Abstract
Background:
Diabetes mellitus is a metabolic disorder characterized by chronic hyperglycemia. Plant extracts and their products are being used as an alternative system of medicine for the treatment of diabetes. Aloe vera has been traditionally used to treat several diseases and it exhibits antioxidant, anti-inflammatory, and wound-healing effects. Streptozotocin (STZ)-induced Wistar diabetic rats were used in this study to understand the potential protective effect of A. vera extract on the pancreatic islets.
Objective:
The aim of the present study was to evaluate the A. vera extract on improvement of insulin secretion and pancreatic β-cell function by morphometric analysis of pancreatic islets in STZ-induced diabetic Wistar rats.
Materials and Methods:
After acclimatization, male Wistar rats, maintained as per the Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines, were randomly divided into four groups of six rats each. Fasting plasma glucose and insulin levels were assessed. The effect of A. vera extract in STZ-induced diabetic rats on the pancreatic islets by morphometric analysis was evaluated.
Results:
Oral administration of A. vera extract (300 mg/kg) daily to diabetic rats for 3 weeks showed restoration of blood glucose levels to normal levels with a concomitant increase in insulin levels upon feeding with A. vera extract in STZ-induced diabetic rats. Morphometric analysis of pancreatic sections revealed quantitative and qualitative gain in terms of number, diameter, volume, and area of the pancreatic islets of diabetic rats treated with A. vera extract when compared to the untreated diabetic rats.
Conclusion:
A. vera extract exerts antidiabetic effects by improving insulin secretion and pancreatic β-cell function by restoring pancreatic islet mass in STZ-induced diabetic Wistar rats.
SUMMARY
Fasting plasma glucose (FPG) and insulin levels were restored to normal levels in diabetic rats treated with Aloe vera extract
Islets of pancreas were qualitatively and quantitatively restored to normalcy leading to restoration of FPG and insulin levels of diabetic rats treated with Aloe vera extract
Morphometric analysis of pancreatic sections revealed quantitative and qualitative gain in terms of number, diameter, volume, and area of the pancreatic islets of diabetic rats treated with Aloe vera extract when compared to the untreated diabetic rats.
Abbreviations Used: A. vera, FPG: Fasting plasma glucose, STZ: Streptozotocin, BW: Body weight
Keywords: Aloe vera, diabetes, insulin, morphometric analysis, pancreas, streptozotocin
INTRODUCTION
Globally, diabetes mellitus is affecting major populations worldwide. It is a metabolic disorder that affects many metabolic pathways. It is characterized by hyperglycemia resulting from absolute insulin deficiency or insufficient insulin secretion and/or insulin sensitivity.[1] Epidemiological studies and clinical trials have shown that hyperglycemia is the principal cause of complications. Effective control of plasma glucose levels is an effective strategy to prevent the diabetic complications and improving quality of life in diabetic patients. Despite important progress in the management of diabetes using synthetic drugs, many traditional plant treatments are still being used throughout the world.[2] Many traditional plants and herbal medicines have been found to possess the antidiabetic activity;[3,4,5,6,7] however, the World Health Organization has recommended that traditional plant treatments for diabetes warrant further evaluation.[8] Search for appropriate antidiabetic agents has been focused on traditional plants because of the leads provided by these plant products that may be considered to be less toxic than currently used drugs.[9,10] Aloe vera contains numerous bioactive compounds/constituents useful for the management of various diseases, including diabetes.[11,12]
A. vera (L.) Burm. fil. (Aloe barbadensis Miller) is a succulent plant which belongs to the family Liliaceae. It has been widely used in traditional medicine as remedy for a variety of ailments. The properties and actions of A. vera documented by traditional uses include its use as the remedy for gastrointestinal ailments, anti-inflammatory and skin diseases,[13] wound healing[14] and burns,[15] antiulcer,[16] and diabetes.[17,18] In our previous studies, streptozotocin (STZ)-induced diabetic Wistar rats treated with A. vera extract led to reduction in fasting plasma glucose (FPG) to normal levels.[18] In the present literature, there is a paucity of the information regarding the efficacy of A. vera extract on morphometric data of pancreatic islets in STZ-induced diabetic rats. Therefore, the present study investigates whether A. vera extract could provide antihyperglycemic activity with improvement in insulin secretion through regeneration of the pancreatic β-cells of STZ-induced diabetic Wistar rats. Morphometric analysis of pancreatic islets was performed to further substantiate the beneficial effect of A. vera extract on pancreatic cell mass.
MATERIALS AND METHODS
Chemicals and reagents
STZ was purchased from Sigma (USA). Glucose oxidase/peroxidase reagent glucose kits were obtained from Span Diagnostics Ltd., Rat insulin ELISA kit (Mercodia, Uppsala, Sweden). All other chemicals used were of analytical grade.
Plant materials
Fresh leaves of A. vera (L.) Burm. fil. were collected from the nursery of VIT University, Vellore, Tamil Nadu, India, between March and April. The leaves were identified by the authorized staff of VIT University. A voucher specimen has been deposited in the center’s herbarium.
Preparation of Aloe vera extract
A. vera extract was prepared from A. vera gel according to published procedure.[18] A greenish-brown powder crude extract of A. vera was obtained. Dried powder was stored at 4°C until further use.
Experimental animals
Three-month-old male albino Wistar rats weighing 150–200 g were used to carry out experiments. Wistar rats were maintained according to the Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines under good laboratory conditions and were allowed free access of food and water ad libitum. The experiments were carried out in accordance with the principles of laboratory animal care, according to the ethical norms approved by Institutional Animal Ethics Committee guidelines of Christian Medical College and Hospital, Vellore, India. During the whole experimental period, animals were fed with a balanced commercial diet (Sai Durga Ltd., Bangalore, India) and water ad libitum.
Induction of diabetes
The Wistar rats were subjected to a 12 h fast. Diabetes was induced by intraperitoneal (i.p.) injection of STZ (Sigma, USA) dissolved in 0.01 M cold sodium citrate buffer (pH 4.4) at a dose of 30 mg/kg body weight (BW).[18] The animals were fasted overnight and blood samples were collected from the tail vein (0.5 ml). Three days after STZ administration, the rats were considered to be diabetic with FPG levels above 250 mg/dL with glycosuria.
Study design and drug administration
A total of 24 male albino Wistar rats were used for the experiment. The rats were divided into four groups of six rats each. The experimental period was 3 weeks. Group I comprised control rats. Group II comprised induced diabetic group. Rats belonging to Groups III and IV (therapeutic group) were given A. vera extract (300 mg/kg BW) and glibenclamide (1 mg/kg BW), respectively, orally after the onset of diabetes daily. The A. vera extract and glibenclamide were administered orally once daily via a steel gavage tube for 3 weeks (28 days) to Group II and Group IV, respectively. The dose of A. vera and glibenclamide was taken from the study done by our previous study.[18] During the course of the experimental study, the rats were weighed every week and observed for any change in the behavior throughout the study period.
Collection of blood and measurement of plasma insulin
After overnight fast, the blood samples for bioassay were collected form the tail vein puncture. Blood was collected in tubes with anticoagulant. FPG and insulin levels were estimated at 1st, 2nd, and 3rd weeks. The FPG levels were estimated using GOD/POD kit (Span diagnostic) at 500 nm. The fasting plasma insulin levels were determined using a rat insulin ELISA kit (Mercodia, Uppsala, Sweden) and absorbance was read with Model 680 series Micro plate Reader S/N 14148 analyzer (Bio-Rad Laboratories, USA) at 450 nm.
Morphometric study
Tissue processing
All the rats after overnight fasting were sacrificed at the end of 3 weeks by inducing anesthesia using thiopentone sodium (40 mg/kg i.p.). Pancreas were removed and fixed in 10% buffered formalin and dehydrated by graded series of alcohol, embedded in paraffin, sectioned at 5 μm in thickness, and were cut using a American Optical microtome (Model 82) and mounted on glass slides. Pancreatic sections were stained with hematoxylin and eosin (H and E). The sections were examined under LEICA Qwin microscope (Leica Qwin, Glattbrugg, Switzerland) with digital camera attached.
Histomorphometric analysis
All the histomorphometric studies were carried out on LEICA Qwin microscope connected to a computer. Five histological sections per slide with the total of 15 sections per animal were taken for the morphometric study at 110 μm intervals between each section with 5-μm thick histological cuts were stained with H and E. The morphometric analysis determined (1) the number of islets in each section of the pancreas, (2) the area of the pancreatic islet (μm2), (3) the volume (μm3), and (4) the diameter of the pancreatic islets (μm) for each islet. The number of islets was expressed as N/10 mm2.[19] The profile diameter (Di) of the islets was calculated from the following equation: profile diameter Di= √ab.[20] Assuming that the islets are of spheroid structures, the formula of Fullman[20] was used to calculate the mean islet diameter (Di) at a magnification of ×200, Di = π/2 × N/1/di1 + 1/di2…….1/diN, where N represents the total profiles measured and (a) and (b) are the major and minor at right angle to (a) axis of the islet, respectively, and they were measured by a computer-based image analysis system (Semiautomatic image analyzer Leica Qwin DC180). The mean radius of the islets (r = D/2) was used to calculate the mean islet area, A = πr2. Islet volume was calculated using the following formula:[20] V = 4 π/3 × (Di/2)3. Images of the histological sections were obtained using ×10 and ×20 magnification and were analyzed by image analysis system (LEICA Qwin), a semi-automatic software program. All measurements for each group were averaged cumulatively and these data were subjected to statistical analysis.
Statistical analysis
Data were expressed as mean ± standard deviation. Significance of the differences in FPG and insulin levels in different groups was assessed by two-way analysis of variance followed by Bonferroni alpha analysis using GraphPad Prism version 6.04 for windows, GraphPad Software, La Jolla California, USA. The significance of difference between and within groups was determined by Student’s t-test. For all the tests, P < 0.05 was considered statistically significant and P ≤ 0.001 as highly statistically significant.
RESULTS
Effect of the 3-week treatment of Aloe vera extract on fasting blood glucose
Control rats (Group 1) fed with water as vehicle were euglycemic and insulin levels were normal throughout the period (3 weeks) of the study [Figure 1a and b]. In a subchronic antidiabetic study, it was observed that, in the untreated group (Group 2), the FPG levels were increased with decrease in insulin levels after induction of diabetes. In Group 3 after A. vera extract (300 mg/g BW) administration, it was noticed that the FPG levels were significantly lower than the diabetic controls. In contrast, the FPG levels of the untreated diabetic rats remained elevated throughout the experimental period. A FPG value of 14.22 mmol/L was observed on the 3rd day (confirmation of diabetes), which subsequently reduced after treatment with A. vera extract to 7.25 mmol/L in the 1st week, which further reduced to normal levels in the 2nd and 3rd weeks at 5.7 and 5.6 mmol/L, respectively [Figure 1a]. The P value was statistically significant (P < 0.001). The standard drug glibenclamide lowered the FPG levels at 8.52, 7.81, and 6.84 mmol/L in the 1st, 2nd, and 3rd weeks, respectively.
Figure 1.
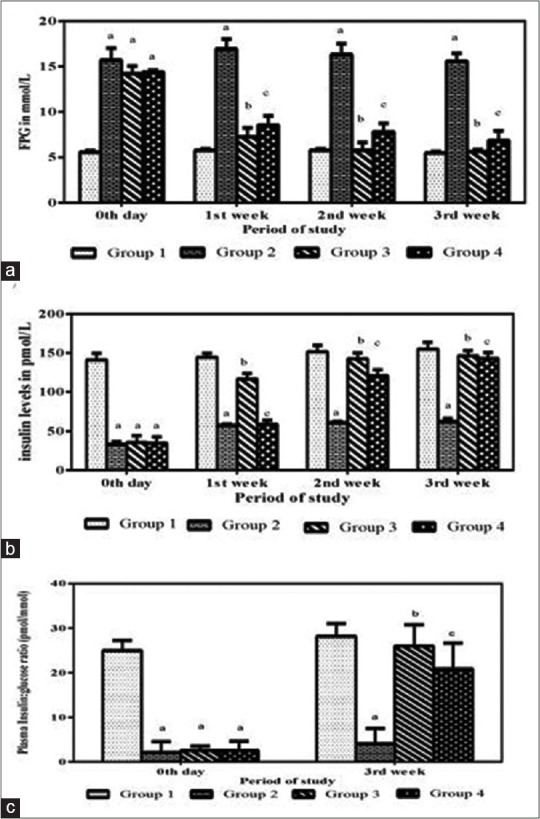
Effect of the 3-week treatment of Aloe vera extract (300 mg/kg) in streptozotocin-induced Wistar diabetic rats. aEffect of Aloe vera on fasting plasma glucose (mmol) levels. bEffect of Aloe vera on serum insulin (pmol) levels. cEffect of Aloe vera treatment on insulin: glucose ratio (pmol/mmol) levels. (a) Statistically significant increase in fasting plasma glucose and decrease in insulin and insulin: glucose ratio levels in Group II compared with Group I (P < 0.0001). (b and c) Statistically significant decrease in fasting plasma glucose and increase in insulin and insulin: glucose ratio in Groups III and IV when compared with Group II (P < 0.0001)
Effect of the 3-week treatment of A. vera extract on fasting serum insulin
The fasting serum insulin levels in Group 1 were normal throughout the experimental period. The serum insulin levels decreased in Group 2 rats when compared to control rats. The serum insulin levels of Group 3 after oral administration of A. vera extract increased from 116.5 pmol/L (1st week) to 146.1 pmol/L (3rd week) [Figure 1b]. The increase in serum insulin levels was found to be statistically significant (P < 0.001). The serum insulin level of Group 4 after oral administration of glibenclamide at 3rd week was 142.6 pmol/L [Figure 1b].
Effect of 3-week treatment of Aloe vera extract on fasting insulin: glucose ratio
The insulin: glucose ratio was calculated from the fasting insulin and glucose values. It was found that 28.2 pmol insulin/mmol glucose was present in control rats; however, only 4.02 pmol insulin/mmol glucose was available in the Group 2 rats [Figure 1c]. After 3 weeks of oral administration of A. vera extract (Group 3) and glibenclamide (Group 4), interestingly there was a 6.4- and 5.2-fold (25.9 and 20.8 pmol insulin/mmol glucose, respectively), respectively, increase in insulin: glucose ratio when compared with the untreated diabetic rats [Figure 1c].
Morphological observations of pancreatic islets
The H and E staining of pancreatic sections upon histological examination is shown in Figure 2. Islets were measured using the formula of Fullman as mentioned in the Methodology section [Figure 2a]. In the control rats (Group 1), the histological appearance of the pancreatic islet cells was normal [Figure 2b]. On the contrary, diabetic pancreatic sections (Group 2) showed reduction of number of pancreatic islets, the islets were damaged and shrunken in size [Figure 2c]. The morphology of the pancreas of Group III and Group IV rats revealed improvement in the pancreatic islets and was comparable with control rats [Figure 2d and e].
Figure 2.
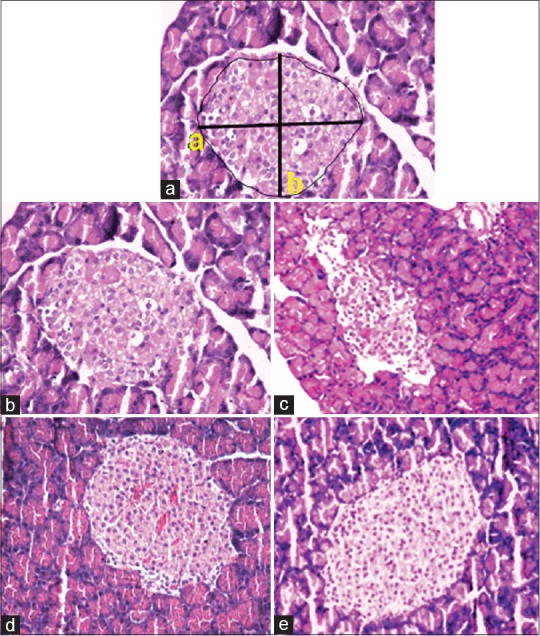
Hematoxylin and eosin-stained sections of pancreas stained after 3-week treatment. (a) Photomicrograph of a pancreatic islet used for morphometric analysis (×20). The major and minor a, bright angle diameter and the surface area of pancreas. (b) Group I islets of Langerhans with native architecture. (c) IN Group II, the islet was damaged and shrunken in size displaying degenerative changes compared to control normal rats. (d) Group III pancreatic islet displaying architecture similar to normal control rat islet. (e) Group IV islet architecture similar to normal control rat islet
Morphometric analysis
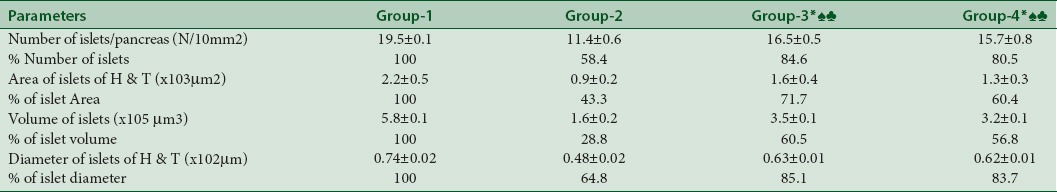
Morphometric examination of the H and E-stained pancreatic sections showed a significant reduction in the total number of islets, diameter, area, and volume of pancreatic islets in STZ-untreated diabetic rats (Group 2) with 58.4% of total number of islets, 64.8% islet diameter, 43.3% islet area, and volume 28.8% when compared with the control rats (Group 1). However, these morphometric parameters were significantly increased in A. vera extract-treated diabetic rat group (Group 3) with 84.6% exhibited recovery in total number of islets, 85.1% in islet diameter, 71.7% of islet area, and 60.5% islet volume. Here, our results suggest that A. vera could influence pancreatic islets in STZ-induced diabetic rats recuperation after A. vera treatment for 3 weeks that may have contributed to the antidiabetic effect [Table 1]. In glibenclamide-treated diabetic rats (Group 4), the recovery was 80.5% in total number of islets, 83.7% in islet diameter, 60.4% of islet area, and 56.8% islet volume.
Table 1.
Morphometric analysis of the pancreatic islets of control, untreated diabetic, Aloe vera-treated diabetic rats and glibenclamide-treated diabetic rats. The data are represented as mean±SD. n=6 rats in each group. ♠Statistically significant increase in mean area of islets (P<0.001), ♣Volume of islets (P<0.001) and *diameter of islets (P=0.008) of Group III and Group IV when compared with untreated Group II rats, respectively. Group I: Control rats; Group II: Untreated diabetic rats; Group III: Aloe vera-treated diabetic rats; Group IV: Glibenclamide-treated diabetic rats; SD: Standard deviation
DISCUSSION
In this study, insulin-producing pancreatic β-cells were degenerated in STZ-induced diabetic rats, leading to an increase in blood glucose concentration and a decrease in insulin secretion [Figure 1a and b]. However, after feeding with the A. vera extract, a significant antihyperglycemic activity was observed in STZ-induced diabetic Wistar rats in comparison to the untreated diabetic group [Figure 1a]. In our preliminary study, we reported the marked amelioration effect of A. vera extract, brings back the FPG levels to normal in STZ-induced diabetic rats with protective effect on pancreas, liver, and small intestine.[18] These observations from this study are also supported by the report of Okyar et al.[21] and Salem and El-Azab[22] who showed that treatment with A. vera extract had blood glucose-lowering effects with possible protective role in pancreatic β-cells of STZ-induced diabetic rats. It has been reported that the active principles from plant sources might act by different mechanisms such as increasing repair/proliferation of pancreatic β-cells, stimulating insulin secretion, and increasing the oxidative capability.[23,24,25] Interestingly, after A. vera extract treatment to the STZ-induced diabetic rats, improved serum insulin levels as well as the insulin: glucose ratio [Figure 1b and c] was observed. The present findings corroborate with the report of Hafizur et al.[26] that the increased serum insulin levels could be related to the upregulation of the insulin synthesis by the pancreatic β-cells or may have the ability in the renewal of β-cells or partial regeneration or preservation of pancreatic β-cell mass in STZ-induced diabetic animals. These observations suggest that A. vera-treated diabetic rats maintain the blood glucose homeostasis. Further, histological studies of pancreas were carried out by morphometric analysis to confirm the rejuvenation of pancreatic β-cells.
The A. vera treatment improved the size of the pancreatic islets in STZ-induced diabetic rats [Figure 2]. To assess the importance of the pancreatic β-cell content for glucose homoeostasis in STZ-induced rats, these diabetic rats were fed with A. vera extract and compared with untreated diabetic rats. The present study is the first one that shows before and after treatment with A. vera change in the total number of islets, islet diameter, islet area, and islet volume with reference to the distribution, area, volume, diameter, and number of islets in each group. The number of pancreatic islets in A. vera-treated diabetic rats was significantly higher than that of the glibenclamide-treated diabetic rats and untreated diabetic rats [Table 1]. These results indicate that A. vera extract markedly increases at 84.6% in the total number of pancreatic islets. These effects could rationalize the improved pancreatic islet number and size and hence better insulin secretion and better glycemic control. This finding further supports the hypothesis of possible rejuvenation of islets, thus attaining the normal levels of insulin. Hence, the balance of the pancreatic islet cells could increase insulin secretion and reverse the diabetes in the A. vera extract-treated diabetic rats, whereas in untreated diabetic rats, the numbers of islets are considerably reduced to 58.4% [Table 1], shrunken, and reduced in size with infiltration of lymphocytes [Figure 2b]. These findings were in line with those of Adeyemi et al.[19] and Ramadan et al.,[27] who also observed with significant reduction in the islet number, area, volume, and diameter in untreated STZ-induced rats [Table 1]. We also noticed that there is difference in number, diameter, area, and volume of pancreatic islets in A. vera, glibenclamide, and untreated diabetic rats when compared with control rats. This difference may be due to variations in the replication from the preexisting surviving β-cells or regeneration of new β-cells. Wang et al.[28] reported that the β-cells which survive STZ destruction presumably belong to a less differentiated, immature cell population which retains a high mitotic potential. Collombat et al.[29] have reported that some kind of plants have been reported to induce regeneration of pancreatic islets in STZ-induced diabetic rats. Our present study shows an increase in the islet number after treatment with A. vera extract (300 mg/kg bw) to STZ-induced diabetic rats. This suggests that A. vera extract-treated group may have a role in the rejuvenation or revitalization of the pancreatic β-cells or in the recovery of the partially destroyed β-cells. It has also been reported that a number of other plants exert antidiabetic activity through these mechanisms.[19,23,26,29,30,31] Some of the literature findings of A. vera’s constituents with particular emphasis on diabetes and other pharmacological actions show the different constituents of A. vera exhibiting antidiabetic activity.[1,32,33,34,35] These studies strongly suggested that A. vera has active components that alleviate diabetes and provide additional health benefits. Further work is under progress to elucidate this hypothesis.
CONCLUSION
A. vera extract exhibited pancreatic islet cell rejuvenation, as evidenced by increased pancreatic islet mass and hence helps in the restoration of insulin levels and therefore improves the glucose metabolism in STZ-induced diabetic rats. Altogether, these findings indicate that A. vera extract has an effective therapeutic effect that helps in the alleviation of diabetes. Further experiments and clinical studies are required to explore the additional mechanisms to establish its clinical utility with adequate ethical and medical background to substantiate the use of A. vera extract as nutraceutical supplement for diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors thank Department of Science Technology (DST) (IR/SO/LU-03/2004), New Delhi, India, for the financial support and also acknowledge the help rendered by Dr. Victoria Job (Department of Clinical Biochemistry, CMC, Vellore) and Mr. A. Soosai Manickam for their help during this study.
REFERENCES
- 1.Maritim AC, Sanders RA, Watkins JB. Diabetes mellitus is a metabolic disorder characterized by hyperglycemia and insufficiency of secretion or action of endogenous insulin. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 2.Bailey CJ, Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12:553–64. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 3.Ranasinghe P, Perera S, Gunatilake M, Abeywardene E, Gunapala N, Premakumara S, et al. Effects of Cinnamomum zeylanicum (Ceylon cinnamon) on blood glucose and lipids in a diabetic and healthy rat model. Pharmacognosy Res. 2012;4:73–9. doi: 10.4103/0974-8490.94719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalaivanan K, Pugalendi KV. Antihyperglycemic effect of the alcoholic seed extract of Swietenia macrophylla on streptozotocin-diabetic rats. Pharmacognosy Res. 2011;3:67–71. doi: 10.4103/0974-8490.79119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kujur RS, Singh V, Ram M, Yadava HN, Singh KK, Kumari S, et al. Antidiabetic activity and phytochemical screening of crude extract of Stevia rebaudiana in alloxan-induced diabetic rats. Pharmacognosy Res. 2010;2:258–63. doi: 10.4103/0974-8490.69128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibegbulem CO, Chikezie PC. Hypoglycemic properties of ethanolic extracts of Gongronema latifolium Aloe perryi Viscum album and Allium sativum administered to alloxan-induced diabetic albino rats (Rattus norvegicus) Pharmacogn Commun. 2013;3:12–6. [Google Scholar]
- 7.Rasineni K, Bellamkonda R, Singareddy SR, Desireddy S. Antihyperglycemic activity of Catharanthus roseus leaf powder in streptozotocin-induced diabetic rats. Pharmacognosy Res. 2010;2:195–201. doi: 10.4103/0974-8490.65523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. WHO expert committee on diabetes mellitus: Second report. World Health Organ Tech Rep Ser. 1980;646:1–80. [PubMed] [Google Scholar]
- 9.Yeh GY, Eisenberg DM, Kaptchuk TJ, Phillips RS. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care. 2003;26:1277–94. doi: 10.2337/diacare.26.4.1277. [DOI] [PubMed] [Google Scholar]
- 10.Chan CH, Ngoh GC, Yusoff R. A brief review on anti-diabetic plants: Global distribution, active ingredients, extraction techniques and acting mechanisms. Pharmacogn Rev. 2012;6:22–8. doi: 10.4103/0973-7847.95854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noor A, Bansal VS, Vijayalakshmi MA. Current update on anti-diabetic biomolecules from key traditional Indian medicinal plants. Curr Sci. 2013;104:721–7. [Google Scholar]
- 13.Langmead L, Makins RJ, Rampton DS. Anti-inflammatory effects of Aloe vera gel in human colorectal mucosa in vitro. Aliment Pharmacol Ther. 2004;19:521–7. doi: 10.1111/j.1365-2036.2004.01874.x. [DOI] [PubMed] [Google Scholar]
- 14.Chithra P, Sajithlal GB, Chandrakasan G. Influence of Aloe vera on the healing of dermal wounds in diabetic rats. J Ethnopharmacol. 1998;59:195–201. doi: 10.1016/s0378-8741(97)00124-4. [DOI] [PubMed] [Google Scholar]
- 15.Pribitkin ED, Boger G. Herbal therapy: What every facial plastic surgeon must know. Arch Facial Plast Surg. 2001;3:127–32. doi: 10.1001/archfaci.3.2.127. [DOI] [PubMed] [Google Scholar]
- 16.Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14:581–91. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Bunyapraphatsara N, Yongchaiyudha S, Rungpitarangsi V, Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice II. Clinical trial in diabetes mellitus patients in combination with glibenclamide. Phytomedicine. 1996;3:245–8. doi: 10.1016/S0944-7113(96)80061-4. [DOI] [PubMed] [Google Scholar]
- 18.Noor A, Gunasekaran S, Manickam AS, Vijayalakshmi MA. Antidiabetic activity of Aloe vera and histology of organs in streptozotocin induced diabetic rats. Curr Sci. 2008;94:1070–6. [Google Scholar]
- 19.Adeyemi DO, Komolafe OA, Adewole OS, Obuotor EM, Abiodun AA, Adenowo TK, et al. Histomorphological and morphometric studies of the pancreatic islet cells of diabetic rats treated with extracts of Annona muricata. Folia Morphol (Warsz) 2010;69:92–100. [PubMed] [Google Scholar]
- 20.Williams MA. Quantitative methods in biology. In: Glauert AM, editor. Practical Methods in Electron Microscopy. Vol. 6. Amsterdam, North-Holland: Elsevier North-Holland Biomedical Press; 1977. pp. 48–62. [Google Scholar]
- 21.Okyar A, Can A, Akev N, Baktir G, Sütlüpinar N. Effect of Aloe vera leaves on blood glucose level in type I and type II diabetic rat models. Phytother Res. 2001;15:157–61. doi: 10.1002/ptr.719. [DOI] [PubMed] [Google Scholar]
- 22.Salem MY, El-Azab NE. The possible protective role of Aloe vera extracts in pancreatic b cells of experimentally induced diabetic rats: A histological and immunohistochemical study. Egypt J Histol. 2014;37:571–8. [Google Scholar]
- 23.Shanmugasundaram ER, Gopinath KL, Radha Shanmugasundaram K, Rajendran VM. Possible regeneration of the islets of Langerhans in streptozotocin-diabetic rats given Gymnema sylvestre leaf extracts. J Ethnopharmacol. 1990;30:265–79. doi: 10.1016/0378-8741(90)90106-4. [DOI] [PubMed] [Google Scholar]
- 24.Ashwini S, Bobby Z, Joseph M, Jacob SE, Padmapriya R. Insulin plant (Costus pictus) extract improves insulin sensitivity and ameliorates atherogenic dyslipidaemia in fructose induced insulin resistant rats: Molecular mechanism. J Funct Foods. 2015;17:749–60. [Google Scholar]
- 25.Verma PR, Itankar PR, Arora SK. Evaluation of antidiabetic antihyperlipidemic and pancreatic regeneration, potential of aerial parts of Clitoria ternatea. Rev Bras Farmacogn. 2013;23:819–29. [Google Scholar]
- 26.Hafizur RM, Kabir N, Chishti S. Asparagus officinalis extract controls blood glucose by improving insulin secretion and β-cell function in streptozotocin-induced type 2 diabetic rats. Br J Nutr. 2012;108:1586–95. doi: 10.1017/S0007114511007148. [DOI] [PubMed] [Google Scholar]
- 27.Ramadan BK, Schaalan MF, Tolba AM. Hypoglycemic and pancreatic protective effects of Portulaca oleracea extract in alloxan induced diabetic rats. BMC Complement Altern Med. 2017;17:37. doi: 10.1186/s12906-016-1530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang RN, Klöppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995;38:1405–11. doi: 10.1007/BF00400600. [DOI] [PubMed] [Google Scholar]
- 29.Collombat P, Xu X, Heimberg H, Mansouri A. Pancreatic beta-cells: From generation to regeneration. Semin Cell Dev Biol. 2010;21:838–44. doi: 10.1016/j.semcdb.2010.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosa MF, Pacheco MR, Girardi AM, Silva MH, Santos E, Baraldi-Altoni SM. Morphometric evaluation of the islets of Langerhans of diabetic rats treated with the extracts of Azadirachta indica (Neem) and streptozotocin 6C. ARS Vet. 2011;27:175–80. [Google Scholar]
- 31.Singh N, Gupta M. Regeneration of beta cells in islets of Langerhans of pancreas of alloxan diabetic rats by acetone extract of Momordica charantia (Linn.) (bitter gourd) fruits. Indian J Exp Biol. 2007;45:1055–62. [PubMed] [Google Scholar]
- 32.Tanaka M, Misawa E, Ito Y, Habara N, Nomaguchi K, Yamada M, et al. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol Pharm Bull. 2006;29:1418–22. doi: 10.1248/bpb.29.1418. [DOI] [PubMed] [Google Scholar]
- 33.Yagi A, Hegazy S, Kabbash A, Wahab EA. Possible hypoglycemic effect of Aloe vera L. High molecular weight fractions on type 2 diabetic patients. Saudi Pharm J. 2009;17:209–15. doi: 10.1016/j.jsps.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huseini HF, Kianbakht S, Hajiaghaee R, Dabaghian FH. Anti-hyperglycemic and anti-hypercholesterolemic effects of Aloe vera leaf gel in hyperlipidemic type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Planta Med. 2012;78:311–6. doi: 10.1055/s-0031-1280474. [DOI] [PubMed] [Google Scholar]
- 35.Yimam M, Zhao J, Corneliusen B, Pantier M, Brownell L, Jia Q, et al. Blood glucose lowering activity of aloe based composition, UP780, in alloxan induced insulin dependent mouse diabetes model. Diabetol Metab Syndr. 2014;6:61. doi: 10.1186/1758-5996-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]


