linn. and Corbichonia decumbens (Forssk.) Exell of Molluginaceae from Thar Desert of Rajasthan, India. Biolife. 2016;4:327–32. [Google Scholar]
- 7.Uma G, Balasubramaniam V, Jagathes KS. Assessment of anti-ulcer activity of Corbichonia decumbens Forssk. Methanolic extract by aspirin plus pyloric ligation model. Int J Pharm Res Sch. 2014;3:757–61. [Google Scholar]
- 8.Uma G, Balasubramaniam V, Jagathes KS. In-vivo screening of anti-inflammatory activity in methanolic extract of Corbichonia decumbens Forssk. using various animal models of paw oedema. Int J Pharm Pharm Sci. 2014;6:146–8. [Google Scholar]
- 9.Arora S, Saini M. Biochemical screening of leaf extract of Corbichonia decumbens (Forssk.) Exell of Molluginaceae from Thar Desert of Rajasthan, India. Adv Plant Sci. 2016;29:275–8. [Google Scholar]
- 10.Rukshana MS, Doss A, Kumari PR. Phytochemical screening and GC-MS ANALYSIS of leaf extract of Pergularia daemia (Forssk) Chiov. Asian J Plant Sci Res. 2017;7:9–15. [Google Scholar]
- 11.Merlin NJ, Parthasarathy V, Manavalan R, Kumaravel S. Chemical investigation of aerial parts of Gmelina asiatica Linn by GC-MS. Phycog Res. 2009;1:152–6. [Google Scholar]
- 12.Zhang C, Qi M, Shao Q, Zhou S, Fu R. Analysis of the volatile compounds in Ligusticum chuanxiong Hort. using HS-SPME-GC-MS. J Pharm Biomed Anal. 2007;44:464–70. doi: 10.1016/j.jpba.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 13.Motamarri SN, Karthikeyan M, Rajasekar S, Gopal V. Indigofera tinctoria Linn-a phytopharmacological review. Int J Res Pharm Biomed Sci. 2012;3:164–9. [Google Scholar]
- 14.Renukadevi KP, Sultana SS. Determination of antibacterial, antioxidant and cytotoxicity effect of Indigofera tinctoria on lung cancer cell line NCI-h69. Int J Pharmacol. 2011;7:356–62. [Google Scholar]
- 15.National Commission of Chinese Pharmacopoeia. Pharmacopoeia of People's Republic of China [Google Scholar]
- 16.Wu C, Guan Q, Wang S, Rong Y. Simultaneous determination of multiple ginsenosides in Panax ginseng herbal medicines with one single reference standard. Phycog Mag. 2017;13(Suppl S1):84–9. doi: 10.4103/pm.pm_274_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohanty S, Cock IE. Bioactivity of Syzygium jambos methanolic extracts: Antibacterial activity and toxicity. Phycog Res. 2010;2:4–9. doi: 10.4103/0974-8490.60577. [DOI] [PMC free article] [PubMed] [Google Scholar]