Summary
Background
Despite effective pharmacotherapy, asthma continues to impair quality of life for most patients. Non-pharmacological approaches, including breathing retraining, are therefore of great interest to patients. However, clinicians rarely advocate breathing retraining and access to this intervention is restricted for most patients due to the limited availability of suitable physiotherapists and poor integration of breathing retraining into standard care. We aimed to assess the effectiveness of a digital self-guided breathing retraining intervention.
Methods
In this randomised controlled trial, we recruited patients from 34 general practices in the UK. Eligibility criteria for patients with asthma were broad, comprising a physician diagnosis of asthma, age of 16–70 years, receipt of at least one anti-asthma medication in the previous year, and impaired asthma-related quality of life (Asthma Quality of Life Questionnaire [AQLQ] score of <5·5). We developed a self-guided intervention, which was delivered as a DVD plus a printed booklet (DVDB). Participants were randomly assigned to receive either the DVDB intervention, three face-to-face breathing retraining sessions, or standard care, in a 2:1:2 ratio, for 12 months. Randomisation was achieved using the Southampton Clinical Trials Unit telephone randomisation service by use of random number generators. The primary outcome was the AQLQ score in the intention-to-treat population at 12 months. The trial was powered to show equivalence between the two active intervention groups, and superiority of both intervention groups over usual care. Secondary outcomes included patient-reported and physiological measures of asthma control, patient acceptability, and health-care costs. This trial was registered with International Standard Randomised Controlled Trial Number registry, number ISRCTN88318003.
Findings
Between Nov 5, 2012 and Jan 28, 2014, invitations to participate in the study were sent to 15 203 patients with general practitioner-diagnosed asthma, of whom 655 were recruited into the study. AQLQ scores at 12 months were significantly higher in the DVDB group (mean 5·40, SD 1·14) than in the usual care group (5·12, SD 1·17; adjusted mean difference 0·28, 95% CI 0·11 to 0·44), and in the face-to-face group (5·33, SD 1·06) than in the usual care group (adjusted mean difference 0·24, 95% CI 0·04 to 0·44); AQLQ scores were similar between the DVDB group and the face-to-face group (0·04, 95% CI −0·16 to 0·24). There were no significant differences between the randomisation groups in FEV1 or fraction of exhaled nitric oxide. 744 adverse events occurred in 272 patients: 101 (39%) of 261 patients in the DVDB group, 55 (42%) of 132 patients in the face-to-face group, and 132 (50%) of 262 in the usual care group, with patients reporting one or more event. 11 (4%) patients in the DVDB group, four (3%) patients in the face-to-face group, and 20 (8%) patients in the usual care group had a serious adverse event.
Interpretation
Breathing retraining programmes improve quality of life in patients with incompletely controlled asthma despite having little effect on lung function or airway inflammation. Such programmes can be delivered conveniently and cost-effectively as a self-guided digital audiovisual programme, so might also reduce health-care costs.
Funding
UK National Institute of Health Research.
Introduction
Asthma is a serious and common global health problem, affecting up to 18% of the world population.1 Although pharmacotherapy can provide full symptom control for some individuals,2 real-life surveys repeatedly show that all outcomes remain suboptimal for many patients, with most having persisting symptoms and quality of life impairment.3 Many patients have concerns about taking regular medication, particularly corticosteroids, and express an interest in non-pharmacological self-management strategies, with up to 30% reporting that they use breathing techniques to help control their symptoms.4 Preliminary randomised trials have reported beneficial outcomes from breathing retraining in asthma, particularly from physiotherapy-based programmes.5, 6 Such programmes are, therefore, now advocated in asthma guidelines1, 7 as an adjuvant for patients whose symptoms remain uncontrolled despite standard pharmacological treatment. However, in National Health Service (NHS) practice, these methods are rarely used because of insufficient access to suitably trained physio therapists. There is clear evidence for the effectiveness of self-management education in asthma management, although current programmes focus mainly on pharmacological strategies and do not include breathing retraining. Self-guided digital programmes have the potential to be accessed easily, conveniently, and inexpensively by large numbers of people with asthma and to deliver standardised interventions.8
Research in context.
Evidence before this study
Asthma is a complex, heterogeneous condition that affects patients in many ways; physically, psychologically, as well as affecting quality of life. Pharmacological treatments are effective, yet most patients continue to have ongoing symptoms and quality of life impairment despite the use of drug treatment. Many patients are interested in non-pharmacological treatment approaches, but the evidence base for most non-pharmacological interventions is inconclusive, with existing studies often underpowered or methodologically flawed. However, breathing retraining exercises have promising supportive evidence and are advocated as an add-on option in several evidence-based asthma guidelines. We identified two extensive recent systematic reviews of breathing retraining in asthma, which have differed in their conclusions; we therefore did not complete a systematic literature search ourselves. The 2012 US Agency for Healthcare Research and Quality analysis study concluded that non-pharmacological techniques can improve asthma symptoms and reduce the need for reliever medication, and the 2013 Cochrane review reported that trends for improvement were encouraging, and advocated further studies. Current National Health Service breathing retraining programmes involve face-to-face attendance with a suitably trained physiotherapist, but access is limited because of the scarcity of therapists and the logistical and financial challenges of making treatment available in routine care.
Added value of this study
We report the largest trial of breathing retraining in asthma to date, which used a pragmatic randomised controlled trial design. The main finding of this study is that asthma-related quality of life impairment is improved by a self-guided programme of physiotherapy-based breathing training delivered by a DVD and booklet, as an alternative model of delivery to the routine face-to-face method, with a magnitude of effect similar to that produced by increasing asthma medication. Patients perceived benefit from the self-guided programme despite little improvement in airway physiology or inflammation. We confirm reports from previous smaller studies that improvements in quality of life were seen with face-to-face physiotherapist-taught programmes compared with usual care. Additionally, we also showed that the DVD and booklet programme resulted in equivalent clinically relevant benefits more conveniently and inexpensively. In keeping with current evidence and meta-analysis, no adverse effects of either intervention were found.
Implications of all the available evidence
Our findings imply that the self-help intervention can be offered conveniently and cost-effectively as an adjuvant non-pharmacological treatment to many patients with asthma in routine care who have persisting impairment in quality of life despite standard pharmacological management.
We hypothesised that breathing retraining delivered as a digital, audiovisual, self-guided programme to patients with mild and moderate asthma, but with persisting symptoms, would improve asthma-related quality of life above usual care, be equivalent to face-to-face physiotherapist instruction, be cost-effective, and be an acceptable intervention for patients.
Methods
Study design and participants
This was a pragmatic 3-group, 12-month, observer-blinded, parallel-group randomised trial comparing the use of a DVD and booklet (DVDB) intervention with face-to-face physiotherapy or with a control group receiving usual care, in adults with asthma who were recruited from 34 UK general practices. Ethical approval for the study was provided by the NHS Health Research Authority South-Central—Hampshire B Research Ethics committee (12/SC/0353). A protocol has been published.9
As a pragmatic, randomised trial,10 participants had baseline characteristics measured, but broad entry criteria (with inclusion of smokers and no requirement for reversibility of airflow obstruction) and a minimally disruptive study procedure were used. To be included in the study, participants had to be aged 16–70 years, be registered at a medical practice for at least 12 months, have asthma diagnosed by a physician, have been prescribed at least 1 asthma medication in the previous year, achieve an Asthma Quality of Life Questionnaire (AQLQ)11 score of less than 5·5, and to provide written informed consent. Patients were excluded if they had concomitant chronic obstructive pulmonary disease (COPD) diagnosis with FEV1 less than 60% predicted.
Randomisation and masking
Patients were randomly assigned (2:1:2) to receive the DVDB intervention, face-to-face physiotherapy, or usual care. This randomisation schedule was used because the face-to-face group was the most financially and logistically challenging to deliver to patients. Randomisation was done after eligibility assessment and informed consent; the research nurse telephoned the randomisation service provided by Southampton Clinical Trials Unit, where staff trained in registration and randomisation procedures accessed the web based Tenalea randomisation system. The research nurse then provided the participant with the appropriate materials and follow-up arrangements for the relevant study group. The research nurses who completed the exit assessments and medical record review were different from those completing the baseline assessment, and had no information about group allocations, with patients requested not to disclose their own treatment group. All analyses were completed by researchers masked to treatment allocation and without access to unmasked data.
Procedures
We developed an evidence-based, self-guided intervention delivered via a DVD plus a printed supporting booklet, which was based on an existing breathing retraining programme taught by physiotherapists and shown to be effective in poorly controlled asthma.5, 6 The DVDB materials were developed by a multidisciplinary team including physicians, physiotherapists, health psychologists, communications technology specialists, and patient representatives, with extensive iterative qualitative patient input to optimise acceptability and effectiveness, and consisted of three elements. First, the DVDB intervention provided a detailed explanation and illustration of how to complete the breathing exercises, with footage showing a physiotherapist teaching these exercises to patients. The exercises comprised an illustration of, and training in, diaphragmatic breathing, nasal breathing, slow breathing, controlled breath holds, and simple relaxation exercises. Second, the DVDB intervention provided motivational components, explaining the rationale and addressing common concerns. Finally, supportive features (such as a daily planner and progress charts) were included for practicing and implementing the techniques in daily life.
The materials were piloted and finalised with 29 members of the target population, purposively sampled for diversity of age, gender, education, and symptom profile, by use of the person-based think aloud methods12 to elicit reactions and modify the materials based on the feedback received.13 The content of the DVD and the supporting booklet have been transferred to an internet-based version, and is freely available through the Breathe Study website.
Potentially eligible patients, identified by computer searches of routine clinical records from general practices, received the study information and the AQLQ by mail. Questionnaires were repeated via post at 3 and 6 months, with a final assessment visit at 12 months done by a study nurse blinded to randomisation group, at which all baseline measures were repeated. If participants were unable to attend the final visit, questionnaire data were obtained by post or telephone.
Participants randomly assigned to the usual care group received usual medical care, with no additional attention to the baseline assessment. Participants randomly assigned to the DVDB intervention group were provided with the DVD and the booklet. Participants randomly assigned to the face-to-face group also received the booklet and worked to a standardised intervention schedule and were seen by a respiratory physiotherapist who was trained and skilled in providing breathing retraining for three one-to-one sessions, each of about 40 min duration, approximately once every 2 weeks after randomisation. The same physiotherapist, working to a structured protocol, delivered all the face-to-face sessions for all patients. Fidelity to treatment delivery was identified by use of a physiotherapist-completed checklist at every session, and a direct observation checklist completed by one author (AB) of a random sample of 5% of sessions. Protocol adherence was predefined as conforming to 90% of the checklist, and was 100% on both the physiotherapist and the observation checklists.
A pre-specified health economic assessment was done by assessing the intervention provision costs, and mean health service use cost per patient by treatment group, aggregated from the costs of asthma-related prescriptions, consultations, and hospital admissions, and is reported in full elsewhere.14
Outcomes
The primary outcome measure was the 12-month AQLQ score adjusted for the baseline values of prespecified covariates during statistical analysis. Secondary patient outcomes were questionnaire measures of asthma control, and measurements of airway physiology (FEV1, peak expiratory flow rate [PEFR], forced vital capacity [FVC]), airway inflammation (fraction of exhaled nitric oxide [FENO] measurements with Nioxx Mino [Circassia, Oxford, UK]), and asthma-related health resource use, in addition to a health-economic assessment (appendix). Validated questionnaires used to assess secondary outcome measures were the Asthma Control Questionnaire (ACQ),15 which measured asthma symptoms; the Nijmegen Questionnaire (NQ),16 which measured symptoms related to hyperventilation and dysfunctional breathing; and the Hospital Anxiety and Depression Score (HADS),17 which had separate domains to measure anxiety and depression.
Patient experiences and engagement were assessed by a study-specific questionnaire and by qualitative interviews with participants selected by purposive sampling until data saturation was achieved (appendix).
Health resource use and prescriptions were obtained from the routine medical records on study exit, with asthma attacks, defined as the use of a course of oral corticosteroids for worsening respiratory symptoms or hospital admission. The economic evaluation report followed the CHEERS guideline.14 Adverse events in the full intention-to-treat population were reported by the local investigators and also gathered from the routine medical record at the end of the study by research nurses masked to the participants randomisation group. All significant (fatal or potentially life-threatening) events were collected, and all adverse events resulting in medical attention that could plausibly be related to the study intervention were included, in the following categories: asthma-related; respiratory (including infections); ear, nose and throat; musculoskeletal; abdominal; neurological; and psychiatric events, detailed in the full study report.14
Statistical analysis
On the basis of a previous breathing retraining trial,6 we assumed an SD of between-group AQLQ change between active treatment and control of 1·03, and a 25% smaller change between the two active intervention groups (SD 0·77). Treatments were deemed equivalent if the 95% CI for the mean difference was included between −0·3 and 0·3, with a two-tailed 5% significance level. Analysable sample sizes of 117 in the face-to-face group and 234 in the usual care and DVDB groups provided 90% power to detect a difference in mean AQLQ of 0·38 between active breathing retraining groups and the usual care group (as observed in the previous trial6 and of similar magnitude to pharmacological interventions in asthma),18 and more than 90% power to show equivalence between the two active intervention groups. Assuming a 10% dropout rate, we therefore aimed to recruit 650 patients.
The primary analysis was a repeated-measures mixed model using the 12-month AQLQ score across the three groups, with adjustments for prespecified covariates comprising baseline AQLQ score, general practitioner (GP) practice, age, gender, smoking status, British Thoracic Society (BTS) asthma treatment step7 (broadly equivalent to GINA step), and HADS and NQ scores. Pair-wise comparisons between DVDB and usual care groups, face-to-face physiotherapy and usual care groups (superiority studies), and DVDB and face-to-face physiotherapy groups (equivalence study) were made. Prespecified sensitivity analyses were analyses of the primary outcome in the per-protocol population (defined below) and used a range of recommended methods for missing data (appendix). Negative binomial regression models were constructed to estimate the incidence rate ratios of asthma attacks and rescue bronchodilator prescriptions in the intervention groups versus the usual care group. No adjustment was made for multiple testing since all tests were prespecified with a priori effect sizes.19 Two statistical packages were used in the analysis, IBM SPSS statistics version 24 and STATA version 14. This trial was registered with International Standard Randomised Controlled Trial Number registry, number ISRCTN88318003.
Role of the funding source
Funding was provided by the UK National Institute of Health Research, Health Technology Assessment funding stream. The funder was not involved in the study design, data collection, data analysis, data interpretation, or in the writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
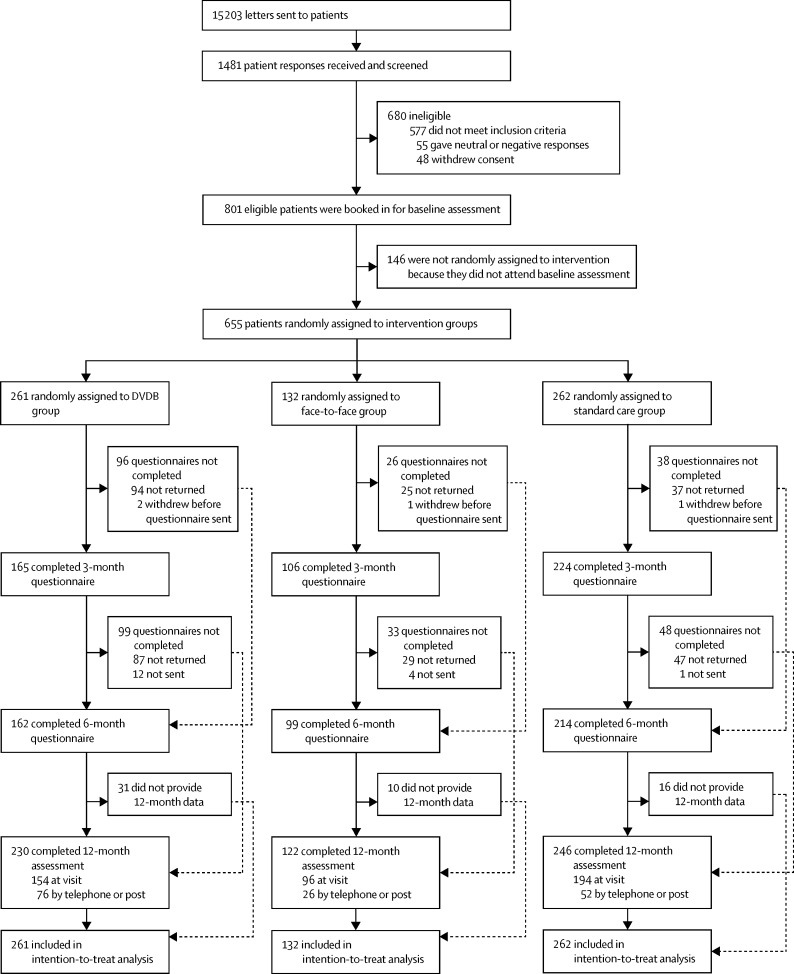
Between Nov 5, 2012, and Jan 28, 2014, patients were recruited from 34 GP practices. Invitations were posted to 15 203 patients and 1481 responses were received (10% response rate). Of those received, 801 (54%) of 1481 patients were eligible to participate in the study, with AQLQ score of more than 5·5 being the commonest ineligibility criterion—with 577 participants being excluded for this reason. 655 (82%) of 801 eligible patients were randomly assigned to treatment: 261 (40%) of 655 to the DVDB group, 262 (40%) to the usual care group, and 132 (20%) to the face-to-face group (figure 1). Baseline data (table 1) were similar between groups. The intention-to-treat population consisted of all participants randomly assigned to intervention, and all analyses were repeated in the per-protocol population, which included all participants with baseline and 12-month primary outcome data. There were few missing data for other questionnaires (<2%; appendix). Spirometry (FEV1, FVC, and FEV1:FVC ratio) was missing in 26 (4%) of 655 participants randomly assigned to treatment and FENO in 49 (8%) of 655, with similar proportions between groups (appendix). 21 (3%) of 655 participants withdrew from the study (13 [5%] of 261 in the DVDB group, 3 [2%] of 132 in the face-to-face group, and 5 [2%] of 262 in the usual care group). The AQLQ was returned at 12 months by 556 (85%) of 655 participants, but all patients submitted a questionnaire at one or more of the follow-up points over the course of the study.
Figure 1.
Trial profile
DVDB=DVD and booklet intervention.
Table 1.
Baseline characteristics
| DVDB intervention (n=261) | Face-to-face intervention (n=132) | Usual care (n=262) | All patients (n=655) | ||
|---|---|---|---|---|---|
| Age, years | 56 (45–65) | 55 (47–63) | 57 (47–65) | 57 (46–64) | |
| Gender | |||||
| Female | 164 (63%) | 91 (69%) | 164 (63%) | 419 (64%) | |
| Male | 97 (37%) | 41 (31%) | 98 (37%) | 236 (36%) | |
| Weight, kg | 79·9 (17·6) | 80·6 (20·2) | 83·1 (18·1) | 81·3 (18·4) | |
| Height, cm | 167·1 (9·0) | 165·7 (8·8) | 166·1 (9·1) | 166·4 (9·0) | |
| Smoking status | |||||
| Current smoker | 16 (6%) | 13 (10%) | 21 (8%) | 50 (8%) | |
| Ex-smoker | 74 (28%) | 43 (33%) | 102 (39%) | 219 (33%) | |
| Never smoker | 169 (65%) | 76 (58%) | 139 (53%) | 384 (59%) | |
| Age at asthma diagnosis | 27 (12–45) | 32 (14–45) | 28 (8–46) | 29 (11–45) | |
| Baseline pulmonary markers | |||||
| FENO, parts per billion | 21 (14–35) | 23 (15–23) | 23 (14–34) | 22 (14–34) | |
| FEV1, L | 2·6 (0·8) | 2·5 (0·7) | 2·6 (0·8) | 2·6 (0·8) | |
| FVC, L | 3·5 (0·9) | 3·3 (0·9) | 3·4 (0·9) | 3·4 (0·9) | |
| FEV1:FVC ratio | 0·8 (0·1) | 0·8 (0·1) | 0·8 (0·1) | 0·8 (0·1) | |
| Predicted FEV1, % | 90·5 (18·8) | 88·8 (18·1) | 91·9 (21·6) | 90·7 (19·8) | |
| PEFR, L/min | 426 (116) | 415 (110) | 423 (120) | 423 (117) | |
| British Thoracic Society treatment step | |||||
| 1 | 19 (7%) | 8 (6%) | 10 (4%) | 47 (7%) | |
| 2 | 65 (25%) | 41 (31%) | 69 (26%) | 175 (27%) | |
| 3 | 107 (41%) | 52 (39%) | 117 (45%) | 276 (42%) | |
| 4 | 26 (10%) | 16 (12%) | 33 (13%) | 75 (12%) | |
| 5 | 0 | 0 | 1 (<1%) | 1 (<1%) | |
| Unknown or unspecified | 44 (17%) | 15 (11%) | 22 (8%) | 81 (12%) | |
| AQLQ | 4·3 (0·9) | 4·2 (0·9) | 4·3 (0·9) | 4·3 (0·9) | |
| ACQ | 1·5 (0·9) | 1·6 (0·8) | 1·5 (0·9) | 1·5 (0·9) | |
| HADS | |||||
| Anxiety | 7·0 (4·0–8·0) | 6·0 (4·0–9·0) | 6·0 (4·0–9·0) | 6·0 (4·0–9·0) | |
| Depression | 3·0 (1·0–5·0) | 2·0 (1·0–5·0) | 3·0 (1·0–5·0) | 3·0 (1·0–5·0) | |
| Nijmegen questionnaire | 19·0 (8·8) | 19·0 (10·5) | 19·4 (9·4) | 19·2 (9·4) | |
Data are mean (SD), n (%), or median (IQR) unless otherwise specified. DVDB=DVD and booklet. FENO=fraction of exhaled nitric oxide. FVC=forced vital capacity. PEFR=peak expiratory flow rate. AQLQ=Asthma Quality of Life Questionnaire. ACQ=Asthma Control Questionnaire. HADS=hospital anxiety and depression questionnaire.
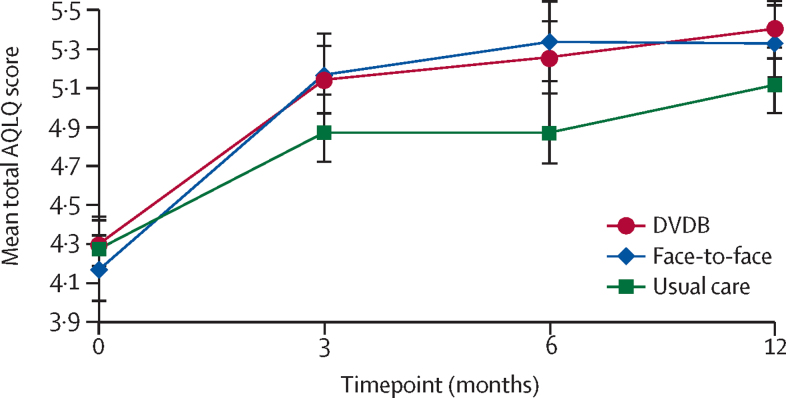
The mean AQLQ scores at 12 months were 5·40 (SD 1·14) in the DVDB group, 5·33 (1·06) in the face-to-face group, and 5·12 (1·17) in the usual care group. In the primary efficacy analysis (table 2, figure 2), the adjusted mean AQLQ score in the DVDB group compared with the usual care group was 0·28 (95% CI 0·11–0·44), and in face-to-face intervention compared with the usual care group was 0·24 (0·04–0·44). The adjusted mean difference between the DVDB and face-to-face interventions was 0·04 (−0·16 to 0·24). In AQLQ subdomains (emotions, symptoms, activities, and en-vironment), the largest improvements were in the emotion subdomain, with the DVDB group (0·38, 95% CI 0·16–0·60) and the face-to-face group (0·43, 0·16–0·71) both showing improvements compared with the usual care group. In the DVDB group, significant improvements over the usual care group were also seen in symptoms (0·24, 95% CI 0·05–0·42), activities (0·21, 0·04–0·41), and environment (0·32, 0·11–0·53) AQLQ subdomains; a similar improvement in the symptom subdomain was also seen in the face-to-face group compared with the usual care group (0·27, 0·04–0·49). There were no significant differences in overall or individual domain scores between the DVDB and face-to-face groups. Significant differences were maintained in the pre-specified sensitivity analyses involving the results from the last observation carried forward, with similar outcomes shown by use of multiple imputation methods for missing data (appendix).
Table 2.
Adjusted mean difference in 12-month primary and secondary outcome measures in the DVDB, face-to-face, and usual care treatment groups in the intention-to-treat and per-protocol populations
|
Intention-to-treat population |
Per-protocol population |
||||||
|---|---|---|---|---|---|---|---|
| Face-to-face intervention vs usual care | DVDB intervention vs usual care | DVDB vs face-to-face interventions | Face-to-face intervention vs usual care | DVDB intervention vs usual care | DVDB vs face-to-face intervention | ||
| AQLQ score | |||||||
| Total score | 0·24 (0·04 to 0·44) | 0·28 (0·11 to 0·44) | 0·04 (−0·16 to 0·24) | 0·22 (0·02 to 0·43) | 0·26 (0·10 to 0·43) | 0·04 (−0·17 to 0·25) | |
| Symptoms | 0·27 (0·04 to 0·49) | 0·24 (0·05 to 0·42) | −0·03 (−0·26 to 0·20) | 0·25 (0·02 to 0·49) | 0·21 (0·02 to 0·40) | −0·04 (−0·27 to 0·19) | |
| Activities | 0·08 (−0·14 to 0·31) | 0·21 (0·04 to 0·41) | 0·13 (−0·10 to 0·36) | 0·08 (−0·15 to 0·30) | 0·21 (0·02 to 0·39) | 0·13 (−0·10 to 0·36) | |
| Emotion | 0·43 (0·16 to 0·71) | 0·38 (0·16 to 0·60) | −0·06 (−0·33 to 0·22) | 0·41 (0·14 to 0·68) | 0·35 (0·13 to 0·58) | −0·05 (−0·33 to 0·22) | |
| Environment | 0·19 (−0·06 to 0·44) | 0·32 (0·11 to 0·53) | 0·13 (−0·12 to 0·39) | 0·18 (−0·07 to 0·44) | 0·32 (0·11 to 0·54) | 0·14 (−0·12 to 0·40) | |
| Pulmonary markers | |||||||
| FEV1, L | −0·04 (−0·11 to 0·04) | −0·001 (−0·07 to 0·07) | 0·03 (−0·05 to 0·12) | 0·02 (−0·06 to 0·11) | −0·01 (−0·08 to 0·07) | −0·03 (−0·12 to 0·06) | |
| FVC, L | −0·04 (−0·16 to 0·08) | −0·03 (−0·14 to 0·07) | 0·01 (−0.12 to 0·13) | 0·03 (−0·09 to 0·16) | 0·02 (−0·09 to 0·13) | −0·01 (−0.14 to 0·12) | |
| FEV1:FVC ratio | −0·01 (−0·02 to 0·01) | 0·0004 (−0·01 to 0·02) | 0·01 (−0·01 to 0·03) | 0·01 (−0·02 to 0·02) | −0·003 (−0·02 to 0·01) | −0·004 (−0·03 to 0·02) | |
| Predicted FEV1, % | 0·44 (−3·23 to 4·12) | 0·53 (−2·75 to 3·81) | 0·09 (−3·81 to 3·99) | −1·49 (−5·33 to 2·36) | 0·98 (−4·35 to 2·39) | 0·51 (−3·55 to 4·57) | |
| PEFR, L/min | −4·8 (−22·4 to 12·8) | −2·0 (−17·8 to 13·9) | 2·8 (−15·9 to 21·5) | 3·2 (−15·4 to 21·8) | 2·9 (−13·7 to 19·5) | −0·3 (−20·1 to 19·5) | |
| FENO*, parts per billion | 1·05 (0·95 to 1·23) | 1·13 (0·98 to 1·29) | 1·07 (0·91 to 1·25) | 1·05 (0·89 to 1·23) | 1·14 (0·98 to 1·31) | 1·08 (0·92 to 1·28) | |
| ACQ | −0·06 (−0·23 to 1·23) | −0·09 (−0·25 to 0·06) | −0·04 (−0·23 to 0·15) | −0·04 (−0·22 to 0·15) | −0·05 (−0·21 to 0·11) | −0·01 (−0·20 to 0·19) | |
| HADS | |||||||
| Anxiety | 0·04 (−0·73 to 0·64) | −0·22 (−0·81 to 0·38) | −0·18 (−0·89 to 0·54) | 0·03 (−0·71 to 0·75) | −0·16 (−0·79 to 0·47) | −0·17 (−0·94 to 0·58) | |
| Depression | −0·55 (−1·14 to 0·04) | −0·56 (−1·07 to −0·05) | −0·01 (−0·63 to 0·60) | −0·58 (−1·19 to 0·04) | −0·56 (−1·10 to 0·03) | 0·02 (−0·62 to 0·66) | |
| Nijmegen questionnaire | 1·28 (−0·55 to 3·12) | 10·90 (−0·71 to 2·51) | −0·38 (−2·30 to 1·55) | 1·41 (−0·50 to 3·32) | 0·99 (−0·67 to 2·65) | −0·42 (−2·42 to 1·58) | |
Data are adjusted mean difference and 95% CI for prespecified list of covariates. DVDB=DVD and booklet. FENO=fraction of exhaled nitric oxide. FVC=forced vital capacity. PEFR=peak expiratory flow rate. AQLQ=Asthma Quality of Life Questionnaire. ACQ=Asthma Control Questionnaire. HADS=hospital anxiety and depression questionnaire.
Geometric mean difference.
Figure 2.
AQLQ scores across all timepoints, by intervention
Data are mean (95% CI). AQLQ=Asthma Quality of Life Questionnaire. DVDB=DVD and booklet.
An analysis of individual patient responses was done that was recommended to quantify the proportion of patients in whom a minimum clinically important difference of 0·5 was met, because this analysis can provide additional information on the spectrum of the response and can be used to calculate numbers needed to treat (NNT).20 Clinically important improvements were seen in 161 (62%) of 261 patients in the DVDB group, 85 (64%) of 132 in the face-to-face group, and 146 (56%) of 262 in the usual care group, with corresponding percentages for deterioration of 14 (5%) in the DVDB group, 5 (4%) in the face-to-face group, and 24 (9%) in the usual care group, providing an NNT of eight for DVDB versus usual care, and seven for face-to-face versus usual care.
We observed no significant differences between groups in any secondary outcome measure, other than small magnitude but significant improvements in the depression component of the HADS in the DVDB group compared with the usual care group (mean adjusted difference −0·56, 95% CI −1·07 to −0·05). In particular, there were no significant within-group or between-group changes in airway obstruction (FEV1, PEFR), inflammation (FENO), ACQ or NQ scores, or in the anxiety component of the HADS (table 2).
Overall, 78 (12%) of 655 participants had one or more asthma attack during the 12 month study (table 3); 24 (9%) of 261 in the DVDB group, 15 (11%) of 132 in the face-to-face group, and 39 (15%) of 262 in the usual care group. The DVDB group showed a non-significant tendency to fewer asthma attacks compared with the usual care group (p=0·062; appendix). In a negative binomial regression model adjusting for baseline asthma attack frequency and prespecified covariates, the incidence of asthma attacks for DVDB versus usual care was 0·68 (95% CI 0·39–1·20) and for face-to-face versus usual was 0·85 (0·43–1·67).
Table 3.
Number of patients having asthma attacks over 12 months in the DVDB, face-to-face, and usual care treatment groups in the intention-to-treat and per-protocol populations, by number of corticosteroid courses used
|
Intention-to-treat population |
Per-protocol population |
|||||||
|---|---|---|---|---|---|---|---|---|
| DVDB (n=261) | Face-to-face (n=132) | Usual care (n=262) | Total (n=655) | DVDB (n=215) | Face-to-face (n=110) | Usual care (n=231) | Total (n=556) | |
| None | 237 (91%) | 117 (89%) | 223 (85%) | 577 (88%) | 193 (90%) | 97 (88%) | 195 (84%) | 485 (87%) |
| 1 | 17 (7%) | 10 (8%) | 26 (10%) | 53 (8%) | 16 (7%) | 9 (8%) | 26 (11%) | 51 (9%) |
| 2 | 2 (1%) | 4 (3%) | 10 (4%) | 16 (2%) | 2 (1%) | 3 (3%) | 8 (3%) | 13 (2%) |
| 3 | 2 (1%) | 0 | 1 (<1%) | 3 (1%) | 2 (1%) | 0 | 1 (<1%) | 3 (1%) |
| 4 or more | 3 (1%) | 1 (1%) | 2 (1%) | 6 (1%) | 2 (1%) | 1 (1%) | 1 (<1%) | 4 (1%) |
DVDB=DVD and booklet.
A negative binomial regression model adjusted for pre-specified covariates showed a non-significant lower incidence of issued bronchodilator prescriptions in the DVDB group versus the usual care group (0·83, 95% CI 0·68–1·03) and in the face-to-face group versus the usual care group (0·81, 0·63–1·04). The incidence of GP respiratory-related consultations was similar in the DVDB group compared with the usual care group (0·93, 95% CI 0·74–1·15), in the face-to-face group compared with the usual care group (0·94, 0·72–1·24), and was also similar in the DVDB group compared with the face-to-face group (0·97, 0·77–1·22). There were only 12 respiratory hospital admissions during the 12 month study period; four in the DVDB group and eight in the usual care group.
The economic assessment is reported in detail elsewhere,14 but showed that both interventions were better than usual care (ie, they resulted in superior outcomes at lower total cost). This finding applied both to AQLQ and quality-adjusted life-year (QALY) comparisons but only the improvement in AQLQ was significant. The mean annual NHS total medical costs (including costs of medication and medical consultations) were numerically lower in the DVDB group (£296) than in both the face-to-face group (£335) and the usual care group (£356), but the result was not statistically significant (appendix). The cost of each intervention (£83·45 per patient for face-to-face group and £2·85 for DVDB group) was offset by reductions in health service use, but the differences in total cost between the intervention groups and usual care group were not significant. The probability of dominance (improved AQLQ at lower cost than control) was 93% for DVDB and 82% for face-to-face interventions. The corresponding figures for improved QALY at lower cost than usual care was 82% for the DVDB group and 51% for the face-to-face group. The low cost of the DVDB intervention meant that it was highly likely to be the most cost-effective option.
744 adverse events occurred in 272 (42%) of 655 patients, with 101 (39%) of 261 patients in the DVDB group, 55 (42%) of 132 patients in the face-to-face group, and 132 (50%) of 262 in the usual care group, with patients reporting one or more event. 11 (4%) patients in the DVDB group, four (3%) patients in the face-to-face group, and 20 (8%) patients in the control group had a serious adverse event. Three deaths occurred that were considered not to be related to the study (one in the DVDB group and two in the usual care group).
Patient experiences of both interventions were favourable in those returning the 3-month questionnaires and in the qualitative interviews, with all of the 132 patients in the face-to-face group returning their questionnaire and 256 (98%) of 261 patients in the DVDB group reporting practising the techniques. Perceived benefits included increased control over breathing, reduced need for medication, feeling more relaxed, and improved quality of life (appendix).
Discussion
In our pragmatic randomised trial, we confirmed that three sessions of face-to-face physiotherapist-taught breathing exercises improve disease-related quality of life for adults with asthma, and we have also shown that equivalent improvements can be achieved from a self-guided digital audiovisual programme. This programme was cost-effective14 and was considered to be an acceptable intervention method by patients.
Breathing retraining exercises are of considerable interest to people with asthma, and are recommended in evidence-based guidelines as possible adjuvant treatment for patients whose symptoms are not adequately controlled by pharmacological treatment.1, 7 The current evidence base for breathing retraining in asthma has been assessed as convincing by some systematic reviews,21 and the most recent Cochrane review22 has reported encouraging trends, but called for further, adequately powered and methodically sound research. Current access to breathing retraining therapy within the NHS is limited by the availability of suitably trained physiotherapists and the logistical and economical challenges to providing face-to-face therapy to a wider population.
To our knowledge, we report the largest trial of breathing retraining in asthma to date. A pragmatic trial design was used, which aimed to recruit a representative patient population (with minimal exclusion criteria) and to minimally disrupt the process of normal care, to maximise generalisability of the results. The main finding of this study is that asthma-related quality-of-life impairment is equivalently improved by the routine face-to-face delivery method for breathing retraining and our alternative intervention of a digital self-guided programme of physiotherapy-based breathing retraining. We confirmed improvements in quality-of-life scores over usual care previously reported in smaller studies for face-to-face physiotherapist-taught programmes, and additionally showed that the DVDB programme results in equivalent clinically relevant benefits more conveniently and less expensively. To show that a new intervention is as effective as an established one, an equivalence margin must be specified for the primary outcome, and the 95% CI for the difference in effect between treatments contained within this margin. Our predefined equivalence margin was −0·3 to 0·3 AQLQ units, on the basis of a previously reported equivalence study.22 We actually observed a small numerical difference favouring the DVDB intervention over face-to-face treatment (0·04), with the 95% CIs being well within our predefined margin (−0·16 to 0·24), giving us confidence that we have demonstrated equivalence.
In agreement with previous studies,6 no significant changes in airway obstruction (as assessed by measures of respiratory physiology such as FEV1 and PEFR) or inflammation (by assessment of FENO) were observed, suggesting that breathing retraining provides a technique for coping better with the consequences and ongoing effects of having asthma, but is not disease-modifying. Therefore this intervention is unlikely to reduce the need for anti-inflammatory medication, so acts as adjuvant rather than replacement for pharmacotherapy. The programme was accepted well and engaged with by patients and could be implemented into routine care with low resource investment.
The importance of a pragmatic trial design to assess the real-world effectiveness of an intervention is recognised.12 Therefore, this randomised trial aimed to recruit a typical asthma population reflecting the heterogeneity of routine practice, and allowed normal care to proceed as much as possible. It was not possible to mask participants to intervention allocation, but observations and analyses were done by researchers masked to group allocation. A limitation of the study is that of the individuals invited to participate, only 10% were enrolled, with a slightly older age profile than the overall UK adult asthma population, and thus might represent an atypical population that might be more prepared to consider breathing retraining. We recruited patients aged 16–70 years, and the median age of participants in the trial was 57 years. UK survey data from 2016 report that the prevalence of asthma in England in the 25–49 year age group is 10%, 11% in the 50–64 year age group, and 13% in the over 65 year age group. There was a modest over-representation of older people in our trial, probably reflecting the fact that people aged over 60 years are more likely to agree to participate in a clinical trial; therefore, further work is required to confirm effectiveness in younger age groups. The requirement to attend study-related visits and to complete questionnaires might have put some people off participating in the study, but preceding qualitative work indicates that the intervention itself would be widely acceptable.10
The mean improvement from baseline AQLQ scores was 1·1 for both the DVDB and face-to-face groups, and 0·8 in the usual care group, with 0·5 equating to a clinically important improvement and 1·0 to a large improvement,20 suggesting that, on average, participants in all groups had clinically relevant improvements in quality of life over the study period. A previous pragmatic active comparator clinical trial in a population of adult patients with suboptimally controlled asthma, reported within-group improvements from baseline in AQLQ scores similar to those observed in our study by the addition of either a long-acting β agonist (mean difference from baseline 1·0) or of a leukotriene receptor antagonist (0·8).23 In our study, there was also a marked improvement from baseline in the usual care group, which is likely to relate to regression to the mean and trial involvement effects, but significant improvements remained in both intervention groups compared with the usual care group.
The qualitative and quantitative process assessments indicated that most participants engaged with breathing retraining, whether it was delivered face-to-face or via DVDB. In the 3-month postal questionnaires, there was an overall response rate of 388 (99%) of 393 patients in the two intervention groups, with high proportions reporting positive engagement with the active interventions and at least some level of practice (256 [98%] of 261 in the DVDB group and 132 [100%] of 132 in the face-to-face group). The amount of practice reported by patients was variable, and higher in the face-to-face group than in the DVDB group, although this was not associated with improved outcomes and there was no apparent evidence of a dose-response association between the amount of practice in the techniques and clinical benefit. The aim of breathing retraining is to teach patients how to adjust their behaviour so that they can embed the techniques into their daily lives. Whether there is a minimum amount of practice required to learn and benefit from the breathing retraining techniques, and whether repeated training is needed at some future time, is not clear from this study and requires future investigation.
Although the significant group mean improvements that we report in both breathing retraining groups are below the minimum clinically important difference for an individual patient, the developers of the AQLQ instrument explicitly state that even if the mean difference between a treatment and a control is appreciably less than the smallest change, treatment might have an important effect on many patients.20 Similarly, one networked meta-analysis of pharmacological randomised trials assessing the magnitude of treatment effects on AQLQ,18 stated that the established within-patient minimum clinically important difference for ACQ and AQLQ is not achievable as a group-wise efficacy threshold between treatment groups in clinical studies. This meta-analysis reported that the mean improvements from the addition of a long-acting β agonist compared with control were 0·35 (95% CI 0·27 to 0·43), 0·20 (0·13 to 0·27) from the addition of leukotriene receptor antagonists, 0·01 (−0·20 to 0·22) from the addition of theophylline, and 0·31 (0·20 to 0·41) from the addition of omalizumab.18 By comparison, improvements in our study from the DVDB intervention compared with usual care were 0·28 (95% CI 0·11 to 0·44), and 0·24 (0·04 to 0·44) for face-to-face physiotherapy compared with usual care. From a patient perspective, the quality of life improvements achieved from both physiotherapy-based programmes in this randomised trial were, therefore, of similar magnitude to those associated with commonly used pharmacological step-up strategies, and could be considered either in addition to, or instead of, increases in drug treatment. Whether improvements associated with pharmacological and non-pharmacological strategies are additive is not known.
The heterogeneity of asthma is increasingly recognised, with evidence of identifiable clinical phenotypes.24, 25 However, a weak association is consistently observed between patient-reported outcome measures and so-called objective parameters measuring airway physiology or inflammation,26, 27 although a stronger association has been reported between outcomes and psychosocial factors.28, 29 Personalised precision medicine aims to target treatments to individuals based on a multi-dimensional assessment incorporating biomarker, phenotypic, and psychosocial characterisation, and an individualised approach based on treatable traits has been advocated in airways disease.30 There is growing evidence that inflammatory biomarkers can identify individuals who will respond to targeted anti-inflammatory treatments in asthma.31, 32 It has been less clear how to meet the needs of symptomatic individuals without objective evidence of an abnormal and pharmacologically modifiable pathway. We believe that it is now possible to offer this simple non-pharmacological intervention as a part of a rational overall asthma treatment strategy.
The use of DVDs has declined since the inception of the study, with a corresponding growth in online and streamed sources for provision of digital information and entertainment. Accordingly, to make this intervention accessible to clinicians, researchers, and people with asthma, we have made the content of the DVD and the supporting booklet freely available online through the Breathe study website. Additionally, we are in the process of producing a web-optimised version of the intervention for online use, in collaboration with our partner patient charity, Asthma UK, and we anticipate that this material will be freely available to patients and professionals in 2018. The low cost of providing an internet-based intervention, the ease of access of content and the absence of adverse outcomes using such approaches indicate that this evidence-based non-pharmacological intervention can now be offered to people with asthma with persisting quality-of-life impairment despite current asthma medication. There is a need to stress to patients that this intervention is in addition to, not instead of, current medication, and that it does not cure asthma, but rather is a means to improve quality of life.
Acknowledgments
Acknowledgments
This article presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Contributors
The original study conception and overall leadership came from MT and AB. The senior statistician was AL, who supervised the trial statistician MT, who did the statistical analyses. Study raw data was also available to the health economic team (JR, SZ, GY) and the chief investigator (MT). LY provided leadership for the development of the digital behavioural intervention and the process assessments. SG, MM, PL, RD, DP, IDP, and STH provided input into the original trial design, supporte d the trial management and advised on interpretation and presentation of results. JR designed the health economic component and was a member of the trial management group, with the health economic analyses done by SZ and GY. EA-C and SK were involved in the digital intervention development process and in the quantitative and qualitative process assessments. JV was involved in the original study conception (at the time being the research lead for Asthma UK) and provided public and patient involvement input, along with MS-W (both of whom attended trial management meetings as PPI representatives). DG was involved in the intervention development. FW and LT were involved in the organisation and delivery of the study. All authors were involved in commenting on the manuscript and have approved the submitted version.
Declaration of interests
AB reports a National Institute for Health Research Senior Research Fellowship. LY reports grants from the National Institute of Health Research during the conduct of the study. JR is a member of the National Institute of Health Research Health Technology Assessment [HTA] programme Editorial Board, outside the submitted work. EA-C reports grants from the National Institute of Health Research during the conduct of the study. MM reports grants from the National Institute of Health Research HTA outside the conduct of the study. RD reports personal fees from Teva Pharmaceuticals; grants and personal fees from Novartis; and personal fees and other support from Synaigen outside the submitted work; he is also Chairman of the European Respiratory Society Clinical Research Collaboration and SHARP. DP reports board membership at Aerocrine, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, Mylan, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; consultancy for Almirall, Amgen, AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Mylan, Mundipharma, Napp, Novartis, Pfizer, Teva Pharmaceuticals, and Theravance; grants from Aerocrine, AKL Research and Development Ltd, AstraZeneca, Boehringer Ingelheim, British Lung Foundation, Chiesi, Mylan, Mundipharma, Napp, Novartis, Pfizer, Respiratory Effectiveness Group, Teva Pharmaceuticals, Theravance, UK National Health Service, and Zentiva; speaking fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, GlaxoSmithKline, Kyorin, Mylan, Merck, Mundipharma, Novartis, Pfizer, Skyepharma, and Teva Pharmaceuticals; payment for manuscript preparation from Mundipharma and Teva Pharmaceuticals; travel grants from Aerocrine, AstraZeneca, Boehringer Ingelheim, Mundipharma, Napp, Novartis, and Teva Pharmaceuticals; funding for patient enrolment from Chiesi, Novartis, Teva Pharmaceuticals, and Zentiva; payment for development of educational materials from Mundipharma and Novartis; and non-financial support from the Efficacy and Mechanism Evaluation programme and Health Technology Assessment outside the submitted work; DP also has stock or stock options from AKL Research and Development Ltd, which produces phytopharmaceuticals; owns 74% of the social enterprise Optimum Patient Care Ltd (Australia, Singapore, and UK); and owns 74% of Observational and Pragmatic Research Institute Pte Ltd (Singapore). IDP reports personal fees from AstraZeneca, Boehringer Ingelheim, Aerocrine, Almirall, Novartis, GlaxoSmithKline, Genentech, Regeneron, Merck & Co, Schering-Plough, Mylan Speciality (Dey Pharma), Napp Pharmaceuticals, and Respivert outside the submitted work. All other authors declare no competing interests.
Supplementary Material
Reference
- 1.Global Initiative for Asthma: global strategy for asthma management and prevention. 2017. http://ginasthma.org/2017-gina-report-global-strategy-for-asthma-management-and-prevention/ No authors listed. (accessed March 6, 2017).
- 2.Bateman ED, Boushey HA, Bousquet J. Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 3.Demoly P, Gueron B, Annunziata K, Adamek L, Walters RD. Update on asthma control in five European countries: results of a 2008 survey. Eur Respir Rev. 2010;19:150–157. doi: 10.1183/09059180.00002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ernst E. Breathing techniques—adjunctive treatment modalities for asthma? A systematic review. Eur Respir J. 2000;15:969–972. doi: 10.1183/09031936.00.15596900. [DOI] [PubMed] [Google Scholar]
- 5.Holloway EA, West RJ. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax. 2007;62:1039–1042. doi: 10.1136/thx.2006.076430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas M, McKinley RK, Mellor S. Breathing exercises for asthma: a randomised controlled trial. Thorax. 2009;64:55–61. doi: 10.1136/thx.2008.100867. [DOI] [PubMed] [Google Scholar]
- 7.Scottish Intercollegiate Guidelines Network British guideline on the management of asthma: a national clinical guideline. 2016. https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-asthma-guideline-2016/ (accessed March 6, 2017).
- 8.McLean G, Murray E, Band R. Interactive digital interventions to promote self-management in adults with asthma: systematic review and meta-analysis. BMC Pulm Med. 2016;16:83. doi: 10.1186/s12890-016-0248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruton A, Kirby S, Arden-Close E. The BREATHE study: breathing retraining for asthma—trial of home exercises. A protocol summary of a randomised controlled trial. Prim Care Respir J. 2013;22:PS1–PS7. doi: 10.4104/pcrj.2013.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roche N, Reddel HK, Agusti A. Integrating real-life studies in the global therapeutic research framework. Lancet Respir Med. 2013;1:e29–e30. doi: 10.1016/S2213-2600(13)70199-1. [DOI] [PubMed] [Google Scholar]
- 11.Juniper EF, Guyatt GH, Epstein RS, Ferrie PJ, Jaeschke R, Hiller TK. Evaluation of impairment of health-related quality of life in asthma: development of a questionnaire for use in clinical trials. Thorax. 1992;47:76–83. doi: 10.1136/thx.47.2.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res. 2015;17:e30. doi: 10.2196/jmir.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arden-Close E, Teasdale E, Tonkin-Crine S. Patients' perceptions of the potential of breathing training for asthma: a qualitative study. Prim Care Respir J. 2013;22:449–453. doi: 10.4104/pcrj.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas M, Bruton A, Little P. A randomised controlled study of the effectiveness of breathing retraining exercises taught by a physiotherapist either by instructional DVD or in face-to-face sessions in the management of asthma in adults. Health Technol Assess. 2017;21:1–162. doi: 10.3310/hta21530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J. 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Dixhoorn J, Duivenvoorden HJ. Efficacy of Nijmegen questionnaire in recognition of the hyperventilation syndrome. J Psychosom Res. 1985;29:199–206. doi: 10.1016/0022-3999(85)90042-x. [DOI] [PubMed] [Google Scholar]
- 17.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 18.Bateman ED, Esser D, Chirila C. Magnitude of effect of asthma treatments on asthma quality of life questionnaire and asthma control questionnaire scores: systematic review and network meta-analysis. J Allergy Clin Immunol. 2015;136:914–922. doi: 10.1016/j.jaci.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 19.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 20.Guyatt GH, Juniper EF, Walter SD, Griffith LE, Goldstein RS. Interpreting treatment effects in randomised trials. BMJ. 1998;316:690–693. doi: 10.1136/bmj.316.7132.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connor E, Patnode CD, Burda BU, Buckley DI, Whitlock EP. Agency for Healthcare Research and Quality; Rockville, MD: 2012. Breathing exercises and/or retraining techniques in the treatment of asthma: comparative effectiveness: AHRQ Publication EHC092-EF. [PubMed] [Google Scholar]
- 22.Freitas DA, Holloway EA, Bruno SS, Chaves GS, Fregonezi GA, Mendonça KMPP. Breathing exercises for adults with asthma. Cochrane Database Syst Rev. 2013;10 doi: 10.1002/14651858.CD001277.pub3. CD001277. [DOI] [PubMed] [Google Scholar]
- 23.Price D, Musgrave SD, Shepstone L. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695–1707. doi: 10.1056/NEJMoa1010846. [DOI] [PubMed] [Google Scholar]
- 24.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 25.Haldar P, Pavord I, Shaw D. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Juniper EF, Wisniewski ME, Cox FM, Emmett AH, Nielsen KE, O'Byrne PM. Relationship between quality of life and measures of clinical status in asthma: a factor analysis. Eur Respir J. 2004;23:287–291. doi: 10.1183/09031936.04.00064204. [DOI] [PubMed] [Google Scholar]
- 27.Shaw D, Green R, Berry M. A cross-sectional study of patterns of airway dysfunction, symptoms and morbidity in primary care asthma. Prim Care Respir J. 2012;21:283–287. doi: 10.4104/pcrj.2012.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rimington LD, Davies DH, Lowe D, Pearson MG. Relationship between anxiety, depression, and morbidity in adult asthma patients. Thorax. 2001;56:266–271. doi: 10.1136/thorax.56.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas M, Bruton A, Moffat M, Cleland J. Asthma and psychological dysfunction. Prim Care Respir J. 2011;20:250–256. doi: 10.4104/pcrj.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agusti A, Bel E, Thomas M. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47:410–419. doi: 10.1183/13993003.01359-2015. [DOI] [PubMed] [Google Scholar]
- 31.Green RH, Brightling CE, McKenna S. Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet. 2002;360:1715–1721. doi: 10.1016/S0140-6736(02)11679-5. [DOI] [PubMed] [Google Scholar]
- 32.Powell H, Murphy VE, Taylor DR. Management of asthma in pregnancy guided by measurement of fraction of exhaled nitric oxide: a double-blind, randomised controlled trial. Lancet. 2012;380:1583–1589. doi: 10.1016/S0140-6736(11)60971-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.