Abstract
Objective:
It has long been known that chickens, like mammals, are capable of producing antigen-specific immunoglobulin Y (IgY), which functions similar to IgG. The present study was performed to investigate the activity of IgY anti-Mycobacterium tuberculosis on proliferation, interleukin (IL)-2, and interferon (IFN)-γ expression of rat peripheral blood mononuclear cells (PBMCs).
Materials and Methods:
The activity of IgY anti-M. tuberculosis in different doses (25, 50, and 100 μg/ml) on rat PBMCs proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay. The production of IL-2 and IFN-γ in the PBMC supernatant was determined using enzyme-linked immunosorbent assay. Investigation was performed on mRNA expression of IL-2 and IFN-γ by reverse transcription-polymerase chain reaction (RT-PCR).
Results:
IgY anti-M. tuberculosis significantly increased the proliferation of rat PBMC. Furthermore, IgY anti-M. tuberculosis dose dependently increased IL-2 and IFN-γ production in PBMC, suggesting that pharmacological activities of IgY anti-M. tuberculosis in PBMC may be mediated by regulating the production of cytokines. In the RT-PCR, expression of cytokines such as IL-2 and IFN-γ in PBMC cultures was increased by IgY anti-M. tuberculosis.
Conclusions:
We concluded that increasing IL-2 and IFN-γ productions in PBMC was related to IgY anti-M. tuberculosis, stimulating the mRNA transcription (gene expression) of these cytokines which can induce proliferation of PBMC.
SUMMARY
Lohman laying hens immunized intramuscularly with antigens of M. tuberculosis can produce specific IgY anti-Mycobacterium tuberculosis complex
IgY anti-M. tuberculosis significantly increased the proliferation of rat peripheral blood mononuclear cell (PBMC)
IgY anti-M. tuberculosis dose dependently increased interleukin 2 (IL-2) and interferon (IFN)-γ production in PBMC
In the reverse transcription-polymerase chain reaction, expression of cytokines such as IL-2 and IFN-γ in PBMC cultures was increased by IgY anti-M. tuberculosis
The increasing IL-2 and IFN-γ productions in PBMC were related to stimulation on mRNA transcription which can induce proliferation of PBMC.
Abbreviations Used: IgY anti-M. tuberculosis: Immunoglobulin Y anti-Mycobacterium tuberculosis; IL-2: Interleukin-2; IFN-γ: Interferon-γ; PBMCs: Peripheral blood mononuclear cells.
Keywords: Immunoglobulin Y anti-Mycobacterium tuberculosis, Interferon-γ, interleukin-2, rat peripheral blood mononuclear cell
INTRODUCTION
Hens’ eggs have long been known as an excellent source of nutrients for humans and also an important source of antibodies. In the eggs from chicken (Gallus domesticus), the major antibody is called immunoglobulin Y (IgY).[1] The laying hens are used to produce polyclonal antibodies as an alternative to the use of mammals, such as rabbits, and since more than two decades, egg yolk antibodies are of low cost and serve as an ethical alternative.[2] Compared to mammalian antibodies, IgY possesses several biochemical advantages such as its simple purification from egg yolk. IgY can be easily obtained noninvasively and prevents a stressful moment in animal handling.[3]
The natural transfer of antibodies that occurs from hen to chick via the egg yolk can be exploited to produce antibodies specific to a given pathogen, simply by immunizing the laying hens with an antigen from this targeted pathogen.[4] Recently, the utilization of IgY from eggs of chickens, which were immunized against certain pathogens, has been the focus of attention in immunotherapy and immunodiagnosis, since IgY antibodies are the predominant serum Ig transferred to egg yolk to confer passive immunity to embryos and neonates. Therefore, research and diagnostic community constantly demands new alternatives and procedures to produce cost-effective antibodies.[5] Sudjarwo et al. (2017) have reported that IgY anti-Mycobacterium tuberculosis was successfully elicited by immunizing the hens with formalin-inactivated M. tuberculosis antigen emulsified in Freund’s adjuvant. The IgY concentration in egg yolk increased during the immunization period until week 6 where it began to increase dramatically at 2 weeks and it reached a plateau at 4 weeks after immunization. After week 6, the levels decreased gradually.[6] The immunization of hens with M. tuberculosis could be a strategy to obtain at low cost a relatively high concentration of anti-M. tuberculosis egg yolk IgY, which could be a useful tool for research, diagnosis, and therapy of M. tuberculosis infection. Considering the epidemiological change in TB disease in developing countries around the world and the rise in the morbidity of the disease, it would be of interest to develop an alternative for the immunotherapy of M. tuberculosis infection.[6]
We developed a new immunotherapy from chickens immunized with the M. tuberculosis antigen that can be used against M. tuberculosis. Evaluations of immunoregulatory agents provide valuable information during assessments of IgY as immunotherapy. Proliferation and cytokine (interleukin 2 [IL-2], interferon-γ [IFN-γ]) productions are two key functions of immune cells, and as such, are important to examine during any evaluation of immunotherapy potential of a given IgY anti-M. tuberculosis. The mitogen-induced proliferation of lymphocyte is often a preferable assay that correlates with the status of cell-mediated immunity in a host after exposure to IgY.[7,8] In this context, immunotherapy serves as a powerful tool to restore the protective immune response. Cytokines are important molecules in innate immunity.[9] CD4 + T helper cells upon antigenic encounter either polarize toward Th-1 phenotype (that produces IFN γ and IL-2) which promotes cell-mediated immunity.[10] The present study was designed to evaluate the activity of IgY anti-M. tuberculosis complex on proliferation, IL-2, and IFN-γ expression of rat peripheral blood mononuclear cells (PBMCs).
MATERIALS AND METHODS
Preparation of rat peripheral blood mononuclear cells
Peripheral blood from rats (5 ml per rat) was collected by cardiac puncture in the presence of heparin as the anticoagulant. PBMC was isolated by the Ficoll-Hypaque gradient density method as described previously.[7] The 20 ml peripheral blood was centrifuged at 2000 rpm, 4°C for 10 min to remove the plasma. Blood cells were diluted with phosphate-buffered saline and then centrifuged in a Ficoll-Hypaque discontinuous gradient at 1500 rpm for 30 min. The PBMC layers were collected and washed with cold distilled water and 10X Hanks’ buffer saline solution to remove red blood cells. The cells were resuspended to a concentration of 2 × 106 cells/ml in RPMI-1640 medium supplemented with 2% fetal calf serum, 100 U/ml penicillin, and 100 μg/ml streptomycin.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide cell viability assay
The effect of the IgY anti-M. tuberculosis on cell viability of PBMC was first determined using a colorimetric technique which is 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.[7] Briefly, 100 μL of RPMI-1640 media with 10% of FBS was added to all the wells except row A in the 96-well plate (TPP, Switzerland). Then, 100 μL of diluted IgY anti-M. tuberculosis at 100 μg/ml was added into row A and row B. A series of 2-fold dilution of the extract was carried out down from row B until row G. Row H was left untouched and the excess solution (100 μl) from row G was discarded. A volume of 100 μl of lymphocyte (from rat peripheral blood) with cell concentration at 5 × 105 cells/ml was added to all wells in the 96-well plate to make up the final volume of 200 μl and thus the extract was diluted into the concentration range of 100–1.56 μg/ml. All the plates were incubated at 37°C, 5% CO2, and 90% humidity incubator for selected period (72 h). After the corresponding period of 72 h, 20 μl of MTT (Sigma, USA) at 5 mg/ml was added to each well in the 96-well plate and incubated for 4 h in 37°C, 5% CO2, and 90% humidity incubator. A volume of 170 μl of medium with MTT was removed from every well and was added to each well of 25, 50, and 100 μg/mL IgY anti-M. tuberculosis and the formazan crystal was solubilized by incubating for 20 min in 37°C, 5% CO2 incubator. Finally, the plate was read at 570 nm wavelength using μ Quant enzyme-linked immunosorbent assay (ELISA) Reader (Bio-tekInstruments, USA). Each IgY anti TB and control was assayed in triplicate in three independent experiments. The percentage of proliferation was calculated by the following formula:
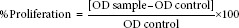
Determination of interleukin 2 and Interferon-γ production in peripheral blood mononuclear cell
PBMCs (2 × 105 cells/well) were cultured with various concentrations of IgY anti-M. tuberculosis (25, 50, and 100 μg/mL) for 72 h. The supernatant samples were collected and stored at −20°C until use. Production of IL-2 and IFN-γ in PBMC supernatant samples was measured using a sandwich ELISA method (Endogen, Boston, MA, USA), according to manufacturer’s instructions.
Extraction of total cellular RNA
Total cellular RNA was extracted from PBMCs using a previously described method.[10] PBMCs (5 × 106 cells) were activated with various concentrations (25, 50, and 100 μg/mL) of IgY anti-M. tuberculosis for 72 h. After incubation, the collected cells were lysed in Tri-Reagent (Molecular Research Center, Cincinnati, OH, USA) as described in the manufacturer’s protocol. After centrifugation, the extracted RNA was precipitated with isopropanol. The mixture was centrifuged, and the total cellular RNA pellet was washed with 75% ethanol. Diethyl pyrocarbonate-treated water was added to re-dissolve the RNA pellet before further processing.
Determination of mRNA expression by reverse transcription-polymerase chain reaction (RT-PCR)
Before the RT-PCR, about 1 μg of total RNA was treated with RNase-free deoxyribonuclease I (DNase I) (Fermentas, Burlington, ON, Canada), according to manufacturer’s instructions. First-strand cDNA was synthesized from about 800 ng DNase I-treated RNA using the ImProm-IITM Reverse Transcription System (Promega) and oligo (dT) 17 primers, as per the manufacturer’s protocol. RT was carried out at 50°C for 65 min. After cDNA synthesis, the desired DNA fragments were amplified for 30–35 cycles using DNA polymerase and specific primers for IL-2, IFN-γ, and β-actin transcripts. Primer sequences for the internal control, actin, were 5-TAC ATG GCT GGG GTG TTG AA-3 for the downstream primer and 5-AAG AGA GGC ATC CTC ACC CT-3 for the upstream primer. Primer sequences for cytokines were as follow: for IL-2, 5-AAC TCC TGT CTT GCA TTG CAC TA-3 for the 5 primer, and 5-TTG CTG ATT AAG TCC CTG GGTC-3 for the 3 primer (Beite Kaito); for IFN-5-AGT TAT ATC TTG GCT TTT CA-3 for the 5 primer, and 5-ACC GAA TAA TTA GTC AGC TT-3 for the 3 primer. The final PCR products were subjected to electrophoresis and stained with EtBr. The DNA bands corresponding to IL-2, IFN-γ, and β-actin transcripts were 229, 435, and 656 bp, respectively.
Statistical analysis
Data were reported as means ± standard deviation, and levels of significance were evaluated using one-way ANOVA with least significant difference test. The differences were considered significant at the level of P < 0.05.
RESULTS
Immunoglobulin Y anti-Mycobacterium tuberculosis effect on rat peripheral blood mononuclear cell proliferation
IgY anti-M. tuberculosis effect on human PBMC proliferation was studied by treating resting cells with 25, 50, and 100 μg/mL IgY anti-M. tuberculosis for 72 h. Cell proliferation was determined using nonradioactive cell proliferation assay or MTT assay. Significant effects on PBMC proliferation were noted after 72 h treatment with 100 μg/mL IgY anti-M. tuberculosis but not 25 and 50 μg/mL IgY anti-M. tuberculosis [Table 1]. Moreover, after 72 h treatment, 100 μg/mL IgY anti-M. tuberculosis increased the viability of PBMCs. IgY anti-M. tuberculosis induced its stimulatory effect on PBMC proliferation in a concentration-dependent manner.
Table 1.
Proliferative effect of immunoglobulin Y anti-Mycobacterium tuberculosis on rat peripheral blood mononuclear cells

Effects of Immunoglobulin Y anti-Mycobacterium tuberculosis on interleukin 2 and interferon-γ levels
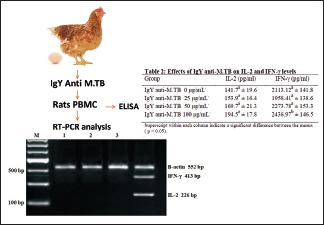
Immunotherapeutic activity of IgY anti-M. tuberculosis can be measured by detecting the changes in the production of immune molecules such as cytokines. To elucidate the molecular mechanisms underlying IgY anti-M. tuberculosis’ biological effect on rat PBMCs, we measured the levels of IL-2 and IFN-γ using ELISA. IL-2 and IFN-γ productions were increased by IgY anti-M. tuberculosis in a dose-dependent manner [Table 2]. IL-2 and IFN-γ productions were significantly increased by 100 μg/mL IgY anti-M. tuberculosis but not 25 and 50 μg/mL IgY anti-M. TBC.
Table 2.
Effects of immunoglobulin Y anti-Mycobacterium tuberculosis on interleukin-2 and interferon-γ levels

Interleukin 2 and interferon-γ mRNA expression of Immunoglobulin Y anti-Mycobacterium tuberculosis complex
To determine whether the stimulation of cytokine production in activated rats PBMCs treated with 25, 50, and 100 μg/mL IgY anti-M. tuberculosis was due to a transcriptional impact, we extracted total RNA from treated cells and determined the levels of IL-2 and IFN-γ mRNA using RT-PCR. The remarkable impact of IgY anti-M. tuberculosis on the mRNA levels of the cytokines is shown in Figure 1. Lane 1: control (0 μg/ml IgY anti-M. tuberculosis), Lane 2: 25 μg/ml IgY anti-M. tuberculosis, Lane 3: 50 μg/ml IgY anti-M. tuberculosis, and Lane 4: 100 μg/ml IgY anti-M. tuberculosis. The RT-PCR results proved that production of cytokines such as IL-2 and IFN-γ in PBMC cultures was increased by IgY anti-M. tuberculosis. By RT-PCR, we have demonstrated that 100 μg/ml but not 25 and 50 μg/ml IgY anti-M. tuberculosis did affect IL-2 and IFN-γ gene expression in PBMC. We suggested that increasing IL-2 and IFN-γ productions in PBMC were related to IgY anti-M. tuberculosis, stimulating the mRNA transcription of these cytokines.
Figure 1.
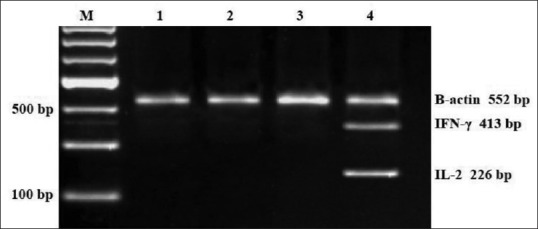
Reverse transcription-polymerase chain reaction analysis of interleukin 2 and interferon-γ expression in rat peripheral blood mononuclear cell treated with immunoglobulin Y anti-Mycobacterium tuberculosis (0, 25, 50, and 100 μg/mL) for 72 h. Representative result is shown on 2% agarose gel electrophoresis displaying mRNA transcripts of interleukin 2, interferon-γ, and β-actin. Lane 1 = control (0 μg/mL immunoglobulin Y anti-Mycobacterium tuberculosis); lane 2, 3, and 4 = Immunoglobulin Y anti M. tuberculosis (25, 50, 100 μg/mL)
DISCUSSION
It has long been known that chickens, like mammals, are capable of producing antigen-specific IgY, which functions similar to IgG, in response to an antigenic stimulus.[11] IgY is continually synthesized, excreted into the blood, and transferred to the egg yolk, where it is accumulated.[12] Recently, considerable research has focused on the use of IgY as an alternative to mammalian antibodies for several applications, including for immunotherapeutic applications, especially for the oral passive immunization against various bacteria and viruses.[13,14] Many researchers have also used IgY as a replacement for IgG in various immunodiagnostic and immunotherapy purposes. The use of IgY offers several advantages over polyclonal antibodies produced in mammals, including providing a much more hygienic, cost-efficient, convenient, humane, and plentiful source of antigen-specific antibodies.[2,15] In the present study, we assessed the potential immunomodulatory properties of IgY anti-M. tuberculosis by analyzing proliferative responses and cytokine profiles in rat PBMCs. The result demonstrates that IgY anti-M. tuberculosis can increase IL-2 and IFN-γ productions and proliferation of rat PBMCs in a dose-dependent manner. This observation in the present work indicates that IgY anti-M. tuberculosis possesses immunological properties in vitro using rat PBMCs as target cells. We suggest that immune stimulatory of IgY anti-M. tuberculosis on PBMC proliferation may have involved the regulation of IL-2 and IFN-γ production in the rat PBMC cultures.
The major role of these cytokines is to control antibody- and cell-mediated immunity.[16] These findings were similar to our previous study results of IgY for immune modulation properties. It has been demonstrated in many previous studies with T-cells that a series of genes such as IL-2 and IFN-γ are pivotal in the proliferation of T-lymphocytes against antigen (bacterial and viral) infection.[7] Regulation of T-lymphocyte activation and proliferation and cytokine production has been shown to be one of the actions of immunomodulatory IgY. Many studies have indicated that the production of cytokines such as IL-2 and IFN-γ is involved in the regulation of PBMC proliferation.[10,17]
IgY anti-M. tuberculosis immunotherapy is thought to enhance T-helper 1 (Th-1) response, resulting in the release of type-1 cytokines (IL-2, IL-12, IL-15, and IFN-γ) and putatively augments cell-mediated immunity. The results of RT-PCR have shown that the levels of IL-2 and IFN-γ expression could be enhanced by IgY anti-M. tuberculosis in rat PBMCs. This study clearly describes that IgY anti-M. tuberculosis can increase IL-2 and IFN-γ expressions which have an important role in the regulation of PBMC proliferation. As we know, interaction of T-cells with antigens initiates a cascade of gene expressions such as IL-2 and IFN-γ mRNA that induce the resting T-cells to enter the cell cycle (G0 to G1 transition) and culminate in the expression of secretion of IL-2 and IFN-γ.[10,17,18] In response to IL-2, the activated T-cells’ progress through the cell cycle and proliferate and differentiate into memory cells or effector cells. It indicates that IgY anti M. tuberculosis is a promoter of Th1 response. We concluded that the enhancements of IL-2 and IFN-γ productions in PBMC were related to IgY anti-M. tuberculosis stimulating the mRNA transcription of these cytokines. The results suggest that IgY anti-M. tuberculosis might be an immunotherapy source that can potentially alter cytokine secretion proliferation in PBMCs.
CONCLUSION
In the present study, IgY anti-M. tuberculosis increased IL-2 and IFN-γ productions and proliferation in rat PBMCs. We concluded that the increasing IL-2 and IFN-γ productions in PBMC were related to IgY anti-M. tuberculosis, stimulating the mRNA transcription (gene expression) of these cytokines which can induce the proliferation of PBMC.
Financial support and sponsorship
This study was supported by the Directorate General of Higher Education, Ministry of Research, Technology and Higher Education of the Republic of Indonesia. Grant number: 004/SP2H/LT/DRPM/IV/2017).
Conflicts of interest
There are no conflicts of interest
Acknowledgement
The authors sincerely thank Prof. Drs. H. Muhammad Nasir, M.Si, Ak, Ph.D, the Minister of Research, Technology and Higher Education of the Republic of Indonesia for funding this work
REFERENCES
- 1.Kovacs-Nolan J, Mine Y. Egg yolk antibodies for passive immunity. Annu Rev Food Sci Technol. 2012;3:163–82. doi: 10.1146/annurev-food-022811-101137. [DOI] [PubMed] [Google Scholar]
- 2.Schade R, Calzado EG, Sarmiento R, Chacana PA, Porankiewicz-Asplund J, Terzolo HR, et al. Chicken egg yolk antibodies (IgY-technology): A review of progress in production and use in research and human and veterinary medicine. Altern Lab Anim. 2005;33:129–54. doi: 10.1177/026119290503300208. [DOI] [PubMed] [Google Scholar]
- 3.Spillner E, Braren I, Greunke K, Seismann H, Blank S, du Plessis D, et al. Avian igY antibodies and their recombinant equivalents in research, diagnostics and therapy. Biologicals. 2012;40:313–22. doi: 10.1016/j.biologicals.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumaran T, Citarasu T. IgYtechnology: Production of antibodies in chickens and use in therapy of infectious diseases. Int J Sci Res Mod Educ. 2016;1:29–35. [Google Scholar]
- 5.Dias da Silva W, Tambourgi DV. IgY: A promising antibody for use in immunodiagnostic and in immunotherapy. Vet Immunol Immunopathol. 2010;135:173–80. doi: 10.1016/j.vetimm.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sudjarwo SA, Eraiko K, Sudjarwo GW, Koerniasari The potency of chicken egg yolk immunoglobulin (IgY) specific as immunotherapy to Mycobacterium tuberculosis infection. J Adv Pharm Technol Res. 2017;8:91–6. doi: 10.4103/japtr.JAPTR_167_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sudjarwo SA, Giftania WS, Koerniasari Proliferator activity, expression interleukin-2 and interferon-γ of immunoglobulin Y anti HIV in peripheral blood mononuclear cells. Int J Chemtech Res. 2015;8:194–9. [Google Scholar]
- 8.Gupta A, Kumar S, Mahindroo N, Saini RV. Bioactive fraction from Datura stramonium linn. promotes human immune cells mediated cytotoxicity towards lung and breast cancer cells. Pharmacogn J. 2016;8:435–9. [Google Scholar]
- 9.Kuo YC, Huang YL, Chen CC, Lin YS, Chuang KA, Tsai WJ, et al. Cell cycle progression and cytokine gene expression of human peripheral blood mononuclear cells modulated by Agaricus blazei. J Lab Clin Med. 2002;140:176–87. doi: 10.1067/mlc.2002.126717. [DOI] [PubMed] [Google Scholar]
- 10.Yeap SK, Mohamed Alitheen NB, Yong Ho W, Omar AR, Ali AM, Beh BK, et al. Immunomodulatory role of Rhaphidophora korthalsii methanol extract on human peripheral blood mononuclear cell proliferation, cytokine secretion and cytolytic activity. J Med Plant Res. 2010;5:958–65. [Google Scholar]
- 11.Dubie T, Yimer S, Adugna M, Sisay T. The potential application of avian egg antibodies with emphasis on immunotherapeutic and immunodiagnostic purpose. Adv Res J Biochem Biotechnol. 2014;1:18–30. [Google Scholar]
- 12.Sudjarwo SA, Indriyani W, Nasronudin Giftania WS, Koerniasari Production and characterization protein of anti-HIV specific immunoglobulin Y for immunotherapy. J App Pharmac Sci. 2014;4:30–4. [Google Scholar]
- 13.Vega CG, Bok M, Vlasova AN, Chattha KS, Fernández FM, Wigdorovitz A, et al. IgY antibodies protect against human Rotavirus induced diarrhea in the neonatal gnotobiotic piglet disease model. PLoS One. 2012;7:e42788. doi: 10.1371/journal.pone.0042788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajan S, Karthika S, Michael A, Gandhimath RS. Generation of egg yolk antibodies in chicken (IgY) against Streptococcus mutans and its in-vitro neutralization efficacy. Arch Appl Sci Res. 2011;3:404–41. [Google Scholar]
- 15.Ferreira Júnior Á, Santiago FM, Silva MV, Ferreira FB, Macêdo Júnior AG, Mota CM, et al. Production, characterization and applications for Toxoplasma gondii-specific polyclonal chicken egg yolk immunoglobulins. PLoS One. 2012;7:e40391. doi: 10.1371/journal.pone.0040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meera S, Nagarjuna CG. Antistress and immunomodulatory activity of aqueous extract of Momordica charantia. Pharmacogn Mag. 2009;5:69–73. [Google Scholar]
- 17.Tsai KD, Lin BR, Perng DS, Wei JC, Yu YW, Cherng JM. Immunomodulatory effects of aqueous extract of Ocimum basilicum (Linn.) and some of its constituents on human immune cells. J Med Plant Res. 2011;5:1873–83. [Google Scholar]
- 18.Nguyen K, Sparks J, Omoruyi F. Effects of Ligusticum porteri (Osha) root extract on human promyelocytic leukemia cells. Pharmacognosy Res. 2017;9:156–60. doi: 10.4103/0974-8490.204641. [DOI] [PMC free article] [PubMed] [Google Scholar]


