Abstract
Cerebral blood flow (CBF) is regulated to secure brain O2 delivery while simultaneously avoiding hyperperfusion; however, both requisites may conflict during sprint exercise. To determine whether brain O2 delivery or CBF is prioritized, young men performed sprint exercise in normoxia and hypoxia (PIO2 = 73 mmHg). During the sprints, cardiac output increased to ∼22 L min−1, mean arterial pressure to ∼131 mmHg and peak systolic blood pressure ranged between 200 and 304 mmHg. Middle-cerebral artery velocity (MCAv) increased to peak values (∼16%) after 7.5 s and decreased to pre-exercise values towards the end of the sprint. When the sprints in normoxia were preceded by a reduced PETCO2, CBF and frontal lobe oxygenation decreased in parallel (r = 0.93, P < 0.01). In hypoxia, MCAv was increased by 25%, due to a 26% greater vascular conductance, despite 4–6 mmHg lower PaCO2 in hypoxia than normoxia. This vasodilation fully accounted for the 22 % lower CaO2 in hypoxia, leading to a similar brain O2 delivery during the sprints regardless of PIO2. In conclusion, when a conflict exists between preserving brain O2 delivery or restraining CBF to avoid potential damage by an elevated perfusion pressure, the priority is given to brain O2 delivery.
Keywords: Exercise, high altitude, hypertension, cerebral blood flow, cerebral haemodynamics
Introduction
Cerebral vascular conductance is continuously adjusted to maintain cerebral oxygen delivery compensating for changes in both arterial O2 content (CaO2) and blood pressure.1,2 When CaO2 and/or blood pressure is reduced cerebral vascular conductance is increased, and vice versa. During exercise arterial blood pressure (i.e. cerebral perfusion pressure) increases as a function of exercise intensity,3 and may surpass the limits of cerebral autoregulation during forceful muscular contractions.4 This imposes a high wall tension in the cerebral arterial tree, which if unchecked could cause cerebrovascular damage.5–8 Although during exercise in hypoxia the brain can compensate for a lower CaO2 by increasing O2 extraction,9,10 this capacity is markedly lower relative to other tissues.10,11 Therefore, to maintain brain oxygen delivery at rest cerebral blood flow is increased in hypoxia.2,12 During dynamic exercise (i.e. cycling, running) in hypoxia the brain faces the combined challenges of reduced CaO2, hypocapnia and increased arterial blood pressure.13 Moreover, arterial blood pressure increases with exercise intensity while CaO2 is progressively reduced due to an impairment of pulmonary gas exchange in hypoxia.13,14 This may lead to inadequate brain oxygenation, central fatigue,15–17 and impaired neural muscle activation.18–20
During exercise in severe acute hypoxia (i.e. equivalent to altitudes above 4500 m) cerebral vascular conductance is much higher than in normoxia,21 implying that the diameter of the resistance vessels should be greater. According to the Laplace Law, the same blood pressure causes a greater wall tension the higher the diameter of vessels. Therefore, the haemodynamic risks for the cerebrovascular system will be greater during high-intensity exercise in hypoxia than in normoxia, unless the level of vasodilation is limited during high-intensity exercise in hypoxia.
During sprint exercise on the cycle ergometer maximal muscle contractions of the lower extremities are coordinated with intense static contractions by the arm muscles to stabilize the trunk. This likely elicits a marked arterial blood pressure elevation,3,4 however, the intra-arterial blood pressure response to sprint exercise has not been measured in humans. Moreover, it remains unknown how cerebral blood flow is regulated when a reduced CaO2 is combined with a high arterial blood pressure as during sprint exercise in severe acute hypoxia.
Therefore, the main aim of this study was to determine the responses of cerebral blood flow and brain oxygenation during sprint exercise and the immediate post-exercise period in humans. We also aimed to determine what is prioritized during sprint exercise: oxygen delivery or the avoidance of hyperperfusion? We hypothesized that cerebral blood flow would be reduced during sprint exercise, and this would depend on the degree of hypocapnia and increase in blood pressure. We further hypothesized that in severe acute hypoxia brain oxygen delivery would be reduced and consequently brain oxygenation would be compromised, leading to lower exercise performance than in normoxia. This information is crucial to provide a better understanding of the physiological responses to high-intensity exercise, currently recommended as a preventive and therapeutic tool in medicine.22–25
Methods
Subjects
This study was part of a larger project designed to address the mechanisms limiting whole body exercise performance in humans.26,27 The present investigation consisted of two studies, one a ‘non-invasive’ study (Study I) and another one with invasive procedures (Study II).26 Twenty physically active young men (age: 24.7 ± 4.7 years, height: 176.3 ± 6.2 cm, body mass: 75.9 ± 9.2 kg, body fat: 18.8 ± 5.1%, VO2max: 3.6 ± 0.6 L min−1 or 48.2 ± 6.8 mL kg−1 min−1) volunteered to participate in Study I. Another 11 comparable subjects (age: 21.5 ± 2.0 years, height: 173.8 ± 8.0 cm, body mass: 72.3 ± 9.3 kg, body fat: 16.1 ± 4.9%, VO2max: 3.6 ± 0.3 L min−1 or 50.7 ± 4.0 mL kg−1 min−1) volunteered to participate in Study II. Subjects received full oral and written information about the experiments and written consent was obtained from each participant. The studies were performed in accordance with the current version of the Helsinki Declaration and approved by the Ethical Committee of the University of Las Palmas de Gran Canaria (CEIH-2010-01 and CEIH-2009-01). Subjects were requested to refrain from ingesting caffeine and taurine-containing beverages and to avoid exercise 24 h before all the experiments.
General overview
After a familiarization process including incremental and sprint exercise (Excalibur Sport 925900, Lode, Groningen, The Netherlands), body composition (Lunar iDXA, General Electric, Wisconsin and Hologic QDR-1500, Hologic Corp., software version 7.10, Waltham, MA) and VO2max were first measured in both studies. On a subsequent day, subjects performed an isokinetic all-out 30 s sprint performed on the cycle ergometer (called Wingate test) at 80 rpm, while MCAv and frontal lobe oxygenation were assessed. During the Wingate test, subjects were required to sprint as fast and hard as possible from the start to the end of the sprint, while being verbally encouraged to give their maximal effort. With the ergometer set in isokinetic mode the resistance offered was continuously adjusted by an automatic servocontrol system that only allows a pedalling rate of 80 (±1) rpm, such that if the subject applies less force on the pedals then the resistance of the ergometer is reduced and vice versa. By using the same pedalling rate in all subjects and conditions we intended to reduce the variability due to differences in pedalling cadence.
In Study II leg VO2, intra-arterial blood pressures, as well as leg blood flow and cardiac output were also measured.
Power output and oxygen uptake (Study I and II)
In Study I, after 3 min recording resting values, subjects started to warm up (2 min at 60 W; 2 min at 100 W and 1 min at 150 W) followed by 5 min at 20 W at 30 rpm. Thirty seconds before the end subjects stopped pedalling and prepared for Wingate. At the fifth minute, subjects began to sprint as hard and fast as possible with the cycle-ergometer set to isokinetic mode at 80 rpm. This warm up resulted in a small reduction of CO2 end-tidal pressure (PETCO2) and cerebral blood flow before the start of the sprints. Once the sprint exercise finished, recovery values were recorded for 1 min.
In Study II, the warm up consisted of 3 min unloaded pedalling before the Wingate test and PETCO2 and PaCO2 were similar to that recorded at rest. In both studies, power output during the sprint is reported as instantaneous peak power (Wpeak-i), and as the mean power output achieved during the full duration of the sprints (Wmean-30). Oxygen uptake was measured with a calibrated metabolic cart (Vmax N29; Sensormedics, Yorba Linda, CA, USA). Respiratory variables were analysed breath-by-breath and averaged every 5 s during the sprint and every 20 s during the incremental exercise tests. The highest 20-s averaged VO2 recorded in normoxia was taken as the VO2max. The same criterion was applied to determine the VO2max in hypoxia.
Cerebral blood flow and oxygenation (Study I and II)
The mean blood flow velocity in the middle cerebral artery (MCAvmean), insonated through the trans-temporal window as described elsewhere,9,28 was determined as an estimate of cerebral blood flow. In Study I, two Doppler 2 MHz transducers were applied bilaterally over the middle transtemporal window29 (Multi Box, DWL, Singen, Germany). Since both Doppler probes yield similar readings these were averaged for further analysis to reduce variability. In Study II, only one Doppler probe was placed on the right side. To minimize potential movement artefacts the Doppler probes where kept in place with a head harness (Figure 1) and subjects were instructed to avoid head movements.
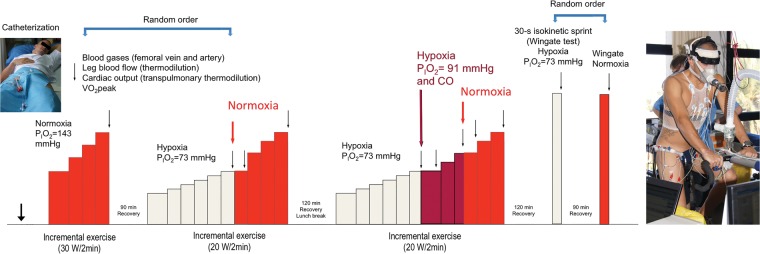
Figure 1.
Study II experimental protocol. After femoral artery and vein catheterization, subjects performed three incremental exercise tests. The first two were applied in random order. After the third incremental exercise test, they rest for two hours: Thereafter they performed 30-s all-out isokinetic sprints in normoxia or hypoxia, followed after 90 min recovery, by another sprint in hypoxia or normoxia, depending on the assignment by randomization. On the right, a picture of one of the volunteers fully instrumented for the experiments. Subjects were instructed to maintain a vertical position during the test and to minimize the movements of the head.
PIO2: inspiratory oxygen pressure; CO: carbon monoxide.
Cerebral oxygenation was assessed using near-infrared spectroscopy (NIRS, NIRO-200, Hamamatsu, Japan) employing spatial resolved spectroscopy to obtain the tissue oxygenation index (TOI) using a pathlength factor of 5.92.30 The NIRS optodes were placed on the right frontoparietal region at 3 cm from the midline and 2–3 cm above the supraorbital crest, to avoid the sagittal and frontal sinus areas. With this optode placement the tissue oxygenation of the superficial frontal cerebral cortex is recorded. This region is irrigated by the anterior cerebral artery, which, like the MCA, receives its flow from the internal carotid artery. Both MCA and anterior cerebral arteries communicate through the circle of Willis. In Study I, an additional optode was placed in the lateral aspect of the thigh at middle length between the patella and the anterosuperior iliac crest, over the middle portion of the m. Vastus lateralis. In Study II, the optodes were placed ipsilaterally to the Doppler probe, that is over the right frontal cortex region of the forehead.
Invasive experiments (Study II)
The experimental protocol for invasive experiments is illustrated in Figure 1. On the experimental day, subjects reported to the laboratory at 07.00 after an overnight fast from 22.00 h. After catheterization (see below) subjects performed three incremental exercise tests: the first two in normoxia or hypoxia (in random order), and a third one always in hypoxia (PIO2 = 73 mmHg), followed by a 2-h resting period, as previously reported.26 Thereafter, the subjects performed in random order two isokinetic Wingate tests, one in normoxia (PIO2 = ∼ 143 mmHg) and another in hypoxia (PIO2 = ∼ 73 mmHg, Altitrainer200, SMTEC, Switzerland) interspaced by a 90 min resting period. No warm up was performed before the Wingate tests apart from 3 min of unloaded pedalling. Subjects breathed hypoxic gas for 4 min before the start of the Wingate in hypoxia. At the end of the Wingate test recovery was always performed in normoxia. Part of the results on oxygen transport and pulmonary gas exchange obtained during the invasive experiments has been reported.26,31,32 Since 7 ml (kg body mass)−1 of carbon monoxide (CO) was administered at exhaustion during the last incremental exercise test, a small amount of CO was present in blood at the start of the Wingate test.26
Catheterization and preparation (Study II)
Prior to the exercise tests, both femoral veins and one femoral artery were catheterized under local anaesthesia (2% lidocaine).26 Catheters and thermistors were used to obtain blood samples, to measure venous and arterial blood temperatures, and to determine leg blood flow (LBF) and cardiac output using the constant infusion thermodilution method.33
Blood sampling (Study II)
Prior to the sprint exercise a blood sample was obtained simultaneously from the right femoral artery and the left femoral vein. Then, during the sprint blood was sampled every 5 s from the left femoral vein and every 10 s from the right femoral artery and used for determination of blood gases and haemoglobin concentrations (ABL90, Radiometer, Copenhagen, Denmark). Blood gases and pH were corrected for blood temperature as previously reported.31
Statistical analysis
Sample size calculation was performed based on previous studies, assuming an effect size of 1.2 for the effect of severe hypoxia on cerebral blood flow21 using free online software G*Power, setting type I error and power to 5% and 80%, respectively. This resulted in a calculated group size of eight subjects. Variables were normally distributed as shown by the Shapiro–Wilks test. Two-way repeated-measures ANOVA for oxygenation (two levels: normoxia vs. hypoxia) and time (six levels: 0, 5, 10, 15, 20, 25, and 30 s) was used to analyse the responses observed during the sprints. The post exercise recovery period was divided in three 5 s averages, which were analysed using also two-way repeated-measures ANOVA for oxygenation (two levels: normoxia vs. hypoxia) and time (three levels: 5, 10, and 15). The Mauchly’s test of sphericity was run before the ANOVAs, and in the case of violation of the sphericity assumption the degrees of freedom were adjusted according to the Huynh and Feldt test. Pairwise comparisons at specific time points were performed with the Student t-test, and adjusted for multiple comparisons with the Holm–Bonferroni method. The relationship between variables was determined using linear regression analysis. Values are reported as the mean ± standard deviation (unless otherwise stated). P < 0.05 was considered significant. All statistical analysis was performed using SPSS v.15.0 for Windows (SPSS Inc., Chicago, IL).
Results
Study I
The responses of ergometric and cardiorespiratory variables are depicted in Figures 2 and 3. During the sprint, subjects reached 92%, 76%, 86%, 77%, 89% and 99% of maximal heart rate (HR), pulmonary ventilation (VE), O2 uptake (VO2), CO2 production (VCO2), VE/VO2 and VE/VCO2 observed during incremental exercise to exhaustion. Middle cerebral artery velocity followed a decreasing curvilinear pattern during the sprint (MCAv = 44.2 − 0.265 t + 0.0026 t2, where MCAv is in cm s−1 and t is in s, R2 = 0.81, P < 0.001; n = 7, each point representing the mean of 20 subjects). From the values recorded just before the start of the sprint to the end of the sprint the MCAv was reduced by 10% (P < 0.05). During the sprints there was dissociation between the changes in MCAv and PETCO2. Frontal lobe cerebral oxygenation (FLO) paralleled the MCAv, that is, it was progressively reduced during the sprint following a curvilinear pattern (FLO = 68.0 − 0.136 t + 0.0004 t2, where FLO is the TOI of the frontal lobe in % and t is the time in s, R2 = 0.97, P < 0.001, n = 7, each point representing the mean of 20 subjects). This resulted in a reduction of TOI from 67.9 ± 7.1 to 64.7 ± 7.9 (P < 0.05). While only small changes in frontal lobe oxygenation were observed during the sprint, Vastus lateralis tissue oxygenation was reduced to minimal values in just 15 s (Figure 3(e)).
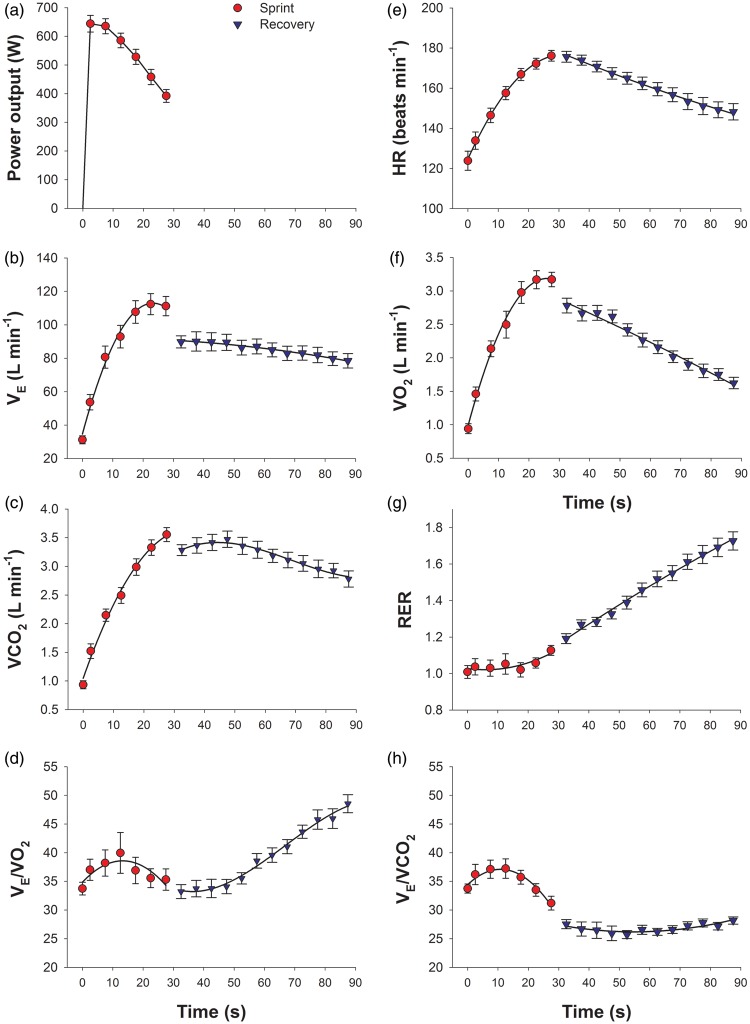
Figure 2.
Ergospirometric variables during the 30 s sprint in normoxia (red circles) and the first minute of the recovery period (blue triangles) (Study I). (a) Power output; (b) pulmonary ventilation (VE); (c) carbon dioxide production (VCO2); (d) ventilatory equivalent for O2 (VE/VO2); (e) heart rate (HR); (f) oxygen consumption (VO2); (g) respiratory exchange ratio (RER); and (h) ventilatory equivalent for CO2 (VE/VCO2); the error bars represent the standard error of the mean, n = 20.
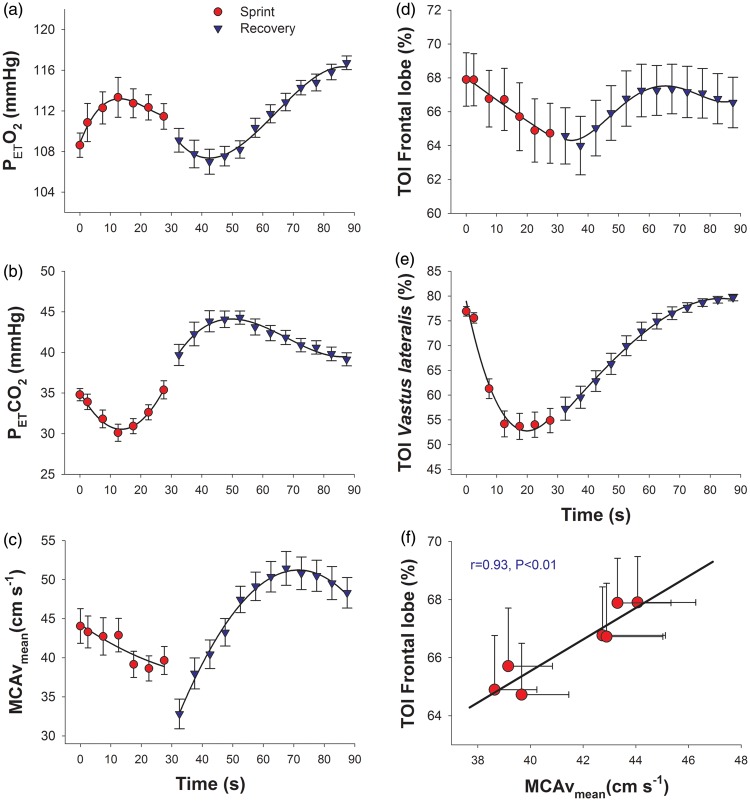
Figure 3.
Respiratory variables brain blood flow and tissue oxygenation during the 30 s sprint in normoxia (red circles) and the first minute of the recovery period (blue triangles) (Study I). (a) End-tidal O2 pressure (PETO2); (b) end-tidal CO2 pressure (PETCO2); (c) middle-cerebral artery mean velocity (MCAvmean); (d) power output; (b) pulmonary ventilation (VE); (c) carbon dioxide production (VCO2); (d) frontal lobe tissue oxygenation index (TOI); and (e) Vastus lateralis tissue oxygenation index; (f) relationship between frontal lobe tissue oxygenation index (TOI) and middle-cerebral artery mean velocity (MCAvmean), each value corresponds to the mean of 20 subjects for the 0, 2.5, 7.5, 12.5, 17.5, and 22.5, 27.5 s time points, and the errors bars represent the standard error of the mean.
With the interruption of contractile activity, 2.5 s after stopping, VE was reduced by 21 L min−1 and VO2 by 390 mL min−1. During the first 20–30 s of the recovery, VE and VCO2 remained almost unchanged, to slowly decrease thereafter. At 2.5 s of the recovery, the MCAv dropped to 32 cm s−1, that is 18% less than observed at the end of exercise. Thereafter, MCAv increased markedly to reach maximal values of 51 cm s−1 at 40 s after the end of exercise that is about 15 s after the peak values of PETCO2 observed during the recovery. Thereafter, MCAv decreased towards the values observed before the start of the sprint. In general, frontal lobe oxygenation reproduced the same pattern observed for MCAv, except at the end of exercise when, despite an abrupt reduction in MCAv, frontal lobe oxygenation remained unchanged.
There was a strong linear association between MCAv and frontal lobe oxygenation during the sprint exercise (r = 0.93, P < 0.01; n = 7, each point representing the mean of 20 subjects) (Figure 3(f)). During recovery the association was less strong but was statistically significant (r = 0.43, P < 0.05; n = 12, each representing the mean of 20 subjects).
Study II
Power output and systemic haemodynamics
In study II, good MCAv recordings were obtained in eight subjects, while a complete set of haemodynamic data during both Wingate test was obtained in nine subjects. Peak power output was not statistically different between normoxia and hypoxia (973 ± 300 and 906 ± 377 W, respectively, P = 0.36). However, the mean power output was reduced by 7%, from 494 ± 47 W in normoxia to 461 ± 52 W in hypoxia (P < 0.05), as previously reported.26
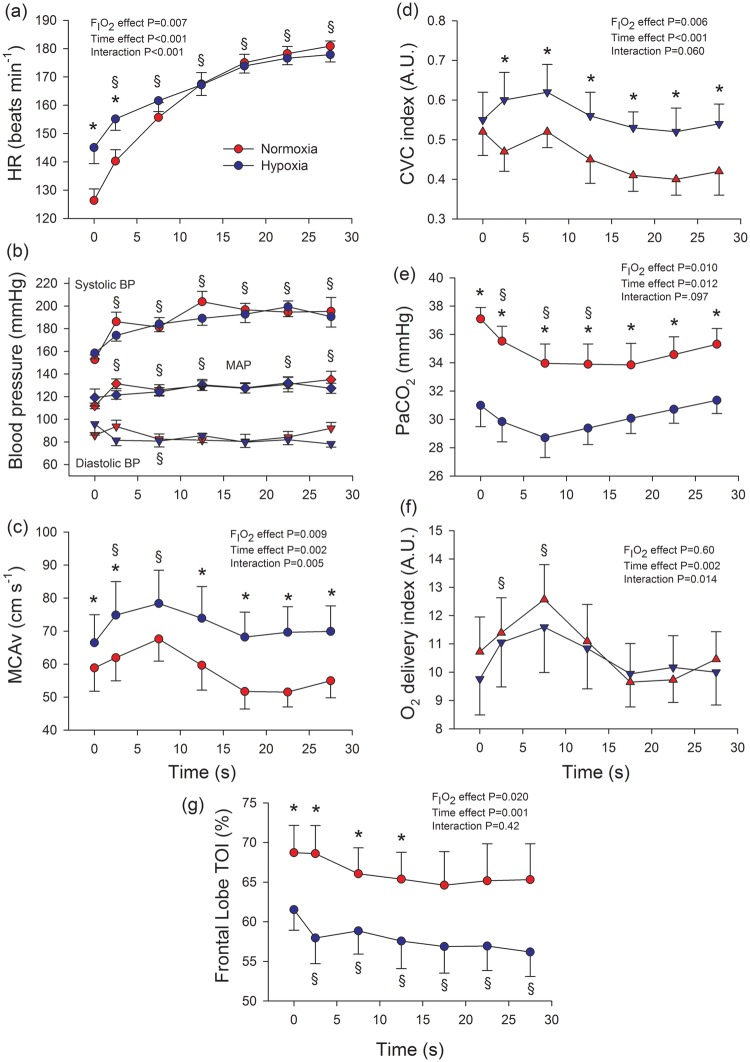
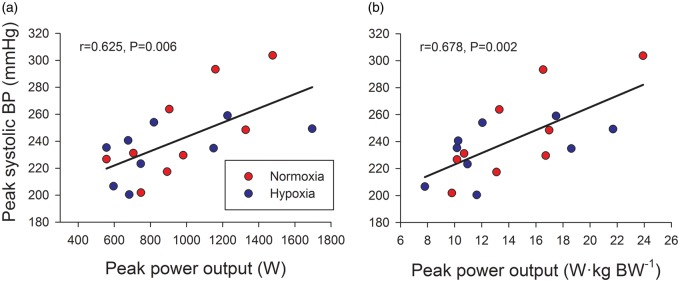
Figure 4 depicts the main hemodynamic variables assessed in a representative subject during a Wingate test in normoxia and hypoxia. As shown in Figure 5(a), heart rate was higher during the first 10 s of the sprint in hypoxia, being similar in both conditions during the last 20 s. Peak cardiac output was 23.2 ± 2.5 and 21.0 ± 2.5 L min−1 in normoxia and hypoxia, respectively (P < 0.05), as previously reported.26 Blood pressure responses are depicted in Figure 5(b). Systolic blood pressure increased during the sprint to mean values close to 200 mmHg (FIO2 effect: P = 0.38; time effect: P < 0.001; FIO2 × time interaction: P = 0.24), while diastolic blood pressure remained at the level observed just before the start of the sprint (80–90 mmHg) (FIO2 effect: P = 0.38; time effect: P = 0.12; FIO2 × time interaction: P = 0.10). Consequently, MAP was increased by approximately 16 mmHg from ∼ 115 to ∼ 131 mmHg (FIO2 effect: P = 0.59; time effect: P = 0.004; FIO2 × time interaction: P = 0.34). The highest systolic blood pressure values recorded were 222 ± 27 (range: 202–304) and 211 ± 24 (range: 200–259) mmHg, in normoxia and hypoxia, respectively (P = 0.197). The highest systolic blood pressure values recorded during the sprints were linearly related to the peak power output expressed in absolute (i.e. in watts) (r = 0.625, P = 0.006) and relative values (i.e. W kg−1 body mass; r = 0.678, P = 0.002) (Figure 6).
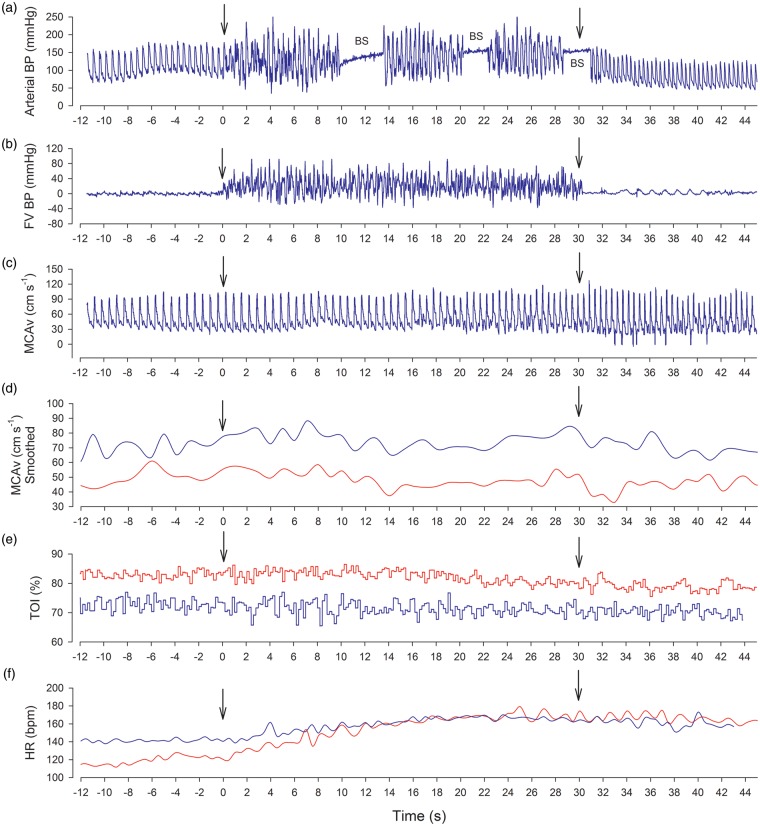
Figure 4.
Raw data obtained from one of the volunteers during 30 s sprints in normoxia (red lines) and severe acute hypoxia (PIO2 = 73 mmHg, blue lines). (a) Intra-arterial blood pressure (FA); (b) blood pressure in the femoral vein (FV); (c) middle-cerebral artery velocity (MCAv); (d) smoothed MCAv (1 s averages); (e) frontal lobe tissue oxygenation index (TOI); (f) heart rate (HR). The first vertical arrow indicates the start of the sprint, and the second the end of the sprint, i.e., the start of the recovery.
BP: blood pressure; BS: blood sample; FV: femoral vein; FA: femoral artery.
Figure 5.
Haemodynamic and cerebral blood flow responses to sprint exercise in normoxia (red circles) and severe acute hypoxia (blue circles; PIO2 = 73 mmHg; study II). (a) heart rate (HR), n = 9; (b) blood pressures, n = 9; (c) middle-cerebral artery mean velocity (MCAvmean), n = 8; (d) cerebrovascular conductance (CVC) index, calculated as the quotient MCAv/MAP, n = 8; (e) arterial partial pressure of carbon dioxide (PaCO2), n = 9; (f) Oxygen delivery index, calculated as the product of arterial oxygen content (CaO2) × MCAv, n = 8; (g) frontal lobe tissue oxygenation index (TOI), n = 9.
BP: blood pressure; MAP: mean arterial pressure. During exercise the Doppler signal was lost in three subjects. *P < 0.05 normoxia versus hypoxia, at the same time point; §P < 0.05 time effect compared to immediately before the start of the sprint; the error bars represent the standard error of the mean.
Figure 6.
Relationships between systolic blood pressure and peak power output during sprint exercise in normoxia (red circles) and severe acute hypoxia (blue circles; PIO2 = 73 mmHg; Study II). The highest blood pressure recorded during the sprints plotted against the peak power output (the highest 1 s average) in absolute values (a) and relative values (b), n = 9.
The systolic blood pressure response to sprint exercise in normoxia was linearly related to the response observed in hypoxia (r = 0.81, P < 0.01). At the end of the sprint in normoxia and hypoxia, the MAP was rapidly and similarly reduced (FIO2 × time interaction: P = 0.773). MAP was reduced by 35, 49, and 57 mmHg at 2.5, 7.5 and 12.5 s into the recovery period (all P < 0.001), compared to the mean MAP recorded during the last 5 s of the sprint in normoxia. Similar MAP declines were observed after the sprint exercise in hypoxia.
Cerebral blood flow and O2 delivery
Seven and a half seconds after the start of the sprint MCAv was increased by 16% (Figure 5c) compared to the values recorded before the start of the sprint (ANOVA time effect P < 0.001). Thereafter, MCAv was decreased to reach values similar to pre-exercise towards the end of the sprint, but this decrease was more marked in normoxia (ANOVA FIO2 × time interaction: P = 0.005) (P < 0.05) (Figure 5(c)). During the sprint in severe hypoxia the mean MCAv was 25% higher than in normoxia (Figure 5(c)). At the end of the sprints, the MCAv dropped in normoxia by 11%, 7%, and 7%, and in hypoxia by 7%, 18% and 20%, at 2.5, 7.5 and 12.5 s into the recovery period, respectively (P < 0.05 compared to the value at the end of the sprint; ANOVA FIO2 × time interaction: P = 0.007).
During the sprints, the cerebral vascular conductance index (MCAv/MAP), paralleled MCAv, being 26% higher in hypoxia than normoxia (Figure 4(d)), despite the markedly lower PaCO2 in hypoxia than normoxia (Figure 5(d)). This level of vasodilation fully compensated for the 22% lower mean CaO2 during the whole sprint in hypoxia than normoxia (187 ± 3 and 146 ± 4 mL L−1, respectively, P < 0.001). Consequently, the O2 delivery index during the sprint was similar in both conditions, with an initial peak 18% above pre-exercise levels at the 7.5th s (P < 0.001) after the start of the sprint, followed by values similar to those observed at rest during the last 15 s of the sprint (Figure 5(f)).
Compared to the last 5 s of sprint, the cerebral vascular conductance index was markedly increased during the first 15 s of recovery, by 20% (P = 0.08), 47% and 61% in normoxia; and by 27%, 32% and 44% in hypoxia (ANOVA FIO2 × time interaction: P = 0.034). The level of cerebral vasodilation was 18% and 29% above pre-exercise values in normoxia and hypoxia, respectively (P < 0.01).
Compared to the values recorded immediately before the start of the sprint, frontal lobe oxygenation was similarly reduced by 4–5 units at the end of the sprint, regardless of FIO2 (Figure 5(g)). However, frontal lobe oxygenation was 8.5 units lower during sprint exercise in normoxia than hypoxia. Despite the marked decrease in MAP at the end of the sprint, during the first 15 s of the recovery period, frontal lobe oxygenation remained at a similar level to that reached during the last 5 s of the sprint in both conditions (i.e. approximately 3–4 units below the level observed immediately before the start of the sprint, P < 0.05).
Study I compared to normoxia in Study II
Before the start of the exercise MCAv was significantly lower in Study I than II (44.1 ± 2.2 and 58.0 ± 6.3 cm s−1, respectively, P = 0.014), due to a lower PETCO2 in Study I (34.8 ± 0.8 and 38.0 ± 1.1 mmHg, respectively, P = 0.025). Frontal lobe oxygenation before the start of the sprints was similar in both studies (67.9 ± 1.6% and 66.8 ± 3.6%, respectively, P = 0.75).
Discussion
The purpose of this study was two-fold. First, we sought to characterise the responses of cerebral blood flow and brain oxygenation during sprint exercise and the immediate post-exercise period. Second, we asked if oxygen delivery or the avoidance of hyperperfusion is prioritized during sprint exercise. We have shown that cerebral blood flow is exquisitely adjusted to maintain a normoxic brain oxygen delivery in severe acute hypoxia, both at rest and during sprint exercise. Moreover, our findings demonstrate that cerebral oxygen delivery is prioritized during sprint exercise when a conflict exists between preserving brain oxygen delivery or restraining cerebral blood flow to avoid potential damage by an elevated blood pressure.
Cardiorespiratory responses to sprint exercise pose a challenge to the brain circulation
During all-out 30 s sprinting peak power output values are reached within the first 5 s. At the end of the sprint VO2 reaches between 80% and 90% of maximum34 and cardiac output approaches peak values.26 This requires a strong central command activation of the medullary vasomotor and cardiorespiratory centres.35 As a part of these integrated responses arterial blood pressure was markedly increased in our study, with mean peak systolic values maintained close to 200 mmHg for the duration of sprint exercise. The blood pressure response we observed is similar or slightly lower than elicited by arm cranking at near-maximal intensities3 and slightly higher than observed close to exhaustion during incremental cycle3 or rowing exercise.36 The combined increase of mean arterial pressure and cardiac output facilitates muscle perfusion in active muscles, but may pose a challenge to the integrity of the blood brain barrier and the brain parenchyma in case of regional or global cerebral hyperperfusion.8,37
Due to the increase in mean arterial pressure, without opposing mechanisms cerebral blood flow would have been increased by ∼20–25% during the sprints, compared to the levels immediately before the start of the sprints. Thus, vasoconstrictor mechanisms must have efficiently opposed to the increase in perfusion pressure in order to avoid brain hyperperfusion during the sprints. The risk of vascular damage during hyperperfusion increases when accompanied by high blood pressure.5,8 Animal studies in lambs have shown a marked sympathetic nerve activity in the superior cervical ganglia in response to hypertension,38 accompanied by vasoconstriction of pial arteries via sympathetic activation combined with a myogenic response preventing or blunting the increase of cerebral blood flow.39,40 Other studies have proposed a role for the carotid and vertebral arteries in sympathetic regulation of cerebral blood flow. Small reductions in their diameter at the entry of the cranium, where they are more tortuous produce a substantial increase in their vascular resistance to limit brain perfusion.1,41,42 Sympathetic and myogenic responses have not been thought to be very important1 but they are probably the most rapid mechanism that could be recruited to prevent an excessive increase of cerebral blood flow during sprint exercise that combines a fast increase of cardiac output with a quick and sustained elevation of blood pressure from the start to the end of the sprint.
Recently, Willie et al.1 combined the results of numerous studies in healthy humans that have reported steady-state changes in mean arterial pressure (MAP) and cerebral blood flow (CBF). From this, they calculated that the slope of the %ΔCBF/%ΔMAP relationship above and below resting mean arterial pressure, has mean values of 0.81 ± 0.77 in the hypotensive range and 0.21 ± 0.47 in the hypertensive range. In our experiments the %ΔCBF/%ΔMAP lied close to 1.08 during the sprint. This is 5-fold higher than observed in other experimental setups, in which the increase in mean arterial pressure occurred more gradually and was not accompanied by an elevation of cardiac output.1 A %ΔCBF/%ΔMAP value close to 1 indicates lack of autoregulation at the start of the sprint or a delayed response, exposing the cerebral circulation to the potential harmful effects of a high blood pressure combined with increased blood flow.5,7,8 The greater degree of hyperventilation and, hence, lower PETCO2, before the start of the sprint exercise in Study I, might have prevented the initial increase in cerebral blood flow during the sprints performed in normoxia in Study I.
A peculiarity of sprint exercise relative to steady-state exercise is the fast, almost instantaneous, reduction of mean arterial pressure upon the cessation of exercise. Several factors to explain the reduction in mean arterial pressure include: a deactivation of the central command, lowered metaboreflex and mechanoreflex feedback after cessation of exercise, and fast reduction of cardiac output caused by the combination of lower central command and venous return.43,44 These post-sprint events pose a substantial challenge to brain O2 delivery homeostasis. According to the estimations by Willie et al.,1 with a 26% drop of mean arterial pressure in just 2.5 s, cerebral blood flow should be reduced by 21%. Indeed, this is in good agreement with the 18% drop in MCAv we observed 2.5 s after the end of the sprint in Study I.
Brain O2 delivery is the main variable determining the regulation of cerebral blood flow during sprint exercise
Brain function is so dependent on O2 that flow arrest leads to loss of consciousness within 5–10 s.45 Therefore, a continuous supply of O2 is required to maintain the cerebral metabolic rate (CMRO2). Sprint exercise causes hypocapnia, which may contribute to the reduction of cerebral blood flow during sprint exercise in normoxia. In fact, the lowest values of MCAv were observed 10 s after the nadir in PETCO2, what corresponds to the expected 5–10 s shift in the MCAv response due to the circulating time.33
For the PaCO2 drop observed during the sprints in severe hypoxia (10 mmHg), a 25% lower cerebral blood flow has been reported in resting humans with the Ketty–Schmidt method.46 However, the vasoconstrictor effect of hypocapnia was completely abrogated during the sprint exercise in severe hypoxia, as reflected by the 25% higher MCAv during the sprint in hypoxia compared to the sprints in normoxia, in Study II. Likewise, the reduction in MCAv observed in normoxia in Study I, was less than expected from the reduction in PETCO2.
The decline of cerebral blood flow during sprint exercise in Study I and during the last 15 s of the sprint in Study II occurred in a context for which an increased brain oxygen demand is expected10 due to the intense neuronal activity required to activate the muscles and cardiorespiratory medullary centres, and to process all afferent signals reaching the CNS.16,47 This neural activity is expected to enhance cerebral blood flow locally by a feed-forward mechanism,48 that is likely counterbalanced or adjusted by neurovascular coupling to provide a good regional match between O2 supply and the O2 demand generated by neural activity.16,49,50 Nevertheless, frontal lobe oxygenation was reduced during sprint exercise implying that O2 extraction likely increased to preserve the appropriate flux of O2 from the brain capillaries to the neurons.9 We interpret our results to mean that during the sprint performed in normoxia, the priority was given to the avoidance of brain hyperperfusion over the maintenance of O2 delivery, by allowing a small decline in cerebral blood flow. However, during sprint exercise in severe acute hypoxia, despite a similar perfusion pressure as in the sprints performed in normoxia, cerebral blood flow was increased to account for the reduction in CaO2 to maintain O2 delivery at levels similar to those observed during the sprint in normoxia. Study II demonstrates that the most critical variable regulated is brain oxygen delivery, to the extent that a greater level of brain perfusion is tolerated despite the increase in perfusion pressure and the reduction in PaCO2 during sprint exercise in severe hypoxia. This secures brain function under conditions of low oxygenation at the expense of increasing haemodynamic risks.51,52
Cerebral blood flow drops markedly upon cessation of sprint exercise
At the end of the sprints in normoxia (Study I) MCAv was abruptly lowered by ∼18 cm s−1 what is more than the 4–5 cm s−1 reduction observed after moderate intensity cycle ergometer exercise in the semi-recumbent position.43 The inability to maintain cerebral perfusion during the first seconds of recovery after sprint exercise likely reflects some impairment of cerebral autoregulation as previously reported for post-exercise recovery in the semi-recumbent position.43 Nevertheless, 20 s after the end of the sprint MCAv and frontal lobe oxygenation values were similar to pre-exercise values indicating a fast re-establishment of normal cerebral perfusion and oxygenation (Figure 3(b)). Our invasive data indicate that, compared to the values observed during the sprint, MAP is 50–60 mmHg lower 12.5 s after cessation of sprint exercise, remaining at this level during the first minute of recovery. Thus, myogenic relaxation (i.e. autoregulation) combined with the vasodilatory effects of increased PaCO2 and neurovascular coupling efficiently counteract the reduction in perfusion pressure that occurs abruptly upon cessation of exercise, although with a small delay.
Methodological considerations
This study has two main methodological limitations related with the use of NIRS to measure brain oxygenation and transcranial Doppler to assess brain blood flow. Although the frontal lobe NIRS signal is contaminated by noise from the skin,53 it has been shown that frontal lobe NIRS oxygenation tracks well changes in brain O2 delivery, during carotid surgery,54,55 postural changes from supine to standing,56 head-up tilt induced presyncope,57 lower-body negative pressure,58 systemic hypoxia,59 exercise,60 prolonged apnoea61 and return of spontaneous circulation during chest compressions.62,63
Since the assessment of cerebral blood flow by inert gasses or dye injection requires steady-state haemodynamic conditions, these techniques cannot be applied to sprint exercise.64 The usage of transcranial Doppler is not without limitations,65 but it does provide a measure of cerebral blood flow16,44 that agrees well with the 133Xenon clearance method.66 The use of transcranial Doppler to measure cerebral blood flow is based on the assumption that the diameter of the MCA does not change with the intervention. This seems to be the case for small fluctuations of PaCO2 (± 8 mmHg).65 Therefore, it is reasonable assuming that the diameter of the MCA was not changed in Study I by the 6 mmHg decrease and increase in PETCO2, during the sprint and recovery, respectively, assuming that PETCO2 is a good surrogate of PaCO2.31
In contrast to hypocapnia, hypoxia may elicit an augmentation of the MCA diameter.21 After 3 h of exposure to acute hypoxia (FIO2 = 0.12), the MCA diameter determined with magnetic resonance angiography was increased from 3.04 to 3.27 mm.21 Using the mean MCAv values recorded during the sprint in normoxia and hypoxia (i.e. 57.9 and 72.5 cm s−1, respectively), we have calculated that had our subjects experienced a similar level of MCA vasodilatation in acute hypoxia as observed by Wilson et al.,21 the estimated cerebral blood flow would have been 35% greater in hypoxia than normoxia. This compares with an estimated 25% increase in cerebral blood flow calculated assuming no change in MCA diameter. Actually, the experimentally observed 25% increase in cerebral blood flow was sufficient to account for the 22% lower CaO2 in hypoxia, indicating that in our experimental conditions most likely the diameter of MCA remained unchanged.
Finally, we only examined blood flow responses in MCA and we cannot rule out the possibility of a different response in other main cerebral arteries. During sub-maximal exercise, blood flow through the vertebral arteries increases more than through the internal carotid artery and MCA.67 Nevertheless, MCA and internal carotid artery flows increase in the same proportion.67 Thus, the haemodynamic risk to the brain barrier could be even higher in the vascular bed irrigated by the vertebral and basilar arteries, particularly in hypoxia.12
Conclusion
During maximal sprint cycle exercise lasting 30 s blood pressure increases markedly and cardiac output reaches values close to maximum at the end of the sprint, regardless of FIO2. When the sprint is performed in normoxia and PETCO2 has been reduced immediately before the beginning of the sprint cerebral blood flow decreases progressively during the sprint. Consequently, frontal lobe oxygenation also decreases in parallel to the changes of cerebral blood flow, indicating that during sprint exercise in normoxia the priority is given to limit brain hyperperfusion even at the expense of a small drop in cerebral blood flow. When the same exercise is performed in severe acute hypoxia, cerebral blood flow is increased to account for the reduction in arterial oxygen content, indicating that the main variable regulated during sprint exercise is oxygen delivery to the brain, which achieves values similar to those observed during sprint exercise in normoxia, regardless of PIO2, PaCO2 and mean arterial pressure. At the end of sprint exercise, mean blood pressure is dramatically reduced by 50–60 mmHg within the first 15 s, causing a drop in cerebral blood flow, which is partly blunted likely by autoregulatory mechanisms. The fast and marked changes in cardiac output and blood pressure during the sprint and the immediate recovery period challenge the regulation of brain circulation. It remains to be determined whether the combination of hypoxia and sprint exercise may be harmful for the integrity of the brain vasculature. New studies are required to establish the safety of sprint exercise for the integrity of the brain vascular tree in patients with hypertension, reduced arterial compliance, dysautonomy or a fragile vasculature.
Acknowledgements
The authors thank José Navarro de Tuero, Jesus Gustavo Ponce-González, Amelia Guadalupe-Grau, Teresa Fuentes and José Losa-Reyna for their technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants from the Ministerio de Educación y Ciencia of Spain (DEP2009-11638 and FEDER) and Ayudas a la Investigación Cátedra Real Madrid-UEM (2015/04RM).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Experiments were carried at the Laboratory of Human Performance in Las Palmas of Gran Canaria. Conception and design of the experiments: JAC. Pre-testing and experimental preparation: DC, DMA, EC, JAC, IPS, MMR, MPV and RTP. Data collection during the main experiments: AWS, CL, CS, DC, DMA, EC, JAC, IPS, MMR, MPV, PR and RTP. Data assembly and analysis: DC, DMA, JAC, MMR, MPV and RTP. The first version of the manuscript was written by DC and JAC. All co-authors read, contributed with comments and approved the final version of the manuscript.
References
- 1.Willie CK, Tzeng YC, Fisher JA, et al. Integrative regulation of human brain blood flow. J Physiol 2014; 592: 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lassen NA. Cerebral blood flow and oxygen consumption in man. Physiol Rev 1959; 39: 183–238. [DOI] [PubMed] [Google Scholar]
- 3.Calbet JA, Gonzalez-Alonso J, Helge JW, et al. Central and peripheral hemodynamics in exercising humans: leg vs arm exercise. Scand J Med Sci Sports 2015; 25(Suppl 4): 144–157. [DOI] [PubMed] [Google Scholar]
- 4.MacDougall JD, Tuxen D, Sale DG, et al. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol 1985; 58: 785–790. [DOI] [PubMed] [Google Scholar]
- 5.van Mook WN, Rennenberg RJ, Schurink GW, et al. Cerebral hyperperfusion syndrome. Lancet Neurol 2005; 4: 877–888. [DOI] [PubMed] [Google Scholar]
- 6.Koh SX, Lee JK. S100B as a marker for brain damage and blood-brain barrier disruption following exercise. Sports Med 2014; 44: 369–385. [DOI] [PubMed] [Google Scholar]
- 7.Lin TW, Wang JN, Kan CD. Cerebral hyperperfusion syndrome after surgical repair of congenital supravalvular aortic stenosis. Ann Thoracic Surgery 2015; 100: e51–e54. [DOI] [PubMed] [Google Scholar]
- 8.Bill A, Linder J. Sympathetic control of cerebral blood flow in acute arterial hypertension. Acta Physiol Scand 1976; 96: 114–121. [DOI] [PubMed] [Google Scholar]
- 9.Rasmussen P, Dawson EA, Nybo L, et al. Capillary-oxygenation-level-dependent near-infrared spectrometry in frontal lobe of humans. J Cereb Blood Flow Metab 2007; 27: 1082–1093. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Alonso J, Dalsgaard MK, Osada T, et al. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J Physiol 2004; 557: 331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calbet JA, Holmberg HC, Rosdahl H, et al. Why do arms extract less oxygen than legs during exercise? Am J Physiol Regul Integr Comp Physiol 2005; 289: R1448–R158. [DOI] [PubMed] [Google Scholar]
- 12.Willie CK, Macleod DB, Shaw AD, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 2012; 590: 3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calbet JA, Lundby C. Air to muscle O2 delivery during exercise at altitude. High Alt Med Biol 2009; 10: 123–134. [DOI] [PubMed] [Google Scholar]
- 14.Calbet JAL, Lundby C, Boushel R. Integrative conductance of oxygen during exercise at altitude. Adv Exp Med Biol 2016; 903: 395–408. [DOI] [PubMed] [Google Scholar]
- 15.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol 2008; 104: 306–314. [DOI] [PubMed] [Google Scholar]
- 16.Phillips AA, Chan FH, Zheng MM, et al. Neurovascular coupling in humans: physiology, methodological advances and clinical implications. J Cereb Blood Flow Metab 2016; 36: 647–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan JL, Kayser B. Fatigue and exhaustion in hypoxia: the role of cerebral oxygenation. High Alt Med Biol 2016; 17: 72–84. [DOI] [PubMed] [Google Scholar]
- 18.Rasmussen P, Nielsen J, Overgaard M, et al. Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J Physiol 2010; 588: 1985–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Torres-Peralta R, Morales-Alamo D, Gonzalez-Izal M, et al. Task failure during exercise to exhaustion in normoxia and hypoxia is due to reduced muscle activation caused by central mechanisms while muscle metaboreflex does not limit performance. Front Physiol 2016; 6: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres-Peralta R, Losa-Reyna J, Morales-Alamo D, et al. Increased PIO2 at exhaustion in hypoxia enhances muscle activation and swiftly relieves fatigue: a placebo or a PIO2 dependent effect? Front Physiol 2016; 7: 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson MH, Edsell ME, Davagnanam I, et al. Cerebral artery dilatation maintains cerebral oxygenation at extreme altitude and in acute hypoxia – an ultrasound and MRI study. J Cereb Blood Flow Metab 2011; 31: 2019–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen BK, Saltin B. Exercise as medicine – evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015; 25(Suppl 3): 1–72. [DOI] [PubMed] [Google Scholar]
- 23.Wisloff U, Coombes JS, Rognmo O. CrossTalk proposal: high intensity interval training does have a role in risk reduction or treatment of disease. J Physiol 2015; 593: 5215–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holloway TM, Spriet LL. CrossTalk opposing view: high intensity interval training does not have a role in risk reduction or treatment of disease. J Physiol 2015; 593: 5219–5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisloff U, Coombes JS, Rognmo O. Rebuttal from Ulrik Wisloff, Jeff Coombes and Oivind Rognmo. J Physiol 2015; 593: 5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calbet JA, Losa-Reyna J, Torres-Peralta R, et al. Limitations to oxygen transport and utilization during sprint exercise in humans: evidence for a functional reserve in muscle O2 diffusing capacity. J Physiol 2015; 593: 4649–4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morales-Alamo D, Losa-Reyna J, Torres-Peralta R, et al. What limits performance during whole-body incremental exercise to exhaustion in humans? J Physiol 2015; 593: 4631–4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willie CK, Colino FL, Bailey DM, et al. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 2011; 196: 221–237. [DOI] [PubMed] [Google Scholar]
- 29.Naqvi J, Yap KH, Ahmad G, et al. Transcranial Doppler ultrasound: a review of the physical principles and major applications in critical care. Int J Vasc Med 2013; 2013: 629378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Zee P, Cope M, Arridge SR, et al. Experimentally measured optical pathlengths for the adult head, calf and forearm and the head of the newborn infant as a function of inter optode spacing. Adv Exp Med Biol 1992; 316: 143–153. [DOI] [PubMed] [Google Scholar]
- 31.González-Henriquez JJ, Losa-Reyna J, Torres-Peralta R, Göran R, Koskolou M, Calbet JAL. A new equation to estimate temperature-corrected PaCO2 from PETCO2 during exercise in normoxia and hypoxia. Scand J Med Sci Sports 2016; 26: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 32.Losa-Reyna J, Torres-Peralta R, González-Henriquez JJ, et al. Arterial to end-tidal PCO2 difference during exercise in normoxia and severe acute hypoxia: importance of blood temperature correction. Physiol Rep 2015; 3: e12512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calbet JA, Mortensen SP, Munch GD, et al. Constant infusion transpulmonary thermodilution for the assessment of cardiac output in exercising humans. Scand J Med Sci Sports 2016; 26: 518–527. [DOI] [PubMed] [Google Scholar]
- 34.Calbet JA, De Paz JA, Garatachea N, et al. Anaerobic energy provision does not limit Wingate exercise performance in endurance-trained cyclists. J Appl Physiol 2003; 94: 668–676. [DOI] [PubMed] [Google Scholar]
- 35.Zouhal H, Rannou F, Gratas-Delamarche A, et al. Adrenal medulla responsiveness to the sympathetic nervous activity in sprinters and untrained subjects during a supramaximal exercise. Int J Sports Med 1998; 19: 172–176. [DOI] [PubMed] [Google Scholar]
- 36.Clifford PS, Hanel B, Secher NH. Arterial blood pressure response to rowing. Med Sci Sports Exerc 1994; 26: 715–719. [DOI] [PubMed] [Google Scholar]
- 37.Deegan BM, Devine ER, Geraghty MC, et al. The relationship between cardiac output and dynamic cerebral autoregulation in humans. J Appl Physiol 2010; 109: 1424–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassaglia PA, Griffiths RI, Walker AM. Sympathetic nerve activity in the superior cervical ganglia increases in response to imposed increases in arterial pressure. Am J Physiol Regul Integr Comp Physiol 2008; 294: R1255–R1261. [DOI] [PubMed] [Google Scholar]
- 39.Thorin-Trescases N, Bartolotta T, Hyman N, et al. Diameter dependence of myogenic tone of human pial arteries. Possible relation to distensibility. Stroke 1997; 28: 2486–2492. [DOI] [PubMed] [Google Scholar]
- 40.Kontos HA, Wei EP, Navari RM, et al. Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol 1978; 234: H371–H383. [DOI] [PubMed] [Google Scholar]
- 41.Schubert T, Santini F, Stalder AF, et al. Dampening of blood-flow pulsatility along the carotid siphon: does form follow function? AJNR Am J Neuroradiol 2011; 32: 1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi S, Karino T. Flow patterns and distributions of fluid velocity and wall shear stress in the human internal carotid and middle cerebral arteries. World Neurosurg 2010; 73: 174–185. discussion e27. [DOI] [PubMed] [Google Scholar]
- 43.Ogoh S, Fisher JP, Purkayastha S, et al. Regulation of middle cerebral artery blood velocity during recovery from dynamic exercise in humans. J Appl Physiol 2007; 102: 713–721. [DOI] [PubMed] [Google Scholar]
- 44.Van Lieshout JJ, Wieling W, Karemaker JM, et al. Syncope, cerebral perfusion, and oxygenation. J Appl Physiol 2003; 94: 833–848. [DOI] [PubMed] [Google Scholar]
- 45.Rossen R, Kabat H, Anderson JP. Acute arrest of cerebral circulation in man. Arch Neurol Psychiatry 1943; 50: 510–528. [Google Scholar]
- 46.Wasserman AJ, Patterson JL., Jr The cerebral vascular response to reduction in arterial carbon dioxide tension. J Clin Invest 1961; 40: 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delp MD, Armstrong RB, Godfrey DA, et al. Exercise increases blood flow to locomotor, vestibular, cardiorespiratory and visual regions of the brain in miniature swine. J Physiol 2001; 533: 849–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin AL, Fox PT, Hardies J, et al. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci U S A 2010; 107: 8446–8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen BR, Kozberg MG, Bouchard MB, et al. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc 2014; 3: e000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamaguchi Y, Kashima H, Fukuba Y, et al. Cerebral blood flow and neurovascular coupling during static exercise. J Physiol Sci 2014; 64: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatzaras I, Tranquilli M, Coady M, et al. Weight lifting and aortic dissection: more evidence for a connection. Cardiology 2007; 107: 103–106. [DOI] [PubMed] [Google Scholar]
- 52.Haykowsky MJ, Findlay JM, Ignaszewski AP. Aneurysmal subarachnoid hemorrhage associated with weight training: three case reports. Clin J Sport Med 1996; 6: 52–55. [DOI] [PubMed] [Google Scholar]
- 53.Ogoh S, Sato K, Okazaki K, et al. A decrease in spatially resolved near-infrared spectroscopy-determined frontal lobe tissue oxygenation by phenylephrine reflects reduced skin blood flow. Anesth Analg 2014; 118: 823–829. [DOI] [PubMed] [Google Scholar]
- 54.Williams IM, Vohra R, Farrell A, et al. Cerebral oxygen saturation, transcranial Doppler ultrasonography and stump pressure in carotid surgery. Br J Surg 1994; 81: 960–964. [DOI] [PubMed] [Google Scholar]
- 55.Ogasawara K, Konno H, Yukawa H, et al. Transcranial regional cerebral oxygen saturation monitoring during carotid endarterectomy as a predictor of postoperative hyperperfusion. Neurosurgery 2003; 53: 309–314. discussion 314–315. [DOI] [PubMed] [Google Scholar]
- 56.Harms MP, Colier WN, Wieling W, et al. Orthostatic tolerance, cerebral oxygenation, and blood velocity in humans with sympathetic failure. Stroke 2000; 31: 1608–1614. [DOI] [PubMed] [Google Scholar]
- 57.Colier WN, Binkhorst RA, Hopman MT, et al. Cerebral and circulatory haemodynamics before vasovagal syncope induced by orthostatic stress. Clin Physiol 1997; 17: 83–94. [DOI] [PubMed] [Google Scholar]
- 58.Olsen KS, Svendsen LB, Larsen FS. Validation of transcranial near-infrared spectroscopy for evaluation of cerebral blood flow autoregulation. J Neurosurg Anesthesiol 1996; 8: 280–285. [DOI] [PubMed] [Google Scholar]
- 59.Sorensen H, Secher NH, Siebenmann C, et al. Cutaneous vasoconstriction affects near-infrared spectroscopy determined cerebral oxygen saturation during administration of norepinephrine. Anesthesiology 2012; 117: 263–270. [DOI] [PubMed] [Google Scholar]
- 60.Rooks CR, Thom NJ, McCully KK, et al. Effects of incremental exercise on cerebral oxygenation measured by near-infrared spectroscopy: a systematic review. Prog Neurobiol 2010; 92: 134–150. [DOI] [PubMed] [Google Scholar]
- 61.Palada I, Obad A, Bakovic D, et al. Cerebral and peripheral hemodynamics and oxygenation during maximal dry breath-holds. Respir Physiol Neurobiol 2007; 157: 374–381. [DOI] [PubMed] [Google Scholar]
- 62.Yagi T, Nagao K, Kawamorita T, et al. Detection of ROSC in patients with cardiac arrest during chest compression using NIRS: a pilot study. Adv Exp Med Biol 2016; 876: 151–157. [DOI] [PubMed] [Google Scholar]
- 63.Koyama Y, Wada T, Lohman BD, et al. A new method to detect cerebral blood flow waveform in synchrony with chest compression by near-infrared spectroscopy during CPR. Am J Emerg Med 2013; 31: 1504–1508. [DOI] [PubMed] [Google Scholar]
- 64.Kety SS, Schmidt CF. The nitrous oxide method for the quantitative determination of cerebral blood flow in man: theory, procedure and normal values. J Clin Invest 1948; 27: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verbree J, Bronzwaer AS, Ghariq E, et al. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol 2014; 117: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 66.Jorgensen LG, Perko G, Secher NH. Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. J Appl Physiol 1992; 73: 1825–1830. [DOI] [PubMed] [Google Scholar]
- 67.Sato K, Ogoh S, Hirasawa A, Oue A, et al. The distribution of blood flow in the carotid and vertebral arteries during dynamic exercise in humans. J Physiol 2011; 589: 2847–2856. [DOI] [PMC free article] [PubMed] [Google Scholar]