Abstract
Purpose
To report the case of an adult female who presented on different occasions with recurrent uveitis provoked by initiating therapy of two recently approved agents, dabrafenib and pembrolizumab, for treatment of metastatic melanoma.
Observations
A 61 year old female presented with bilateral anterior uveitis after initiating therapy with dabrafenib for advanced metastatic melanoma. Her symptoms resolved and exam improved with oral and topical steroid therapy. Months later, she was started on pembrolizumab and transitioned off dabrafenib. Within days of starting pembrolizumab, she developed recurrent bilateral uveitis. This responded to escalating doses of topical and oral corticosteroid therapy and resolved following discontinuation of pembrolizumab. Nine months later, our patient received her third dose of pembrolizumab due to further progression of melanoma and within three days developed blurry vision, photophobia and subsequent ophthalmologic exam demonstrated bilateral panuveitis.
Conclusions and importance
Dabrafenib and pembrolizumab therapy have both previously been associated with uveitis. Here, we document a case of a woman who developed acute uveitis in response to beginning therapy with dabrafenib and then later developed acute uveitis soon after initiating pembrolizumab. To our knowledge, this is the first time this uncommon side-effect has been reported in the same patient after receiving sequential targeted agents and checkpoint inhibitors.
Keywords: Dabrafenib, Pembrolizumab, Uveitis, Vasculitis
1. Introduction
The treatment of metastatic cutaneous melanoma has traditionally been challenging and often associated with a poor prognosis [1]. However, over the past five years multiple agents have been developed that show promise in successfully treating this aggressive neoplasm. One of these medications, dabrafenib (Tafinlar™; Novartis, Basel, Switzerland), is a reversible adenosine triphosphate-competitive inhibitor that selectively blocks BRAF kinase, resulting in inhibition of the MAPK pathway in melanoma cells [2]. Another novel drug used for treatment of advanced melanoma is pembrolizumab (Keytruda™; Merck, Kenilworth, New Jersey, USA), a monoclonal antibody against the T-cell co-inhibitory receptor programmed death (PD-1) protein. While dabrafenib decreases cancer cell proliferation by inhibiting the oncogene BRAFV600E within tumor cells [2], pembrolizumab functions by increasing the immune systems T-cell response to tumor antigens [3]. Side-effects are pervasive with both therapies and thought to occur in approximately 40–50% of patients [3], [4], [5]. Eye specific complications of therapy, however, are infrequent and rarely reported in the literature. Although ocular effects are uncommon and despite the differences in their mechanism of action, here we report a case of a 61 year old female who developed bilateral uveitis shortly after initiating therapy with dabrafenib and then later developed this same reaction when given pembrolizumab. The patient provided written consent for publication of personal information including medical record details and photographs.
2. Case report
A 61 year old Caucasian female undergoing treatment for metastatic melanoma was initially referred to our eye clinic in 2013 for an ocular screening exam prior to beginning an experimental therapy. She was originally diagnosed with stage I malignant melanoma of the lateral aspect of her right chest wall following a shave biopsy in May 2007. Subsequent wide local excision failed to eliminate the tumor and in October 2007, positron emission tomography (PET) scan demonstrated a possible mass lesion in the right upper lung. Unfortunately, the patient was lost to follow-up until November 2012 when she developed fatigue and weight loss. Repeat imaging in April 2013 showed lesions concerning for metastases to the inguinal, axillary, and pectoralis regions. Axillary biopsy at that time confirmed metastatic melanoma and her tumor tested BRAFV600E positive by Cobas 4800 assay (Roche Molecular Diagnostics).
Over the next several months, she was enrolled in a randomized controlled trial with a combination therapy of vemurafenib (Zelboraf™; Roche, Basel, Switzerland) and cobimetinib (Cotellic™; Roche, Basel, Switzerland) or placebo. Her eye exam at that time was unremarkable and she denied a prior history of uveitis. Due to drug-induced liver injury her therapy was discontinued August 2013 but staging computed tomography (CT) scans demonstrated reduction in overall tumor burden. When CT scans in January 2014 off therapy demonstrated tumor progression with worsening axillary and sub-pectoral adenopathy, the patient began commercial dabrafenib/trametinib (Mekinist™; GlaxoSmithKline, Research Triangle Park, North Carolina, USA) combination therapy. Within a month, she again developed a drug-induced liver injury and so this therapy was discontinued and ipilimumab (Yervoy™; Bristol-Myers Squibb, New York, New York, USA) was initiated in May 2014. Despite four doses of ipilimumab, imaging in August 2014 showed metastatic progression and brain magnetic resonance imaging (MRI) showed interval development of multiple punctate intracranial metastases. Ipilimumab was discontinued and she was restarted on single agent dabrafenib.
Within days of beginning dabrafenib therapy, the patient presented to our clinic in September 2014 with a sensation of flashes of light and floaters in the left greater than right eye. Examination revealed a hemorrhagic posterior vitreous detachment of the left eye but no cell or flare was noted. Two weeks later, she was noted to have 1 + cell by standardized criteria [6] in the left anterior chamber and few keratic precipitates on the corneal endothelium and she was started on topical prednisolone acetate four times daily to the left eye. At her subsequent follow-up appointment October 2014, slit-lamp exam demonstrated vitreous debris and trace cell in both eyes along with a single keratic precipitate in the right eye and so prednisolone acetate was added to the right eye four times daily. Given the bilateral nature of her uveitis, systemic evaluation for an underlying etiology was pursued and showed a negative RPR, FTA-ABS, HLA-B27, and QuantiFERON-Tb Gold. Her ACE was elevated to 105 but this was determined to be secondary to the effect of immunomodulatory therapy as opposed to underlying sarcoidosis. Throughout this period, our patient's vision was never worse than 20/40 in either eye and she was maintained on dabrafenib therapy at the approved dose of 150 mg by mouth twice daily. In November 2014, she had obtained complete resolution of symptoms, her visual acuity was back at its baseline, and she had no evidence of active inflammation in the eye. Her prednisolone acetate was tapered to twice daily in both eyes. Repeat MRI of the brain in November 2014 also showed resolution of the previously noted punctate intracranial metastases.
On 12/16/2014, our patient started therapy with pembrolizumab and dabrafenib was stopped at the beginning of January 2015. Within three days of starting pembrolizumab, she noted decreased vision in both eyes accompanied by floaters and flashes of light. Visual acuity at her subsequent appointment in January 2015 was noted to be 20/70 and 20/125 in the right and left eyes, respectively. She was further noted to have 1–2+ vitreous cell as well as small keratic precipitates in both eyes. Topical prednisolone acetate was increased to four times daily and two weeks later she had symptomatic and clinical improvement. She continued cancer therapy and was given her second dose of pembrolizumab on 1/28/2015. At a follow-up appointment two weeks later she was noted to have worsening vitreous cell. Prednisone 40 mg oral daily was added to treat her uveitis as well as for the concern of a developing grade two autoimmune colitis. Her topical and oral prednisone were successfully tapered and by May 2015 she was off all steroids, her exam was negative for signs of recurrent uveitis, and her vision had returned to 20/25 and 20/30 in the right and left eyes, respectively. Follow-up brain MRI showed no evidence of recurrence of intracranial metastases and her sub-pectoral adenopathy had resolved. Consequently, the decision was made to continue monitoring the patient without giving additional anti-tumorigenic agents.
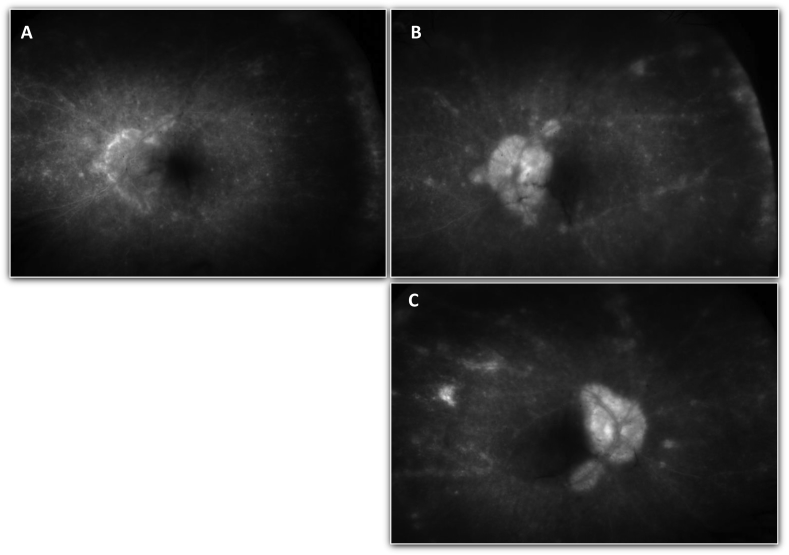
In November 2015, surveillance CT showed progression of disease and pembrolizumab monotherapy was re-initiated. In an effort to prevent recurrent uveitis our patient was also started on a prophylactic dose of topical prednisolone acetate four times daily to both eyes. Within days of starting therapy, though, she developed flashes of light and floaters in her vision and when evaluated in clinic was noted to have 20/80 and 20/250 vision in the right and left eyes, respectively. Exam demonstrated 1 + anterior chamber cell and 2 + vitreous cell in both eyes. Fluorescein angiography obtained at this appointment also showed retinal vasculitis in both eyes (Fig. 1). Given her presentation with panuveitis, treatment with prednisone 40 mg oral daily was once again initiated. At a follow-up appointment two weeks later, her vision had improved to 20/50 in both eyes, the anterior chamber reaction resolved, but she still had persistent vitreous inflammation and retinal vasculitis. A decision was made to continue with pembrolizumab therapy as long as her uveitis could be managed with corticosteroid therapy.
Fig. 1.
Fluorescein angiogram of both eyes, taken two weeks after re-starting pembrolizumab therapy, with serial images capturing contrast transit through the left eye. A) Early laminar venous phase of the left eye. B) Late phase angiogram of the left eye showing diffuse vasculitis and RPE disturbance. C) Late phase angiogram of the right eye showing diffuse vasculitis and RPE disturbance.
3. Discussion
Ocular complications of dabrafenib and pembrolizumab therapy are uncommon. Dry eye syndrome, endophthalmitis, uveitis, optic neuritis, and papillitis have all been previously reported with pembrolizumab [3], [4], [7]. In patients taking dabrafenib in combination with trametinib, chorioretinopathy and uveitis infrequently occur [5], [8], [9], [10]. To our knowledge, here we present the first case of a patient who developed uveitis as a response to initiating both dabrafenib and, later, pembrolizumab. The temporal relationship between chemotherapy initiation and uveitis onset strongly suggests medication induced uveitis as the cause of her findings. However, it should be noted both of these anti-tumorigenic medications have different mechanisms of action. Dabrafenib exerts its effect on cancer cells as an oral serine kinase inhibitor. In contrast, pembrolizumab, an immune checkpoint blocking antibody, increases the immune response to metaplasia. Furthermore, pembrolizumab is a humanized IgG4 antibody while dabrafenib is a methanesulfonate salt. Despite these differences, our patient unfortunately had the propensity of developing acute uveitis soon after initiating therapy with these agents on multiple occasions.
Other potential etiologies to our patient's presentation were also considered. She had no prior history of inflammatory disease and a normal screening eye exam prior to initiating chemotherapy. Serologic work-up failed to demonstrate an infectious etiology such as tuberculosis or syphilis. Furthermore, her presentation was not thought to represent drug-induced sarcoidosis as she didn't have accompanying systemic findings of sarcoidosis. A case of metastatic melanoma to the vitreous has also previously been reported in a patient taking pembrolizumab [11], but we did not think this likely in our patient as she presented with bilateral findings and her exam normalized with corticosteroids and cessation of cancer therapy. Another possibility is that the uveitis was secondary to an immune response to melanoma antigens released by the anti-tumorigenic therapy.
While unable to definitely prove the cause of our patient's uveitis, the temporal relationship of chemotherapy initiation and onset of eye symptoms on three different occasions strongly suggest an inflammatory effect of pembrolizumab and dabrafenib on the eyes. Ongoing safety surveillance of these medications will better elucidate the frequency of uveitis in pembrolizumab and dabrafenib and also clarify the potential link between pembrolizumab-associated and dabrafenib-associated uveitis.
References
- 1.Bhatia S., Tykodi S.S., Thompson J.A. Treatment of metastatic melanoma: an overview. Oncology. May 2009;23(6):488–496. [PMC free article] [PubMed] [Google Scholar]
- 2.Menzies A.M., Long G.V., Murali R. Dabrafenib and its potential for the treatment of metastatic melanoma. Drug Des. Dev. Ther. 2012;6:391–405. doi: 10.2147/DDDT.S38998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naidoo J., Page D.B., Li B.T. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. Dec 2015;26(12):2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topalian S.L., Hodi F.S., Brahmer J.R. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. Jun 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaherty K.T., Infante J.R., Daud A. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med. Nov 2012;367(18):1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jabs D.A., Nussenblatt R.B., Rosenbaum J.T. Standardization of uveitis nomenclature working group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. Sep 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abu Samra K., Valdes-Navarro M., Lee S., Swan R., Foster C.S., Anesi S.D. A case of bilateral uveitis and papillitis in a patient treated with pembrolizumab. Eur. J. Ophthalmol. Apr 2016;26(3):e46–e48. doi: 10.5301/ejo.5000724. [DOI] [PubMed] [Google Scholar]
- 8.Draganova D., Kerger J., Caspers L., Willermain F. Severe bilateral panuveitis during melanoma treatment by Dabrafenib and Trametinib. J. Ophthalmic. Inflamm. Infect. Jun 2015;5:17. doi: 10.1186/s12348-015-0049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huillard O., Bakalian S., Levy C. Ocular adverse events of molecularly targeted agents approved in solid tumours: a systematic review. Eur. J. Cancer. Feb 2014;50(3):638–648. doi: 10.1016/j.ejca.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 10.Joshi L., Karydis A., Gemenetzi M., Shao E.H., Taylor S.R. Uveitis as a result of MAP kinase pathway inhibition. Case Rep. Ophthalmol. Nov 2013;4(3):279–282. doi: 10.1159/000357060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manusow J.S., Khoja L., Pesin N., Joshua A.M., Mandelcorn E.D. Retinal vasculitis and ocular vitreous metastasis following complete response to PD-1 inhibition in a patient with metastatic cutaneous melanoma. J. Immunother. Cancer. Dec 2014;2(1):41. doi: 10.1186/s40425-014-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]