Abstract
Background:
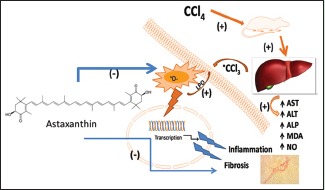
Astaxanthin is of carotenoids group which possess strong antioxidant properties. The present study was conducted to evaluate the hepatoprotective effects of astaxanthin in carbon tetrachloride (CCl4)-treated rats.
Materials and Methods:
Female Long-Evans rats were administered with CCl4 orally (1 ml/kg) twice a week for 2 weeks and were treated with astaxanthin (10 mg/kg) every day for 2 weeks. Blood plasma samples were isolated from each group and were analyzed for alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase activities. Oxidative stress parameters such as malondialdehyde (MDA), nitric oxide (NO), and advanced protein oxidation product (APOP) were measured. Several enzyme functions such as myeloperoxidase (MPO), superoxide dismutase (SOD), and catalase (CAT) activities in the plasma and liver tissues were also analyzed. Moreover, inflammation and tissue fibrosis were also confirmed by histological staining of liver tissues.
Results:
This investigation revealed that CCl4 administration in rats increased plasma AST, ALT, and ALP activities which were normalized by astaxanthin treatment. Moreover, CCl4 administration increased as MDA, NO, and APOP level both in plasma and tissues compared to control rats. Astaxanthin also exhibited a significant reduction of those parameters in CCl4-administered rats. Astaxanthin treatment also restored the CAT and SOD activities and lowered MPO activity in CCl4-administered rats. Histological assessment also revealed that the astaxanthin prevented the inflammatory cells infiltration, decreased free iron deposition, and fibrosis in liver of CCl4-administered rats.
Conclusion:
These results suggest that astaxanthin protects liver damage induced by CCl4 by inhibiting lipid peroxidation and stimulating the cellular antioxidant system.
SUMMARY
Carbon tetrachloride (CCl4) administration increased oxidative stress-mediated hepatic damage and inflammation in rats
Astaxanthin, a potent antioxidant, prevents oxidative stress and inflammatory cells infiltration in CCl4-administered rats
Astaxanthin also ameliorated the progression of hepatic fibrosis in CCl4-administered rats.
Abbreviations Used: APOP: Advanced protein oxidation product; AST: Aspartate aminotransferase; ALT: Alanine aminotransferase; ALP: Alkaline phosphatase; CAT: Catalase; CCl4: Carbon tetrachloride; CVD: Cardiovascular disease; HSCs: Hepatic stellate cells; H2O2: Hydrogen peroxide; MDA: Malondialdehyde; MMP2: Matrix metalloproteinase2; MPO: Myeloperoxidase; NF-κB: Nuclear factor kappa B; NO: Nitric oxide; Nrf2: Nuclear factor erythroid 2-related factor 2; ·ONOO−: Peroxynitrate; ROS: Reactive oxygen species; SOD: superoxide dismutase; TCA: Trichloroacetic acid; TBA: Thiobarbituric acid; TGF-1: Transforming growth factor 1, TGF-β: Transforming growth factor-β; TIMP1: Tissue inhibitor of metalloproteinase 1; TNF-α: Tumor necrosis factor-alpha;·CCl3: Trichloromethyl free radical; CCl3O2−: Trichloroperoxyl radical
Keywords: Carbon tetrachloride, fibrosis, inflammation, lipid peroxidation
INTRODUCTION
Liver is the most important internal organ within the human frame playing a vital role in metabolism and detoxification of a variety of drugs and xenobiotics. Thus, liver is susceptible to a wide range of toxic, microbial, metabolic, circulatory, and neoplastic insults.[1] Liver diseases are very common worldwide problem. Liver cirrhosis is an irreversible stage in the process of liver damage that occurs after liver fibrosis. Liver fibrosis is attributed to inflammation, excessive accumulation of extracellular matrix (ECM), and tissue remodeling under wound healing.[2] Chronic hepatitis and liver cirrhosis are positively associated with the occurrence of hepatocellular carcinoma.[3,4] Therefore, the inhibition of hepatic inflammation and fibrosis is crucial in preventing the occurrence of liver cirrhosis and hepatocellular carcinoma. Several researches reported that free radicals and reactive oxygen species (ROS) play a pivotal role in the various steps that initiate and regulate the progression of liver fibrosis independently.[5,6] Various xenobiotics are known to cause hepatotoxicity; one among them is carbon tetrachloride (CCl4).[7] CCl4 is usually used experimentally to induce liver injury, fibrosis, and carcinoma in rodents. A single dose of CCl4 leads to centrizonal necrosis and steatosis,[8] while repeated administration leads to liver fibrosis, cirrhosis, and hepatocellular carcinoma.[9] CCl4 induces the production of several types of ROS.[10] Hepatotoxicity of CCl4 involves its biotransformation into free radicals such as trichloromethyl free radical (CCl3) and trichloroperoxyl radical, which may increase lipid peroxidation.[11] The current researches are now directed toward finding naturally occurring antioxidants, which might help prevent oxidative damage. Astaxanthin, a kind of carotenoid pigment naturally produced by algae, bacteria, and phytoplankton, contains conjugated double bonds, hydroxyl and ketone groups, involved in electron transfer and possesses free radicals scavenging activity.[12] Earlier reports showed that astaxanthin can scavenge peroxyl radicals and destroys peroxides, thereby protecting biological membranes from lipid peroxidation.[13,14] Several studies also suggest that astaxanthin has a protective effect against oxidative stress, inflammation, and metabolic disorders, such as type 2 diabetes and cardiovascular diseases, in experimental animals[15,16,17] and humans.[18] It was previously demonstrated that astaxanthin could attenuate both lung fibrosis and renal fibrosis.[19] A recent report also suggests that astaxanthin ameliorated liver damage and fibrosis by decreasing the expression of nuclear factor kappa B (NF-κB) and transforming growth factor 1 (TGF-1) and maintained the balance between matrix metalloproteinase 2 and tissue inhibitor of metalloproteinase 1 in the liver of mice.[20] In this investigation, liver fibrosis was induced by bile duct ligation in mice and using CCl4 administration intraperitoneally for a long period (2 months). Long-term CCl4 administration in animal developed fibrosis of liver; however, time frame for the initiation of fibrosis is important and needs to be addressed. Moreover, astaxanthin effect on antioxidant defense in damaged liver tissue by short-term CCl4 administration was not addressed properly before. Our recent investigations showed that antioxidant-rich fraction of plant powder ameliorated the oxidative stress, inflammatory insult, and fibrosis in liver of CCl4-administered rats.[21,22] However, the effect of astaxanthin on oxidative stress, iron deposition, and inflammation in liver damage was poorly understood. Therefore, the present study was conducted to elucidate the effect of astaxanthin on oxidative stress and hepatic damage in CCl4-induced rats. Moreover, the effect of astaxanthin on inflammatory cells infiltration, iron deposition, and prevention of early development of fibrosis in liver of CCl4-induced rats was also addressed.
MATERIALS AND METHODS
Chemicals
CCl4 was obtained from Merck (Germany) and astaxanthin was obtained from the General Pharmaceutical Limited (Dhaka, Bangladesh). Thiobarbituric acid (TBA) was purchased from Sigma Chemical Company (USA). Trichloroacetic acid (TCA) was purchased from J. I. Baker (USA). Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) assay kits were obtained from DCI Diagnostics (Budapest, Hungary). Sodium hydroxide was collected from Merck (Germany). All other chemicals and reagents used were of analytical grade.
Animals and treatment
Twenty-four 10–12-week-old Long-Evans female rats (150–180 g) were obtained from the Animal Production Unit of Animal House at the Department of Pharmaceutical Sciences, North South University, and were kept in individual cages at room temperature of 25°C ± 3°C with a 12-h dark/light cycles. They have free access to standard laboratory feed (pellet food crushed to coarse powder) and water, according to the study protocol approved by the Ethical Committee of Department of Pharmaceutical Sciences, North South University, for animal care and experimentation.
To study the hepatoprotective effects of astaxanthin, rats were equally divided into four groups (six rats in each group).
Group I (control): Animals of Group I were treated with 1 mL/kg of saline (0.85%) and olive oil (1 mL/kg) intragastrically twice a week for 2 weeks
Group II (CCl4): Animals of Group II were treated with CCl4 (1:3 in olive oil) at a dose of 1 mL/kg intragastrically twice a week for 2 weeks
Group III (control + astaxanthin): Animals of Group III were treated with astaxanthin 10 mg/kg (dissolved in olive oil) orally every day for 2 weeks
Group IV (CCl4 + astaxanthin): Rats of Groups IV were treated with CCl4 (1:3 in olive oil) at a dose of 1 mL/kg intragastrically twice a week for 2 weeks. Animals of Group IV were also treated with astaxanthin 10 mg/kg (dissolved in olive oil) orally every day for 2 weeks.
The weighed quantity of astaxanthin was dissolved in olive oil. Olive oil was chosen as the vehicle because astaxanthin is soluble in olive oil. The dose of astaxanthin (10 mg/kg) was selected on the basis of previously published literature survey, which showed a wide range of doses used in various investigations. Animals were checked for the body weight gain and measured the food and water intake on a daily basis. After 2 weeks, all animals were anesthetized using ketamine and pentobarbitone, sacrificed, and collected the blood and organs such as heart, kidney, spleen, and liver. Immediately after collection of the organs, they are weighted and stored at −20°C for further studies. Blood was drawn through syringe and centrifuged at 8000 rpm for 15 min at 4°C. Then, serum was transferred using a micropipette into microcentrifuge tubes (Eppendorf, Tarsons Products Pvt., Ltd., Kolkata, India) and stored at −20°C until analyzed.
Assessment of hepatotoxicity
Liver marker enzymes (ALT, AST, and ALP) were estimated in plasma using Diatec Diagnostic Kits (Hungary) according to the manufacturer’s protocol.
Preparation of tissue sample for the assessment of oxidative stress markers
For determination of oxidative stress markers, liver tissue was homogenized in 10 volumes of phosphate buffer containing pH 7.4 and centrifuged at 8000 rpm for 15 min at 4°C. The supernatant was collected and used for the determination of protein and enzymatic studies as described below.
Estimation of lipid peroxidation
Lipid peroxidation in the liver was estimated calorimetrically measuring TBA reactive substances, followed by previously described method.[23] In brief, 0.1 ml of tissue homogenate (Tris-HCl buffer, pH 7.5) was treated with 2 ml of (1:1:1 ratio) TBA-TCA-HCl reagent (0.37% TBA, 0.25 N HCl, and 15% TCA) and placed in hot water bath for 15 min and cooled. The absorbance of clear supernatant was measured against reference blank at 535 nm.
Assay of nitric oxide
Nitric oxide (NO) was determined according to the method described by Tracey et al. as nitrate.[24] In this study, Griess-Illosvoy reagent was modified using naphthyl ethylene diamine dihydrochloride (0.1% w/v) instead of 1-naphthylamine (5%). The reaction mixture (3 mL) containing brain homogenates (2 mL) and phosphate buffer saline (0.5 mL) was incubated at 25°C for 150 min. Rest of process was followed as described in previous experiment of NO scavenging assay of the extract. A pink-colored chromophore was formed in diffused light. The absorbance of these solutions was measured at 540 nm against the corresponding blank solutions. NO level was measured using standard curve and expressed as nmol/g of tissue.
Advanced protein oxidation products assay
Determination of advanced protein oxidation products (APOPs) level was performed by modification of the method of Witko-Sarsat et al.[25] and Tiwari et al.[26] Two milliliters of plasma was diluted 1:5 in phosphate buffered saline (PBS); 0.1 mL of 1.16 M potassium iodide was then added to each tube, followed by 0.2 mL acetic acid after 2 min. The absorbance of the reaction mixture was immediately read at 340 nm against a blank containing 2 mL of PBS, 0.1 mL of KI, and 0.2 mL of acetic acid. The chloramine-T 7 absorbance at 340 nm was found linear within the range of 0–100 nmol/mL, AOPP concentrations were expressed as nmol/mL chloramine-T equivalents.
Catalase assay
Catalase (CAT) activities were determined using previously described method by Khan.[27] The reaction solution of CAT activities contained 2.5 ml of 50 mmol phosphate buffer (pH 5.0), 0.4 ml of 5.9 mmol H2O2, and 0.1 ml enzyme extract. Changes in absorbance of the reaction solution at 240 nm were determined after 1 min. One unit of CAT activity was defined as an absorbance change of 0.01 as units/min.
Estimation of superoxide dismutase activity
Superoxide dismutase (SOD) was assayed in plasma and tissue homogenates using previously described method.[28] Three milliliters reaction mixture consisted of aliquot of enzyme preparation and PBS to make up the volume to 2.94 ml. The reaction was started by addition of 0.06 ml of 15 mM epinephrine. Change in absorbance was recorded at 480 nm for 1 min at 15 s interval. Control consisting of all the ingredients, except enzyme preparation, was run simultaneously. One unit of enzyme activity has been defined to cause 50% inhibition of autooxidation of epinephrine present in the assay system.
Estimation of myeloperoxidase activity
Myeloperoxidase (MPO) activity was determined by a dianisidine-H2O2 method,[29] modified for 96-well plates. Briefly, plasma samples (10 μg protein) were added in triplicate. o-dianisidine dihydrochloride (Sigma) (0.53 mM) and H2O2 (0.15 mM) in potassium phosphate buffer (50 mM) (pH 6.0) were also added to the sample mixture. The change in absorbance was measured at 460 nm. Results were expressed as units of MPO/mg protein.
Histopathological determination
For microscopic evaluation, liver tissues were fixed in neutral buffered formalin and embedded in paraffin, sectioned at 5 μm, and subsequently stained with hematoxylin and eosin, and Sirius red staining was also done to evaluate the inflammation, necrosis, and fibrosis in liver. Moreover, Prussian blue staining was also conducted to evaluate the iron deposition in liver section of rats. Sections were studied under light microscope at ×40 magnifications.
Statistical analysis
All values are expressed as mean ± standard error of mean. Statistical analysis was conducted by one-way analysis of variance followed by Newman–Keuls post hoc test using GraphPad Software, Inc. 7825 Fay Avenue, Suite 230 La Jolla, CA 92037 USA. Significant changes were considered as P < 0.05 in all cases.
RESULTS
Effect of astaxanthin on body weight, food, and water intake
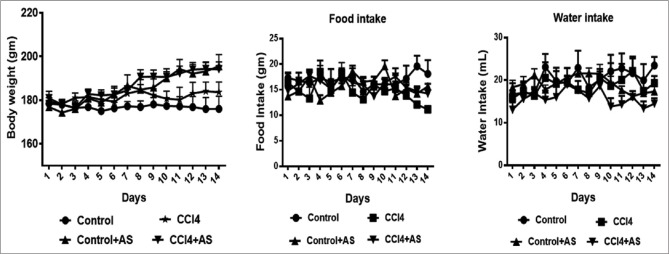
Body weight of each rat was recorded every day during the experiment, and the percentage change was calculated for all groups. It was found that the body weight did not decrease significantly in CCl4-intoxicated rat group compared to the control rats. On the other hand, treatment of CCl4-intoxicated group with astaxanthin showed a significant increase in body weight compared to other groups [Figure 1]. Besides, CCl4-intoxicated group showed decreased food and water intake compared to control rats [Figure 1 and Table 1]. However, reduction of food and water intake was not improved by astaxanthin in CCl4-intoxicated group [Figure 1 and Table 1].
Figure 1.
Effect of astaxanthin on body weight, food, and water intake in carbon tetrachloride-induced rats. Data are presented as mean ± standard error of mean, n = 6–7 or otherwise stated. Statistical analysis was conducted by one-way analysis of variance followed by Newman–Keuls post hoc test; significance was considered at P < 0.05 in all cases
Table 1.
Effect of astaxanthin on body weight, food, and water intake and organ weight of carbon tetrachloride-treated rats
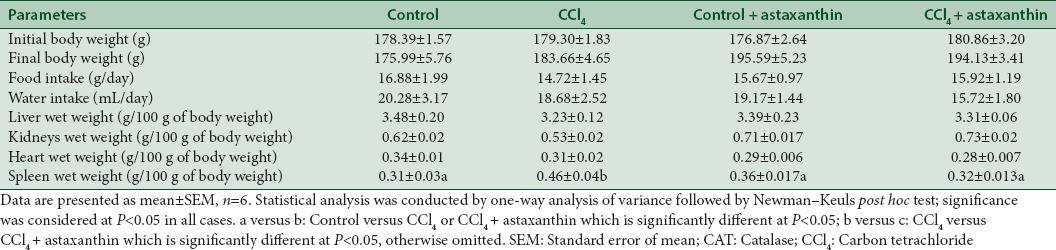
Effect of astaxanthin on organ wet weights
Table 1 shows the effect of CCl4 treatments on the rats’ organs wet weights. CCl4-treated rats showed slight decrease in liver wet weight compared to control rats; however, astaxanthin-treated rats did not improve in decreased liver wet weight compared to the control [Table 1]. The wet weight of kidneys was not significantly changed due to CCl4 administration and astaxanthin treatment among the groups studied [Table 1]. The heart wet weight was also not changed significantly among the groups tested in this study. However, the wet weight of spleen was changed significantly in CCl4-treated rats compared to control rats (P < 0.05). Astaxanthin treatment in CCl4-treated rats normalized the spleen wet weight compared to CCl4-treated rats (P < 0.05).
Effect of astaxanthin on biochemical parameter of liver functions
To confirm the hepatic damage due to CCl4 administration, we measured the liver marker enzyme activities in plasma. Biochemical measurement of liver functions revealed that CCl4 induced a significant increase in plasma AST, ALT, and ALP activities compared to control rats (P < 0.05) [Table 2]. Treatment of animals with astaxanthin every day for 2 weeks along with CCl4 administration significantly counteracted the increased plasma AST, ALT, and ALP activities compared to the CCl4-intoxicated group [Table 2]. In addition, treatment of animals with astaxanthin alone for 2 weeks did not show any significant change in liver enzymes compared to the control group, which signifies a nontoxic effect of this compound on liver [Table 2].
Table 2.
Effect of astaxanthin on biochemical parameter in plasma and liver
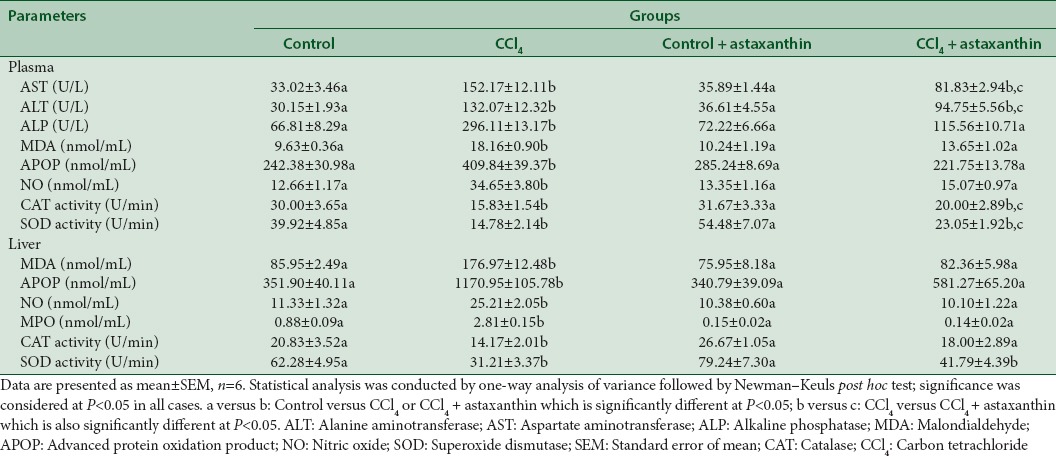
Effect of astaxanthin on oxidative stress markers and antioxidant enzymes
CCl4 administration leads to the formation of free radicals after metabolism in the liver. Thus, increased lipid peroxidation and oxidative stress parameters required to be measured. To determine the oxidative stress in our study, we evaluated the malondialdehyde (MDA), NO, and APOP content in plasma and liver homogenates.
CCl4-induced rats showed significant (P < 0.05) increased lipid peroxidation product MDA concentration both in plasma and liver homogenates compared to control group [Table 2]. In addition, astaxanthin co-treatment significantly normalized the elevated level of MDA compared to CCl4-intoxicated group [Table 2]. Astaxanthin treatment alone in Group III did not alter the MDA concentration compared to control rats [Table 2].
In physiological system, NO is considered as beneficial; however, in oxidative stress, NO level increased significantly and may turn into peroxynitrate (·ONOO−) production. In this study, NO was measured as nitrate, which was increased significantly both in plasma and liver homogenates compared to control rats (P < 0.05) [Table 2]. Astaxanthin treatment in CCl4-intoxicated group normalized the elevated NO content in the plasma and liver homogenates [Table 2]. Astaxanthin treatment alone in Group III did not alter the NO concentration compared to control rats [Table 2].
Further, CCl4 administration has profound effect on APOP development in plasma and liver homogenates. CCl4 challenge in rats significantly increased the APOP concentration in plasma and liver compared to control rats, which was decreased significantly due to astaxanthin treatment in CCl4-intoxicated rats (P < 0.05) [Table 2].
Increased oxidative stress parameters could be result of decreased antioxidant defense in tissue level. Thus, tissue antioxidants such as SOD and CAT activities were measured in this study. CCl4-induced rats showed a significant decrease in liver antioxidant enzyme CAT and SOD activities, respectively, compared to the control rats (P < 0.05) [Table 2]. It was also found that SOD and CAT activities were restored to near-normal by astaxanthin treatment in CCl4-intoxicated group compared to CCl4-toxicated group (P < 0.05) [Table 2].
To determine inflammation and inflammatory cells infiltration in liver, we measured myeloperoxidase (MPO) activity in liver tissues. CCl4-intoxicated group rats showed significant increased MPO activity in liver compared to control rats [Table 2]. Astaxanthin treatment significantly normalized the MPO activity compared to CCl4-intoxicated group (P < 0.05) [Table 2]. These data are further supported by the histological assessment of tissue sections of liver.
Effect of astaxanthin on inflammation and fibrosis in the liver
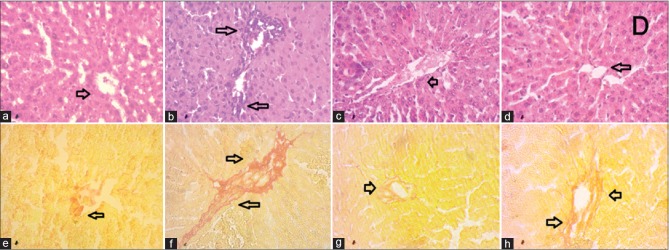
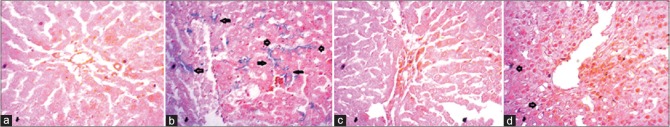
Control rats showed normal structure and orientation of liver tissue with normal hepatic vein and bile ducts [Figure 2a]. Similar, histoarchitechture was also seen in liver of rats treated with astaxanthin alone [Figure 2c]. However, inflammation in the liver was seen in rats treated with CCl4 [Figure 2b]. Massive serge of inflammatory cells was found in the centilobular part of liver section stained with hematoxylin and eosin staining in CCl4-treated rats group [Figure 2b, upper panel]. Necrotized tissue scar and ballooning of the hepatocytes were also seen in the liver of CCl4-treated rats. Treatment with astaxanthin attenuated the inflammatory cell infiltration and necrosis in the liver tissues of CCl4-treated rats [Figure 2d].
Figure 2.
Effect of astaxanthin on hepatic inflammation and hepatic fibrosis in CCl4-treated rats. (a and e) Control; (b and f) CCl4; (c and g) control + astaxanthin; and (d and h) CCl4 + astaxanthin, (×40). CCl4: Carbon tetrachloride
Liver fibrosis was evaluated histologically by visualizing the red color of collagen fibers deposition using Sirius red stain. Control rats showed baseline collagen around portal tracts and central veins [Figure 2e, lower panel]. The collagen fibers were found heavily deposited around portal tracts and central veins in CCl4-intoxicated group and extended from central vein to portal tract, resulting in the formation of pseudolobules which were not seen in control rats [Figure 2f, lower panel]. Treatment with astaxanthin prevented the initiation of fibrosis in the liver tissues of CCl4-treated rats [Figure 2h]. Astaxanthin treatment in control rats also did not show any collagen deposition in liver [Figure 2c].
Furthermore, Prussian blue staining of liver sections was carried out to evaluate the free iron deposition in liver tissues. CCl4-induced rats showed that heavy iron droplets were deposited in liver section [Figure 3a] compared to control rats [Figure 3b], which was further normalized by astaxanthin treatment [Figure 3d]. Astaxanthin treatment in control rats also did not show any iron deposition in liver [Figure 3c].
Figure 3.
Effect of astaxanthin on hepatic iron deposition in CCl4-treated rats. (a) Control; (b) CCl4; (c) control + astaxanthin; and (d) CCl4 + astaxanthin, (×40). CCl4: Carbon tetrachloride
DISCUSSION
Liver injury induced by CCl4 is one of the best-characterized systems of xenobiotic-induced hepatotoxicity and commonly used model for the screening of hepatoprotective activity of different drugs.[30] Oxidative stress has been postulated as major molecular mechanism in hepatic damage induced by CCl4.[31] It is well known that CCl4 is biotransformed by the cytochrome P450 system in the endoplasmic reticulum. The preliminary metabolite is the CCl3, which when combined with cellular lipids and proteins in the presence of oxygen forms trichloromethyl peroxy radical that may assault lipids at the membrane of endoplasmic reticulum quicker than CCl3. Consequently, trichloromethyl peroxy-free radical leads to cause lipid peroxidation.[30,32] Free radicals formation and lipid peroxidation are the potent cellular mechanisms involved in the development of fatty liver caused by CCl4.[33] Antioxidants have been shown to possess a protective effect on liver fibrosis both in animal models and clinical trials.[21,34]
Our results suggest that astaxanthin possesses protective action against hepatic damages induced by CCl4. Serum hepatobiliary enzymes such as AST, ALT, and ALP are present in high concentrations under the disease conditions. When there is hepatocyte necrosis or membrane damage, these enzymes will be released into the circulation as indicated by elevated serum enzyme levels.[35] In the present study, the elevated levels of all these marker enzymes observed in CCl4-treated rats indicate liver damage. Treatment of astaxanthin to CCl4-induced rats ameliorated the toxic effects of CCl4 and the above markers restored toward the normal level. This protective effect may be attributed to the free radical scavenging activity of astaxanthin, and results obtained in this study are in agreement with earlier findings.[21,36]
Hepatic damage and increased serum hepatobiliary enzymes activities are the end results of free radical-mediated oxidative stress in the liver. Increased oxidative stress has been attributed to the formation of reactive metabolites due to biotransformation of CCl4 by cytochrome P4502E1.[37] Once formed, free radicals trigger a cascade of reactions that culminate in lipoperoxidation.[38] Lipid peroxidation product MDA and APOP will be generated eventually. Our investigations showed that MDA concentration was significantly increased in CCl4-treated group which was also reported in the previous studies.[21,22] Astaxanthin treatment in CCl4-administered rats significantly declined the MDA concentration both in plasma and liver homogenates indicates antilipid peroxidative effects. In contrast to other carotenoids, 13 conjugated double bonds are present in astaxanthin structure which gives it a significantly greater antioxidant capacity. Polar hydroxyl groups are also present in the 3 and 3’ positions of astaxanthin allowing it to sit near the lipid/water interface of the cell membrane bilayers where free radicals attack occur predominantly.[39] Thus, it can prevent chain reactions that occur when a fatty acid is oxidized.
Increased free radicals may also trigger oxidation of other important components of cells such as proteins. Our investigation also showed that APOP concentration was also increased significantly in CCl4-treated rats, which was further normalized by astaxanthin treatment. This finding is in agreement with the previous report which suggests that antioxidant-rich food supplements can prevent protein oxidation and lowers APOP concentration in plasma and tissues of CCl4-administered rats.[21] Moreover, this investigation also suggests that astaxanthin treatment in CCl4-intoxicated group reduced the elevated NO concentration in plasma and liver of CCl4-treated rats. NO has been reported to be increased in liver cirrhosis.[40,41] Some authors also proposed that a high level of NO is associated with CCl4-induced acute liver injury.[42] In presence of other free radicals such as superoxide radicals [·O2−], NO converts into peroxynitrite radicals (·ONOO−) which can react directly with thiol groups or with tyrosine hydroxyl groups on cellular enzymes and inactivates these enzymes.[43] Astaxanthin-mediated inhibition of NO production was also supported by the previous study.[16]
Increased lipid peroxidation and oxidative stress are direct results of the diminishing antioxidant defense of the tissue. It was observed that the antioxidant SOD and CAT enzymes function lower significantly in chronic oxidative stress condition in liver of CCl4-administered rats.[44,45]
SOD is the main antioxidant enzyme that protects cells and tissues from the ROS generated from endogenous and exogenous sources. SOD also catalyzes mainly the conversion of superoxide anion (O2•− to H2O2). On the other hand, CAT largely localized in subcellular organelles such as peroxisomes and is heme-containing enzyme. CAT converts H2O2 to water and O2. It is thus protecting the cell from oxidative damage by H2O2 and OH. Restoration of antioxidant enzyme activity (SOD and CAT) and decreased NO production by astaxanthin have been reported in cell lines undergone oxidative stress.[16,46] Astaxanthin treatment in this study also improved and restored the SOD and CAT activities in plasma and liver significantly in CCl4-administered rats. Astaxanthin treatment also showed protecting effect of tissue damage by stimulating the cellular antioxidant enzymes, lowering lipid peroxidation, and protein oxidation against 2,3,7,8-tetrachloride benzo-p-dioxin-induced toxicity in rats.[47] This investigation suggests that carotenoid such as astaxanthin is useful in the prevention of oxidative stress-mediated tissue damage by upregulating the antioxidant enzymes. The nuclear factor erythroid 2-related factor 2 (Nrf2) is an important regulator of cellular resistance to oxidants and regulating transcription of antioxidants enzymes such as SOD and CAT.[48] Previous reports suggest that astaxanthin increased the nuclear translocation of Nrf2 and promoted the expression of antioxidant enzymes against oxidative stress.[49,50] We propose that astaxanthin-mediated upregulation of antioxidant enzymes follows Nrf2-mediated pathways which need to be addressed properly in the future.
Oxidative stress and lipid peroxidation-mediated hepatic cells damages may generate an inflammatory response, which was observed by infiltrating inflammatory cells alongside the blood vessels in the liver.[21,34] Previous literature suggests that the infiltrating inflammatory cells are mainly monocyte and neutrophil in CCl4-induced liver damage in rats.[51,52] CCl4 metabolism-mediated free radicals’ generation also stimulates the activation of Kupffer cells which release various inflammatory mediators such as tumor necrosis factor alpha (TNF-α) and interleukins.[53] These cytokines serve several aspects of inflammation such as synthesis of prostaglandins, macrophage activation, and the infiltration of neutrophil in inflamed area.[54,55] The current study also showed that CCl4 administration in rats develops inflammatory cells infiltration and an accumulation of extracellular collagen matrix around the scar site. The current observation is also supporting the biochemical finding which showed that MPO activity in liver tissue homogenates is also increased in CCl4-administered rats, which was further normalized by astaxanthin treatment. MPO is an important enzyme, released after recruitment and activation of neutrophils, and catalyze the formation of hypochlorous acid/hypochlorite and other oxidizing species.[56]
In addition to the chronic inflammation, reactive oxidative species also play a critical role in the activation of hepatic stellate cells (HSCs) during liver fibrogenesis. Various cytokines, including TGF-β, TNF-α, and platelet-derived growth factor promote signaling for HSCs activation.[57] Activated Kupffer cells and ROS are other factors that are also responsible for the HSCs activation.[57,58] Fibrogenic stimuli from TGF-β and ROS to activated HSCs result to excess production and deposition of abnormal ECM components in liver tissue.[58] Our investigation also revealed that CCl4-administered rats showed ECM deposition alongside the bile duct and central veins in liver which was further normalized by astaxanthin treatment. This finding in agreement with the recent report suggested that astaxanthin prevents hepatic fibrosis by decreasing the expression of NF-κB and TGF-β1.[20] Moreover, hepatic iron deposition was also found in CCl4-administered rats, which was further normalized by astaxanthin treatment. Free iron is a source of profibrogenic response in progressive liver fibrosis toward end-stage liver disease.[59] In fact, free iron deposition can trigger Fenton-like reaction in hepatocytes which produces notorious hydroxyl (·OH) free radicals.
CONCLUSION
From the above discussion, it can be concluded that astaxanthin contains significant hepatoprotective effect against CCl4-induced hepatotoxicity in rats. Astaxanthin-mediated protection against hepatic fibrosis, oxidative stress, and inflammation depends on the restoration of antioxidant enzymes, lowering lipid peroxidation, and decreased free iron deposition in hepatic tissues. Further investigations are warranted to elucidate the molecular mechanism of astaxanthin which may upregulate the antioxidant enzymes synthesis probably by Nrf2-mediated pathway.
Financial support and sponsorship
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We gratefully acknowledge the logistic support provided by the Department of Pharmaceutical Sciences, North South University, Bangladesh.
REFERENCES
- 1.Taub R. Liver regeneration: From myth to mechanism. Nat Rev Mol Cell Biol. 2004;5:836–47. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 2.Neubauer K, Saile B, Ramadori G. Liver fibrosis and altered matrix synthesis. Can J Gastroenterol. 2001;15:187–93. doi: 10.1155/2001/870205. [DOI] [PubMed] [Google Scholar]
- 3.Kew MC, Popper H. Relationship between hepatocellular carcinoma and cirrhosis. Semin Liver Dis. 1984;4:136–46. doi: 10.1055/s-2008-1040653. [DOI] [PubMed] [Google Scholar]
- 4.Lu SN, Su WW, Yang SS, Chang TT, Cheng KS, Wu JC, et al. Secular trends and geographic variations of hepatitis B virus and hepatitis C virus-associated hepatocellular carcinoma in Taiwan. Int J Cancer. 2006;119:1946–52. doi: 10.1002/ijc.22045. [DOI] [PubMed] [Google Scholar]
- 5.Kamel H, Azza H, Walaa A, Ahmed M, Mohamed A. Protective effect of some antioxidants against CCl4-induced toxicity in liver cells from BRL3A cell line. J Am Sci. 2010;6:992–1003. [Google Scholar]
- 6.Vitaglione P, Morisco F, Caporaso N, Fogliano V. Dietary antioxidant compounds and liver health. Crit Rev Food Sci Nutr. 2004;44:575–86. doi: 10.1080/10408690490911701. [DOI] [PubMed] [Google Scholar]
- 7.Kodavanti PR, Joshi UM, Young RA, Meydrech EF, Mehendale HM. Protection of hepatotoxic and lethal effects of CCl4 by partial hepatectomy. Toxicol Pathol. 1989;17:494–505. doi: 10.1177/019262338901700304. [DOI] [PubMed] [Google Scholar]
- 8.Pierce RA, Glaug MR, Greco RS, Mackenzie JW, Boyd CD, Deak SB. Increased procollagen mRNA levels in carbon tetrachloride-induced liver fibrosis in rats. J Biol Chem. 1987;262:1652–8. [PubMed] [Google Scholar]
- 9.Pérez Tamayo R. Is cirrhosis of the liver experimentally produced by CCl4 and adequate model of human cirrhosis? Hepatology. 1983;3:112–20. doi: 10.1002/hep.1840030118. [DOI] [PubMed] [Google Scholar]
- 10.Tada S, Nakamoto N, Kameyama K, Tsunematsu S, Kumagai N, Saito H, et al. Clinical usefulness of edaravone for acute liver injury. J Gastroenterol Hepatol. 2003;18:851–7. doi: 10.1046/j.1440-1746.2003.03064.x. [DOI] [PubMed] [Google Scholar]
- 11.Feng Y, Siu KY, Ye X, Wang N, Yuen MF, Leung CH, et al. Hepatoprotective effects of berberine on carbon tetrachloride-induced acute hepatotoxicity in rats. Chin Med. 2010;5:33. doi: 10.1186/1749-8546-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications –A review. Mar Drugs. 2014;12:128–52. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goto S, Kogure K, Abe K, Kimata Y, Kitahama K, Yamashita E, et al. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim Biophys Acta. 2001;1512:251–8. doi: 10.1016/s0005-2736(01)00326-1. [DOI] [PubMed] [Google Scholar]
- 14.Kurashige M, Okimasu E, Inoue M, Utsumi K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol Chem Phys Med NMR. 1990;22:27–38. [PubMed] [Google Scholar]
- 15.Yang Y, Seo JM, Nguyen A, Pham TX, Park HJ, Park Y, et al. Astaxanthin-rich extract from the green alga Haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J Nutr. 2011;141:1611–7. doi: 10.3945/jn.111.142109. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Bai SK, Lee KS, Namkoong S, Na HJ, Ha KS, et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I (kappa) B kinase-dependent NF-kappaB activation. Mol Cells. 2003;16:97–105. [PubMed] [Google Scholar]
- 17.Uchiyama K, Naito Y, Hasegawa G, Nakamura N, Takahashi J, Yoshikawa T. Astaxanthin protects beta-cells against glucose toxicity in diabetic db/db mice. Redox Rep. 2002;7:290–3. doi: 10.1179/135100002125000811. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida H, Yanai H, Ito K, Tomono Y, Koikeda T, Tsukahara H, et al. Administration of natural astaxanthin increases serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia. Atherosclerosis. 2010;209:520–3. doi: 10.1016/j.atherosclerosis.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Zhang J, Song X, Liu W, Zhang L, Wang X, et al. Astaxanthin ameliorates lung fibrosis in vivo and in vitro by preventing transdifferentiation, inhibiting proliferation, and promoting apoptosis of activated cells. Food Chem Toxicol. 2013;56:450–8. doi: 10.1016/j.fct.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Shen M, Chen K, Lu J, Cheng P, Xu L, Dai W, et al. Protective effect of astaxanthin on liver fibrosis through modulation of TGF-ß1 expression and autophagy. Mediators Inflamm. 2014;2014:954502. doi: 10.1155/2014/954502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chowdhury MR, Sagor MA, Tabassum N, Potol MA, Hossain H, Alam MA. Supplementation of Citrus maxima peel powder prevented oxidative stress, fibrosis, and hepatic damage in carbon tetrachloride (CCl4) treated rats. Evid Based Complement Alternat Med. 2015;2015:10. doi: 10.1155/2015/598179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagor AT, Chowdhury MR, Tabassum N, Hossain H, Rahman MM, Alam MA. Supplementation of fresh ucche (Momordica charantia L. var. muricata Willd) prevented oxidative stress, fibrosis and hepatic damage in CCl4 treated rats. BMC Complement Altern Med. 2015;15:115. doi: 10.1186/s12906-015-0636-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niehaus WG, Jr, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 24.Tracey WR, Tse J, Carter G. Lipopolysaccharide-induced changes in plasma nitrite and nitrate concentrations in rats and mice: Pharmacological evaluation of nitric oxide synthase inhibitors. J Pharmacol Exp Ther. 1995;272:1011–5. [PubMed] [Google Scholar]
- 25.Witko-Sarsat V, Friedlander M, Capeillère-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–13. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 26.Tiwari BK, Kumar D, Abidi AB, Rizvi SI. Efficacy of composite extract from leaves and fruits of medicinal plants used in traditional diabetic therapy against oxidative stress in alloxan-induced diabetic rats. ISRN Pharmacol. 2014;2014:608590. doi: 10.1155/2014/608590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan RA. Protective effects of Sonchus asper (L.) Hill, (Asteraceae) against CCl4- induced oxidative stress in the thyroid tissue of rats. BMC Complement Altern Med. 2012;12:181. doi: 10.1186/1472-6882-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5. [PubMed] [Google Scholar]
- 29.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–9. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 30.Recknagel RO, Glende EA, Jr, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–54. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 31.Sun F, Hamagawa E, Tsutsui C, Ono Y, Ogiri Y, Kojo S. Evaluation of oxidative stress during apoptosis and necrosis caused by carbon tetrachloride in rat liver. Biochim Biophys Acta. 2001;1535:186–91. doi: 10.1016/s0925-4439(00)00098-3. [DOI] [PubMed] [Google Scholar]
- 32.Rumack BH. Acetaminophen hepatotoxicity: The first 35 years. J Toxicol Clin Toxicol. 2002;40:3–20. doi: 10.1081/clt-120002882. [DOI] [PubMed] [Google Scholar]
- 33.Tribble DL, Aw TY, Jones DP. The pathophysiological significance of lipid peroxidation in oxidative cell injury. Hepatology. 1987;7:377–86. doi: 10.1002/hep.1840070227. [DOI] [PubMed] [Google Scholar]
- 34.Reza HM, Tabassum N, Sagor MA, Chowdhury MR, Rahman M, Jain P, et al. Angiotensin-converting enzyme inhibitor prevents oxidative stress, inflammation, and fibrosis in carbon tetrachloride-treated rat liver. Toxicol Mech Methods. 2016;26:46–53. doi: 10.3109/15376516.2015.1124956. [DOI] [PubMed] [Google Scholar]
- 35.Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol. 1978;1:163–71. doi: 10.3109/01480547809034433. [DOI] [PubMed] [Google Scholar]
- 36.Kang JO, Kim SJ, Kim H. Effect of astaxanthin on the hepatotoxicity, lipid peroxidation and antioxidative enzymes in the liver of CCl4-treated rats. Methods Find Exp Clin Pharmacol. 2001;23:79–84. doi: 10.1358/mf.2001.23.2.627931. [DOI] [PubMed] [Google Scholar]
- 37.Dai Y, Cederbaum AI. Inactivation and degradation of human cytochrome P4502E1 by CCl4 in a transfected HepG2 cell line. J Pharmacol Exp Ther. 1995;275:1614–22. [PubMed] [Google Scholar]
- 38.Nissar AU, Farrukh MR, Kaiser PJ, Rafiq RA, Afnan Q, Bhushan S, et al. Effect of N-acetyl cysteine (NAC), an organosulfur compound from Allium plants, on experimentally induced hepatic prefibrogenic events in Wistar rat. Phytomedicine. 2013;20:828–33. doi: 10.1016/j.phymed.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Shibata A, Kiba Y, Akati N, Fukuzawa K, Terada H. Molecular characteristics of astaxanthin and beta-carotene in the phospholipid monolayer and their distributions in the phospholipid bilayer. Chem Phys Lipids. 2001;113:11–22. doi: 10.1016/s0009-3084(01)00136-0. [DOI] [PubMed] [Google Scholar]
- 40.Arkenau HT, Stichtenoth DO, Frölich JC, Manns MP, Böker KH. Elevated nitric oxide levels in patients with chronic liver disease and cirrhosis correlate with disease stage and parameters of hyperdynamic circulation. Z Gastroenterol. 2002;40:907–13. doi: 10.1055/s-2002-35413. [DOI] [PubMed] [Google Scholar]
- 41.El-Sherif AM, Abou-Shady MA, Al-Bahrawy AM, Bakr RM, Hosny AM. Nitric oxide levels in chronic liver disease patients with and without oesophageal varices. Hepatol Int. 2008;2:341–5. doi: 10.1007/s12072-008-9077-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tipoe GL, Leung TM, Liong E, So H, Leung KM, Lau TY, et al. Inhibitors of inducible nitric oxide (NO) synthase are more effective than an NO donor in reducing carbon-tetrachloride induced acute liver injury. Histol Histopathol. 2006;21:1157–65. doi: 10.14670/HH-21.1157. [DOI] [PubMed] [Google Scholar]
- 43.Hrabarova E, Juranek I, Soltes L. Pro-oxidative effect of peroxynitrite regarding biological systems: A special focus on high-molar-mass hyaluronan degradation. Gen Physiol Biophys. 2011;30:223–38. doi: 10.4149/gpb_2011_03_223. [DOI] [PubMed] [Google Scholar]
- 44.Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, et al. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16:26087–124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rao AR, Sarada R, Shylaja MD, Ravishankar GA. Evaluation of hepatoprotective and antioxidant activity of astaxanthin and astaxanthin esters from microalga-Haematococcus pluvialis. J Food Sci Technol. 2015;52:6703–10. doi: 10.1007/s13197-015-1775-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Franceschelli S, Pesce M, Ferrone A, De Lutiis MA, Patruno A, Grilli A, et al. Astaxanthin treatment confers protection against oxidative stress in U937 cells stimulated with lipopolysaccharide reducing O2-production. PLoS One. 2014;9:e88359. doi: 10.1371/journal.pone.0088359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turkez H, Geyikoglu F, Yousef MI. Beneficial effect of astaxanthin on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced liver injury in rats. Toxicol Ind Health. 2013;29:591–9. doi: 10.1177/0748233711434959. [DOI] [PubMed] [Google Scholar]
- 48.Ma Q. Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Dong X, Liu H, Chen X, Shi H, Fan Y, et al. Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt. Mol Vis. 2013;19:1656–66. [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Q, Zhang XS, Wang HD, Zhang X, Yu Q, Li W, et al. Astaxanthin activates nuclear factor erythroid-related factor 2 and the antioxidant responsive element (Nrf2-ARE) pathway in the brain after subarachnoid hemorrhage in rats and attenuates early brain injury. Mar Drugs. 2014;12:6125–41. doi: 10.3390/md12126125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zimmermann HW, Trautwein C, Tacke F. Functional role of monocytes and macrophages for the inflammatory response in acute liver injury. Front Physiol. 2012;3:56. doi: 10.3389/fphys.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brempelis KJ, Crispe IN. Infiltrating monocytes in liver injury and repair. Clin Transl Immunol. 2016;5:e113. doi: 10.1038/cti.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kiso K, Ueno S, Fukuda M, Ichi I, Kobayashi K, Sakai T, et al. The role of Kupffer cells in carbon tetrachloride intoxication in mice. Biol Pharm Bull. 2012;35:980–3. doi: 10.1248/bpb.35.980. [DOI] [PubMed] [Google Scholar]
- 54.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–75. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 55.Xu R, Huang H, Zhang Z, Wang FS. The role of neutrophils in the development of liver diseases. Cell Mol Immunol. 2014;11:224–31. doi: 10.1038/cmi.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klebanoff SJ. Myeloperoxidase: Friend and foe. J Leukoc Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 57.Weiskirchen R, Tacke F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg Nutr. 2014;3:344–63. doi: 10.3978/j.issn.2304-3881.2014.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gandhi CR. Oxidative stress and hepatic stellate cells: A paradoxical relationship. Trends Cell Mol Biol. 2012;7:1–10. [PMC free article] [PubMed] [Google Scholar]
- 59.Ramm GA, Ruddell RG. Hepatotoxicity of iron overload: Mechanisms of iron-induced hepatic fibrogenesis. Semin Liver Dis. 2005;25:433–49. doi: 10.1055/s-2005-923315. [DOI] [PubMed] [Google Scholar]