Abstract
Cocaine, a powerful vasoconstrictor, induces immune responses including cytokine elevations. Chronic cocaine use is associated with functional brain impairments potentially mediated by vascular pathology. Although the Crack-Cocaine epidemic has declined, its vascular consequences are increasingly becoming evident among individuals with cocaine use disorder of that period, now aging. Paradoxically, during the period when prevention efforts could make a difference, this population receives psychosocial treatment at best.
We review major postmortem and in vitro studies documenting cocaine-induced vascular toxicity. PubMed and Academic Search Complete were used with relevant terms.
Findings consist of the major mechanisms of cocaine-induced vasoconstriction, endothelial dysfunction, and accelerated atherosclerosis, emphasizing acute, chronic, and secondary effects of cocaine. The etiology underlying cocaine's acute and chronic vascular effects is multifactorial, spanning hypertension, impaired homeostasis and platelet function, thrombosis, thromboembolism, and alterations in blood flow. Early detection of vascular disease in cocaine addiction by multimodality imaging is discussed. Treatment may be similar to indications in patients with traditional risk-factors, with few exceptions such as enhanced supportive care and use of benzodiazepines and phentolamine for sedation, and avoiding β-blockers.
Given the vascular toxicity cocaine induces, further compounded by smoking and alcohol comorbidity, and interacting with aging of the crack generation, there is a public health imperative to identify pre-symptomatic markers of vascular impairments in cocaine addiction and employ preventive treatment to reduce silent disease progression.
Keywords: Cocaine, Vascular disease, Endothelial dysfunction, Atherosclerosis, Cardiotoxicity, Neurotoxicity
1. Phenomenology contributing to vascular damage
Cocaine, compared to other illicit drugs, poses a particular risk for vascular disease and is most involved in emergency room visits (40.3%), with highest rates for men aged 35–44 years, amounting to a vast social and economic burden [1]. Cocaine-induced damage to the cardiovascular and cerebrovascular systems is widely reported, and is linked with hypertension, tachycardia, ventricular arrhythmias [2],myocardial infarction [3,4], stroke [4,5], resulting in severe functional impairments or sudden mortality [6–10].
Vast efforts are geared toward psychosocial rehabilitation of cocaine use disorder (CUD). However, the accelerated development of vascular disease remains mostly undetected and asymptomatic presentation of vascular pathology in CUD results in silent disease progression.
“Crack-Cocaine” was introduced in the mid-1980s involving a new route of administration, smoking (as opposed to sniffing), which enhances vascular toxicity. Furthermore, the phenomenology of CUD consists of repeated drug use leading to tolerance, withdrawal, and compulsive drug-seeking behavior with inability to abstain, despite adverse effects to medical, social and occupational functioning. Underlying this addiction is CUD's association with abnormal brain morphology [11] and function involving inefficiencies in circuits that coordinate reward and self-control processes [12].
Despite advances in characterization of addiction, knowledge about the contribution of vascular aging to brain impairments in human CUD is scarce. We review the mechanisms underlying the vascular damage associated with cocaine use and possible treatment directions.
2. Search strategy
We present the main mechanisms of acute and chronic cocaine-induced toxicity on vessels, brain and heart (Fig. 1) and the common vascular and systemic effects of cocaine use in humans (Fig. 2). Particular attention was given to the imaging studies that measured cocaine-induced changes to the human heart, brain, and arteries (Table 1), since these methods are gaining a central role as markers of inflammatory disease. Review methodology included search in two electronic databases (PubMed and Academic Search Complete) using relevant search terms (cocaine, inflammation, cardiovascular, cerebrovascular, carotid artery, MRI, magnetic resonance spectroscopy, PET, CT, and ultrasound) from 1978 to 2017; results are presented in Table 1 and Fig. 2. Major findings in circulating pathology are noted as well.
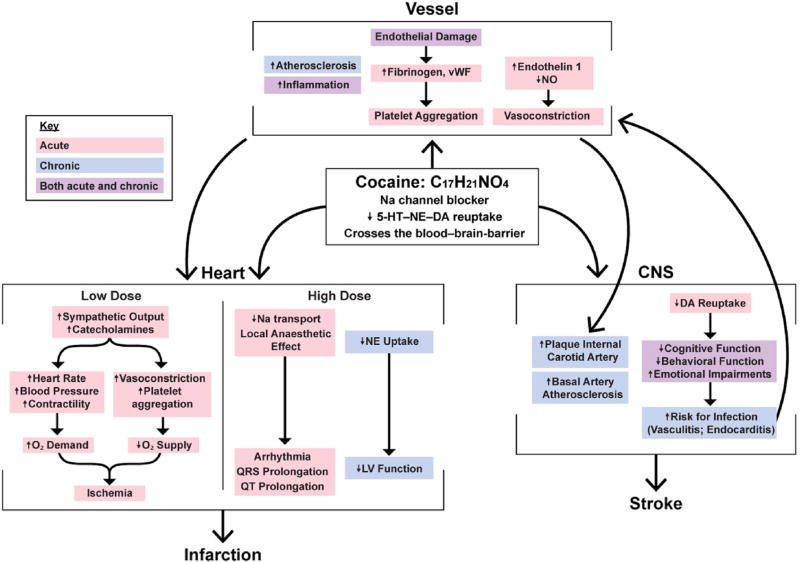
Fig. 1. Cocaine's acute and chronic toxicity mechanisms.
Cocaine's acute and chronic toxicity mechanisms on the vessel, heart, and the central nervous system (CNS), and their interactions. Carbon (C). Hydrogen (H). Nitrogen (N). Oxygen (O). Sodium (Na). Serotonin (5-HT). Norepinephrine (NE). Dopamine (DA). Nitric oxide (NO). Von Willebrand factor (vWF). Dioxygen (O2). Left ventricular (LV). Heart mechanisms adapted from Ref. [13]; figure based on following references [2,5,6,10,13,15–19,23–44].
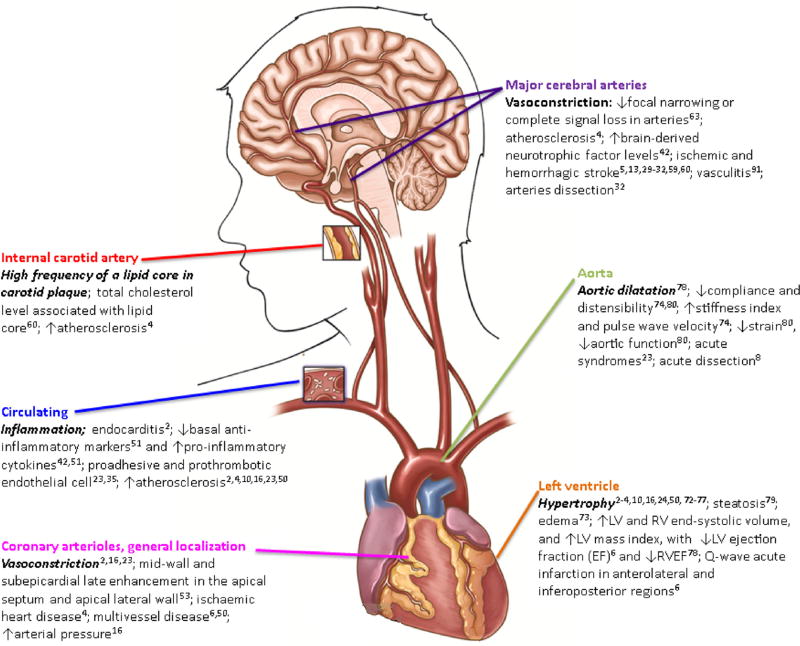
Fig. 2. Cocaine-induced major pathophysiological load to the cardiovasculature, cerebrovasculature, and arteries.
The main clinical pathology associated with cocaine (and its citations) is listed according to localization. Left ventricular (LV). Right ventricular (RV).
Table 1.
Cardiovascular, cerebrovascular and arterial pathology in cocaine users as imaged by magnetic resonance imaging and positron emission tomography.a
| Localization | Methodology | Sample | Selected findings |
|---|---|---|---|
| Internal carotid artery [60] | 1.5 Tesla (T) scanner, 3-dimensional time-of- flight angiogram, black blood images pre-post injection with intravenous gadodiamide | 37 asymptomatic African American with or without HIV and CUD (mean age 38.7 ± 4.9) | A high frequency (24.3%, 95% CI = 0.12–0.41) of a lipid core in carotid plaque. Only total cholesterol level associated with presence of lipid core. |
| Left ventricular (from the mitral plane valve to LV apex) [73] | 1.5T scanner, a T2-weighted STIR and LGE sequences | 30 asymptomatic CUD 48 h after drug cessation (mean age 39 ± 7) | Structural damage in 83% of CUD: delayed enhancement (73%) and focal edema of the LV (47%). 37% showed both edema and fibrosis; 32% had ischemic patterns of fibrosis and 68% had non-ischemic patterns of fibrosis. Edema correlated with cocaine dose-related effect in 86% of CUD. |
| Left ventricular [3] | T2-STIR and LGE sequences | 11 asymptomatic CUD and 11 matched healthy controls, without cardiovascular disease (age not provided) | Discrete LGE with non-ischemic pattern in 27% of CUD but not in controls. Global extracellular volume fraction significantly larger in CUD vs. controls (31 ± 6% vs. 26 ± 2%, p < 0.05). No significant difference found between groups in LV EF or edema. |
| Left ventricular [79] | MRI/Proton magnetic resonance spectroscopy (1H-MRS) 3T scanner and a retrospectively gated TrueFisp sequence | 44 females (32 CUD, mean age 45.7 ± 6.7; 12 non-users. Mean age 42.8 ± 9.5) | Cardiac steatosis in CUD (65.7%) vs. controls (16.7%), a 14-fold risk of cardiac steatosis, controlling for age, gender, glucose, triglycerides, and systolic blood pressure. |
| Left ventricular (anterior descending coronary artery) [71] | 1.5T scanner. Cine images with a steady-state free precession pulse sequence. Delayed-enhancement images acquired after a bolus injection of 0.2 mmol kg−1, with an inversion recovery fast gradient-echo pulse sequence. | 24 CUD (22 male; mean age 29.7) with history of cocaine associated chest pain, without emergency department admission | Regional ventricular function of 17 segments was normal in all patients. Only one participant (4%) had calcified plaques at the anterior descending coronary artery (proximal and medium segments). |
| Left and right ventricles. Aorta (the oblique sagittal plane and thoracic aorta) [78] | 3T scanner, high resolution Balanced Steady-State Free Precession cine, and LGE sequences | 94 asymptomatic CUD (81 male; mean age 36.6 ± 7; 13.9 ± 9 years of cocaine use) and 80 age-matched healthy controls | CUD had increased LV and RV end-systolic volume, and LV mass index, with decreased LVEF and RVEF vs. healthy controls; 30% of CUD had LGE; CVD was detected in 71% of CUD and mean duration of cocaine use was related to probability of LV systolic dysfunction and aortic dilatation. |
| Aorta (transverse plane; right pulmonary artery in proximal descending aorta). Left and right ventricles [74] | CMR using a 1.5T scanner, a Balanced Steady-State Free Precession cine, a prospectively gated cine, and LGE sequences and high resolution black blood images | 20 regular cocaine users (17 male; mean age 37 ± 7), at least monthly during the last year, and 20 healthy control subjects (19 male; mean age 33 ± 7), without CVD | CUD had increased arterial stiffness - reduced aortic compliance, decreased distensibility, increased stiffness index, and higher pulse wave velocity, independent of vessel wall thickness. LV mass was 18% higher in CUD, and had larger left atrial diameter than healthy controls, as associated with duration and frequency of cocaine use. |
| Aorta (ascending and descending) [80] | MRI using an automated contours detection method applied to images of a phase-contrast acquisition perpendicular to the ascending aorta. Blood pressure was measured by a brachial cuff during MRI | 33 long term CUD (20 female; mean age 46 ± 7, and 13 healthy controls (7 female; mean age 43 ± 9) | Aortic strain and distensibility were lower in CUD vs. controls, with relationship to duration of cocaine use as an independent predictor of descending aortic dysfunction with a significant average decrease in strain of 2.5% and a trend for a decrease in aortic distensibility for 1 year of cocaine use. |
| Major cerebral arteries [63] | 1.5T scanner, T1-weighted sagittal localizer images, the 3-dimensional sequence, with magnetization transfer, flow compensation and saturation imaging options, pre-post double-blind intravenous administration of cocaine or placebo | 24 healthy and neurologically normal men (mean age 29 ± 5) with median cocaine use of 8 lifetime exposures (range 3 to >40) | Vasoconstriction in 62.5% and 33.3% subjects receiving 0.4- and 0.2-mg/kg cocaine, respectively, compared with 14.2% subjects administered placebo. Alterations from reduced arterial caliber to focal narrowing or complete signal loss detected in posterior, middle arteries, vertebral arteries, and the anterior and posterior communicating arteries. |
| Cortical and subcortical regions [105] | 3T MRI scan for determination of regions of interest, using an automated segmentation tool; PET using the ECAT EXACT HR camera after an intravenous bolus injection of [11C]PBR28 | 15 recently abstinent crack-cocaine abusers (8 female; mean age 39.9 ± 9; 17 ± 7 yrs of use) and 17 healthy controls (9 female; mean age 38.4 ± 8.1) matched on ethnicity, age, smoking | No significant differences in [11C]PBR28 VT were observed in the cortical and subcortical regions in cocaine abusers compared with healthy controls. The results of this in vivo study do not support increased 18 kDa translocator protein expression and, by extension, microglial activation in chronic cocaine-abusing humans. |
Excluding case reports. Ejection fraction (EF). Late gadolinium enhancement (LGE). Left and right ventricles (LV, RV).
3. Pharmacodynamics of cocaine
Cocaine's main vasoactive metabolite benzoylmethylecgonine, a tropane alkaloid, is a sodium channel blocker, which produces enhanced sympathetic activity at low doses [13,14] (Fig. 1, center box). At high doses, cocaine is markedly more dangerous than other central nervous system stimulants, including amphetamines [15], and can cause sudden cardiac death through its effect on sodium channels and local anesthetic actions [13,14,16]. Cocaine crosses the blood–brain-barrier perhaps better than other psychoactive chemicals and may even induce its breakdown [17,18]. In addition, cocaine blocks reuptake of catecholamines in the presynaptic neurons in the central and peripheral nervous systems, resulting in increased catecholamines, sympathetic output and stimulation [2,19]. There is also evidence that the cardiovascular actions of cocaine are mediated in part by dopamine [20], via central and peripheral mechanisms [21]. Stimulation of dopamine cells in the ventral tagmental area increases blood pressure and this effect is antagonized by the dopamine D2 receptor blockers [22].
4. Acute effects of cocaine
Cocaine's acute hematological effects on the vessel (Fig. 1, upper box) [10,23,24] center on the loss of the endothelium's protective functions, a common denominator in the pathogenesis of ischemic vascular disease [35,36]. Cocaine releases endothelin-145, which is found to be elevated in CUD and declines with detoxification [36,46,47]. When vessels are stressed, endothelin-1 (a vasoconstrictor protein produced by vascular endothelial cells) is elevated and nitric oxide (a blood vessel dilator) decreases, leading to vasoconstriction [35,36]. It was recently demonstrated that cocaine elicits autophagy involving nitric oxide and glyceraldehyde-3-phosphate dehydrogenase signaling cascade [48]. Additional mechanisms implicated in cocaine induced vasoconstriction include increases in calcium [49]. In cases of acute damage, when stress leads to rupture in vessels, endothelial damage promotes the increase of fibrinogen (glycoprotein, which helps in formation of blood clots) and Von Willebrand factor (glycoprotein signaling endothelium changes), leading to platelet aggregation and ultimately the formation of blood clots [38]. This cascade reduces blood flow following cocaine use and can lead to acute organ damage.
Additionally, inflammation and atherosclerosis are substantial potentially lethal vascular effects of cocaine use that have acute and chronic systemic impact [2,4,10,13,16,23,35,37,38,42,50]. Cocaine creates an elevated immune system inflammatory state with decreased basal anti-inflammatory markers (e.g., interleukin-10) [42,51], and increased pro-inflammatory cytokines (e.g., tumor necrosis factor alpha, Interleukin 1β) [42,51], all contributing to vascular disease (e.g., endocarditis).
Cocaine induces acute cardiotoxicity through multiple pathways (Fig. 1, left box). While smoking crack or sniffing cocaine, there is a vast accumulation of the drug in the heart affecting myocardial tissue directly [26]. Sympathomimetic effects generate a rise of heart rate, blood pressure and myocardial contractility, which enhance myocardial oxygen demand, whereas myocardial oxygen supply is decreased by coronary vasoconstriction and enhanced thrombosis. These mechanisms underlie inadequate myocardial oxygen equilibrium, which may lead to ischemia and manifest as angina or infarction [2,13]. Ischemia is suggested as the main mechanism of acute damage responsible for various clinical presentations [25]. Other known mechanisms of cardiotoxicity, include cocaine's blockage of sodium channels and a subsequent increase in calcium flux and a vasoconstrictor response [28]. For example, cocaine-induced blockade of cardiac sodium channel Nav1.5 affects myocardial electrical impulses critical for action potential generation and propagation in the heart (by increasing the sympathomimetic stimulation frequency, cocaine produces a progressive reduction in Nav1.5 current amplitude for successive pulses within a stimulation train by a mechanism commonly referred to as use-dependent inhibition) [52]. In addition to ischemic heart disease [4], other complications include multiple foci of mid-wall and subepicardial late enhancement in the apical septum and apical lateral wall [53] and coronary vasoconstriction [2,16,23]. Acute aortic syndromes [23] and acute aortic dissection [2,8] are other frequent findings associated with cocaine use (Fig. 2).
The full etiology underlying cocaine's acute neurotoxicity is multifactorial (Fig. 1, right box), with similar mechanisms that also underlie its cardiotoxicity effects, spanning hypertension, impaired homeostasis and platelet function (aggregation), thrombosis, thromboembolism, decreased cerebral blood flow (CBF), and focal perfusion deficits [29–32,54,55]. Cerebral vasospasm is pharmacologically induced via cocaine's potent sympathomimetic properties [32] and an increase of endothelin-132,34. In addition, cocaine induces disruption of cerebral autoregulation of blood flow (maintaining relatively constant blood flow despite changes in perfusion pressure) and global reduction in cerebral glucose metabolism [31]. Abnormalities in the expression of transcription factors in cells and changes of brain neurotransmitter systems have been reported [31]. For example, vasoconstriction, a main underlying cause of ischemic strokes, may result from the increased availability of epinephrine, norepinephrine, and serotonin (especially in large and medium-sized brain vessels) in the vasculature due to cocaine blockade of their reuptake [55–57]. Furthermore, vasoconstriction at presynaptic nerve terminals increases the release of calcium from the sarcoplasmic reticulum in cerebral vascular smooth muscle cells [32,33]. Sudden increases in arterial pressure can induce aneurysms (a localized widening of an artery or vein, resulting from weakening of vessel wall), arteriovenous malformations (abnormal connection between arteries and veins, bypassing the capillary system) and hemorrhagic strokes [55]. Massive strokes can also be caused by arterial dissection and hemispheric infarcts [32]. Carotid artery dissection might also be caused by cocaine mediated apoptosis of vascular cells leading to ischemic stroke, although the mechanism is not fully understood [58].
The major cerebrovascular effects of cocaine consist of ischemic and hemorrhagic (including subarachnoid and intracerebral hemorrhages) strokes [5,13,29–32,59–61] (Fig. 2). In particular, crack cocaine seems to be associated with both ischemic strokes and hemorrhage strokes, whereas cocaine hydrochloride causes mainly intracerebral and subarachnoidal bleeding [61,62]. Individuals with underlying arteriovenous malformation or aneurysm are at greater risk for such events [31]. Additional findings point to reduced arterial caliber, focal narrowing in the anterior (and middle cerebral arteries) and posterior cerebral circulation as well as communicating arteries [63].
Volkow et al. [64] were the first to document that CUD had profound decreases in CBF as evidenced by decreased brain uptake of water. Cocaine doses within the range self-administered by drug abusers can markedly decrease CBF (approximately by 70%) within 2–3 min after administration, lasting in some arteriolar branches for over 45 min [5]. Thus, cocaine induces microischemia in various types of vessels and arteriolar branches that is exacerbated with repeated use and is likely to be a contributor to its neurotoxic effects [5]. Furthermore, chronic cocaine-use reduces capillary flows in brain and may be responsible for cerebrovascular small-vessel ischemic disease (e.g. cocaine-induced leukoaraiosis), possibly involving genetic factors [65,66].
Cocaine-induced acute neurotoxic mechanisms are further revealed by uncoupling between vascular effects [i.e., changes in: CBF, oxygenated (HbO2) and deoxygenated hemoglobin (HbR), and cerebral blood volume] from the cellular effects (i.e., changes in intracellular calcium [Ca2+], an indicator of neuronal activation). Such an examination in a rat cortical brain identified a 2.9 ± 0.5 min lag time between the peak neuronal and vascular responses to cocaine [67]. Specifically, a multimodality imaging study revealed that cocaine caused an immediate decrease in local oxygen content and CBF (t < 4 min) followed by a longer lasting overshoot (7.1 ± 0.2 min) in these measures (up to 40 min) while Ca2+ increases were immediate (peaked at 4.1 ± 0.4 min) and remained elevated over 20 min [67].
Cocaine's immediate increases in neuronal activity and abrupt decrease in CBF and HbO2 could underlie cerebrovascular complications associated with cocaine use, such as ischemic stroke [67,68]. Furthermore, Ca2+ increases could also underlie the reported enhanced hemodynamic and field potential responses to sensory stimulation after acute cocaine administration [67,69].
5. Chronic effects of cocaine
Cocaine's chronic effects on the vessel (Fig. 1, upper box) [10,23,24] consist of repeated endothelial damage leading to premature and severe atherosclerosis in various organs [10,19].
In the heart, the significant interaction of cocaine with norepinephrine transporters [26,27] can lead to left ventricular dysfunction by effect of dilation, reduction of ejection phase and reduced contractility [70]. Despite some controversy [3,53,71], left ventricular hypertrophy (as shown in Fig. 2) is among the most prevalent chronic morphological findings associated with cocaine toxicity in patients with cocaine-associated chest pain [2–4,10,16,24,50,72–77]. Structural and functional damage has been reported in both ventricles including decreased ejection fraction and increased end-systole [6,78] and steatosis [79] (abnormal retention of lipids within cells) that is associated with impaired left ventricle function [6]. These effects, as well as others (e.g., myocardial edema), may show a cocaine dose-related response [73]. Other reports document aortic damage including dilatation [78], reduced strain, compliance and distensibility [74,80], and increased stiffness index and pulse wave velocity [74]. Furthermore, asymptomatic or subclinical cardiovascular disease is shown in coronary calcification, plaque and stenosis and found to be independently associated with cocaine use [81]. In a longitudinal study, after 6 months of cocaine, use >50% coronary stenosis developed [46].
Cocaine-induced chronic neurotoxicity consists of monoamine re-uptake inhibition, anti-cholinergic activity, and alpha-adrenergic stimulation [19]. Advanced atherosclerosis of intracranial vessels [32] is noted as the cause of cocaine-induced stroke in numerous studies [4,29].
Atherosclerosis of the carotid arteries is of particular relevance to CUD because these arteries supply blood to the brain regions that are implicated in the cognitive impairments documented in CUD [39–41]. Studies in healthy populations reveal association between cognitive deficiencies and atherosclerosis, indicating that there is an inflammatory pathway that reduces the brain's executive control network efficiency [82–84]. For example, carotid arteries thickness has been associated with diminished attention-executive–psychomotor network functioning in elderly humans [85] and levels of atherosclerosis correlate with functioning of cognitive control networks in healthy individuals [82–84]. Thus, atherosclerosis may impact cognitive and behavioral functioning even before arterial narrowing results in a stroke. Literature that characterizes atherosclerosis in the carotid arteries in asymptomatic cocaine users is scarce. Additional in vivo examinations are clearly required to solidify knowledge concerning early vascular disease detection in CUD, especially, the assessment of carotid plaque composition for determining risk profiles and predicting future clinical events in CUD.
Additional reports on chronic cocaine use impact, document orbitofrontal cortex inflammation associated with intranasal cocaine use resulting in mutilations such as: panhypopituitarism [86] (inadequate or absent production of the anterior pituitary hormones); optic nerve dysfunction, and diffuse white-matter lesions in downstream regions including the basal ganglia [87]; proptosis (forward displacement of the eye) and double vision generated by osteolytic destruction (dissolution of bone) of the sinuorbital barriers [88]; sinonasal inflammatory condition involving severe destructive lesions of the nasal cavities and facial structures [89]; and extensive bony destruction of the orbital walls with associated orbital cellulitis, and nasolacrimal duct obstruction [90].
6. Secondary effects of cocaine
In addition to cocaine-specific effects, there are secondary harms resulting from synergetic effects between multiple environmental, psychosocial and behavioral factors comprising the addiction phenomenology that could in turn enhance potential vascular damage. These factors include the life-course and complexity of CUD, comprised of years (often, decades) of concomitant alcohol and/or tobacco and/or other drug use, potentiating vascular toxicity. The issue is complicated further by the fact that contaminants such as procainamide, quinidine and antihistamines, which are often mixed with the cocaine, may contribute to the effects seen and influence the underlying pathophysiology [30]. Furthermore, compromised decision making underlying having unprotected sex or using drugs intravenously can increase exposure to infections that activate the immune and inflammatory systems [42], potentially of additive impact in accelerating aging of the vasculature [91]. The prevailing low socio-economic status, limited awareness of health issues, lack of sleep, and poor nutrition, could further hasten vascular disease [43,44]. Additionally, genetic factors leading to variability in reaction to cocaine can enhance hemodynamic responsiveness, incidence of coronary vasoconstriction, and vascular damage [16].
7. Prevention and treatment
Cocaine use promotes vascular disease, while also influencing the course of disease management, and therapy. Here too, it is helpful to briefly review prevention and treatment recommendations separately for acute vascular events.
Preventing acute events in pre-symptomatic individuals must include special consideration. First, in terms of risk detection, studies with CUD document that Framingham risk scores label the majority of CUD as low risk, underestimating the indications for preventive action. The use of beta-blockers show mixed results. Notably, traditional cardiac biomarkers, such as myeloperoxidase and c-reactive protein are not useful as biomarkers for CUD [92], since imaging evidence reveal that the relationships between myocardial fat and body mass index in CUD is different than non-drug users [93].
Medication to reduce inflammation (e.g., recombinant IL-10, soluble receptor medication such as Etanercept) may be helpful to control cocaine induced inflammatory cascade [51]. As nitric oxide/glyceraldehyde-3-phosphate dehydrogenase pathway mediates cocaine induced autophagy, glyceraldehyde-3-phosphate dehydrogenase can be tested for use (see clinical trials in Parkinson's disease [48,94]). Finally, and perhaps most importantly, cocaine abstinence or even reduced use promotes reduction in endothelial-1 damage [45,46]. Thus, prevention of secondary harms and halting of further disease progression in CUD mandates cessation of cocaine use and cigarette smoking, limitation of alcohol consumption, as well as enhancing healthy life routines (e.g., regular health monitoring, physical activity, sleep, diet, stress management).
Treatment for cocaine-induced acute vascular events may be similar to indications in patients with traditional risk-factors, with few exceptions. For example, enhanced supportive care and use of benzodiazepines and phentolamine for sedation; and avoiding β-blockers, which can lead to severe hyper-tension and coronary vasoconstriction resulting from the interaction of β-blockers with cocaine (for review see Ref. [13]). Notably, there is no specific pharmacological antidote for cocaine overdose, yet the administration of benzodiazepines can help alleviate some of the stress that is placed on the heart and may greatly reduce the risk of heart attack, stroke or serious heart damage arising from the overdose.
The first line of treatment for cocaine induced sodium channel blockade is alkalization with hypertonic sodium bicarbonate. Class IB antiarrhythmic agents are indicated and class IA should be avoided. Direct acting vasodialators as nitroglycerin can be helpful though these are rarely used. In patients with acute manifestation of cerebrovascular events it is essential to perform a toxicological drug screening also in presence of normal blood pressure and with spontaneous subcortical hemorrhagic stroke and negative anamnesis for drug abuse at admission [95].
Chronic treatments for CUD with cardiovascular problems include antiplatelet and antithrombin agents, statins and diuretics.
8. Future directions: urgent need for early detection of a complex disease process in a vulnerable and aging population
As evident from this review, there is ample data on cocaine-induced endothelial dysfunction, vasoconstriction, and accelerated atherosclerosis. Given the known vascular toxicity cocaine induces [13,23], further compounded by cigarette smoking and alcohol comorbidity [32,73,96] and interacting with the progressing age of the crack generation [97,98], there is a public health imperative to identify presymptomatic markers of vascular impairments in CUD.
Chest pain [8,13,76] and cerebrovascular events [5,31,63] may occur within minutes to just a few hours from cocaine use. Atherosclerosis, however, develops during prolonged periods of chronic cocaine use and in its early stages usually does not create symptoms or signs. Indeed, silent disease progression is particularly pronounced in CUD who remain asymptomatic until they reach the emergency room with acute events [8,24,73]. Therefore, the ability to identify plaques before luminal stenosis develops is fundamental for early disease detection [99].
Multimodality imaging studies could promote the identification of CUD with silent pre-symptomatic atherosclerosis in the brain, heart and arteries [100–104]. Advances in imaging help to detect morphology of blood vessels and the composition of the vessel walls, facilitating observation of atherosclerosis-associated abnormalities in the arteries [99]. For example, 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) and magnetic resonance imaging (MRI), PET/MR, allow simultaneous investigation and tracking of brain, cardiac and the carotid arteries function and structure in the same individuals. The PET with 18F-FDG can quantify vessel-wall inflammation in atherosclerotic plaques [101,102] and three dimensional black-blood dynamic contrast-enhanced MRI [103] can characterize carotid wall morphology (plaque microvessels, composition and burden).
9. Conclusion
With the proliferation of coronary artery and vascular disease among cocaine users, more procedures are required for early detection and prevention of cardiovascular and cerebrovascular associated morbidity and mortality in this population. Prevention of cocaine-induced systemic complications could be considered as part of a harm reduction strategy. Consequently, cocaine use should be included in protocols and guidelines as a risk factor for cardiovascular, cerebrovascular and other vascular and arterial disease. Furthermore, guidelines of pharmacological management of addictions should consider preventive treatment for vascular damage in cocaine users, and hopefully this will reduce severe impairment and sudden premature mortality in this population.
Acknowledgments
We thank Nora D. Volkow, Salvador Sierra, and Jill Gregory, for their input on this manuscript.
Financial support
NIDA T32-DA007135-31, Department of Preventive Medicine support, Icahn School of Medicine at Mount Sinai (KB); NIDA R21DA034954 (RZG); NIH/NHLBI R01 HL071021 (ZAF).
Abbreviations
- CUD
cocaine use disorder
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- CT
computed tomography
- CBF
cerebral blood flow
- 18F-FDG
18F-fluorodeoxyglucose
Footnotes
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
Author contributions
Keren Bachi: literature search, literature analysis and interpretation, writing first and consecutive drafts, and flow chart and figure design.
Venkatesh Mani: vascular imaging commentary.
Devi Jeyachandran: medical terminology and content commentary.
Zahi A Fayad: vascular imaging, and treatment commentary.
Rita Z Goldstein: cocaine use disorder commentary.
Nelly Alia-Klein: study design, figure design, interpretation, and writing.
References
- 1.Substance Abuse and Mental Health Services Administration. NSDUH Series H-48, HHS Publication No. (SMA) 14–4863. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2014. Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. [Google Scholar]
- 2.Afonso L, Mohammad T, Thatai D. Crack whips the heart: a review of the cardiovascular toxicity of cocaine. Am. J. Cardiol. 2007;100:1040–1043. doi: 10.1016/j.amjcard.2007.04.049. http://dx.doi.org/10.1016/j.amjcard.2007.04.049. [DOI] [PubMed] [Google Scholar]
- 3.Radunski UK, et al. Increased extracellular volume in asymptomatic cocaine abusers detected by cardiovascular magnetic resonance imaging. J. Cardiovasc. Magn. Reson. (BioMed Central) 2013;15:1–2. http://dx.doi.org/10.1186/1532-429X-15-S1-E101. [Google Scholar]
- 4.Darke S, Kaye S, Duflou J. Comparative cardiac pathology among deaths due to cocaine toxicity, opioid toxicity and non-drug-related causes. Addict. (Abingd. Engl.) 2006;101:1771–1777. doi: 10.1111/j.1360-0443.2006.01601.x. http://dx.doi.org/10.1111/j.1360-0443.2006.01601.x. [DOI] [PubMed] [Google Scholar]
- 5.Ren H, et al. Cocaine-induced cortical microischemia in the rodent brain: clinical implications. Mol. Psychiatry. 2012;17:1017–1025. doi: 10.1038/mp.2011.160. http://dx.doi.org/10.1038/mp.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrillo X, et al. Acute coronary syndrome and cocaine use: 8-year prevalence and inhospital outcomes. Eur. Heart J. 2011;32:1244–1250. doi: 10.1093/eurheartj/ehq504. http://dx.doi.org/10.1093/eurheartj/ehq504. [DOI] [PubMed] [Google Scholar]
- 7.McCord J, et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation. 2008;117:1897–1907. doi: 10.1161/CIRCULATIONAHA.107.188950. http://dx.doi.org/10.1161/circulationaha.107.188950. [DOI] [PubMed] [Google Scholar]
- 8.Hsue PY, Salinas CL, Bolger AF, Benowitz NL, Waters DD. Acute aortic dissection related to crack cocaine. Circulation. 2002;105:1592–1595. doi: 10.1161/01.cir.0000012524.44897.3a. [DOI] [PubMed] [Google Scholar]
- 9.Magnano AR, et al. Effect of acute cocaine administration on the QTc interval of habitual users. Am. J. Cardiol. 2006;97:1244–1246. doi: 10.1016/j.amjcard.2005.11.046. http://dx.doi.org/10.1016/j.amjcard.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Dressler FA, Malekzadeh S, Roberts WC. Quantitative analysis of amounts of coronary arterial narrowing in cocaine addicts. Am. J. Cardiol. 1990;65:303–308. doi: 10.1016/0002-9149(90)90292-9. [DOI] [PubMed] [Google Scholar]
- 11.Ersche KD, Jones PS, Williams GB, Robbins TW, Bullmore ET. Cocaine dependence: a fast-track for brain ageing? Mol. Psychiatry. 2013;18:134–135. doi: 10.1038/mp.2012.31. http://www.nature.com/mp/journal/v18/n2/suppinfo/mp201231s1.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. http://dx.doi.org/10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122:2558–2569. doi: 10.1161/CIRCULATIONAHA.110.940569. http://dx.doi.org/10.1161/circulationaha.110.940569. [DOI] [PubMed] [Google Scholar]
- 14.Egashira K, Morgan KG, Morgan JP. Effects of cocaine on excitation-contraction coupling of aortic smooth muscle from the ferret. J. Clin. Investig. 1991;87:1322–1328. doi: 10.1172/JCI115135. http://dx.doi.org/10.1172/jci115135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nutt D, King LA, Saulsbury W, Blakemore C. Development of a rational scale to assess the harm of drugs of potential misuse. Lancet. 2007;369:1047–1053. doi: 10.1016/S0140-6736(07)60464-4. http://dx.doi.org/10.1016/s0140-6736(07)60464-4. [DOI] [PubMed] [Google Scholar]
- 16.Knuepfer MM. Cardiovascular disorders associated with cocaine use: myths and truths. Pharmacol. Ther. 2003;97:181–222. doi: 10.1016/s0163-7258(02)00329-7. http://dx.doi.org/10.1016/S0163-7258(02)00329-7. [DOI] [PubMed] [Google Scholar]
- 17.Sharma HS, Muresanu D, Sharma A, Patnaik R. Cocaine-induced breakdown of the blood-brain barrier and neurotoxicity. Int. Rev. Neurobiol. 2009;88:297–334. doi: 10.1016/S0074-7742(09)88011-2. http://dx.doi.org/10.1016/s0074-7742(09)88011-2. [DOI] [PubMed] [Google Scholar]
- 18.Dietrich JB. Alteration of blood-brain barrier function by methamphetamine and cocaine. Cell Tissue Res. 2009;336:385–392. doi: 10.1007/s00441-009-0777-y. http://dx.doi.org/10.1007/s00441-009-0777-y. [DOI] [PubMed] [Google Scholar]
- 19.Bansal S, Morgan JP. Vascular toxicity of cocaine. Vasc. Dis. Prev. 2009;6:30–35. [Google Scholar]
- 20.Tella SR, Goldberg SR. Monoamine transporter and sodium channel mechanisms in the rapid pressor response to cocaine. Pharmacol. Biochem. Behav. 1998;59:305–312. doi: 10.1016/s0091-3057(97)00448-6. [DOI] [PubMed] [Google Scholar]
- 21.van den Buuse M. Role of the mesolimbic dopamine system in cardiovascular homeostasis. Stimulation of the ventral tegmental area modulates the effect of vasopressin on blood pressure in conscious rats. Clin. Exp. Pharmacol. Physiol. 1998;25:661–668. doi: 10.1111/j.1440-1681.1998.tb02273.x. [DOI] [PubMed] [Google Scholar]
- 22.Mannelli M, et al. Dopamine and sympathoadrenal activity in man. Clin. Exp. Hypertens. (New York, NY.:1993) 1997;19:163–179. doi: 10.3109/10641969709080813. [DOI] [PubMed] [Google Scholar]
- 23.Finkel JB, Marhefka GD. Rethinking cocaine-associated chest pain and acute coronary syndromes. Mayo Clin. Proc. 2011;86:1198–1207. doi: 10.4065/mcp.2011.0338. http://dx.doi.org/10.4065/mcp.2011.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diercks DB, et al. Illicit stimulant use in a United States heart failure population presenting to the emergency department (from the Acute Decompensated Heart Failure National Registry Emergency Module) Am. J. Cardiol. 2008;102:1216–1219. doi: 10.1016/j.amjcard.2008.06.045. http://dx.doi.org/10.1016/j.amjcard.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 25.De Giorgi A, et al. Cocaine and acute vascular diseases. Curr. Drug Abuse Rev. 2012;5:129–134. doi: 10.2174/1874473711205020129. [DOI] [PubMed] [Google Scholar]
- 26.Volkow ND, Fowler JS, Ding Y-S. Cardiotoxic Properties of Cocaine: Studies with Positron Emission Tomography. The National Institute on Drug Abuse. 1996:159–174. [PubMed] [Google Scholar]
- 27.Fowler JS, et al. PET studies of cocaine inhibition of myocardial norepinephrine uptake. Synapse. 1994;16:312–317. doi: 10.1002/syn.890160407. http://dx.doi.org/10.1002/syn.890160407. [DOI] [PubMed] [Google Scholar]
- 28.Kloner RA, Hale S, Alker K, Rezkalla S. The effects of acute and chronic cocaine use on the heart. Circulation. 1992;85:407–419. doi: 10.1161/01.cir.85.2.407. [DOI] [PubMed] [Google Scholar]
- 29.Sordo L, et al. Cocaine use and risk of stroke: a systematic review. Drug Alcohol Depend. 2014;142:1–13. doi: 10.1016/j.drugalcdep.2014.06.041. http://dx.doi.org/10.1016/j.drugalcdep.2014.06.041. [DOI] [PubMed] [Google Scholar]
- 30.Treadwell SD, Robinson TG. Cocaine use and stroke. Postgrad. Med. J. 2007;83:389–394. doi: 10.1136/pgmj.2006.055970. http://dx.doi.org/10.1136/pgmj.2006.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Büttner A, Mall G, Penning R, Sachs H, Weis S. The neuropathology of cocaine abuse. Leg. Med. 2003;5:S240. doi: 10.1016/s1344-6223(02)00122-0. [DOI] [PubMed] [Google Scholar]
- 32.Farooq MU, Bhatt A, Patel M. Neurotoxic and cardiotoxic effects of cocaine and ethanol. J. Med. Toxicol. Off. J. Am. Coll. Med. Toxicol. 2009;5:134–138. doi: 10.1007/BF03161224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du C, et al. Cocaine increases the intracellular calcium concentration in brain independently of its cerebrovascular effects. J. Neurosci. Off. J. Soc. Neurosci. 2006;26:11522–11531. doi: 10.1523/JNEUROSCI.3612-06.2006. http://dx.doi.org/10.1523/jneurosci.3612-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fandino J, Sherman JD, Zuccarello M, Rapoport RM. Cocaine-induced endothelin-1-dependent spasm in rabbit basilar artery in vivo. J. Cardiovasc. Pharmacol. 2003;41:158–161. doi: 10.1097/00005344-200302000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Sáez CG, et al. Atorvastatin reduces the proadhesive and prothrombotic endothelial cell phenotype induced by cocaine and plasma from cocaine consumers in vitro. Arterioscler. Thromb. Vasc. Biol. 2014 doi: 10.1161/ATVBAHA.114.304535. http://dx.doi.org/10.1161/atvbaha.114.304535. [DOI] [PubMed]
- 36.Sáez CG, et al. Increased number of circulating endothelial cells and plasma markers of endothelial damage in chronic cocaine users. Thromb. Res. 2011;128:e18–23. doi: 10.1016/j.thromres.2011.04.019. http://dx.doi.org/10.1016/j.thromres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 37.Kolodgie FD, Virmani R, Cornhill JF, Herderick EE, Smialek J. Increase in atherosclerosis and adventitial mast cells in cocaine abusers: an alternative mechanism of cocaine-associated coronary vasospasm and thrombosis. J. Am. Coll. Cardiol. 1991;17:1553–1560. doi: 10.1016/0735-1097(91)90646-q. [DOI] [PubMed] [Google Scholar]
- 38.Siegel AJ, Sholar MB, Mendelson JH, et al. Cocaine-induced erythrocytosis and increase in von willebrand factor: evidence for drug-related blood doping and prothrombotic effects. Arch. Intern. Med. 1999;159:1925–1929. doi: 10.1001/archinte.159.16.1925. http://dx.doi.org/10.1001/archinte.159.16.1925. [DOI] [PubMed] [Google Scholar]
- 39.Alia-Klein N, Parvaz MA, Woicik PA, et al. Gene × disease interaction on orbitofrontal gray matter in cocaine addiction. Arch. General Psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. http://dx.doi.org/10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldstein RZ, et al. Is decreased prefrontal cortical sensitivity to monetary reward associated with impaired motivation and self-control in cocaine addiction? Am. J. Psychiatry. 2007;164:43–51. doi: 10.1176/appi.ajp.164.1.43. http://dx.doi.org/10.1176/appi.ajp.164.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narvaez JC, et al. Peripheral toxicity in crack cocaine use disorders. Neurosci. Lett. 2013;544:80–84. doi: 10.1016/j.neulet.2013.03.045. http://dx.doi.org/10.1016/j.neulet.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 43.Yusuf S, Reddy S, Ôunpuu S, Anand S. Global burden of cardiovascular diseases: part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. http://dx.doi.org/10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 44.Janković S, Stojisavljević D, Janković J, Erić M, Marinković J. Association of socioeconomic status measured by education, and cardiovascular health: a population-based cross-sectional study. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005222. http://dx.doi.org/10.1136/bmjopen-2014-005222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pradhan L, Mondal D, Chandra S, Ali M, Agrawal KC. Molecular analysis of cocaine-induced endothelial dysfunction: role of endothelin-1 and nitric oxide. Cardiovasc. Toxicol. 2008;8:161–171. doi: 10.1007/s12012-008-9025-z. http://dx.doi.org/10.1007/s12012-008-9025-z. [DOI] [PubMed] [Google Scholar]
- 46.Lai H, et al. Cocaine abstinence and reduced use associated with lowered marker of endothelial dysfunction in African Americans: a Preliminary study. J. Addict. Med. 2015;9:331–339. doi: 10.1097/ADM.0000000000000140. http://dx.doi.org/10.1097/adm.0000000000000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tai H, Lai H, Jani J, Lai S, Kickler TS. HIV infection and cocaine use induce endothelial damage and dysfunction in African Americans. Int. J. Cardiol. 2012;161:83–87. doi: 10.1016/j.ijcard.2011.04.034. http://dx.doi.org/10.1016/j.ijcard.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guha P, Harraz MM, Snyder SH. Cocaine elicits autophagic cytotoxicity via a nitric oxide-GAPDH signaling cascade. Proc. Natl. Acad. Sci. 2016;113:1417–1422. doi: 10.1073/pnas.1524860113. http://dx.doi.org/10.1073/pnas.1524860113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He GQ, Zhang A, Altura BT, Altura BM. Cocaine-induced cerebrovasospasm and its possible mechanism of action. J. Pharmacol. Exp. Ther. 1994;268:1532–1539. [PubMed] [Google Scholar]
- 50.Lucena J, et al. Cocaine-related sudden death: a prospective investigation in south-west Spain. Eur. Heart J. 2010;31:318–329. doi: 10.1093/eurheartj/ehp557. http://dx.doi.org/10.1093/eurheartj/ehp557. [DOI] [PubMed] [Google Scholar]
- 51.Fox HC, et al. Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum. Psychopharmacol. 2012;27:156–166. doi: 10.1002/hup.1251. http://dx.doi.org/10.1002/hup.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Leary ME, Hancox JC. Role of voltage-gated sodium, potassium and calcium channels in the development of cocaine-associated cardiac arrhythmias. Br. J. Clin. Pharmacol. 2010;69:427–442. doi: 10.1111/j.1365-2125.2010.03629.x. http://dx.doi.org/10.1111/j.1365-2125.2010.03629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buchholz S, Figtree GA, Grieve S. Cocaine-induced myocardial injury identified as multiple mid-wall foci of enhancement by contrast-enhanced cardiac MRI and large troponin rise. J. Cardiovasc. Magn. Reson. (BioMed Central) 2010;12:1–2. doi: 10.1093/eurheartj/ehq036. http://dx.doi.org/10.1186/1532-429X-12-S1-P114. [DOI] [PubMed] [Google Scholar]
- 54.Kilts CD, Gross RE, Ely TD, Drexler KPG. The neural correlates of cue-induced craving in cocaine-dependent women. Am. J. Psychiatry. 2004;161:233–241. doi: 10.1176/appi.ajp.161.2.233. http://dx.doi.org/10.1176/appi.ajp.161.2.233. [DOI] [PubMed] [Google Scholar]
- 55.Bolla KI, Cadet J-L, London ED. The neuropsychiatry of chronic cocaine abuse. J. Neuropsychiatry Clin. Neurosci. 1998;10:280–289. doi: 10.1176/jnp.10.3.280. http://dx.doi.org/10.1176/jnp.10.3.280. [DOI] [PubMed] [Google Scholar]
- 56.Klonoff DC, Andrews BT, Obana WG. Stroke associated with cocaine use. Arch. Neurol. 1989;46:989–993. doi: 10.1001/archneur.1989.00520450059019. [DOI] [PubMed] [Google Scholar]
- 57.Hardebo JE, Edvinsson L, Owman C, Svendgaard NA. Potentiation and antagonism of serotonin effects on intracranial and extracranial vessels. Possible implications in migraine. Neurology. 1978;28:64–70. doi: 10.1212/wnl.28.1.64. [DOI] [PubMed] [Google Scholar]
- 58.Dabbouseh NM, Ardelt A. Cocaine mediated apoptosis of vascular cells as a mechanism for carotid artery dissection leading to ischemic stroke. Med. Hypotheses. 2011;77:201–203. doi: 10.1016/j.mehy.2011.04.011. http://dx.doi.org/10.1016/j.mehy.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 59.Petitti DB, Sidney S, Quesenberry C, Bernstein A. Stroke and cocaine or amphetamine use. Epidemiol. (Camb. Mass.) 1998;9:596–600. [PubMed] [Google Scholar]
- 60.Du J, et al. Cholesterol is associated with the presence of a lipid core in carotid plaque of asymptomatic, young-to-middle-aged African Americans with and without HIV infection and cocaine use residing in inner-city Baltimore, Md., USA. Cerebrovasc. Dis. (Basel, Switz.) 2012;33:295–301. doi: 10.1159/000334661. http://dx.doi.org/10.1159/000334661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Siniscalchi A, et al. Cocaine dependence and stroke: pathogenesis and management. Curr. Neurovascular Res. 2015;12:163–172. doi: 10.2174/1567202612666150305110144. [DOI] [PubMed] [Google Scholar]
- 62.Brust JC. Clinical, radiological, and pathological aspects of cerebrovascular disease associated with drug abuse. Stroke J. Cereb. Circ. 1993;24:I129–I133. discussion I134-125. [PubMed] [Google Scholar]
- 63.Kaufman MJ, Levin JM, Ross MH, et al. Cocaine-induced cerebral vasoconstriction detected in humans with magnetic resonance angiography. JAMA. 1998;279:376–380. http://dx.doi.org/10.1001/jama.279.5.376. [PubMed] [Google Scholar]
- 64.Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br. J. Psychiatry J. Ment. Sci. 1988;152:641–648. doi: 10.1192/bjp.152.5.641. [DOI] [PubMed] [Google Scholar]
- 65.Siniscalchi A, et al. Editorial: cocaine and cerebral small vessel: is it a negative factor for intravenous thrombolysis? Curr. Vasc. Pharmacol. 2016;14:304–306. doi: 10.2174/1570161114999160204151620. [DOI] [PubMed] [Google Scholar]
- 66.Baud MO, Brown EG, Singhal NS, Hemphill JC. Immediate hemorrhagic transformation after intravenous tissue-type plasminogen activator injection in 2 cocaine users. Stroke; a J. Cereb. Circ. 2015;46:e167–e169. doi: 10.1161/STROKEAHA.115.008687. http://dx.doi.org/10.1161/strokeaha.115.008687. [DOI] [PubMed] [Google Scholar]
- 67.Yuan Z, Luo Z, Volkow ND, Pan Y, Du C. Imaging separation of neuronal from vascular effects of cocaine on rat cortical brain in vivo. NeuroImage. 2011;54:1130–1139. doi: 10.1016/j.neuroimage.2010.08.045. http://dx.doi.org/10.1016/j.neuroimage.2010.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson BA, Devous MD, Sr, Ruiz P, Ait-Daoud N. Treatment advances for cocaine-induced ischemic stroke: focus on dihydropyridine-class calcium channel antagonists. Am. J. Psychiatry. 2001;158:1191–1198. doi: 10.1176/appi.ajp.158.8.1191. [DOI] [PubMed] [Google Scholar]
- 69.Devonshire IM, et al. Haemodynamic responses to sensory stimulation are enhanced following acute cocaine administration. Neuroimage. 2004;22:1744–1753. doi: 10.1016/j.neuroimage.2004.03.042. http://dx.doi.org/10.1016/j.neuroimage.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 70.Movahed A, et al. Norepinephrine-induced left ventricular dysfunction in anesthetized and conscious, sedated dogs. Int. J. Cardiol. 1994;45:23–33. doi: 10.1016/0167-5273(94)90051-5. http://dx.doi.org/10.1016/0167-5273(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 71.Paraschin K, Guerra de Andrade A, Rodrigues Ps J. Assessment of myocardial infarction by CT angiography and cardiovascular MRI in patients with cocaine-associated chest pain: a pilot study. Br. J. Radiol. 2012;85:e274–e278. doi: 10.1259/bjr/52001979. http://dx.doi.org/10.1259/bjr/52001979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brickner ME, Willard JE, Eichhorn EJ, Black J, Grayburn PA. Left ventricular hypertrophy associated with chronic cocaine abuse. Circulation. 1991;84:1130–1135. doi: 10.1161/01.cir.84.3.1130. [DOI] [PubMed] [Google Scholar]
- 73.Aquaro GD, et al. Silent myocardial damage in cocaine addicts. Heart (Br. Card. Soc. 2011;97:2056–2062. doi: 10.1136/hrt.2011.226977. http://dx.doi.org/10.1136/hrt.2011.226977. [DOI] [PubMed] [Google Scholar]
- 74.Kozor R, et al. Regular cocaine use is associated with increased systolic blood pressure, aortic stiffness and left ventricular mass in young otherwise healthy individuals. PLoS One. 2014;9:1–8. doi: 10.1371/journal.pone.0089710. http://dx.doi.org/10.1371/journal.pone.0089710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee HO, Eisenberg MJ, Drew D, Schiller NB. Intraventricular thrombus after cocaine-induced myocardial infarction. Am. Heart J. 1995;129:403–405. doi: 10.1016/0002-8703(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 76.Hollander JE, Hoffman RS, Burstein JL, et al. Cocaine-associated myocardial infarction: mortality and complications. Arch. Intern. Med. 1995;155:1081–1086. http://dx.doi.org/10.1001/archinte.1995.00430100117013. [PubMed] [Google Scholar]
- 77.Chakko S, et al. Cardiac manifestations of cocaine abuse: a cross-sectional study of asymptomatic men with a history of long-term abuse of “crack” cocaine. J. Am. Coll. Cardiol. 1992;20:1168–1174. doi: 10.1016/0735-1097(92)90374-v. http://dx.doi.org/10.1016/0735-1097(92)90374-V. [DOI] [PubMed] [Google Scholar]
- 78.Maceira AM, et al. Long term effects of cocaine on the heart assessed by cardiovascular magnetic resonance at 3T. J. Cardiovasc. Magn. Reson. Off. J. Soc. Cardiovasc. Magn. Reson. 2014;16:26. doi: 10.1186/1532-429X-16-26. http://dx.doi.org/10.1186/1532-429x-16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu C-Y, et al. Cocaine use as an independent predictor of cardiac steatosis: initial experience by 1H spectroscopy. J. Cardiovasc. Magn. Reson. (BioMed Central) 2010;12:1–2. http://dx.doi.org/10.1186/1532-429X-12-S1-O90. [Google Scholar]
- 80.Redheuil A, et al. Long-term cocaine use is associated with premature alterations in regional aortic strain and distensibility measured by magnetic resonance imaging. J. Cardiovasc. Magn. Reson. (BioMed Central) 2010;12:1–2. http://dx.doi.org/10.1186/1532-429X-12-S1-P146. [Google Scholar]
- 81.Lai S, et al. Effect of cocaine use on coronary calcium among black adults in Baltimore, Maryland. Am. J. Cardiol. 2002;90:326–328. doi: 10.1016/s0002-9149(02)02475-x. http://dx.doi.org/10.1016/S0002-9149(02)02475-X. [DOI] [PubMed] [Google Scholar]
- 82.Mangiafico RA, Sarnataro F, Mangiafico M, Fiore CE. Impaired cognitive performance in asymptomatic peripheral arterial disease: relation to Creactive protein and D-dimer levels. Age Ageing. 2006;35:60–65. doi: 10.1093/ageing/afi219. http://dx.doi.org/10.1093/ageing/afi219. [DOI] [PubMed] [Google Scholar]
- 83.Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004;39:687–699. doi: 10.1016/j.exger.2004.01.009. http://dx.doi.org/10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Singh-Manoux A, Britton AR, Marmot M. Vascular disease and cognitive function: evidence from the Whitehall II study. J. Am. Geriatr. Soc. 2003;51:1445–1450. doi: 10.1046/j.1532-5415.2003.51464.x. http://dx.doi.org/10.1046/j.1532-5415.2003.51464.x. [DOI] [PubMed] [Google Scholar]
- 85.Haley AP, et al. Carotid artery intima-media thickness and cognition in cardiovascular disease. Int. J. Cardiol. 2007;121:148–154. doi: 10.1016/j.ijcard.2006.10.032. http://dx.doi.org/10.1016/j.ijcard.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.de Lange TE, Simsek S, Kramer MH, Nanayakkara PW. A case of cocaine-induced panhypopituitarism with human neutrophil elastase-specific antineutrophil cytoplasmic antibodies. Eur. J. Endocrinol./Eur. Fed. Endocr. Soc. 2009;160:499–502. doi: 10.1530/EJE-08-0941. http://dx.doi.org/10.1530/eje-08-0941. [DOI] [PubMed] [Google Scholar]
- 87.Goldberg RA, et al. Orbital inflammation and optic neuropathies associated with chronic sinusitis of intranasal cocaine abuse: possible role of contiguous inflammation. Arch. Ophthalmol. 1989;107:831–835. doi: 10.1001/archopht.1989.01070010853028. http://dx.doi.org/10.1001/archopht.1989.01070010853028. [DOI] [PubMed] [Google Scholar]
- 88.Neugebauer P, Fricke J, Neugebauer A, Kirsch A, Russmann W. Sinuorbital complications after intranasal cocaine abuse. Strabismus. 2004;12:205–209. doi: 10.1080/09273970490515900. http://dx.doi.org/10.1080/09273970490515900. [DOI] [PubMed] [Google Scholar]
- 89.Trimarchi M, et al. Sinonasal osteocartilaginous necrosis in cocaine abusers: experience in 25 patients. Am. J. Rhinol. 2003;17:33–43. [PubMed] [Google Scholar]
- 90.Alexandrakis G, Tse DT, Rosa RH, Jr, Johnson TE. Nasolacrimal duct obstruction and orbital cellulitis associated with chronic intranasal cocaine abuse. Arch. Ophthalmol. (Chic. Ill.:1960) 1999;117:1617–1622. doi: 10.1001/archopht.117.12.1617. [DOI] [PubMed] [Google Scholar]
- 91.Bachi K, Sierra S, Volkow ND, Goldstein RZ, Alia-Klein N. Is biological aging accelerated in drug addiction? Curr. Opin. Behav. Sci. 2017;13:34–39. doi: 10.1016/j.cobeha.2016.09.007. http://dx.doi.org/10.1016/j.cobeha.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.O'Conor K, Chang AM, Wu AH, Hollander JE. Myeloperoxidase and Creactive protein in patients with cocaine-associated chest pain. Am. J. Emerg. Med. 2013;31:664–669. doi: 10.1016/j.ajem.2012.11.026. http://dx.doi.org/10.1016/j.ajem.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 93.Lai S, et al. Chronic cocaine use and its association with myocardial steatosis evaluated by 1H magnetic resonance spectroscopy in African Americans. J. Addict. Med. 2015;9:31–39. doi: 10.1097/ADM.0000000000000078. http://dx.doi.org/10.1097/adm.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Olanow CW, et al. TCH346 as a neuroprotective drug in Parkinson's disease: a double-blind, randomised, controlled trial. Lancet. Neurol. 2006;5:1013–1020. doi: 10.1016/S1474-4422(06)70602-0. http://dx.doi.org/10.1016/s1474-4422(06)70602-0. [DOI] [PubMed] [Google Scholar]
- 95.Siniscalchi A, Lentidoro W, Pisanil E, De Sarro G, Gallelli L. Intracerebral hemorrhage in a middle-aged cocaine user despite normal blood pressures. Am. J. Emerg. Med. 2016 doi: 10.1016/j.ajem.2016.09.003. http://dx.doi.org/10.1016/j.ajem.2016.09.003. [DOI] [PubMed]
- 96.D'Agostino RB, et al. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am. heart J. 2000;139:272–281. doi: 10.1067/mhj.2000.96469. [DOI] [PubMed] [Google Scholar]
- 97.The European Monitoring Centre for Drugs and Drug Addiction, European Drug Report 2013: Trends and Developments. The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA); Portugal: Lisbon: May, 2013. [PubMed] [Google Scholar]
- 98.Cornish JW, O'Brien CP. Crack cocaine abuse: an epidemic with many public health consequences. Annu. Rev. Public Health. 1996;17:259–273. doi: 10.1146/annurev.pu.17.050196.001355. http://dx.doi.org/10.1146/annurev.pu.17.050196.001355. [DOI] [PubMed] [Google Scholar]
- 99.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 100.Fuster V, Jiménez-Borreguero L-J, Sanz G, Ibáñez B. HIV and cardiovascular disease: urgent need for sub-clinical detection. Nat. Rev. Cardiol. (CNIC Ed.) 2010;7:17–21. [Google Scholar]
- 101.Fayad ZA, et al. Rationale and design of dal-PLAQUE: a study assessing efficacy and safety of dalcetrapib on progression or regression of atherosclerosis using magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Am. Heart J. 2011;162:214–221. doi: 10.1016/j.ahj.2011.05.006. http://dx.doi.org/10.1016/j.ahj.2011.05.006 e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fayad ZA, et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547–1559. doi: 10.1016/S0140-6736(11)61383-4. http://dx.doi.org/10.1016/s0140-6736(11)61383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wong SK, et al. Atherosclerosis imaging using 3D black blood TSE SPACE vs 2D TSE. World J. Radiol. 2014;6:192–202. doi: 10.4329/wjr.v6.i5.192. http://dx.doi.org/10.4329/wjr.v6.i5.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parvaz MA, Alia-Klein N, Woicik PA, Volkow ND, Goldstein RZ. Neuroimaging for drug addiction and related behaviors. Rev. Neurosci. 2011;22:609–624. doi: 10.1515/RNS.2011.055. http://dx.doi.org/10.1515/RNS.2011.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Narendran R, et al. Cocaine abuse in humans is not associated with increased microglial activation: an 18-kDa translocator protein positron emission tomography imaging study with [11C]PBR28. J. Neurosci. Off. J. Soc. Neurosci. 2014;34:9945–9950. doi: 10.1523/JNEUROSCI.0928-14.2014. http://dx.doi.org/10.1523/jneurosci.0928-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]