Abstract
Background:
The assessment of the nutritional composition and phytochemical screening of banana pseudostem (PB) and flower (FB) advocate this nonconventional food source for routine consumption, considering its various health benefits.
Objectives:
The aim is to assess the proximate nutrient composition, fatty acids, minerals, amino acid profile, and global antioxidant response (GAR) of PB and FB.
Methods:
Standard analytical procedures were used to determine the nutritional quality and GAR of PB and FB.
Results:
The chemical analysis illustrated that functional profile (water holding capacity, oil holding capacity, swelling power, and solubility), and proximate (ash, moisture, protein, fat, dietary fiber, and carbohydrate) contents were substantially high in FB than PB. With a well-proportionate amino acid profile, PB (0.56) and FB (0.54) comprised of a high ratio of essential to nonessential amino acids than those of FAO/WHO requirement (0.38). The mineral analysis revealed that PB and FB were rich in macro and micro minerals in the order K > Ca > Mg > P > Na and K > Mg > Na > Ca > P, respectively. Linoleic acid was found to be the major component in PB and FB. Besides, total antioxidant activity conducted for PB and FB by GAR method, measuring both bio-accessible and insoluble fractions, revealed that the soluble fraction fared better than the chemical extracts.
Conclusion:
The results revealed high nutritional qualities of the byproducts of banana and the low cost of its production promotes their use as a prospective nonconventional food resource with high nutraceutical value.
SUMMARY
AOAC: Association of Analytical Communities
FAO/WHO: Food and Agriculture Organization of the United Nations/World health organization
Abbreviations Used: Banana flower was more potent than banana pseudostem in terms of its nutritional quality and total antioxidant capacity affirming their usefulness (of both the secondary products) in the pharmaceutical sector as a nutritional supplement due to the health-related properties of dietary fibre and associated bioactive compounds.
Keywords: Amino acid, fatty acid, global antioxidant response, mineral element, proximate composition
INTRODUCTION
The population explosion has exemplified a substantial rise in the demand for food resources which has shifted the attention of the global food market to nonconventional food resources.[1] Several by-products of cultivation, once discarded as wastes, are studied for their nutritive values to advocate their use as routine food sources meeting the expanding demands of the industry. Such by-products have proved to be economical and hence are well-accepted in the global market considering the present scenario.[2] India contributes a major portion of the total banana production in the world, whereas it is a conventional form of food and commercial food as well. Banana cultivation comprises of the secondary products, banana pseudostem (PB), and banana flower (FB) which have been discarded as wastes, fed to cattle or used for composting. More recently, some studies have reported its use in the production of alcohol, methane, food for livestock, or adsorbents for water purification. A massive quantity (about 40%) of the total fresh weight of banana plant comprises of PB and FB, and hence, it can be useful as an alternative food resource.[3] However, to accomplish the potential health benefits of these, a detailed study of its nutritional value needs to be carried out with special emphasis on the influence of a particular variety on its nutritional composition. In vitro and in vivo studies using the extracts have proven PB and FB as antihyperglycemic,[4,5] antimicrobial, hypolipidemic, and anti-hypertensive agents thus upholding their health beneficial properties.[6] The dietary fiber content, antioxidant compounds and several other macro- and micro-nutrients are responsible for these health benefits, and thus, the present study was designed to assess the composition of PB and FB in terms of its nutritional value.
Further, it is well-known that diseases either acute or chronic induce the generation of reactive oxygen species which are also the main factors responsible for tissue damage and aging.[7] To ameliorate these damages, a mode of treatment which also has antioxidant properties has improved the condition and hence, this study also aims to evaluate the antioxidant potential of PB and FB. Several procedures are available to assess the antioxidant properties of food, and its potency depends upon either the method of assessment or the method of extraction of samples. In this regard, the total antioxidant capacity (TAC) can be determined after analyzing the mode of action likely to be either radical scavenging or metal ion chelating activity.[8] In addition, the extraction procedure plays a significant role for its TAC since irrespective of the extraction procedure; some fraction of the extract always remains insoluble and not involved in the activity. More recently, a method developed by Vural et al.[9] known as Quencher is being widely accepted for TAC evaluation since the method is carried out without extraction and thus the entire sample is tested in its solid state. The acceptance of this method is also affirmed because it resembles the condition similar to the physiological conditions where antioxidants are not extracted and administered directly, but instead, it needs to be released from the food source during digestion. Similar to the physiological conditions, after the enzymatic digestion, the extractable antioxidants play their role, and the nonextracted materials enter the intestine where they are acted on by the intestinal microflora and continue the digestion process. The extracted antioxidants are estimated by conventional methods, and the undigested material is evaluated for its antioxidant capacity using the Quencher method thus attaining a the global antioxidant response (GAR). This method provides a summation of the complete antioxidant potential of the food source the same way as it exists in vivo and hence, the method is widely accepted.[10]
With this background, the objectives of this study are an evaluation of the nutritional composition and TAC of PB and FB to advocate this nonconventional food source for routine consumption, considering its various health benefits.
MATERIALS AND METHODS
Samples
Flawless inflorescences and pseudostems of Musa sp. cv. Nanjangud rasa bale were harvested from the banana cultivating farms of Nanjangud, Karnataka, India. The specimen was identified by the Department of Horticulture, Government of Karnataka, Mysore, India. Peeling the thick outer leaf-sheath of the tender pseudostems, the inner pith region was collected and flowers were separated from the inflorescences by discarding the spathe. For isolation, pseudostems (PB) and FBs were gutted, chopped and allowed to dry in an oven (40°C). This was pulverized, using a homogenizer and further stored at 4°C until use.
Proximate analysis
Moisture (method 44-15A), ash (method 08–01), crude fiber (method 32–10), fat (method 30–25), and carbohydrate content of PB and FB were determined according to the AACC method.[11] Total carbohydrates were expressed as residual percent weight by the formula: [100-(moisture + ash + fat + fibre + protein)]. Crude protein (method 46–13) was estimated by the procedure described by Kjeldahl.[12] The total dietary fiber content (method 991.43) in PB and FB was estimated by food-enzymatic-gravimetric method.[13] The procedure described by Thomas et al.[14] was used to determine neutral detergent fiber (NDF), acid detergent fiber (ADF), lignin, hemicellulose, and cellulose content in PB and FB. Hemicellulose and cellulose were estimated according to the formula: [Hemicellulose = NDF– ADF]; and [Cellulose = ADF– lignin], respectively. Water holding capacity (WHC), oil holding capacity (OHC), swelling power (g of swollen granules/g of dry weight of sample), and solubility (%) of PB and FB were performed as per the method described by Noor et al.[15] WHC and OHC were expressed as grams of water or oil/grams of dry weight of samples, respectively. Starch and uronic acid content were determined by the method described by Jamuna et al.[16] The phytochemical analysis and the vitamin content of PB and FB were determined.[17,18,19] The sugar composition was performed according to AACC[20] for PB and FB using high-performance liquid chromatography (HPLC) with differential refractive index detector (RID-10A, Shimadzu, Japan).
Fatty acid profile by gas chromatography-mass spectrometry
Before gas chromatography-mass spectrometry (GC-MS) analysis, derivatization was performed for PB and FB samples using BF3- methanol as derivatizing reagent.[21] Once the conversion of non-volatile fatty acids into volatile fatty acid methyl esters (FAMEs) through methylation[22] was achieved, the samples were subjected to GC (Clarus 500, Perkin Elmer, AOC-20i autosampler; Perkin Elmer, California, USA) interfaced with a mass spectrometer equipped with an Elite-5MS (5% diphenyl/95% dimethyl polysiloxane) fused to a capillary column (30 nm × 0.25 mm ID × 0.25 μm film thickness, DF). To achieve a good resolution of FAMEs, chromatographic parameters were optimized as per Ramith et al.[5] with slight modifications in the oven temperature. It was programmed at the rate of 10°C/min (no hold) up to 200°C, later at the rate of 5°C/min up to 280°C for a 9 min hold. In comparison to the acquired mass spectra of PB and FB with the standard mass spectra of NIST Library (NIST 05), the phytocomponents present were recognized.
Mineral analysis
PB and FB were assessed for comprehensive mineral analysis (Li, B, Na, Mg, Al, K, Ca, Cr, Mn, Fe, Cu, Ni, Cs, Zn, and Pb) using inductively coupled plasma atomic emission spectrometry (ICP-AES, Varian Vista MPX, USA) as per the official method 985.01.[23] Ahead of subjecting to ICP-AES, the dry samples were ashed in a muffle furnace at 400°C–500°C and acid digested.[23,24] On the other hand, the concentrations of Mo, Se, P, As, Cd, and Sb were determined using flame atomic absorption spectroscopy (AAS, Varian 240, USA) according to the method described by Vikas et al.[25] Phosphoric acid and boric acid were measured according to the method described by Pearson[26] and method 970.33.[27] The elemental analysis of PB and FB was performed on a Perkin Elmer 2400 elemental analyzer.
Amino acid composition
Amino acid composition of PB and FB was analyzed according to the standard AOAC procedure (method 994.12).[28] For hydrolysis, methods of Wong and Peter[29] were employed. Before the analysis of the samples through automated amino acid analyzer (L 8900, Hitachi, Japan), filtration was performed using a 0.45 mm nylon membrane filter. Subsequently, following the prehydrolysis oxidation with performic acid, cysteine, and methionine (sulfur-containing amino acids) were determined.[30] In comparison with the FAO/WHO (1985)[31] reference amino acid pattern, the composition of different amino acids recovered was presented as mg/g of protein. The essential amino acid (EAA) score was evaluated using the equation of FAO/WHO described: (Score of EAA = mg of EAA in 1 g of test protein/mg of EAA in 1 g of reference protein) × 100.
Antioxidant potential
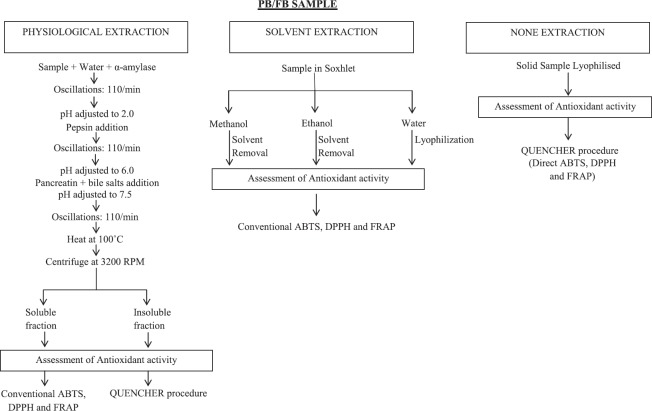
To measure antioxidants in PB and FB, different procedures were established using (i) GAR method (GAR); (ii) Sequential solvent extraction method and (iii) without extraction. In GAR, physiological extraction by the means of in vitro gastrointestinal digestion was done as described by Pastoriza et al.[7] The in vitro GAR method is performed to mimic digestion (through gastrointestinal tract) to discharge antioxidants from foods into soluble (bio-accessible) and insoluble (nonaccessible) fractions, which is summarized in Figure 1. Three diverse conventional protocols (DPPH, ABTS, and FRAP) defined by Cristina et al.[32] for the soluble fractions obtained by gastrointestinal digestion was followed. In all cases, the results were expressed as mmol equivalents of Trolox per kg of sample.[32] To determine the antioxidant activity of lyophilized insoluble fractions obtained by gastrointestinal digestion, the Quencher procedure was conducted as described by Vural et al.[9] Calibration curve was obtained using Trolox solutions and microcrystalline cellulose as the blank. Results were expressed as mmol equivalents of Trolox per kg of sample. Second the coarse powder was subjected to successive extraction with methanol, ethanol and water in a Soxhlet apparatus. Extraction was done twice with each of the solvents (500 ml) followed by the filtering of individual extracts from solvents. The three filtrates were then stored at −20°C until used for the analysis of total phenolic content and antioxidant activity. All the samples were analyzed in triplicates. The phenolic component separation of PB and FB extracts was performed on a reverse phase C18 (250 mm × 4.6 mm, Supelco) and the compounds were monitored by PDA (photodiode array) detector HPLC system (Agilent Technologies Inc., USA). Column temperature was maintained at 37°C and flow rate was set to 0.8 ml/min. The solvent system used was 0.1% formic acid (solvent A) and methanol (solvent B). The solvent gradient elution program was: 0–55 min 85% of A and 15% of B; 55–57 min 20% of A and 80% of B; 57–60 min 85% of A and 15% of B. A volume of 20 μl of the sample was injected (auto-injection) into the column and the phenolic acids were detected at 280 nm. The sample was quantified by comparing the retention time/peak areas with those of standards, namely, gallic acid, p-hydroxybenzoic acid, chlorogenic acid, sinapic acid, caffeic acid, vanillin, p-coumaric acid, quercetin, catechin, and epicatechin. The Quencher procedure described by Vural et al.[9] was employed to determine antioxidant activity of the solid sample. In addition, enzymatic dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), and catalase (CAT) activities were determined by following the method of Moaed et al.[33] and Manoj et al.[1]
Figure 1.
A procedure to determine global antioxidant response of banana pseudostem and flower
Statistical analysis
All data were expressed as mean ± standard deviation (n = 3). Results were determined using one-way analysis of variance (ANOVA), followed by Duncan’s multiple range test using SPSS Software (version 21.0, Chicago, USA). The results were considered as statistically significant if the P < 0.05.
RESULTS AND DISCUSSION
Dietary fiber composition
The proximate composition of PB and FB are defined in Table 1 which suggests a high total dietary fiber content. A diet comprising of high dietary fiber is efficient in generating early satiety signal by increasing the food retention time in the stomach and also reduces risk towards the development of gastric ulcers. Of the total dietary fibers, while the soluble fibers possess the property of higher expansion volume rendering bulk density of the food materials, insoluble fibers swell on encounter with water promoting the elimination of waste materials by increasing bowel movement. Thus, a fiber-rich diet facilitates digestion, as well as elimination of wastes and also prevents constipation.[34] In this study, the IDF was found to be more than the soluble dietary fiber in both the byproducts tested and on the whole, dietary fiber in FB (70.1%) was found to be higher than PB (61.1%). In support to the present findings, a previous study suggested IDF as the major fraction in the dietary fiber composition of banana (Musa acuminata × balbisiana Colla cv. Awak) pseudostem flour and boiled tender core of the PB flour.[15] As well, a similar trend was observed in banana and plantain peels.[14] In summary, despite the difference in the fiber content in comparison to other studies, the dietary fiber content of PB from Musa sp. cv. Nanjangud rasa bale was higher than PB from Musa sp. cv. elakki bale, which had a value of 28.8%.[16] Such differences may be attributed to the different botanical origins, geographical conditions such as soil, climate, and collection time. These results indicate the potential of banana by-products soon to replace oats and sorghum as fiberenriched food source.
Table 1.
Nutritional composition of banana pseudostem and banana flower
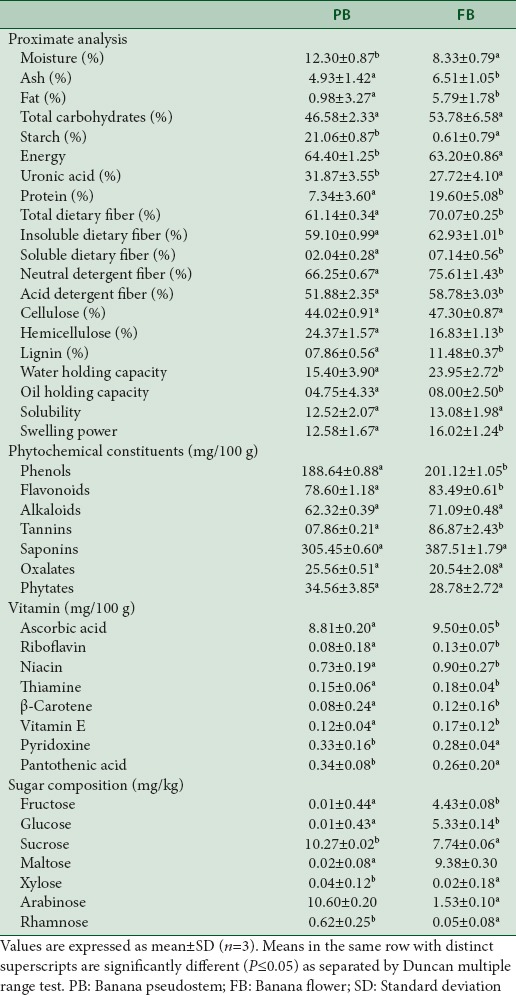
Contrary to Insoluble dietary fiber (IDF), NDF includes a complex of cellulose, lignin and insoluble hemicellulose. Accordingly, NDF values are always higher than those of the IDF and present study results support this as it is clear from Table 1. ADF and NDF content of FB (58.78 and 75.61, respectively) was more than PB (51.88 and 61.25, respectively) while both were higher than Musa acuminata x balbisiana Colla cv. Awak pseudostem tender core flour (ADF: 32.02; NDF: 43.89) reported by Thomas et al.[14] The present study shows that cellulose was the most abundant component, followed by hemicellulose and then lignin in both PB and FB. These components are considered insoluble and thus are not digested. Overall, the relative amounts of hemicellulose and cellulose in the study were higher than those published by Samrat et al.[35] (14.98% hemicellulose and 31.27% cellulose) and Thomas et al.[14](18% hemicellulose and 42% cellulose) PB fibers of Musa sapientum and Musa acuminata x balbisiana Colla cv. Awak, respectively. Cellulose constitutes the major component or primary and secondary cell walls, thus explaining their presence at high levels. However, the cellulose content of PB and FB reported in this study is less than the cellulose content in the outer bark material of pseudostems of M. acuminate Colla (40.2%) and pseudostem tender core flour as reported by Cordeiro et al.[36] and Thomas et al.[14] respectively. On the contrary, the lignin content of both PB and FB was higher than banana pulp (6.0%), wheat (0.88%), and soy meal (0.58%), as reported by Jrgen[37] and are considerably lower with other wood-based materials such as sawdust (20.33%), Musa sapientum species (15.07%) and plantain (green banana) (14.3%).
Functional properties
The functional properties such as WHC, OHC, solubility, and swelling capacity (SWC) of banana by-products were measured and are presented in Table 1. From the physiological standpoint, the ability of any material to retain water when subjected to an external centrifugal gravity force or compression is its WHC. The study suggests that FB exhibited highest WHC (23.9 g water/g dry weight of samples) compared to PB (15.4 g water/g of dry weight of samples). Meanwhile, PB and FB exhibited greater WHC than those of cereals which showed <5.5 g water/g, such as rice bran (5.21 g water/g) and durum wheat (1.5–2.1 g water/g). This minute disparity may be due to the structural differences in cell wall components between the stem and flower fibers. Subsequently, the SWC of PB and FB were assessed which is directly attributed to the amount of cellulose in the dietary fiber. The extent of water retained in the swollen granules of FB (16.02 g of swollen granules/g of dry weight of sample) was significantly (P < 0.05) greater than PB (12.58 g of swollen granules/g of dry weight of sample) with no statistically significant (P < 0.05) differences in solubility between them, suggesting them to be more potent than some of the exotic fruits such as pineapple and mango concentrates (7.2, 6.6 and 4.60 ml water/g sample, respectively). Furthermore, the OHC of PB and FB were assessed which is attributed to the chemical and physical structure of the plant polysaccharides. With an OHC value of 4.75 of oil/g of dry weight in PB and 8.0 g of oil/g of dry weight of sample in FB, they fared better than dietary fibres obtained from commercial preparations (1.29 g of oil/g of dry matter) and other fibrous residues, such as coconut fibre (5.3 g oil/g fibre or banana fibre-rich powder (2.2 g oil/g fibre). With these results, PB and FB can be advocated for use in stabilizing emulsions and as a dietary fiber reservoir.[14,38]
Sugars
Sugars are the main source of bio-available energy, and hence, it is important to assess the sugar content as well as the type of sugars present in the food. The digestible sugar content in PB was 21.57 mg/kg, and FB was 28.48 mg/kg samples [Table 1] and was not as high as banana fruit and other tropical fruits.[18] Further, the sugar profile of PB revealed the presence of sucrose and arabinose, which contributed 47.6% and 49.1% to total sugars, respectively while FB revealed the presence of several types of sugars, namely, maltose, sucrose, glucose, fructose, and arabinose. Despite the quick metabolism of these sugars, their presence at low levels prevents the use of PB and FB as an alternative energy resource. While the present study revealed the absence of galactose and rhamnose, a previous GC-MS study of polysaccharide fractions of Musa sp. cv. elakki bale suggested their presence[16] which might be due to the difference in the banana cultivars used in the study. In addition, the level of sucrose was higher than glucose in both PB and FB. Other studies with banana peel exhibited high fructose while banana pulp showed the presence of glucose, fructose, and sucrose with lower sucrose levels as compared to other sugars.[39]
Phytochemicals
A class of alkaloids, flavonoids, tannins, saponins and more complex phenolic, phytosterols, oxalates, and phytates are collectively known as phytochemicals which not only impart color to the fruits and vegetables but also possess several physiological functions, including antioxidant properties.[40] Table 1 elaborates on the phytochemicals and their amount in PB and FB which reveal that the most abundant phytochemical in this study are phenols and saponins. Furthermore, high tannin content (86.9 mg/100 g) in FB as compared to PB was witnessed. All these phytochemicals are proven to possess antimicrobial, antioxidant, and hormone modulatory activities. The study also revealed high amounts of flavonoids, which are well-known for their antioxidant properties. The higher flavonoids and saponins were present in PB and FB than in banana flowers of two cultivars[41] Baxijiao (saponins: 0.11 g/100 g and flavonoids: 5.90 mg/100 g) and Paradisiacal (saponins: 0.12 g/100 g and flavonoids: 5.27. mg/100 g) and considering these benefits, the potential of PB and FB for their health beneficiary properties is upheld.
Vitamins
Vitamins are the micronutrients required in minute amounts to the body, deficiency of which adversely affect the metabolism of the body. In the present study, Vitamin C (ascorbic acid) was present in the highest quantity with a mean content of 9.50 and 8.81 mg/100 g of FB and PB, respectively. While ascorbic acid is among the most important antioxidants involved in the prevention or minimization of the formation of carcinogenic substances from dietary material by preventing the oxidation of nitrate, its deficiency causes impaired functioning of the intracellular substances in the body including collagen, bone matrix, and tooth dentine.[40] In addition to ascorbic acid, riboflavin, niacin, thiamine, β-carotene, vitamin E, pyridoxine, and pantothenic acid were observed in PB and FB in quantities significant to create a nutritional impact by the food source [Table 1]. Vitamin C content in FB and PB was lower than banana fruit (10 mg/100 g) but higher than other tropical fruits, namely, blueberries (6 mg/100 g) pears (3 mg/100 g), and grapes (3 mg/100 g). Furthermore, the vitamin B complex was present in a significant amount which emphasizes these by-products for their potential in the treatment of various diseases including prostate cancer.[18]
Fatty acids
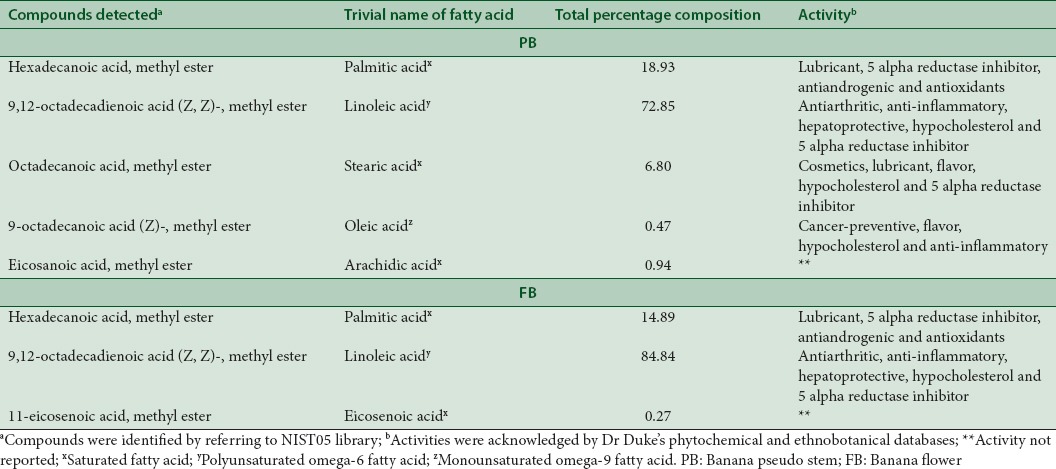
Fatty acid composition as given in Table 2 suggests that linoleic acid and palmitic acid were the major components in both the parts of the banana. FB contained 84.8% linoleic acid of its total fatty acid content, while PB contained 72.8%, which was followed by palmitic acid, which was high in PB (18.9%) compared to FB (14.8%). These results were similar to banana fruit peels of the Musa Genus: French Clair (FC), Grande Naine (GN), Big Ebanga (BE), pelipita (PPT), Yankambi Km5 (YKm5), and CRBP039039 had high proportions of unsaturated fatty acid, especially linoleic acid.[40] Linoleic acid is a precursor fatty acid for cell membrane components as well as other compounds involved in physiological responses and its presence in this study proves beneficial. Further, some of the less common fatty acids in PB were stearic acid and arachidic acid and FB was eicosenoic acid. Further, the polyunsaturated fatty acid levels were greater in this study as against Musa spp. Baxijiao and Paradisiacal flowers.[41] Such variations may be attributed to the stage of ripening at harvest, changes in the climate, soil conditions, and genetic variations between the sources. The online Dr. Duke’s phytochemical and ethnobotanical database-assisted in ascertaining the biological activity of the compounds and the same are tabulated in Table 2.
Table 2.
Fatty acid profile of banana pseudostem and banana flower by gas chromatography-mass spectrometry
Minerals
Based on the amount required for the human body, minerals are classified as macro- and micro-elements. The minerals present in PB and FB are given in Table 3 which suggests the presence of Na, K, Ca, Mg, and P with K being the major mineral in both PB and FB. However, minerals in FB were about 2–5-fold higher than the levels in PB. The levels of minerals in FB were in the order K > Mg > Na > Ca > P while that of PB was in the order K > Ca > Mg > P > Na. While Na and K are involved in the ion pumps in several metabolic pathways, Mg regulates over 300 metabolic reactions by acting as cofactors to several enzymes, P is involved in almost every chemical reaction taking place in the body in the form of ATP and Ca along with P forms Ca3 (PO4)2 and are essential for bone and teeth formation.[42] Overall, the levels of these minerals were in agreement with that of Musa spp. Baxijiao and Paradisiacal flower variety,[41] but slightly lower than the limiting contents found in banana peels and pulps determined by Shaida et al.[43] In summary, the peel had a higher content of minerals than the pulp and the potassium content was lower to banana fruits and other tropical fruits such as pears, blueberries, and grapes.[18]
Table 3.
Mineral composition of banana pseudostem and banana flower
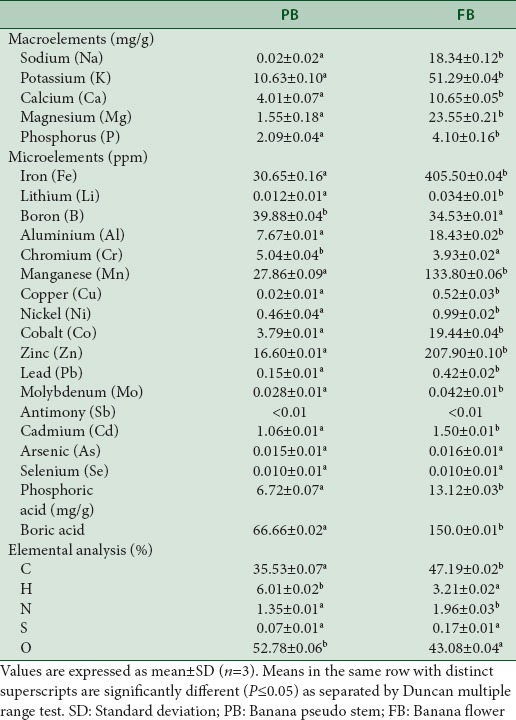
Along with these macro minerals, the micro minerals in PB and FB were also evaluated which showed the presence of Fe, Mn, Zn, Cu, Al, and several others are listed in Table 3. The levels of these elements in FB were found to be higher than PB and overall higher than those of other tropical fruits, including banana when compared with the data given by The Department of Health (2013).[18] Similar to the various macro-elements, microelements also have several vital biological functions. Zn is involved in various reactions of the body to construct and maintain DNA, required for the growth and repair of body tissues and iron along with manganese, copper, and zinc are constituents of various important proteins and enzymes involved in macro-nutrient metabolism and body function.[42] Considering the several vital functions of the macro- and micro-elements, their high contents in FB and PB could contribute to explain their use in folk medicine.
Further, a thorough analysis to evaluate the elements present in the banana byproducts was performed, and the results are tabulated in Table 3. Carbon was present in the highest amounts in FB (47.1%) compared to PB (35.5%) which was contrary to the hydrogen content which was higher in PB (6.01 ± 0.02) as against FB (3.21 ± 0.02). The present findings were on par with the composition of principal elements of banana (Musa acuminate) pseudostem by Ketty et al.,[44] i. e., (carbon: 36.83%, hydrogen: 5.19%, and nitrogen: 0.93%). The composition of hydrogen was higher in PB than FB and this is due to the high moisture composition of that compared to the FB [Table 1]. The moisture content of the present study was higher in case of PB (13.3%) over FB (8.33%) suggesting the difference in the hydrogen content of both the byproducts. Overall, the moisture content of both PB and FB were lower than commercial wheat flour, which had a value of 12.36%[44] and PB and FB of elakki bale cultivar as reported by Jamuna et al.[16]
In support to the above findings, the ash content that is directly proportional to the mineral content was also estimated which suggested the presence of it at high levels. It is clear from our studies that the highest levels of ash content were recorded for Musa sp. cv. Nanjangud rasa bale (PB and FB of 4.9 and 6.5%, respectively), and was comparatively higher than those Musa sp. cv. elakki bale (0.3 and 0.5%, respectively),[16] banana fruit of 1.1%.[18] Whereas it was comparable with banana (Musa acuminata x balbisiana Colla cv. Awak) pseudostem flour (3.03%)[15] and lower than banana peel and pulps (6.4%–12.8%).[39]
Amino acids
The quality of EAAs suggests the nutritional value of dietary proteins, and hence the amino acid content in PB and FB were tested. An overall picture of the amino acid content present in PB and FB is given in Table 4 suggest that all the EAAs according to the FAO classification[31] are present in them with FB having a major amino acid content compared to PB. A high glutamic acid content (63.8 and 152.9 mg/g of protein) followed by aspartic acid, leucine, alanine, proline, arginine, cysteine, serine, and lysine was witnessed in both FB and PB, respectively. The importance of glutamine is learnt during critical illness where it acts as a prime carrier of ammonia to the splanchnic area and the immune system. In addition while the sulfur-containing amino acids were above the FAO/WHO,[31] requirement (score ranged from 99 to 240), the other EAAs met FAO/WHO,[31] requirement pattern. Further, the concentration of the amino acids that are lower than the FAO standard protein value is considered as limiting concentration, and in this context, in the present study, lysine was at the limiting concentration and the same has been reported by Thomas et al.[39] in fruit peels of the Musa Genus: FC, GN, BE, PPT, YKm5, and 039, obtained at three different stages of ripeness, namely, stage 1 (Green), stage 5 (More yellow than green), stage7 (yellow/a few brown spots). The ratio of essential to non-EAAs for PB and FB were 0.56 and 0.54, respectively, which was substantially higher than their requirement in adults (0.38) as recommended by the WHO. In addition, the protein values of PB (7.3%) and FB (19.3%) in the present study were marginally higher than the values reported for Musa spp. Baxijiao and Paradisiaca flowers (1.62%–2.7%), elakki bale cultivar (PB: 2.5 and FB: 12.5%), banana fruit peels (ranged from 8.3%–10.2%), banana (Musa acuminata x balbisiana Colla cv. Awak) pseudostem flour (0.89%–3.52%), banana peels of yelakki bale (7.7%), pachabale (6.7%) and nendrabale (4.6%) and green banana Cavendish (AAA) flour (4.1%). Proteins being the source for the supplementation of amino acids, it can thus be suggested that PB and FB are potent sources of EAAs.[3,14,15,16]
Table 4.
Amino acid profile of banana pseudostem and banana flower
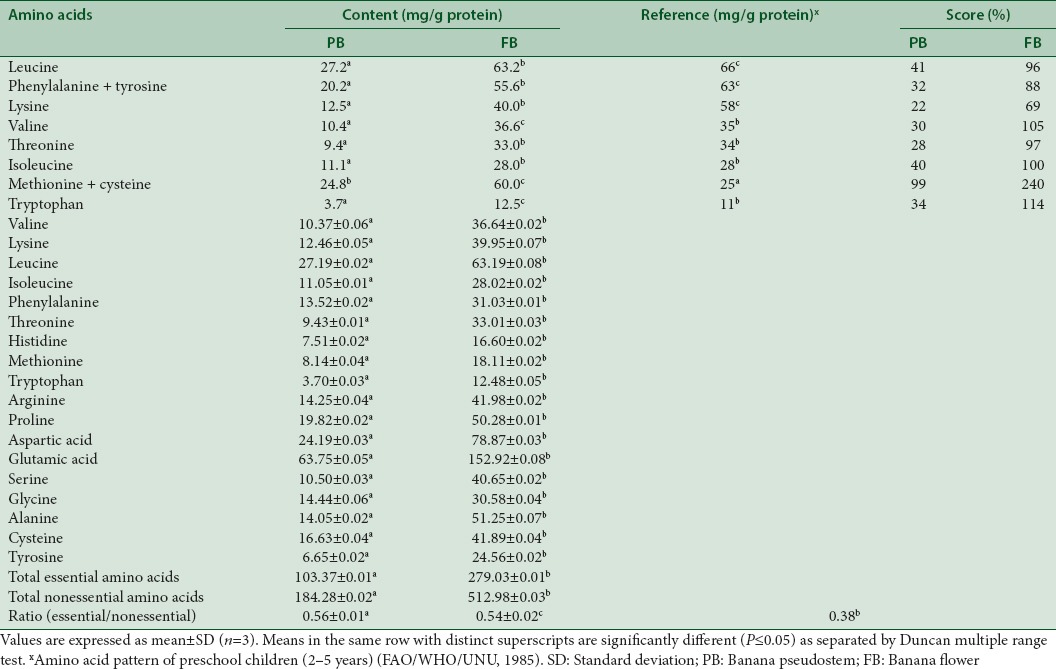
Antioxidants
Antioxidant adjuncts have proven beneficiary in many diseases where they play a protective role in the prevention of ROS mediated damage to the cells and tissues. Hence, in the present study, we have evaluated the antioxidant potential of PB and FB and the results thus obtained are tabulated in Table 5a. Studies have suggested both PB[45] and FB[4,16] of banana as potent antioxidants extractable with aqueous and organic solvents. Most antioxidant studies involve its evaluation using the extractable form which creates a lacuna in assessing the nonextractable substance for their antioxidant capacity and hence, we evaluated the GAR according to Pastoriza et al.[7] Further, antioxidant activity using a single assay does not give conclusive evidence hence, three common radical scavenging assays namely ABTS which determines the single electron-transfer capabilities, DPPH which evaluates the hydrogen-donating potency and Fe + 3 (FRAP) which reflects the reductive antioxidant power of the antioxidant compounds[9] were carried out to assess in vitro antioxidant activity of PB and FB [Figure 5b]. As mentioned previously, along with the method of evaluation, another factor contributing to the antioxidant potential of the samples is the method of extraction and hence, a conventional solvent extraction (with different solvents), a direct measure using the QUENCHER procedure, an in vitro gastrointestinal digestion, and the combination of the latter with the application of the QUENCHER procedure[10] to the insoluble fraction [termed henceforth the GAR method] are the methods of extraction employed in the present study. The results provide promising evidence for the need for employing such methods of antioxidant estimation since the chemical extraction method (solvents and aqueous) gave lower results, ranging from 2 to 2.5 times lower in comparison with the Quencher and GAR methods. They are in agreement with the previous reports for 27 fresh and cooked foods, estimated by Pastoriza et al.[7] On the other hand, with regard to the chemical extraction method, both PB and FB extracted with the solvent ethanol fared better than methanol and aqueous counterparts. They were also higher than the Quencher method, but lower than GAR method of antioxidant evaluation. The previous phytochemical analysis also reports high amounts of total phenolic content in the ethanolic extract of PB and FB[4,5] which are well-known as the major phytochemicals (phenolic acids and flavonoids) to possess antioxidant activities in fruits and vegetables.
Table 5.
Enzymatic antioxidant potential and global antioxidant response of banana pseudostem and banana flower using different methods and distribution of antioxidant activity in soluble and insoluble fractions after in vitro digestion (a); yield, total phenolic content and antioxidant activity of banana pseudostem and banana flower sequential solvent extracts (b) and phenolic acids identification (c)
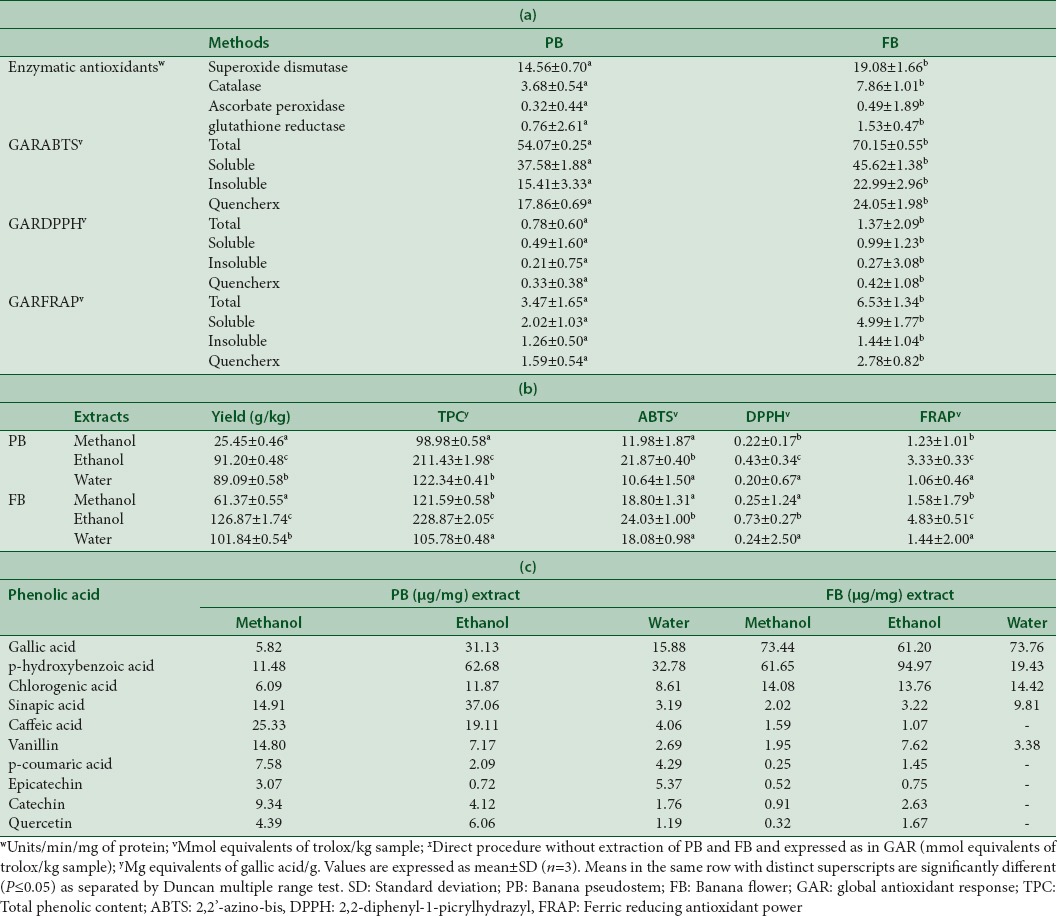
Further, to acquire a detailed phenolic composition of the extracts, HPLC analysis was performed and the results are detailed in Table 5c suggesting the presence of diverse phenolic acids, namely, gallic acid, p-hydroxybenzoic acid, chlorogenic acid, sinapic acid, caffeic acid, vanillin, p-coumaric acid, quercetin, catechin, and epicatechin at different concentrations. Ethanol extracts in both PB and FB were found to contain high concentrations of phenolic acids in comparison to methanol and aqueous extract. p-hydroxybenzoic acid was the most predominant phenolic acid recorded in PB and FB (62.7 μg/mg and 95 μg/mg), followed by gallic acid (31.1 and 61.3 μg/mg, respectively) with varying concentrations. Caffeic acid was predominant in the methanol extract of PB (23.3 μg/mg), whereas gallic acid was predominant in that of FB (73.4 μg/mg). Although methanol and aqueous extract had phenolic acids, the concentration was lesser than the ethanol extract [Table 5c]. However, under physiological conditions, these results cannot be reproduced by administering the extracted antioxidants directly. Irrespective of the extraction method, some amount of the sample always remains insoluble in one or the other solvent and hence Arda et al.[46] developed a direct procedure (QUENCHER) to evaluate the TAC of foods without an extraction step. Since, this method cannot differentiate between the physiologically active fraction and the insoluble one, a combination of enzymatic digestion step for the soluble fraction and the Quencher method for the insoluble fraction thus furnish an optimal antioxidant potential of the given sample.
The results of the antioxidant activity using Quencher method [Table 5a] for PB and FB (ABTS: 17.8 and 24; DPPH: 0.33 and 0.42; FRAP: 1.59 and 2.78 mmol equivalents of the standard Trolox per kg of sample, respectively) were in accordance with the results obtained by Arda et al.[46] for different cereal products. The order of magnitude was same as the GAR method for PB and FB samples despite a 2–3 times reduction in most parts of the results. Such a reduction could be attributed to the absence of the enzymatic digestion step which could otherwise result in different compounds obtained after the enzymatic reactions. Overall, the best results were obtained by the GAR method which exhibited highest antioxidant activity with FB faring better than PB. In particular, the insoluble fraction exhibited about 40%–50% of the total antioxidant activity and since this fraction is excluded during the extraction process, this is the most recommended method for the measurement of TAC. Although the antioxidant role of the insoluble fraction is questioned since they are not extractable, they are expected to exert their effect by the surface reaction phenomenon. Furthermore, some part of the insoluble fraction may undergo digestion by the intestinal microflora thus releasing some substances which can also exert antioxidant properties and considering these; it would be essential to measure the antioxidant capacity of even the insoluble fraction of the digested food.[47]
Further, with respect to the antioxidant assays, the different affinities of the radicals to scavenge various antioxidant groups present in different samples suggest the need to use more than a single assay to determine the antioxidant potential of a particular sample. In this regard, in the present study, the TAC as measured with two radical scavenging assays (ABTS and DPPH) fared differently for both the byproducts. In support of these results, Roger et al.[48] demonstrated that the macromolecules are seldom attacked by the hydrophobic radicals, which could be the reason for the lower activity in DPPH as compared to the ABTS assay wherein DPPH is a hydrophobic radical while ABTS is more of a hydrophilic probe. Furthermore, DPPH being more selective in the reaction with H-donors, it could also be the reason for its lower TAC values in this assay. Further, the FRAP activity which is based on the reduction of the Fe + 3–TPTZ complex in the ferrous form at low pH, exhibited 6.5 mmol Trolox Eq./Kg for FB and for PB with a statistically significant difference in the values (P > 0.05). The results, however, in comparison with ABTS were lower, but better than the DPPH assay.[49]
In addition, enzymatic (SOD, CAT, APX, and GR) antioxidant potential has been evaluated for the FB and PB. As evident from Table 5a, FB showed maximum activity of SOD (19.1 U/min/mg protein) followed by catalase (7.9 U/min/mg protein), GR (1.5 U/min/mg protein) and APX (0.49 U/min/mg protein). On the other hand, PB also exhibited enzymatic activities for SOD (14.6 U/min/mg protein) followed by catalase (3.7 U/min/mg protein), GR (0.76 U/min/mg protein), APX (0.32 U/min/mg protein) and found was to be lower in comparison to FB. Higher SOD, APX, and GR enzymatic antioxidant activities in PB and FB clearly indicates their greater ability to detoxify ROS such as superoxide, hydroxyl, and peroxide radicals formed in human cell by endogenous and exogenous factors which in turn could lead to geriatric degenerative conditions, cancer and a wide range of other human diseases.
CONCLUSION
In summary, the present study manifests that both PB and FB possess rich nutraceutical properties because of the presence of various bioactive ingredients with numerous benefits. It provides evidence that the two banana byproducts are rich in proximate nutrient composition, minerals, fatty acids, and antioxidants (both enzymatic and nonenzymatic) and hence could be used in the human diet. The beneficiary properties are mainly derived from their minerals, carbohydrates, dietary fibers and proteins together with the low content of fat and calories. Furthermore, as a rich source of phytochemicals, minerals and vitamins reside in PB and FB they can be further evaluated for use as a key ingredient for valuable drugs. To add to these, the high total dietary fiber content and a balanced ratio between insoluble dietary fiber and soluble dietary fiber in both PB and FB are attractive targets for the food industry. These could be used in the development of a nutritional supplement because of their health-related properties of dietary fiber and associated bioactive compounds.
In addition to the strong basis provided by the nutritional aspects of PB and FB, their potential as antioxidants are also confirmed by a series of studies which included different methods of extraction as well as different assays to determine their antioxidant potential. It is demonstrated that the GAR method exhibited antioxidant activity higher than that reported with traditional procedures, which asserts the role of both insoluble as well as soluble fractions of the digested food to possess antioxidant properties. To summarize on the whole, this paper reinforces the concept that PB and FB are potent sources of several biologically active ingredients and also possess rich antioxidant property.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Manoj K, Vishal G, Puja K, Reddy CR, Jha B. Assessment of nutrient composition and antioxidant potential of Caulerpaceae seaweeds. J Food Comp Anal. 2011;24:270–8. [Google Scholar]
- 2.Afshar MA, Naser MS. Nutritive value of some agro-industrial By-products for Ruminants –A review. World J Zoo. 2008;3:40–6. [Google Scholar]
- 3.Debabandya M, Sabyasachi M, Namrata S. Banana and its by-product utilization: An overview. J Sci Ind Res. 2010;69:323–9. [Google Scholar]
- 4.Ramith R, Prithvi SS, Farhan Z, Lakshmi VR, Nagendra PM. Inhibitory effect of banana (Musa sp. var. Nanjangud rasa bale) flower extract and its constituents Umbelliferone and Lupeol on α-glucosidase, aldose reductase and glycation at multiple stages. S Afr J Bot. 2014;95:54–63. [Google Scholar]
- 5.Ramu R, Shirahatti PS, Zameer F, Prasad MN. Investigation of antihyperglycaemic activity of banana (Musa sp. Var. Nanjangud rasa bale) pseudostem in normal and diabetic rats. J Sci Food Agric. 2015;95:165–73. doi: 10.1002/jsfa.6698. [DOI] [PubMed] [Google Scholar]
- 6.Jamuna JB. Effect of Banana Flower and Pseudostem on Advanced Glycation end Products and Glucose Transporters in Kidney During Diabetes. Ph.D. Thesis. University of Mysore, India. 2015 [Google Scholar]
- 7.Pastoriza S, Delgado AC, Haro A, Rufian HJ. A physiologic approach to test the global antioxidant response of foods. The GAR method. Food Chem. 2011;129:1926–32. [Google Scholar]
- 8.Jara PJ, Sara A, Maria T, Elena DR, Jose S, Isabel G, et al. Update methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res Int. 2008;41:274–85. [Google Scholar]
- 9.Vural G, Arda S, Vincenzo F. Direct measurement of the total antioxidant capacity of foods: The ‘QUENCHER’approach. Trends Food Sci Tech. 2009;20:278–88. [Google Scholar]
- 10.Arda S, Vural G, Vincenzo F. Solvent effects on total antioxidant capacity of foods measured by direct QUENCHER procedure. J Food Comp Anal. 2012;26:52–7. [Google Scholar]
- 11.American Association of Cereal Chemists (AACC). Approved Methods of Analysis. 10th ed. St. Paul, MN, USA: American Association of Cereal Chemists; 2000. [Google Scholar]
- 12.Kjeldahl JZ. A new method for the determination of nitrogen in organic matter. Anal Chem. 1883;22:366. [Google Scholar]
- 13.Association of Official Analytical Chemists (AOAC). AOAC Method 991.43. Total, Insoluble and Soluble Dietary Fibre in Food-Enzymatic-Gravimetric Method, MES-TRIS Buffer. In: Official Methods of Analysis. 16th ed. Gaithersburg, MD: AOAC International; 1995. pp. 71–2. [Google Scholar]
- 14.Happi Emaga T, Robert C, Ronkart SN, Wathelet B, Paquot M. Dietary fibre components and pectin chemical features of peels during ripening in banana and plantain varieties. Bioresour Technol. 2008;99:4346–54. doi: 10.1016/j.biortech.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Noor AA, Lee HH, Baharin A, Rajeev B, Lai HC, Mohamad NM. Chemical and functional properties of the native banana (Musa acuminatax Balbisiana Colla cv. Awak) pseudo-stem and pseudo-stem tender core flours. Food Chem. 2011;128:748–53. [Google Scholar]
- 16.Bhaskar JJ, Mahadevamma S, Chilkunda ND, Salimath PV. Banana (Musa sp. Var. Elakki bale) flower and pseudostem: Dietary fiber and associated antioxidant capacity. J Agric Food Chem. 2012;60:427–32. doi: 10.1021/jf204539v. [DOI] [PubMed] [Google Scholar]
- 17.Sofowora A. Medicinal Plants and Traditional Medicine in Africa. 1st ed. Chichester, New York: John Wiley and Sons; 1982. p. 256. [DOI] [PubMed] [Google Scholar]
- 18.Department of Health 2013. Nutrient Analysis of Fruit and Vegetables: Analytical Report. [Last accessed on 2014 May 24]. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/167944/Nutrient_analysis_of_fruit_and_vegetables_-_Analytical_Report.pdf .
- 19.Trease GE, Evans WC. Pharmacognsy. 11th ed. UK: Macmillian Publishers; 1989. [Google Scholar]
- 20.American Association of Cereal Chemists (AACC). Determination of Simple Sugars in Cereal Products—HPLC Method (Method 80-04), MN. St. Paul, USA: American Association of Cereal Chemists; 1994. [Google Scholar]
- 21.Ahmad S, Ahmad S, Bibi A, Ishaq MS, Afridi MS, Kanwal F, et al. Phytochemical analysis, antioxidant activity, fatty acids composition, and functional group analysis of heliotropium bacciferum. ScientificWorldJournal. 2014;2014:829076. doi: 10.1155/2014/829076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harborn JB. Phytochemical Method. AOAC 991.39. 17th ed. Ch. 41. FAO; 2000. [Google Scholar]
- 23.Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International. 17th ed. Gaithersburg, MD, USA: Association of Official Analytical Chemists; 2000. [Google Scholar]
- 24.Hue NV, Uchida R, Ho MC. Sampling and analysis of soils and plant tissues. Plant Nutrient Management in Hawaii's Soils. In: Silva JA, Uchida RS, editors. College of Tropical Agriculture and Human Resources. Honolulu: University of Hawaii; 2000. pp. 23–6. [Google Scholar]
- 25.Vikas N, Sarkara BC, Sharmaa HK, Bawa AS. Physico-chemical properties and estimation of mineral content in honey produced from different plants in Northern India. J Food Comp Anal. 2003;16:613–9. [Google Scholar]
- 26.Pearson D. The Chemical Analysis of Foods. 7th ed. London: Churchill Living Stone; 1976. [Google Scholar]
- 27.Association of Official Analytical Chemists. Boric Acid and borates in food, qualitative test, final action. A.O.A.C. International. Method: no. 970.33, Procedure 47.3.07. 16th ed. Vol. 2. Arlington, VA, USA: Association of Official Analytical Chemists; 1996. p. 11. [Google Scholar]
- 28.Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International. 18th ed. Gaithersburg, MD, USA: Association of Official Analytical Chemists; 2005. [Google Scholar]
- 29.Wong KH, Peter CK. Nutritional evaluation of some subtropical red and green seaweeds Part I –Proximate composition, amino acid profiles and some physico-chemical properties. Food Chem. 2000;71:475–82. [Google Scholar]
- 30.Ronny H, Navam H, Kannan A, Pengyin C. Proximate composition and amino acid and mineral contents of Mormordica charantia L. pericarp and seeds at different maturity stages. Food Chem. 2010;122:1111–5. [Google Scholar]
- 31.FAO/WHO/UNU. Energy and Protein Requirements, Report of a Joint FAO/WHO/UNU Expert Consultation. World Health Organisation and United Nations University. Technical Rep. Ser. 724. Geneva, Switzerland: WHO; 1985. [PubMed] [Google Scholar]
- 32.Cristina DA, Jose AC, Ana H, Silvia PD, Jose AR. A combined procedure to evaluate the global antioxidant response of bread. J Cereal Sci. 2010;52:239–46. [Google Scholar]
- 33.Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006;171:382–8. doi: 10.1016/j.plantsci.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JW, Baird P, Davis RH, Jr, Ferreri S, Knudtson M, Koraym A, et al. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205. doi: 10.1111/j.1753-4887.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 35.Samrat M, Raul F, Yusuf A, Ulku S. Banana fibers –Variability and fracture behaviour. J Eng Fibers Fabr. 2008;3:39–45. [Google Scholar]
- 36.Cordeiro N, Belgacem MN, Torres IC, Moura JC. Chemical composition and pulping of banana pseudo-stems. Ind Crops Prod. 2004;19:147–54. [Google Scholar]
- 37.Möller J. Gravimetric determination of acid detergent fiber and lignin in feed: Interlaboratory study. J AOAC Int. 2009;92:74–90. [PubMed] [Google Scholar]
- 38.Martínez R, Torres P, Meneses MA, Figueroa JG, Pérez-Álvarez JA, Viuda-Martos M, et al. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012;135:1520–6. doi: 10.1016/j.foodchem.2012.05.057. [DOI] [PubMed] [Google Scholar]
- 39.Thomas HE, Rado HA, Bernard W, Jean TT, Michel P. Effects of the stage of maturation and varieties on the chemical composition of banana and plantain peels. Food Chem. 2007;103:590–600. [Google Scholar]
- 40.Okwu DE. Phytochemicals, Vitamins and mineral contents of two Nigerian medicinal plants. Int J Mol Med Adv Sci. 2005;1:375–81. [Google Scholar]
- 41.Zhan WS, Wei HM, Zhi QJ, Yang B, Zhi GS, Hua TD, et al. Investigation of dietary fiber, protein, Vitamin E and other nutritional compounds of banana flower of two cultivars grown in China. Afri J Biotech. 2010;9:3888–95. [Google Scholar]
- 42.Joseph M, Charles A, Alex RV. Phytochemical screening and bioactivity studies of Phyllanthus wightianus. J Pharm Res. 2013;6:188–92. [Google Scholar]
- 43.Shaida FS, Nor AM, Ibrahim ME, Eng MS, Azliana AB, Supriatno KL. Correlation between total phenolic and mineral contents with antioxidant activity of eight Malaysian bananas (Musa sp.) J Food Comp Anal. 2011;24:1–10. [Google Scholar]
- 44.Bilba K, Arsene MA, Ouensanga A. Study of banana and coconut fibers botanical composition, thermal degradation and textural observations. Bioresour Technol. 2007;98:58–68. doi: 10.1016/j.biortech.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 45.Saravanan K, Aradhya SM. Polyphenols of pseudostem of different banana cultivars and their antioxidant activities. J Agric Food Chem. 2011;59:3613–23. doi: 10.1021/jf103835z. [DOI] [PubMed] [Google Scholar]
- 46.Arda S, Vural G, Nicoletta P, Vincenzo F. Direct measurement of the total antioxidant capacity of cereal products. J Cereal Sci. 2008;48:816–20. [Google Scholar]
- 47.Serpen A, Gökmen V, Fogliano V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012;90:60–5. doi: 10.1016/j.meatsci.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Dean RT, Hunt JV, Grant AJ, Yamamoto Y, Niki E. Free radical damage to proteins: The influence of the relative localization of radical generation, antioxidants, and target proteins. Free Radic Biol Med. 1991;11:161–8. doi: 10.1016/0891-5849(91)90167-2. [DOI] [PubMed] [Google Scholar]
- 49.Serpen A, Capuano E, Fogliano V, Gökmen V. A new procedure to measure the antioxidant activity of insoluble food components. J Agric Food Chem. 2007;55:7676–81. doi: 10.1021/jf071291z. [DOI] [PubMed] [Google Scholar]