Abstract
Post-translational protein modification by small ubiquitin-like modifier (SUMO) regulates a myriad of homeostatic and stress responses. The SUMOylation pathway has been extensively studied in brain ischemia. Convincing evidence is now at hand to support the notion that a major increase in levels of SUMOylated proteins is capable of inducing tolerance to ischemic stress. Therefore, the SUMOylation pathway has emerged as a promising therapeutic target for neuroprotection in the face of brain ischemia. Despite this, it is prudent to acknowledge that there are many key questions still to be addressed in brain ischemia related to SUMOylation. Accordingly, herein, we provide a critical review of literature within the field to summarize current knowledge and in so doing highlight pertinent translational implications of the SUMOylation pathway in brain ischemia.
Keywords: Brain ischemia, cell-therapy, drug repurposing, hypothermia, neuroprotection, stroke, SUMOylation
Introduction
Small ubiquitin-like modifier (SUMO) conjugation (SUMOylation) has been demonstrated to be massively upregulated in the brains of 13-lined ground squirrels (Ictidomys tridecemlineatus) during hibernation torpor, a state when both brain temperature and cerebral blood flow (CBF) are reduced to levels that would be otherwise lethal for non-hibernating animals.1 This seminal observation has attracted a significant amount of attention and inspired a series of powerful experiments in the field of brain ischemia. Two key aspects associated with the physiological process of mammalian hibernation are of particular clinical interest: brain ischemia and hypothermia. Brain ischemia is associated with conditions such as cardiac arrest and cerebrovascular accidents (e.g. ischemic stroke), while states of mild hypothermia are currently employed within the clinic to protect organs from ischemic damage during major surgeries that require cardiopulmonary bypass.
Beyond hibernation, transient brain ischemia has been demonstrated to involve a dramatic increase in the levels of SUMO-conjugated proteins; increases in SUMO2/3 are particularly pronounced. This increase in SUMOylation begins immediately after reperfusion, when cells are attempting to recover from the severe metabolic stress induced by ischemia.2–5 While numerous stress response pathways are activated within post-ischemic brains, SUMO-conjugation stands out with regard to the extent of activation and the potential consequences for post-ischemic tissue.2 Critically, many known SUMO target proteins are transcription factors and other nuclear proteins that modulate gene expression. Considering the massive increase in levels of SUMO2/3-conjugated proteins (an energy-requiring process) shortly after the onset of reperfusion in viable neurons but not in infarcted tissue,3 it is most likely that SUMOylation is an endogenous neuroprotective stress response capable of shielding neurons from damage induced by ischemia/reperfusion.
As such, the SUMOylation pathway has been actively investigated in a variety of brain ischemia models over the last decade, and convincing evidence is now at hand supporting the notion that a major increase in levels of SUMOylated proteins induces tolerance to ischemic stress. However, there are still many outstanding questions, including the elucidation of the exact mechanisms underlying activation of SUMOylation in post-ischemic brains, how exactly SUMOylation effects neuroprotection, and what are optimal approaches towards translating our knowledge of SUMOylation-dependent neuroprotection into the clinical setting.
Here, we present a critical review of the current SUMO/brain ischemia literature. We begin with an overview of the SUMOylation pathway, followed by a comprehensive summary of what is currently known and unknown about SUMOylation in brain ischemia and hypothermia. We then closely examine the problems associated with clarification of the molecular/cellular mechanisms underlying the neuroprotection induced by SUMO-conjugation, and end with a discussion on what strategies can be envisioned to translate the neuroprotective potential of the SUMO-conjugation pathway into the clinical setting to improve patient outcomes.
SUMOylation pathway
Three distinct SUMO isoforms have been well characterized in mammals: SUMO2 and SUMO3, which share 96% homology; and SUMO1, which shares only 45% homology with SUMO2 and SUMO3. Despite the similarities between SUMO2 and SUMO3, differences in their expression levels can have a profound impact on physiologic outcomes; e.g. deletion of Sumo2 is embryonic lethal, but deletion of Sumo3 shows no overt phenotype. Notably, SUMO2 is the predominantly expressed SUMO isoform, while SUMO3 is expressed at a much lower level.6 SUMO1 knockout mice display normal gross phenotypes, as SUMO2/3 can compensate for loss of SUMO1 during mouse development.7,8 A more detailed analysis of SUMO1 knockout mice, however, has revealed differences in body weight, adipogenesis, and inflammatory responses.9,10 This suggests that while SUMO2/3 may be able to compensate for many of the developmental roles of SUMO1, there are subtler isoform-specific functions which require SUMO1.11 Of note, the existence of a fourth isoform (SUMO4), which possesses 86% homology with SUMO2, has been confirmed; however, its functional role remains unclear.11
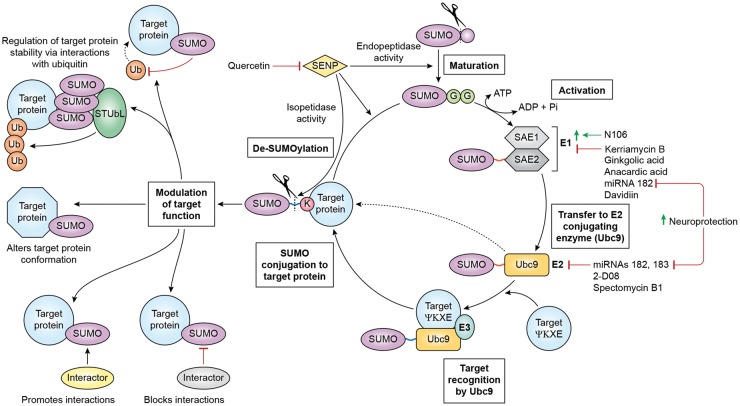
Like ubiquitin, SUMO is synthesized as an inactive precursor. This is subsequently processed by the endopeptidase activity of SUMO-specific proteases (SENPs) to produce the mature ∼11 kDa form (Figure 1). To initiate conjugation, the heterodimeric E1 enzyme, SUMO activating enzyme (SAE)1/2, adenylates SUMO to form a covalent thioester E1-SUMO intermediate in an ATP-dependent step. This E1-SUMO intermediate is then transferred to the catalytic cysteine of Ubc9, the sole E2 conjugase, thereby catalyzing the formation of an isopeptide linkage between the C-terminal di-glycine (GG) motif of SUMO and the ɛ amino-group of a lysine within the target substrate.12 Depending on the identity of a candidate SUMO substrate, a target-specific E3 ligase, such as the protein inhibitor of activated STAT (PIAS) proteins, may assist its interaction with Ubc9.13
Figure 1.
SUMO conjugation-deconjugation cycling, known modulators thereof, and functional consequences of SUMOylation. Maturation of the SUMO precursors is catalyzed by the endopeptidase activity of SENPs, which exposes a GG motif. In the activation step, the SAE1/SAE2 heterodimeric E1 enzyme binds to SUMO in an ATP-dependent process forming a thioester bond (red). A number of compounds have been identified as modulators of the activation step: N106 is an activator, while kerriamycin B1, gingkolic acid, anacardic acid, miRNA-182, and davidiin are inhibitors. SUMO is then transferred to the E2 ligase Ubc9. Ubc9 is inhibited by miRNAs 182 and 183, 2-D08, and spectomycin B1. Critically, inhibition of miRNAs 182 and 183 is neuroprotective in cell culture. With the assistance of a substrate-specific E3 protein, Ubc9 targets SUMO to a substrate protein, conjugates it to the target’s ɛ-amino-group lysine, thereby forming an isopeptide bond (blue). SUMO is deconjugated via the isopeptidase activity of SENPs, which may be inhibited by small molecules such as quercetin to effect neuroprotection. SUMOylation may regulate target protein stability/degradation through interactions with ubiquitin (e.g. blocking the ubiquitination site or recruiting STUbLs), induce conformational changes and thus functional alterations in target proteins, or promote or inhibit interactions between the target protein and other proteins.
Importantly, the regulation of SUMOylation emerges from a complex balance between SUMO conjugation and deconjugation, which is effected by the isopeptidase activity of the SENP proteins (Figure 1).14 In mammals, the SENP family comprises six members (SENPs1–3 and SENPs5–7).13 SENP1 in vitro demonstrates broad specificity for all SUMO isoforms and processes SUMO precursors, though recent evidence suggests that it may be specific to SUMO1-conjugated proteins in vivo.15,16 SENP2 similarly demonstrates broad specificity for all SUMO isoforms and processes SUMO precursors, but has a noted preference for SUMO2/3-conjugated proteins.15 SENP3 and SENP5-7 appear to have a preference for SUMO2/3-conjugated proteins, but only SENP5 can process SUMO precursors.15 Among all the SENPs, SENP1 and SENP2 have been shown to exhibit the highest catalytic activity in vitro.17 Few other mammalian SUMO proteases exist: deSUMOylating isopeptidase 1 and 2 (DeSI-1/2) and ubiquitin-specific protease-like 1 (USPL1) have been identified. However, as they show specificity to particular SUMO substrates, their role in global SUMOylation regulation appears to be limited.18,19 Taking the above into account, it is evident that SENP1 and SENP2 are the most prominent actors in global de-SUMOylation.
The consequences associated with the conjugation of SUMO to protein targets include the blocking or facilitation of interacting proteins with the target protein (e.g. via the provision of SUMO interacting motifs (SIMs)), alterations in target protein stability (e.g. via the recruitment of SUMO-Targeted Ubiquitin Ligases (STUbLs) or hindrance of ubiquitin conjugation), and/or regulation of activity (e.g. via a modulation of protein conformation) (Figure 1).13,20 Detailed information about the effects of SUMOylation on target proteins can be found in recent reviews.13,20
SUMOylation in experimental brain ischemia/stroke
A substantial body of evidence has shown that both global and focal transient cerebral ischemia dramatically increase the levels of SUMO-conjugated proteins (especially SUMO2/3 conjugation) in rodent brains shortly after reperfusion, and that this process may be neuroprotective (summarized in Table 1).3–5,21–23 However, it is prudent to note that evidence of SUMO activation in post-ischemic human brain tissue has yet to be put forward and should therefore be actively pursued.
Table 1.
SUMO pathway in various biological contexts.
| Context | Change and effect of SUMOylation | Ref. | |
|---|---|---|---|
| Hibernation | ↑ | SUMO-conjugation and Ubc9 in multiple organs of the 13-lined ground Squirrel during hibernation torpor | 1,62 |
| Hypothermia In vitro | ↑ | SUMO-conjugation in immortal cells and primary neurons after hypothermia | 1,35 |
| – | additional protective effect of preconditioning at 4℃ in resistance to OGD in SHSY5Y cells overexpressing Ubc9 | 1 | |
| In vivo (rodents) | ↑ | SUMO-conjugation in multiple organs after hypothermia (18℃) | 31 |
| ↑ | SUMO-conjugation and nuclear translocation of Ubc9 in the brain after varying hypothermic temperatures ranging from 18℃–30℃ | 37 | |
| – | Additional protective effect of hypothermia in stroke in transgenic mice overexpressing Ubc9 | 32 | |
| Brain ischemia In vitro | ↑ | SUMOylation in immortal cells and primary neurons after OGD | 37,40,41,56 |
| ↑ | Resistance to OGD in immortal cells and primary neurons with increased SUMOylation (overexpressing Ubc9 or SUMO1, or silencing SENP3) | 1,36,40 | |
| ↓ | Resistance to OGD in immortal cells and primary neurons with decreased SUMOylation (overexpressing dominant negative Ubc9, silencing SUMO1 or SUMO2/3, or overexpressing SENP1) | 1,36,37,41 | |
| In vivo (rodents) | ↑ | SUMO-conjugation in the brain after transient forebrain ischemia | 4,5 |
| ↓ | SUMO-conjugation in the brain during transient forebrain ischemia | 5 | |
| ↑ | SUMO-conjugation in the brain after transient ischemic stroke | 3,23,39 | |
| ↑ | Stroke outcomes in transgenic mice overexpressing Ubc9 | 37 | |
| ↓ | Functional outcomes post-forebrain ischemia in transgenic mice with silenced neuronal SUMO1-3 expression | 22 |
The temporal profiles and potential mechanisms of ischemia/reperfusion-induced SUMOylation within the brain are quite different from those reported to occur during the physiologic process of hibernation. Specifically, in transient brain ischemia, SUMOylation markedly decreased during the ischemic period (i.e. when ATP is depleted), but dramatically increased during reperfusion when metabolism was restored.5 The observation that 10 min of forebrain ischemia was sufficient to essentially de-SUMOylate the majority of SUMO-conjugated proteins suggests that SUMOylation/de-SUMOylation cycling is highly active within the brain. In contrast, SUMOylation within the brains of hibernating squirrels was massively increased during hibernation torpor,1 when CBF is extremely low,24 and SUMOylation returned to baseline levels after arousal when the body temperature returned to euthermic levels.1 Further, while increases in SUMO-conjugation during hibernation torpor were associated with elevated Ubc9 protein levels, Ubc9 levels after transient brain were unaltered (in the cortex) or even decreased (in the hippocampus).1,4 Together, these data suggest that during hibernation torpor energy stores are preserved to allow for the activation of SUMO-conjugation; this is indeed what has been shown for other hibernating mammals.25
After transient focal cerebral ischemia, SUMO2/3 conjugation is activated in neurons located in the ischemic penumbra but not within the infarcted core; a brief period of ischemia is sufficient to activate this process.3 This suggests that post-ischemic increases in SUMOylation are indeed a neuroprotective stress response, as has been proposed for SUMOylation during the physiological state of hibernation torpor.1 However, the precise mechanisms responsible for SUMOylation-induced neuroprotection in hibernation torpor or brain ischemia are poorly defined, and it remains to be clarified whether such increases result from activation of SUMO-conjugation, the inhibition of de-SUMOylation, and/or the inhibition of the proteasome.26,27
Considering that transient brain ischemia triggers a massive increase in both levels and nuclear accumulation of proteins involved in gene expression,5,28 it is very likely that modified gene expression contributes to neuroprotection provided by post-ischemic SUMOylation. Indeed, of the many genes that are activated in the post-ischemic brains of wild-type animals, a significant number are instead suppressed in mice that have been genetically modified to silence SUMO1-3 (SUMO-knockdown (SUMO-KD) mice).22
SUMOylation and hypothermia
Therapeutic hypothermia, also known as targeted temperature management, is currently employed in the clinic for perioperative organ protection in major surgeries requiring cardiopulmonary bypass. Although evidence supporting the utility of hypothermia in acute stroke has accumulated,29,30 there have been no large-scale randomized studies proving efficacy; clinical trials such as EuroHYP-1 (NCT01833312) and iCOOL 2 (NCT01584167) are ongoing.
Understanding the connection between SUMOylation, hibernation, and hypothermia-induced neuroprotection (Table 1), it was of interest to clarify whether hypothermia-induced SUMOylation could occur in mammals subjected to clinically relevant hypothermia. In rats subjected to cardiopulmonary bypass at 18℃, the levels of SUMO-conjugated proteins were increased in all organs tested.31 Follow-up studies showed that levels and nuclear accumulation of SUMO2/3-conjugated proteins were markedly increased in forebrain neurons of animals subjected to cardiopulmonary bypass at 18℃ or clinically relevant hypothermic conditions (24℃ or 30℃) vs. normothermia. Notably, moderate hypothermia (30℃) was sufficient to stimulate SUMOylation. Hypothermia also triggered the nuclear translocation of Ubc9 in neurons, suggesting that hypothermia-induced SUMOylation is an active process and not the result of inhibition of SENP-dependent de-SUMOylation.32 It is also noteworthy that hibernation-like conditions induced by opioid receptor agonists, such as (D-Ala2, D-Leu5) enkephalin (DADLE), protected the brain from ischemic injury.33 While the exact molecular mechanisms underlying this neuroprotective effect remain to be clarified, SUMOylation may be involved via the regulation of interactions between opioid receptors and the regulators of G-protein signaling proteins (RGS).34
The observation that hypothermia did not provide an additional level of neuroprotection to Ubc9 overexpression-induced SUMOylation supports the notion that activation of SUMOylation is a key component of hypothermia-induced neuroprotection.35 Thus, pharmacological activation of SUMOylation may be useful for perioperative brain protection, avoiding the potential adverse effects of hypothermia and the extra time required to cool down and rewarm patients. Furthermore, such work may ultimately be leveraged in the event of an acute cerebrovascular accident to provide clinically relevant emergent neuroprotection.
Effects of SUMOylation in brain ischemia/stroke
Considering the dramatic increase in the levels of SUMOylated proteins in the brain after ischemic stress followed by reperfusion, it is of prime importance to clarify the significance of this process with regard to neurological function/outcomes. In vitro and in vivo experimental approaches have been used to determine whether post-ischemic SUMOylation is indeed a neuroprotective stress response that shields neurons from the damage induced by ischemia, or is simply an epiphenomenon that is not directly related to ischemia-induced pathobiology (summarized in Table 1).1,22,36–40
Cell-based studies have been conducted by exposing primary neuronal cells or neuroblastoma cells to transient oxygen/glucose deprivation (OGD), which is considered to be an acceptable in vitro model of ischemia. The effect of SUMOylation on outcomes after OGD has been assessed by genetically manipulating the SUMO pathway in the cells, such as by increasing the levels of SUMOylated proteins via the overexpression of SUMO isoforms or the conjugating enzyme Ubc9,1,36 or suppressing SUMOylation by microRNA-induced silencing of SUMO isoforms or through overexpressing SENP1.36,37,41 These studies have confirmed a protective role for SUMOylation in transient OGD, with better outcomes in conditions associated with increased SUMOylation and worse outcomes in conditions associated with decreased SUMOylation.
Two genetically modified SUMO mouse models were developed to further clarify the role of SUMOylation in brain ischemia.22,38 In the first transgenic mouse model, mice overexpressing Ubc9 in all cells have higher levels of SUMOylated proteins in the brain, are more tolerant to ischemic stress, and have smaller infarcts after permanent middle cerebral artery occlusion (MCAO).38 The second transgenic mouse model uses the neuron-specific Thy-1 promoter to express three distinct microRNAs that silence SUMO1, SUMO2, and SUMO3 expression. These SUMO-KD mice have worse functional outcomes compared to wild-type mice after transient forebrain ischemia.22 Together, results from these in vivo studies support the idea that the post-ischemic increases in SUMOylation are neuroprotective.
SUMOylation in brain ischemia/stroke: The effect of age
SUMOylation is also involved in the aging process and in the pathogenesis of age-related diseases such as Alzheimer’s.42–44 This involvement is largely due to the critical functions played by SUMO in maintaining genome integrity and protein homeostasis. Many protein components of DNA repair pathways are in fact SUMO targets. It has been proposed that SUMO functions as a protein glue that facilitates assembly of large DNA repair complexes via covalent and/or non-covalent protein-protein interactions.45 To maintain protein homeostasis and prevent the harmful accumulation of misfolded/unfolded proteins, cells have established a sophisticated protein quality control system in which the functional interplay between ubiquitination and SUMOylation is a key component.46 Therefore, defects in the SUMO-conjugation process would be expected to compromise the cells’ capacity to repair damaged DNA and sustain proteostasis in response to stress, and, as a result, aggravate the progression of age-related diseases.
Aging is a key risk factor for stroke, with the majority of patients being more than 60 years old. Both short- and long-term experimental stroke studies have shown that aged animals exhibit worse stroke outcomes compared to young animals.47,48 This disparity in stroke outcomes between young and aged animals has been attributed to many factors, including post-stroke immune responses and spreading depressions (SDs).49,50 For example, in aged brains, MCAO generated prolonged SDs over a larger area, which is believed to accelerate brain damage after stroke.50 Recently, we provided evidence that worse outcomes after brain ischemia in aged animals may also be due to the impaired activation of several potentially protective stress responses, including the unfolded protein response, O-GlcNAcylation, ubiquitination, and SUMOylation.2,51,52 Indeed, after global forebrain ischemia, the increase in levels of both SUMO1- and SUMO2/3-conjugated proteins was significantly less in aged mouse brains than in young brains.2 Such impairments in SUMO activation may lead to decreased efficiency in DNA repair and, concurrently, impede recovery of proteostasis caused by ischemic injury in aged brains, thereby contributing to the worse functional outcomes reported in aged animals versus young controls.
SUMO targets in brain ischemia/stroke
The molecular mechanisms underlying the SUMOylation-mediated neuroprotective effect have yet to be fully elucidated. This is due primarily to our limited understanding of the SUMO-modified proteome involved in brain ischemia. Many recent reviews have speculated on the potential effects on cerebral stroke/ischemia outcomes of proteins known to be SUMO-targets in general53,54; however, here we wish to focus only on those proteins that have been experimentally demonstrated to be SUMOylated in post-ischemic brains.
Identification and verification of SUMOylated proteins are challenging, as only a small fraction of SUMO target proteins are SUMOylated at a given time. Furthermore, since SUMOylated proteins interact with other proteins via SIMs, stringent isolation procedures are required to avoid co-precipitation of proteins interacting with SUMOylated proteins through SIMs. In addition, experimental ischemia models used to investigate the mechanisms underlying SUMO-dependent neuroprotection need to mimic SUMOylation patterns present in vivo. This is characterized by nearly complete de-SUMOylation during ischemia and a dramatic increase in levels of SUMOylated proteins after ischemia, during reperfusion.5
Two types of approaches have been utilized to identify proteins that are SUMO-conjugated in response to brain ischemia in vitro (transient OGD) or in vivo: (1) a focused approach analyzing a specific protein of interest, and (2) an unbiased approach based on systematic screening. The latter usually employs affinity-purification of SUMOylated proteins followed by liquid chromatography (LC)-tandem mass spectrometry (MS/MS) analysis. Notably, it was recently reported that SUMO1-conjugated proteins identified at synapses using a focused approach could not be verified under highly stringent conditions.55 This suggests that SUMO targets identified in experimental ischemia studies using a focused approach must still be verified. Thus far, only two published studies have reported on the identification of SUMOylated proteins in brain ischemia or ischemia-like conditions using an unbiased proteomics approach that is in line with the stringent criteria outlined by Daniel et. al.55 In the first cell culture study, B35 neuroblastoma cells expressing hemagglutinin (HA)-tagged SUMO3 were exposed to transient OGD.56 In the second study, mice expressing epitope-tagged SUMO isoforms were subjected to transient forebrain ischemia.5 The findings from both studies suggest significant crosstalk between the SUMOylation and ubiquitin conjugation pathways after OGD or brain ischemia. Furthermore, a large fraction of identified SUMO targets were transcription factors and nuclear proteins involved in post-transcriptional RNA modification. Perhaps the most prominent SUMO target identified in post-ischemic brains to date is the glucocorticoid receptor (GR). Interestingly, while immunoprecipitation of epitope-tagged SUMO3 was required to identify SUMOylated proteins in post-ischemic brains, SUMOylated GR was detected in whole cell lysates without immunoprecipitation, and was found predominantly in nuclei. This is particularly relevant in our efforts to understand the mechanisms that link SUMOylation to neuroprotection, because stress-induced GR activation results in more pronounced ischemia-induced cell damage and SUMO-conjugation may actually repress GR activity.57,58 Thus, GR SUMOylation may represent a major component of the post-ischemic neuroprotection provided by SUMO-conjugation.
While relatively few proteins have been definitively verified as SUMO-conjugation targets after brain ischemia, the general importance of SUMOylation in both neuronal homeostasis and pathology has been extensively examined.53,54 Such studies, primarily utilizing focused approaches, have identified many putative SUMO targets (e.g. transcriptional factors and other nuclear proteins, extranuclear proteins such as membrane receptors, transporters and channels, mitochondrial proteins, and scaffolding and signaling proteins). These putative SUMO targets have been demonstrated to play diverse roles in myriad cellular processes within neurons beyond the modulation of gene expression (e.g. mitochondrial dynamics, mRNA trafficking, metabolism, and synaptic function); of note, neuronal SUMOylation has been extensively reviewed elsewhere.11,59 However, the contributions of these potential SUMO targets and their effects with regard to SUMOylation-mediated neuroprotection remain unknown.
Translational approaches targeting SUMOylation
Increasing global SUMOylation
As evidenced above, SUMOylation is a promising therapeutic target for brain ischemia/stroke. To potentially translate this knowledge into clinical practice, the first step is to identify small molecules that can increase global SUMOylation by either activating SUMOylation or inhibiting de-SUMOylation (Figure 1; Table 2), followed by testing of these compounds in experimental models of brain ischemia/stroke.
Table 2.
Small-molecule targeting of the SUMO pathway.
| Target | Small molecule(s) | Effect on global SUMOylation | Ref. |
|---|---|---|---|
| SAE1/SAE2 | Ginkgolic acid | Decrease | 67 |
| Anacardic acid | 67 | ||
| Kerriamycin B | 68 | ||
| Davidiin | 69 | ||
| N106 | Increase | 60 | |
| Ubc9 | 2-D08 | Decrease | 70 |
| Spectomycin B1 | 71 | ||
| miR-182 | HDAC inhibitors | Increase | 61 |
| miR-183 | Synthetic retinoids | Increase | 61 |
| SENP1/2 | Quercetin | Increase | 66 |
| Unknown | Topotecan | Decrease | 81 |
In line with such thinking, a recent study using SUMO E1/E2 enzymes as screen targets identified a lead compound (N106) capable of directly activating the E1 component of the SUMO pathway; if permeable to the blood–brain barrier, this compound should certainly be studied in preclinical stroke models.60 We have also identified specific inhibitors of microRNAs (miRNAs)-182 or 183 (Figure 1).61 Levels of the miRNA-200 family and the miRNA-182 family are decreased in the squirrel brain during the torpor phase when global SUMOylation is massively increased, and inhibition of these miRNAs increases global SUMOylation in cell culture.61,62 Importantly, a number of compounds (e.g. histone de-acetylase (HDAC) inhibitors and synthetic retinoids) have been identified that can increase global SUMOylation and ultimately provide protective effects after OGD in vitro.61 Since the screening libraries used in this study are collections of pharmacologically active compounds that are in clinical use, the repurposing potential of these compounds in brain ischemia is high providing that those compounds cross the blood–brain barrier.
In the field of drug discovery and development, however, it is more common to screen for drug candidates that act through the inhibition of specific enzyme targets. Therefore, strategies for increasing SUMOylation may also center on screens for compounds that prevent de-SUMOylation via the inhibition of the isopeptidase activity of SENPs (Figure 1). Major efforts have been invested in screening for specific SENP inhibitors via both in silico and conventional high throughput screening (HTS) methods.63,64 However, few of these screens have identified candidates with medicinal properties and in most cases, previous screens have employed artificial SENP substrates.
In light of such considerations, we have developed an AlphaScreen-based HTS compatible assay designed to screen for inhibitors of the isopeptidase activity of SENPs using a SUMO-conjugated protein as a physiologically relevant SENP substrate.65 This platform can therefore be used in future HTS studies to screen for new classes of SENP inhibitors. Our recent work demonstrated for the first time that quercetin is a putative SENP inhibitor, and protects neurons from OGD in part via increased global SUMOylation,66 supporting the feasibility of such an approach.
Decreasing global SUMOylation
While activators of SUMOylation are sought as potential neuroprotective agents, inhibitors of SUMOylation are considered useful tools to study the role of SUMOylation in brain ischemia (Table 2). Furthermore, such molecules may eventually be developed as therapeutic drugs for various types of cancer, including glioblastoma (GBM).
A few small molecule inhibitors of SUMOylation have been reported. Ginkgolic acid, its structural analog anacardic acid, kerriamycin B, and davidiin are inhibitors of the E1 proteins.67–69 2-D08 and spectomycin B1 were identified as inhibitors of the E2 conjugase Ubc9 (Figure 1).70,71 Recent work has focused on developing novel approaches that center on small-molecule microarrays to identify inhibitors of the notoriously difficult-to-drug E2 conjugases.72 Such studies may ultimately lead to the discovery of novel/clinically relevant inhibitors of Ubc9.
Notably, pharmacologically induced decreases in protein SUMOylation have been shown to inhibit tumor growth.73,74 Indeed, a substantial body of evidence has demonstrated that SUMOylation promotes cancer initiation, progression, metastasis, and chemoresistance.74–80 In human astrocytic brain tumors, for example, SUMO-conjugation is enhanced, with markedly elevated levels of both SUMO1- and SUMO2/3-conjugated proteins in GBM.80 Thus, SUMOylation inhibitors may be considered novel drugs for cancer therapy, as evidenced by our recent repositioning of topotecan (a novel inhibitor of global SUMOylation) in GBM.81
SUMOylation and stem cells
Modulators of SUMOylation also have the potential to impact stem cell-based therapies. Mounting evidence has demonstrated that SUMOylation directly modulates the activity, stability, and localization of crucial factors involved in pluripotency, propagation, and/or differentiation of stem cells (e.g. Oct4, Nanog, and SALL4).82–84 Furthermore, other studies have shown that SUMOylation is essential for cancer stem cell maintenance and self-renewal, is required for stem cell survival in the small intestine and bone marrow, and is involved in neural stem cell differentiation.85–88 Collectively, these data suggest that stem cell-based therapies may benefit from modulating (i.e. increasing or decreasing) the levels of global SUMOylation. Indeed, recent data have shown that increased SUMOylation is associated with increased pathotropism and proliferation of mesenchymal stem cells.89
In stroke, transplanted stem cells have been shown to exert beneficial effects via structural replacement of damaged tissue and a litany of other immunomodulatory and neurotrophic actions.90–92 Unfortunately, the clinical translation of such promising therapies continues to remain elusive, in part due to the limited persistence/survivability of grafted cells within the hostile ischemic microenvironment.91,93 Given the roles of SUMOylation in stem cells as noted above, and SUMOylation’s protective effects in ischemia, increasing SUMOylation may be a promising strategy in aiding the development of stem-based therapies in stroke.91
Conclusions and future directions
SUMOylation plays a role in numerous cellular processes including signal transduction, gene expression, chromatin remodeling, and protein translocation,12,20 and is critically involved in maintaining cellular homeostasis from early embryonic development to advanced age. An intact SUMOylation pathway is critically important during early embryonic development6,94; for basic brain functions, including episodic and fear memory processes95; as well as in pathological states to help cells withstand stress conditions, as expounded upon in this review. As discussed, SUMO-conjugation is dramatically increased in post-ischemic brains early in reperfusion, when energy metabolism is beginning to normalize. Thus, the idea that SUMOylation helps cells to recover from ischemic stress was conceived, and is supported by a variety of observations. Outcomes are improved when the SUMOylation machinery is activated, and impaired when it is downregulated.
The scientific community is still in the early stages of elucidating the potential mechanisms that link SUMOylation to brain ischemia/stroke outcomes. Many putative protective proteins and pathways that are regulated by SUMO and ischemia have been identified based on results from unbiased proteomics analysis of post-ischemic brains. These include the GR, SUMOylation-dependent ubiquitin conjugation, and proteins involved in RNA processing.5 Further characterization of the proteins/pathways that are modulated by SUMO-conjugation in post-ischemic brains is of major interest, and will clarify the mechanisms that link SUMOylation to improved outcomes/neuroprotection. Recent reviews have perhaps been too optimistic regarding the extent of our knowledge about the mechanisms that link ischemia-induced activation of SUMOylation to post-ischemic outcomes.53,54 For example, putative SUMO target proteins have been discussed as potential major players in post-ischemic recovery, when in fact, their SUMOylation status after brain ischemia has not yet been established.
Most of the studies on neuroprotective effects of SUMOylation after ischemia have been conducted in genetically modified cells and animals, such models are akin to pre-conditioning strategies (Table 1). For example, Ubc9 has recently been shown to be involved in isoflurane-induced preconditioning against ischemic neuronal injury.96 These findings, together with those derived from therapeutic hypothermia, strongly support a pre-treatment paradigm wherein SUMOylation is increased prior to high risk procedures (e.g. carotid endarterectomy), in order to prevent secondary ischemic damage within the brain and/or other end organs. However, to be of translational significance in the management of acute brain ischemia/stroke, we must begin to employ additional experimental models to verify that post-treatment strategies centered on pharmacologically-induced SUMO-mediated neuroprotection are ultimately effective; accordingly, the search for potent activators of SUMOylation continues to be an area of active research. Finally, if SUMOylation is eventually harnessed (via preconditioning or cellular engineering) to improve stem-based therapies in stroke, this pathway may become relevant in regenerative medicine.
We feel it is important to highlight that the role of SUMOylation in brain ischemia/stroke is inherently challenging to study. This is due to the active nature of SUMOylation/de-SUMOylation cycling, as well as the low abundance of individual SUMO-conjugated proteins. Measures must be taken to avoid de-SUMOylation during sample preparation,61 which may limit the study of human samples to specimens taken during surgery and immediately snap-frozen or chemically fixed.80 As such, various lysis buffers (e.g. those containing sodium dodecyl sulfate (SDS) and/or N-ethylmaleimide (NEM)) have been employed in studies analyzing the levels of SUMOylated proteins in post-ischemic brains. The low abundance of SUMOylated proteins presents an additional challenge to identification and verification. This can be mitigated with the employment of focused approaches that seek to analyze a specific protein of interest, or unbiased approaches designed to identify SUMOylated proteins via proteomic analysis.55 Both approaches require stringent techniques to avoid false positive hits.
In summary, SUMOylation is an endogenous protective pathway with strong potential as a target for novel therapeutic strategies. Novel strategies that provide neuroprotection are urgently needed for patients undergoing major cardiovascular operations that require a period of circulatory arrest and for patients who suffer acute brain ischemia/stroke. Currently, hypothermia is used in the clinic for perioperative organ protection. Considering that hypothermia activates SUMOylation,31,32 and that this activation is believed to be a molecular mechanism underlying hypothermia-induced tolerance to ischemic stress,35 a drug that activates SUMOylation and provides levels of tolerance to ischemic stress similar to hypothermia, would be of great clinical interest. Notably, high-throughput drug screening identified drugs that activate SUMOylation and provide protection of neurons exposed to ischemia-like stress.61 We are therefore confident that SUMOylation is a promising target for neuroprotection, and are continuing our search for small molecules that trigger an increase in levels of SUMOylated proteins by a variety of different means. We are furthermore working on strategies to make this strategy suitable for clinical use, which may ultimately help improve quality of life for patients and their families.
Acknowledgements
The authors apologize to the colleagues/researchers whose contributions were not cited here owing to space constraints.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: JDB, YJL, DY and JMH are supported by the Intramural Research Program of the NINDS/NIH. JDB is also supported by a NIH-OxCam Fellowship. WP is supported by NIH R01 grants NS081299 and NS097554. WY is supported by NIH R01 grant NS099590 and American Heart Association grant 16GRNT30270003.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lee YJ, Miyake S, Wakita H, et al. Protein SUMOylation is massively increased in hibernation torpor and is critical for the cytoprotection provided by ischemic preconditioning and hypothermia in SHSY5Y cells. J Cereb Blood Flow Metab 2007; 27: 950–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu S, Sheng H, Yu Z, et al. O-linked beta-N-acetylglucosamine modification of proteins is activated in post-ischemic brains of young but not aged mice: implications for impaired functional recovery from ischemic stress. J Cereb Blood Flow Metab 2016; 36: 393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang W, Sheng H, Warner DS, et al. Transient focal cerebral ischemia induces a dramatic activation of small ubiquitin-like modifier conjugation. J Cereb Blood Flow Metab 2008; 28: 892–896. [DOI] [PubMed] [Google Scholar]
- 4.Yang W, Sheng H, Warner DS, et al. Transient global cerebral ischemia induces a massive increase in protein sumoylation. J Cereb Blood Flow Metab 2008; 28: 269–279. [DOI] [PubMed] [Google Scholar]
- 5.Yang W, Sheng H, Thompson JW, et al. Small ubiquitin-like modifier 3-modified proteome regulated by brain ischemia in novel small ubiquitin-like modifier transgenic mice: putative protective proteins/pathways. Stroke 2014; 45: 1115–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, Wansleeben C, Zhao S, et al. SUMO2 is essential while SUMO3 is dispensable for mouse embryonic development. EMBO Rep 2014; 15: 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evdokimov E, Sharma P, Lockett SJ, et al. Loss of SUMO1 in mice affects RanGAP1 localization and formation of PML nuclear bodies, but is not lethal as it can be compensated by SUMO2 or SUMO3. J Cell Sci 2008; 121: 4106–4113. [DOI] [PubMed] [Google Scholar]
- 8.Zhang FP, Mikkonen L, Toppari J, et al. Sumo-1 function is dispensable in normal mouse development. Mol Cell Biol 2008; 28: 5381–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikkonen L, Hirvonen J, Janne OA. SUMO-1 regulates body weight and adipogenesis via PPARgamma in male and female mice. Endocrinology 2013; 154: 698–708. [DOI] [PubMed] [Google Scholar]
- 10.Venteclef N, Jakobsson T, Ehrlund A, et al. GPS2-dependent corepressor/SUMO pathways govern anti-inflammatory actions of LRH-1 and LXRbeta in the hepatic acute phase response. Genes Dev 2010; 24: 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henley JM, Craig TJ, Wilkinson KA. Neuronal sumoylation: mechanisms, physiology, and roles in neuronal dysfunction. Physiol Rev 2014; 94: 1249–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gareau JR, Lima CD. The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nature Rev Mol Cell Biol 2010; 11: 861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flotho A, Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem 2013; 82: 357–385. [DOI] [PubMed] [Google Scholar]
- 14.Mukhopadhyay D, Dasso M. Modification in reverse: the SUMO proteases. Trends Biochem Sci 2007; 32: 286–295. [DOI] [PubMed] [Google Scholar]
- 15.Hickey CM, Wilson NR, Hochstrasser M. Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol 2012; 13: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P, Yamada S, Lualdi M, et al. Senp1 is essential for desumoylating sumo1-modified proteins but dispensable for Sumo2 and Sumo3 deconjugation in the mouse embryo. Cell Rep 2013; 3: 1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendes AV, Grou CP, Azevedo JE, et al. Evaluation of the activity and substrate specificity of the human SENP family of SUMO proteases. BBA Molec Cell Res 2016; 1863: 139–147. [DOI] [PubMed] [Google Scholar]
- 18.Shin EJ, Shin HM, Nam E, et al. DeSUMOylating isopeptidase: a second class of SUMO protease. EMBO Rep 2012; 13: 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz S, Chachami G, Kozaczkiewicz L, et al. Ubiquitin-specific protease-like 1 (USPL1) is a SUMO isopeptidase with essential, non-catalytic functions. EMBO Rep 2012; 13: 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nature Rev Mol Cell Biol 2007; 8: 947–956. [DOI] [PubMed] [Google Scholar]
- 21.Cimarosti H, Lindberg C, Bomholt SF, et al. Increased protein SUMOylation following focal cerebral ischemia. Neuropharmacology 2008; 54: 280–289. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Liu X, Sheng H, et al. Neuron-specific SUMO knockdown suppresses global gene expression response and worsens functional outcome after transient forebrain ischemia in mice. Neuroscience 2017; 343: 190–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hochrainer K, Jackman K, Benakis C, et al. SUMO2/3 is associated with ubiquitinated protein aggregates in the mouse neocortex after middle cerebral artery occlusion. J Cereb Blood Flow Metab 2015; 35: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frerichs KU, Kennedy C, Sokoloff L, et al. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. J Cereb Blood Flow Metab 1994; 14: 193–205. [DOI] [PubMed] [Google Scholar]
- 25.Lust WD, Wheaton AB, Feussner G, et al. Metabolism in the hamster brain during hibernation and arousal. Brain Res 1989; 489: 12–20. [DOI] [PubMed] [Google Scholar]
- 26.Kamikubo T, Hayashi T. Changes in proteasome activity following transient ischemia. Neurochem Int 1996; 28: 209–212. [DOI] [PubMed] [Google Scholar]
- 27.Asai A, Tanahashi N, Qiu JH, et al. Selective proteasomal dysfunction in the hippocampal CA1 region after transient forebrain ischemia. J Cereb Blood Flow Metab 2002; 22: 705–710. [DOI] [PubMed] [Google Scholar]
- 28.Golebiowski F, Matic I, Tatham MH, et al. System-wide changes to SUMO modifications in response to heat shock. Sci Signal 2009; 2: ra24. [DOI] [PubMed] [Google Scholar]
- 29.Yenari MA, Hemmen TM. Therapeutic hypothermia for brain ischemia: where have we come and where do we go? Stroke 2010; 41(10 Suppl): S72–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 2012; 13: 267–278. [DOI] [PubMed] [Google Scholar]
- 31.Yang W, Ma Q, Mackensen GB, et al. Deep hypothermia markedly activates the small ubiquitin-like modifier conjugation pathway; implications for the fate of cells exposed to transient deep hypothermic cardiopulmonary bypass. J Cereb Blood Flow Metab 2009; 29: 886–890. [DOI] [PubMed] [Google Scholar]
- 32.Wang L, Ma Q, Yang W, et al. Moderate hypothermia induces marked increase in levels and nuclear accumulation of SUMO2/3-conjugated proteins in neurons. J Neurochem 2012; 123: 349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowley MG, Liska MG, Lippert T, et al. Utilizing delta opioid receptors and peptides for cytoprotection: implications in stroke and other neurological disorders. CNS Neurol Disord Drug Targets 2017; 16: 414–424. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez-Munoz M, Bermudez D, Sanchez-Blazquez P, et al. Sumoylated RGS-Rz proteins act as scaffolds for Mu-opioid receptors and G-protein complexes in mouse brain. Neuropsychopharmacology 2007; 32: 842–850. [DOI] [PubMed] [Google Scholar]
- 35.Lee YJ, Mou Y, Klimanis D, et al. Global SUMOylation is a molecular mechanism underlying hypothermia-induced ischemic tolerance. Front Cell Neurosci 2014; 8: 416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YJ, Castri P, Bembry J, et al. SUMOylation participates in induction of ischemic tolerance. J Neurochem 2009; 109: 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Datwyler AL, Lattig-Tunnemann G, Yang W, et al. SUMO2/3 conjugation is an endogenous neuroprotective mechanism. J Cereb Blood Flow Metab 2011; 31: 2152–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee YJ, Mou Y, Maric D, et al. Elevated global SUMOylation in Ubc9 transgenic mice protects their brains against focal cerebral ischemic damage. PLoS One 2011; 6: e25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cuomo O, Pignataro G, Sirabella R, et al. Sumoylation of LYS590 of NCX3 f-Loop by SUMO1 participates in brain neuroprotection induced by ischemic preconditioning. Stroke 2016; 47: 1085–1093. [DOI] [PubMed] [Google Scholar]
- 40.Guo C, Hildick KL, Luo J, et al. SENP3-mediated deSUMOylation of dynamin-related protein 1 promotes cell death following ischaemia. EMBO J 2013; 32: 1514–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cimarosti H, Ashikaga E, Jaafari N, et al. Enhanced SUMOylation and SENP-1 protein levels following oxygen and glucose deprivation in neurones. J Cereb Blood Flow Metab 2012; 32: 17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee L, Sakurai M, Matsuzaki S, et al. SUMO and Alzheimer's disease. Neuromolecular Med 2013; 15: 720–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steffan JS, Agrawal N, Pallos J, et al. SUMO modification of Huntingtin and Huntington's disease pathology. Science 2004; 304: 100–104. [DOI] [PubMed] [Google Scholar]
- 44.Bischof O, Dejean A. SUMO is growing senescent. Cell Cycle 2007; 6: 677–681. [DOI] [PubMed] [Google Scholar]
- 45.Psakhye I, Jentsch S. Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 2012; 151: 807–820. [DOI] [PubMed] [Google Scholar]
- 46.Tatham MH, Matic I, Mann M, et al. Comparative proteomic analysis identifies a role for SUMO in protein quality control. Sci Signal 2011; 4: rs4. [DOI] [PubMed] [Google Scholar]
- 47.Liu F, Yuan R, Benashski SE, et al. Changes in experimental stroke outcome across the life span. J Cereb Blood Flow Metab 2009; 29: 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suenaga J, Hu X, Pu H, et al. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol 2015; 272: 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manwani B, Liu F, Scranton V, et al. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp Neurol 2013; 249: 120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clark D, Institoris A, Kozak G, et al. Impact of aging on spreading depolarizations induced by focal brain ischemia in rats. Neurobiol Aging 2014; 35: 2803–2811. [DOI] [PubMed] [Google Scholar]
- 51.Jiang M, Yu S, Yu Z, et al. XBP1 (X-Box-Binding Protein-1)-Dependent O-GlcNAcylation is neuroprotective in ischemic stroke in young mice and its impairment in aged mice is rescued by thiamet-G. Stroke 2017; 48: 1646–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang W, Paschen W. Is age a key factor contributing to the disparity between success of neuroprotective strategies in young animals and limited success in elderly stroke patients? Focus on protein homeostasis. J Cereb Blood Flow Metab 2017; 37: 3318–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silveirinha V, Stephens GJ, Cimarosti H. Molecular targets underlying SUMO-mediated neuroprotection in brain ischemia. J Neurochem 2013; 127: 580–591. [DOI] [PubMed] [Google Scholar]
- 54.Peters M, Wielsch B, Boltze J. The role of SUMOylation in cerebral hypoxia and ischemia. Neurochem Int 2017; 107: 66–77. [DOI] [PubMed] [Google Scholar]
- 55.Daniel JA, Cooper BH, Palvimo JJ, et al. Analysis of SUMO1-conjugation at synapses. eLife 2017; 6: pii: e26338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang W, Thompson JW, Wang Z, et al. Analysis of oxygen/glucose-deprivation-induced changes in SUMO3 conjugation using SILAC-based quantitative proteomics. J Proteome Res 2012; 11: 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davies L, Karthikeyan N, Lynch JT, et al. Cross talk of signaling pathways in the regulation of the glucocorticoid receptor function. Mol Endocrinol 2008; 22: 1331–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balkaya M, Prinz V, Custodis F, et al. Stress worsens endothelial function and ischemic stroke via glucocorticoids. Stroke 2011; 42: 3258–3264. [DOI] [PubMed] [Google Scholar]
- 59.Wilkinson KA, Nakamura Y, Henley JM. Targets and consequences of protein SUMOylation in neurons. Brain Res Rev 2010; 64: 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kho C, Lee A, Jeong D, et al. Small-molecule activation of SERCA2a SUMOylation for the treatment of heart failure. Nat Commun 2015; 6: 7229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bernstock JD, Lee YJ, Peruzzotti-Jametti L, et al. A novel quantitative high-throughput screen identifies drugs that both activate SUMO conjugation via the inhibition of microRNAs 182 and 183 and facilitate neuroprotection in a model of oxygen and glucose deprivation. J Cereb Blood Flow Metab 2016; 36: 426–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee YJ, Johnson KR, Hallenbeck JM. Global protein conjugation by ubiquitin-like-modifiers during ischemic stress is regulated by microRNAs and confers robust tolerance to ischemia. PLoS One 2012; 7: e47787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kumar A, Zhang KY. Advances in the development of SUMO specific protease (SENP) inhibitors. Comput Struct Biotechnol J 2015; 13: 204–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang W, Sheng H, Wang H. Targeting the SUMO pathway for neuroprotection in brain ischaemia. Stroke Vasc Neurol 2016; 1: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang W, Wang L, Paschen W. Development of a high-throughput screening assay for inhibitors of small ubiquitin-like modifier proteases. J Biomol Screen 2013; 18: 621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee YJ, Bernstock JD, Nagaraja N, et al. Global SUMOylation facilitates the multimodal neuroprotection afforded by quercetin against the deleterious effects of oxygen/glucose deprivation and the restoration of oxygen/glucose. J Neurochem 2016; 138: 101–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukuda I, Ito A, Hirai G, et al. Ginkgolic acid inhibits protein SUMOylation by blocking formation of the E1-SUMO intermediate. Chem Biol 2009; 16: 133–140. [DOI] [PubMed] [Google Scholar]
- 68.Fukuda I, Ito A, Uramoto M, et al. Kerriamycin B inhibits protein SUMOylation. J Antibiot 2009; 62: 221–224. [DOI] [PubMed] [Google Scholar]
- 69.Takemoto M, Kawamura Y, Hirohama M, et al. Inhibition of protein SUMOylation by davidiin, an ellagitannin from Davidia involucrata. J Antibiot 2014; 67: 335–338. [DOI] [PubMed] [Google Scholar]
- 70.Kim YS, Nagy K, Keyser S, et al. An electrophoretic mobility shift assay identifies a mechanistically unique inhibitor of protein sumoylation. Chem Biol 2013; 20: 604–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirohama M, Kumar A, Fukuda I, et al. Spectomycin B1 as a novel SUMOylation inhibitor that directly binds to SUMO E2. ACS Chem Biol 2013; 8: 2635–2642. [DOI] [PubMed] [Google Scholar]
- 72.Zlotkowski K, Hewitt WM, Sinniah RS, et al. A small-molecule microarray approach for the identification of E2 enzyme inhibitors in ubiquitin-like conjugation pathways. SLAS Discov 2017; 22: 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bossis G, Sarry JE, Kifagi C, et al. The ROS/SUMO axis contributes to the response of acute myeloid leukemia cells to chemotherapeutic drugs. Cell Rep 2014; 7: 1815–1823. [DOI] [PubMed] [Google Scholar]
- 74.Bogachek MV, Chen Y, Kulak MV, et al. Sumoylation pathway is required to maintain the basal breast cancer subtype. Cancer Cell 2014; 25: 748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moschos SJ, Smith AP, Mandic M, et al. SAGE and antibody array analysis of melanoma-infiltrated lymph nodes: identification of Ubc9 as an important molecule in advanced-stage melanomas. Oncogene 2007; 26: 4216–4225. [DOI] [PubMed] [Google Scholar]
- 76.Li R, Wei J, Jiang C, Liu D, et al. Akt SUMOylation regulates cell proliferation and tumorigenesis. Cancer Res 2013; 73: 5742–5753. [DOI] [PubMed] [Google Scholar]
- 77.Kessler JD, Kahle KT, Sun T, et al. A SUMOylation-dependent transcriptional subprogram is required for Myc-driven tumorigenesis. Science 2012; 335: 348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim JH, Choi HJ, Kim B, et al. Roles of sumoylation of a reptin chromatin-remodelling complex in cancer metastasis. Nature Cell Biol 2006; 8: 631–639. [DOI] [PubMed] [Google Scholar]
- 79.Driscoll JJ, Pelluru D, Lefkimmiatis K, et al. The sumoylation pathway is dysregulated in multiple myeloma and is associated with adverse patient outcome. Blood 2010; 115: 2827–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang W, Wang L, Roehn G, et al. Small ubiquitin-like modifier 1-3 conjugation [corrected] is activated in human astrocytic brain tumors and is required for glioblastoma cell survival. Cancer Sci 2013; 104: 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernstock JD, Ye D, Gessler FA, et al. Topotecan is a potent inhibitor of SUMOylation in glioblastoma multiforme and alters both cellular replication and metabolic programming. Sci Rep 2017; 7: 7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang F, Yao Y, Jiang Y, et al. Sumoylation is important for stability, subcellular localization, and transcriptional activity of SALL4, an essential stem cell transcription factor. J Biol Chem 2012; 287: 38600–38608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wei F, Scholer HR, Atchison ML. Sumoylation of Oct4 enhances its stability, DNA binding, and transactivation. J Biol Chem 2007; 282: 21551–21560. [DOI] [PubMed] [Google Scholar]
- 84.Wu Y, Guo Z, Wu H, et al. SUMOylation represses Nanog expression via modulating transcription factors Oct4 and Sox2. PLoS One 2012; 7: e39606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Du L, Li YJ, Fakih M, et al. Role of SUMO activating enzyme in cancer stem cell maintenance and self-renewal. Nat Commun 2016; 7: 12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Demarque MD, Nacerddine K, Neyret-Kahn H, et al. Sumoylation by Ubc9 regulates the stem cell compartment and structure and function of the intestinal epithelium in mice. Gastroenterology 2011; 140: 286–296. [DOI] [PubMed] [Google Scholar]
- 87.Hasegawa Y, Yoshida D, Nakamura Y, et al. Spatiotemporal distribution of SUMOylation components during mouse brain development. J Comp Neurol 2014; 522: 3020–3036. [DOI] [PubMed] [Google Scholar]
- 88.Yuan H, Zhang T, Liu X, et al. Sumoylation of CCAAT/enhancer-binding protein alpha is implicated in hematopoietic stem/progenitor cell development through regulating runx1 in zebrafish. Sci Rep 2015; 5: 9011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ciria M, Garcia NA, Ontoria-Oviedo I, et al. Mesenchymal stem cell migration and proliferation are mediated by hypoxia-inducible factor-1alpha upstream of notch and SUMO pathways. Stem Cells Dev 2017; 26: 973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hermann DM, Peruzzotti-Jametti L, Schlechter J, et al. Neural precursor cells in the ischemic brain - integration, cellular crosstalk, and consequences for stroke recovery. Front Cell Neurosci 2014; 8: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bernstock JD, Peruzzotti-Jametti L, Ye D, et al. Neural stem cell transplantation in ischemic stroke: a role for preconditioning and cellular engineering. J Cereb Blood Flow Metab 2017; 37: 2314–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borlongan MC, Kong S, Hess DC. Lab-to-clinic application of stem cell therapy for stroke. Chin Neurosurg J 2016; 2: 42. [Google Scholar]
- 93.Wei L, Wei ZZ, Jiang MQ, et al. Stem cell transplantation therapy for multifaceted therapeutic benefits after stroke. Prog Neurobiol 2017; 157: 49–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nacerddine K, Lehembre F, Bhaumik M, et al. The SUMO pathway is essential for nuclear integrity and chromosome segregation in mice. Dev Cell 2005; 9: 769–779. [DOI] [PubMed] [Google Scholar]
- 95.Wang L, Rodriguiz RM, Wetsel WC, et al. Neuron-specific Sumo1-3 knockdown in mice impairs episodic and fear memories. J Psychiatry Neurosci 2014; 39: 130148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tong L, Wu Z, Ran M, et al. The role of SUMO-conjugating enzyme Ubc9 in the neuroprotection of isoflurane preconditioning against ischemic neuronal injury. Mol Neurobiol 2015; 51: 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]