Abstract
Peroxisome proliferator-activated receptors are regulators of inflammatory signaling. This has fostered hope that PPAR agonists might have neuroprotective potential. We hypothesized that PPARδ activation by the novel orally administered lipophilic PPARδ agonist SAR145 may improve short- and long-term outcome after focal brain ischemia. We induced ischemia by transient filamentous middle cerebral artery occlusion (MCAo) in 227 C57BL/6 mice and administered SAR145 in varying doses and time windows post-injury. Outcome was assessed by three functional tests and histologically determining ischemic lesion sizes. In a second experiment, we tested SAR145 treatment in 40 PPARδ-knockout mice using the same procedures. Three independent groups treated with 10 mg/kg bodyweight SAR145 directly after filament removal showed a mean reduction in lesion sizes of 18 ± 10% compared to vehicle-treated groups. We did not observe a consistent improvement in the long-term functional outcome by SAR145-treatment. PPARδ-knockout mice showed a significantly higher mortality after MCAo. As expected, we did not find a reduction of lesion size by SAR145-treatment in PPARδ-knockout mice. In summary, we found no evidence of a long-term neuroprotective effect of post-injury SAR145 treatment in cerebral ischemia. However, PPARδ appears to play a pathophysiologic role in acute infarct development and overall mortality after brain ischemia.
Keywords: Animal studies, brain ischemia, focal ischemia, neuroprotection, stroke
Introduction
The quest for neuroprotective therapies allowing for treatment beyond a narrow time window, has largely resulted in the failure to translate promising preclinical findings into successful clinical trials.1
Cerebral ischemia induces an inflammatory reaction in the affected area, which remains activated for days to weeks after the onset of symptoms and which contributes to the progression of ischemic brain injury.2,3 Therefore, interventions aiming at suppression of post-ischemic inflammation represent an attractive avenue for the treatment of ischemic stroke patients, with a potentially wide therapeutic window. To date, specific treatment is limited to intravenous tissue plasminogen activator administration within 4.5 h after symptoms onset4,5 and to intraarterial recanalization in patients with large cerebral artery occlusion.6
Recent animal studies on cerebral ischemia have suggested that activation of PPARs suppresses acute and protracted post-ischemic inflammation in the brain and thereby reduces ischemic brain injury.7,8 Concordantly, mice deficient for different PPARs show larger infarct volumes.8–12 PPARs are nuclear receptors and belong to a family of ligand-activated transcription factors consisting of at least three subtypes – PPARα, PPARδ and PPARγ.13 To date, most studies on the role of PPARs in ischemic brain injury have focused on PPARα7 and PPARγ.14 The role of PPARδ and its activation by synthetic ligands in experimental cerebral ischemia has only been recently investigated.12 Three studies testing PPARδ agonists in models of focal cerebral ischemia reported neuroprotection induced by PPARδ activation resulting in a reduction in infarct sizes by around 30%.8,10,12 All studies have tested a parenteral, mostly intracerebroventricular administration route of PPARδ agonists prior to induction of cerebral ischemia.8,10,12 To date, there are no reports testing long-term effects of PPARδ agonist treatment after cerebral ischemia. To assess cumulative treatment effects on lesion development and functional outcome, long-term observation is pivotal. Therefore, we sought to characterize the novel orally administrable PPARδ agonist SAR145 and assessed behavioural and morphological outcomes up to 28 days after MCAo.
Materials and methods
Animals
All experimental procedures were approved by the “Landesamt für Gesundheit und Soziales Berlin” and were in accordance to national and institutional guidelines for the care and use of laboratory animals and the directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010. Reporting conforms to the ARRIVE guidelines.15 A total of 227 male C57Bl6 (10 weeks old, mean weight 25 ± 2 g) and 40 male PPARδ-knockout (ko) mice (8–11 weeks old, mean weight 22 ± 2 g) were used (Table 1). C57Bl6 mice were purchased from Charles-River, PPARδ-ko mice backcrossed on C57Bl6 strain were kindly supplied by Frank J Gonzales.16 Animals were housed in groups of three to five animals per cage with a standard light-dark cycle (12 h–12 h) and ad libitum access to chow and water.
Table 1.
Animals used in the study.
| Experiment | Included | Total | Died | Excluded | Mean lesion size % of contralateral hemisphere |
|---|---|---|---|---|---|
| Dose | after 72 h | ||||
| Vehicle | 45 | 51 | 3 | 3 | 44 |
| 1 mg/kg | 13 | 16 | 2 | 1 | 41 |
| 10 mg/kg | 13 | 15 | 0 | 2 | 35 |
| 30 mg/kg | 13 | 13 | 0 | 0 | 35 |
| 100 mg/kg | 14 | 15 | 1 | 0 | 41 |
| Time point | after 72 h | ||||
| Vehicle | 21 | 24 | 2 | 1 | 46 |
| 0 h | 8 | 8 | 0 | 0 | 43 |
| 3 h | 12 | 12 | 0 | 0 | 37 |
| 6 h | 12 | 12 | 0 | 0 | 48 |
| 12 h | 11 | 11 | 0 | 0 | 55 |
| 24 h | 10 | 10 | 0 | 0 | 54 |
| Knock-out | after 72 h | ||||
| KO Vehicle | 15 | 20 | 5 | 0 | 50 |
| KO 10 mg/kg | 13 | 20 | 4 | 3 | 56 |
| WT Vehicle | 8 | 10 | 1 | 1 | 49 |
| WT 10 mg/kg | 8 | 10 | 1 | 1 | 35 |
| Long-term outcome | after 28 days | ||||
| Vehicle | 6 | 10 | 4 | 0 | 28 |
| 10 mg/kg | 9 | 10 | 1 | 0 | 37 |
| Total | 231 | 267 | 24 | 12 | |
Randomization, blinding, exclusion criteria
For randomization, wildtype (wt) and ko animals were numbered and randomly assigned to treatment or vehicle groups, respectively, using a randomization software (Randomness and Integrity Services Ltd, Dublin, Ireland). For all protocols and outcome assessments using PPARδ-ko animals, the experimenter (sk) was blinded for treatment group and for genotype for the entire duration of the experiment. All outcome assessments were performed by one experimenter (sk) who was blinded for treatment groups. For behavioural assessments, the experimenter was blinded regarding group allocation for the entire duration of the experiment. All animals assigned to one of the experimental groups are reported. Animals that had died before the end of the experiment or that did not show an infarct were excluded from further analysis (Table 1). All other animals, including animals killed before the end of the experiment and animals with unexpectedly small lesions, were included in the analyses.
Drug
The specific PPARδ agonist SAR145, kindly provided by Sanofi, was dissolved in vehicle solution (aqua destillata with 0.6% Methylcellulose, 0.5% Tween-80 (Sigma-Aldrich Corp., Saint Louis, MO, USA) using a Teflon tissue grinder (Sigma-Aldrich). Treatment with the compound was performed by oral gavage using a 22 Gauge feeding needle (Fine Science Tools GmbH, Heidelberg, Germany) by a trained experimenter (sk). A total amount of 0.1 ml of SAR145 or the vehicle solution was administered once daily after induction of transient middle cerebral artery occlusion (MCAo) until animals were killed.
Measurement of SAR145 concentration in the brain
To validate the application method and confirm blood–brain barrier penetration of the compound, three mice were treated orally with SAR145 on seven consecutive days; 24 h after the last application, mice were killed in deep isoflurane anaesthesia and were perfused transcardially with saline. Brains were removed from the skull, snap frozen in Methylbutane on dry ice and stored at −20℃. For each animal, three tissue samples of 12–31 mg from the cortex and striatum were used for further analyses. A mixture of ethylacetat/methanol was used for elution. To obtain a solid residue, the eluate was evaporated under a stream of nitrogen. Residues were then dissolved in 100 μl acetonitrile. High-performance liquid chromatography (HPLC) measurement was performed using an Agilent 1290 HPLC (Agilent Technologies, Santa Clara, CA) system with binary pump, autosampler and column thermostat equipped with a Zobrax Eclipse Plus – column 50 × 2.1 mm, 1.8 µm. The solvent system consisted of aqueous ammoniumacetat (10 mM) and acetonitrile. The gradient elution was started with 50% acetonitrile, increased to 95% acetonitrile within 5 min and stopped after 7 min. A flow rate of 0.4 ml/min and an injection volume of 1 µl was used. HPLC was coupled with an Agilent 6490 triple quadruple mass spectrometer with electrospray ionization source operated in positive mode. The source parameters were as follows: Drying gas 140℃/18 L/min; sheath gas 380℃/10 L/min; nebulizer pressure 40 psi; capillary voltage 3500 V; nozzle voltage 0 V. The results were calculated compared with SAR145. Mass spectrometry measurements were performed by a commercial partner (LIPIDOMIX Gmbh, Berlin, Germany).
Model of middle cerebral artery occlusion in mice
All experimental procedures were conducted by a trained experimenter (sk) following published standard operating procedures.17 A filament model of transient MCAo was used as described previously.18 Briefly, mice were anaesthetized using 2.5% (vol/vol) isoflurane for induction and 1.5% (vol/vol) isoflurane for maintaining anaesthesia in a mixture with 30% O2 and ∼68% N2O. To obtain a reproducible positioning, animals were placed in a stereotactic frame (David Kopf, Tujunga, CA, USA). Cerebral ischemia was induced by introducing a 7-0 silicon-rubber-coated MCAo suture (Doccol Corp., Sharon MA, USA) into the left internal carotid artery and by advancing it up to the anterior cerebral artery, thereby occluding the middle cerebral artery. The filament was withdrawn after an occlusion time of 45 min.19 Rectal temperature was maintained at 37.5 ± 0.5℃ throughout the experiment using a feed-back controlled heating pad with a rectal probe (Fine Science Tools GmbH, Heidelberg, Germany). During MCAo and for at least 2 h after reperfusion, animals were allowed to recover in a heated cage (Peco Services, Cumbria, UK). Afterwards, they were transferred to the home cage. For the first three to five days after MCAo, animals had access to soft food and were treated with daily subcutaneous saline injections to ensure adequate hydration after surgery.
Experimental design
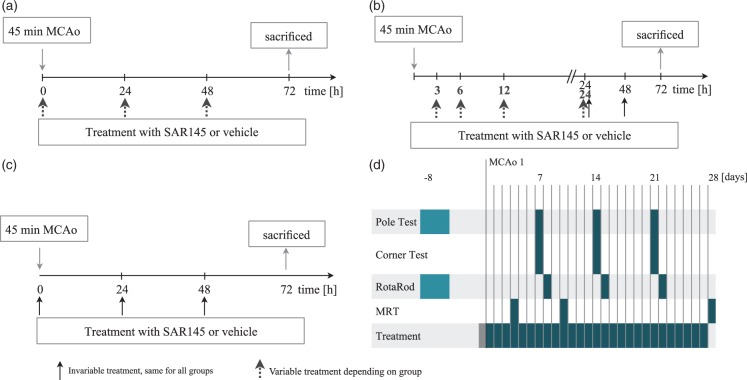
Four different experimental protocols were utilized in this study (Figure 1). To assess the efficacy of SAR145, histological and behavioural outcome parameters were analysed. Effects were studied in wt and ko animals. All experiments were designed according to the STAIR criteria.20,21 Focal cerebral ischemia was induced by 45 min of MCAo. SAR145 or vehicle treatment and neurological assessment after MCAo were performed daily.
Figure 1.
Experimental protocols.(a) Dose response. Animals were either treated orally with 1 mg/kg bodyweight (BW) (n = 16), 10 mg/kg BW (n = 15), 30 mg/kg BW (n = 13), 100 mg/kg BW (n = 15) SAR145 or vehicle (n = 51) followed by 72 h of reperfusion.(b) Time window. Animals were treated with 10 mg/kg BW or vehicle (n = 24) at 3 h (n = 12), 6 h (n = 12), 12 h (n = 11) or 24 h (n = 10) after reperfusion followed by 72 h of reperfusion.(c) PPARδ knockout mice and wild type (C57Bl6, Charles River) were treated with 10 mg/kg BW SAR145 (20 knockout animals; 10 wild type animals, n = 8 included) or vehicle (20 knockout animals; 10 wild type animals) at the time of reperfusion followed by 72 h of reperfusion. Ko and wt animals were phenotypically identical.(d) Animals were randomly assigned to a treatment (n = 10) and a vehicle group (n = 10). Starting two weeks before MCAo, animals were handled daily in preparation for behavioural tests. Training for behavioural analysis was commenced eight days prior to MCAo. Animals were treated with 10 mg/kg BW SAR145 or vehicle beginning at 3 h after reperfusion and then once daily. MRI infarct volume assessment was performed at 4, 10 and 28 days after MCAo. Behavioural tests were conducted on a weekly basis. Pole and corner tests were performed at 7, 14 and 21 days after MCAo, RotaRod assessment was conducted on postoperative days 8, 15, and 22.
In the first protocol, we characterized the effect of treatment with SAR145 at the time of reperfusion on the infarct volume. Increasing doses of SAR145 were administered to evaluate the dose-response effect. In the second set of experiments, we evaluated the effect of delayed administration of SAR145 on the infarct volume in a time window up to 24 h after reperfusion using the most effective dose obtained in the first set of experiments. In a third set, we evaluated the effect of SAR145 treatment on the infarct volume in PPARδ-ko animals.
In the first three protocols, animals were killed in deep isoflurane anaesthesia 72 h after MCAo. Infarct volume was assessed as described below. In a fourth protocol, we assessed long-term effects of SAR145 treatment. In a clinical setting, treatment initiation at the time of ischemia onset is not feasible. To increase applicability of our findings to the clinical setting, a delayed treatment protocol beginning 3 h after reperfusion was selected. Effects on infarct volume and on behavioural outcome were studied for up to 28 days after MCAo.
Infarct volume measurement
Histological assessment
Animals were killed in deep isoflurane anaesthesia. Brains were removed from the skull and snap frozen in methylbutane on dry ice and stored at −20℃ for cryostat sectioning. Coronal serial 20 µm sections were collected at 0.6 mm intervals (13–14 sections/brain) and haematoxylin & eosin (H&E)-stained for infarct volume assessment. Infarct volume was assessed as described in detail previously.22,23 In brief, in each section, areas of ipsilateral and contralateral hemispheres and the infarct area were manually classified using a computer system (MCID CoreTM 7.0 Rev.2.0, InterFocus Imaging Ltd, Cambridge, UK). Lesion volume relative to the contralateral hemisphere and corrected for acute brain swelling was determined by the following formula:
Magnetic resonance imaging
Magnetic resonance imaging was conducted on a 7 Tesla rodent scanner (Pharmascan 70/16, Bruker BioSpin, Ettlingen, Germany) with a 16 cm horizontal bore magnet and a 9 cm (inner diameter) shielded gradient with a H-resonance-frequency of 300 MHz and a maximum gradient strength of 300 mT/m. For imaging, a 1H-RF quadrature-volume resonator with an inner diameter of 20 mm was used. For data acquisition and image processing, the Bruker software Para vision 4.0 was employed. Mice were anaesthetized as described above. To ensure constant body temperature of 37℃ during the entire experiment, animals were placed on an MRI compatible heating blanket (Small Animal Monitoring & Gating System, SA Instruments, Stony Brook, New York, USA). T2 relaxation time in the brain was determined with a T2 MSME sequence (imaging parameters TR = 2500/16 Echoes with TE 10–160 ms). Eight axial slices with a slice thickness of 1.0 mm, a field of view of (FOV) 2.56 × 2.56 cm and a matrix of 256 × 256 were positioned over the brain from olfactory bulb to cerebellum. Calculation of the lesion volume was performed with Analyze 10.0 (AnalyzeDirect, Inc., Lenexa USA) using a region of interest tool to delineate the hyperintense ischemic areas in T2-weighted images. A 3D object map of the stroke region was constructed by connecting all pixels within a specified threshold range about a selected seed pixel. Subsequently, the total volume of the whole object map was automatically calculated. Atrophy corrected relative lesion volume in the long-term outcome group was calculated by the following formula:
Behavioural assessment
Rotarod
The Rotarod (TSE systems) is an established test to assess motor function in rodents and was performed as described previously.24 It consists of a rotating and accelerating bar (4 to 40 rounds per minute over 300 s) in a box subdivided into five chambers to allow for multiple simultaneous testing. Animals were placed on the bar and the time until the animal dropped was automatically recorded. In each recording session, three trials per animal were recorded and the mean was used for further statistical analysis. Between each single trial, animals were allowed to rest for 15 min. Animals were trained on four consecutive days preoperative with three daily trials. The last three trials served as a preoperative baseline.
Pole test
The pole test has been shown to be useful to assess motor function in rodents after cerebral ischemia25 and was used as described previously.24 The test apparatus consists of a vertical 55 cm tall steel pole coated with tape to create a rough surface and a diameter of 8–10 mm. At the top of the pole, a 7 × 7 cm measuring cardboard was attached to avoid animals from climbing over the top instead of turning around. Animals were placed at the top of the bar and the time until the mouse performed a complete turn (T turn) as well as the time to make a complete descend (T down) were measured. Preoperatively animals were trained on four consecutive days with four daily trials. A preoperative baseline was recorded on the last training day. Postoperative recording sessions consisted of four trials per animal. The mean of these four trials was used for further statistical analysis. All trials were recorded with a video camera and analysed using Avidemux (MEAN) video analysing software.
Corner test
The corner test was developed to assess sensorimotor function in rats but has been established for functional evaluation in mice after cerebral ischemia.26 The test consists of a diamond-shaped arena made from four 30 × 30 cm measuring cardboards joined in 30° and 150° angles. Animals are placed in the middle of the arena and will typically run in one of the 150° corners. Once their whiskers touch the wall, mice rear and turn to either side. A trial was ended after 10 turning movements to the left or right. The laterality index was calculated as [Turns(left) – Turns(right)]/[Turns(left) + Turns(right)]. Healthy animals do not show a side preference and thus the laterality index of a healthy animal is 0.26
Statistical evaluation
Data are represented as mean ± standard deviation (SD). Comparison between two groups was performed using Student’s t-test for independent samples. Differences between more than two groups were evaluated using one-way, two-way ANOVA or a mixed linear model according to the test requirements. For post hoc comparisons, Bonferroni or Tukey-HSD tests were used as recommended by Schlattmann and Dirnagl.27
Sample size calculation
Sample size calculation was based on an assumed effect size of 30% and a SD of 25%. A power of .8 at an alpha criterion of .05 was targeted. For studies comparing more than two groups, calculated group sizes varied between n = 12 and 15 animals per group depending on assumed distribution of treatment effects. For studies comparing two groups, the calculated group size equalled 10 animals per group. Because of an a priori assumed higher mortality among PPARδ, null mice group sizes for these two groups were doubled.
Results
Concentration of SAR145 in the brain tissue
After oral treatment (24 h), mass spectroscopic measurement showed a concentration of 2.23 ± 0.57 µg/g (∼4.4 µM) in the brain parenchyma which is well above the EC50 values of SAR145 at murine PPARδ of 0.005 μM. These findings are in line with data supplied by the supplier (Sanofi) reporting a peak concentration of ∼4 µg/g (8.2 µM) SAR145 8 h after oral administration (tmax) in the brain and a half-life of 23 h (personal communications).
Studies 1 and 2: Determining the most effective dose and time of administration in wt mice
Study 1: Treatment with SAR145 at the time of reperfusion results in a smaller mean infarct volume compared to vehicle-treated animals
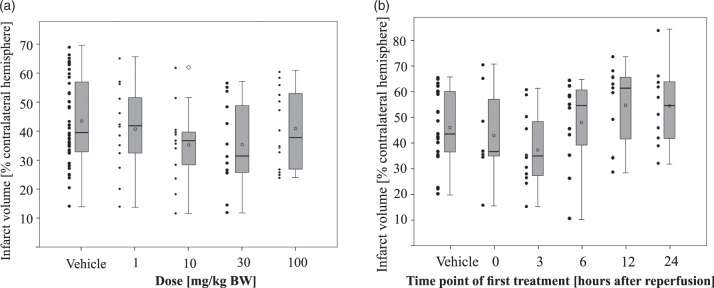
Animals received treatment of SAR145 in different doses (1, 10, 30, and 100 mg/kg body weight) at the time of reperfusion after 45 min MCAo followed by a daily treatment of the respective dose. In animals treated with 10 mg/kg (n = 15, n = 13 included, 2 no infarct), or 30 mg/kg (n = 13, n = 13 included) SAR145, we observed smaller mean infarct volumes 72 h after MCAo compared to vehicle-treated controls (n = 51, n = 45 included, 3 died, 3 no infarct) (Figure 2(a)). Compared to the controls in these two groups, we observed a mean relative reduction in infarct volumes by 19%. In animals treated with 1 mg/kg (n = 16, n = 13 included, 2 died, 1 no infarct), or with 100 mg/kg (n = 15, n = 14 included, 1 died) SAR145, mean infarct volumes were comparable to vehicle-treated animals. However, inferential statistical testing with one-way ANOVA did not show a significant difference between these groups.
Figure 2.
Effects of SAR145 treatment at different doses and with different treatment delays on infarct size.(a) Infarct volumes determined at 72 h after ischemia were reduced by 19% in animals treated with SAR145 at the time of reperfusion with a dose of 10 mg/kg BW (35 ± 13%; n = 13) or 30 mg/kg BW (35 ± 15%; n = 13) compared to animals treated with vehicle (44 ± 15%; n = 45). An only negligible reduction in infarct sizes was observed in animals treated with 1 mg/kg BW (41 ± 15%; n = 13) or 100 mg/kg BW (41 ± 13%; n = 14). There was no statistically significant difference between these groups, ANOVA F(4, 93) = 1.312, p = .271. Data are presented as Tukey boxplot with means (circle in box) and dotplot of all included values. Edema-corrected infarct volumes as percentage of the contralateral hemisphere ± SD and number of animals included in the study are given in brackets. (b) Treatment with 10 mg/kg BW SAR145 enabled a reduction in infarct size in animals treated at the time of reperfusion (43 ± 18%; n = 8) or 3 h after (37 ± 14%; n = 12). Initial treatment with longer delays of 6 h (48 ± 17%; n = 12), 12 h (55 ± 16%; n = 11) or 24 h (54 ± 16%; n = 10) did not reduce infarct volume compared to vehicle treatment (46 ± 15%, n = 21). Effects were not statistically significant at p < .05 in ANOVA testing, F(5, 68) = 2.049, p = .083. Data are presented as Tukey boxplot with means (circle in box) and dotplot of all included values. Edema-corrected infarct volumes as percentage of the contralateral hemisphere ± SD and number of animals included in the study are given in brackets.
Study 2: Delayed administration of SAR145 up to 3 h after of reperfusion reduces infarct volumes
To assess the efficacy of delayed administration of SAR145 after 45 min MCAo, we commenced treatment 3 h, 6 h, 12 h, and 24 h after reperfusion followed by daily treatment. We used a constant dose of 10 mg/kg SAR145 for these experiments because it had shown largest relative mean infarct reduction compared to controls in study 1. Administration of 10 mg/kg SAR145 at 3 h (n = 12, n = 12 included) after reperfusion resulted in 19% smaller mean infarct volumes after 72 h compared to controls (n = 24, n = 21 included, 2 died, 1 no infarct) (Figure 2(b)). Treatment initiation at later time points 6 h (n = 12, n = 12 included), 12 h (n = 11, n = 11 included) and 24 h (n = 10, n = 10 included) resulted in slightly increased infarct volumes. These effects did not reach significance in inferential statistics with one-way ANOVA, F(5, 68) = 2.049, p = .083
Study 3: Effects of SAR145 treatment in PPARδ null mice
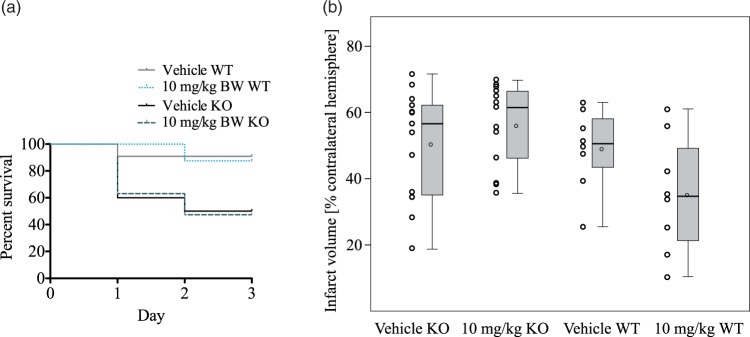
To assess the effects of the PPARδ agonist SAR145 in PPARδ null mice, we treated PPARδ −/− and +/+ mice according to the paradigm described in Study 1 with 10 mg/kg SAR145 (20 ko animals, n = 13 included, 4 died, 2 no infarct, 1 died during MCAo procedure; 10 wt animals, n = 8 included, 1 died, 1 no infarct) or vehicle (20 ko animals, n = 15 included, 5 died; 10 wt animals, n = 8 included, 1 died, 1 no infarct) at the time of reperfusion followed by daily treatment. Infarct volumes were evaluated 72 h after MCAo (45 min).
PPARδ null mice have a higher mortality 72 h after MCAo
In a Kaplan–Meier survival analysis of PPARδ −/− and +/+ mice after MCAo, survival of PPARδ null mice 72 h after transient cerebral ischemia was significantly reduced compared to wt animals irrespective of treatment (Figure 3(a)). There was no statistically significant difference in mortality between treatment groups within each genotype.
Figure 3.
Effects of SAR145 treatment in PPARδ-knockout and wild type animals.(a) Kaplan–Meier curve of percentage of knockout (ko) and wild-type (wt) animals surviving. A smaller percentage PPARδ-ko animals survived the full length of the experiment. In both ko groups; 50% of the animals died or had to be killed prematurely within the first 72 h, whereas only 10% of the wt animals did not survive the entire length of the experiment. This difference was statistically significant (vehicle ko vs. vehicle wt: Gehan-Breslow-Wilcoxon test χ2 = 4.608, p = .0318; 10 mg/kg ko vs. 10 mg/kg wt Gehan-Breslow-Wilcoxon-Test χ2 = 4,05, p = .0442).(b) PPARδ-ko animals and wt mice were randomly allocated to SAR145 and vehicle groups and were operated in a random order. SAR145 was administered at the time of reperfusion in a dosage of 10 mg/kg BW. Mean infarct sizes between vehicle (50 ± 16%, n = 15) and SAR145- (56 ± 12%, n = 13) treated ko animals did not differ significantly (two-tailed t-test for independent samples: p = .324). In wt animals, mean infarct sizes in vehicle (49 ± 12%, n = 8) and SAR145- (35 ± 18%, n = 8) treated groups were similar to previous results in this study. Data are presented as Tukey boxplot with means (circle in box) and dotplot of all included values. Edema-corrected infarct volumes as percentage of the contralateral hemisphere ± SD and number of animals included in the study are given in brackets.
PPARδ null mice killed prematurely show larger infarct volumes than mice surviving the full length of the experiment
To analyse if increased mortality in PPARδ nulls was related to bigger ischemic infarcts, we compared infarct volumes of null mice killed prematurely according to predefined animal welfare criteria to null mice surviving the full length of the experiment. In PPARδ null SAR145 treated and vehicle groups, animals killed prematurely showed significantly larger infarct volumes (null mice killed prematurely (n = 11) vs. null mice killed after 72 h (n = 17) in vehicle and SAR145 groups, one-way ANOVA and Tukey post hoc test; F(3, 24) = 7.669, p = .001; vehicle: p = .004; SAR145: p = .043). Therefore, increased mortality in PPARδ-ko mice after MCAo was likely associated with larger infarcts in ko animals.
SAR145 treatment in PPARδ null mice does not reduce mean infarct volumes
Distribution of infarct volumes in PPARδ null mice treated with SAR145 was similar to vehicle-treated nulls (Figure 3(b)). Overall, mean infarct volumes were slightly larger in PPARδ null mice compared to wt animals (Figure 3). In wt mice, mean infarct volumes were 28% smaller after SAR145 treatment compared to vehicle-treated controls (two-tailed t-test for independent samples: p = .089). These data implicate a role of PPARδ in infarct formation after MCAo.
Study 4: Long-term functional and histological outcome
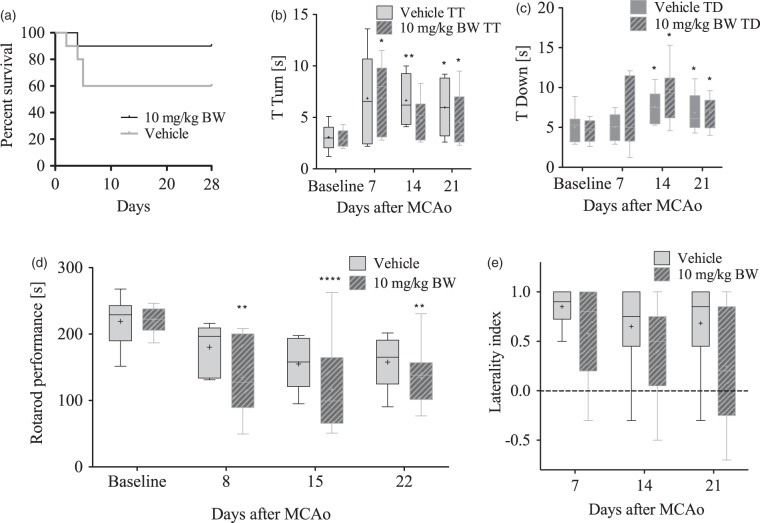
To assess the long-term functional and histological outcome, we used behavioural tests and MR imaging at three time points for up to 28 days after MCAo. Mice received delayed treatment with 10 mg/kg SAR145 (n = 10, n = 9 included, 1 died) or vehicle (n = 10, n = 6 included, 4 died) beginning at 3 h after reperfusion followed by daily treatment with SAR145 or vehicle. Mortality in these long-term experiments was 10% in SAR145 and 40% in vehicle-treated groups (Gehan-Breslow-Wilcoxon p = .14) (Figure 4(a)).
Figure 4.
Longer term functional outcome measurements.(a) Kaplan–Meier curve of long-term survival of animals treated with SAR145 or vehicle: Mortality in SAR145-treated animals was 10% after 28 days, whereas 40% of vehicle-treated animals died. This difference was not significant (Gehan-Breslow-Wilcoxon, p = .14).(b and c) Long-term functional outcome measured with the pole test: (b) Time to turn make a complete turn (s): Animals took significantly more time to make a complete turn on the pole after MCAo. No significant difference between vehicle and SAR145-treated groups. (c) Time to reach the ground (s). Animals took significantly more time to reach the ground after MCAo (*p < .05 vs. baseline). No significant difference between vehicle and SAR145-treated groups. Data are presented as Tukey boxplot with means (plus sign in box). A mixed linear mixed model with Bonferroni post hoc test was employed as we were unable to obtain valid data from all surviving animals at all time points. T turn Vehicle n = 6–10; 10 mg/kg n = 7–10. T down, Vehicle n = 6–10; 10 mg/kg n = 7–10. Time turn: Effect of treatment group: F(1, 14) = 0.379, p = .55; Effect of time point: F(3, 18) = 8.95, p = .001; Group × time point interaction: F(3, 18) = 2.26, p = .115; Time down: Effect of treatment group F(1, 19) = 0.9, p = .35; Effect of time point: F(3, 20) = 7.84, p = .001; Group × time point interaction: F(3, 20) = 0.722, p = .55). *p < .05 versus baseline.(d and e) Long-term functional outcome measured with the Rotarod and corner test. (d) Rotatrod performance was diminished in animals after MCAo. No significant difference between vehicle and SAR145-treated groups. Data are presented as Tukey boxplot with means (plus sign in box). *p < .05, **p < .01, ***p < .0001 versus baseline; two-way repeated measures ANOVA and Bonferroni post hoc test (vehicle n = 6; 10 mg/kg n = 9). Effect of treatment group: F(1, 13) = 2.4, p = .14; Effect of time point: F(4, 13) = 14.9, p = <.0001; Group × time point interaction: F(4, 13) = 0.55, p = .69. E) There was no significant difference in laterality index after MCAo between vehicle (n = 6) and 10 mg/kg SAR145- (n = 9) treated animals. Two-way repeated measures ANOVA and Bonferroni post hoc test. Effect of treatment group: F(1, 13) = 2.1, p = .17; Effect of time point: F(2, 13) = 2.5, p = .09; Group × time point interaction F(2, 13) = 0.39, p = .67.
Delayed SAR145 treatment does not improve functional outcome assessed for up to 28 days after MCAo
In pole test, post-ischemic animals in both treatment groups needed significantly longer to completely turn and to reach the floor as compared to baseline values. There was no significant difference in the performances of vehicle vs. SAR145-treated animals at any time point throughout the 28-days behavioural observation period (Figure 4(b) and (c)). Similarly, in rota rod test, time spent on the rod was decreased after MCAo in both groups as compared to baseline values (Figure 4(d)). No significant difference between SAR145 and vehicle-treated groups was observed. Similarly, the corner test did also not reveal any differences between vehicle or SAR145-treated animals (Figure 4(e)).
Delayed SAR145 treatment does not reduce infarct size in the long-term observation
To assess long-term effects of SAR145 on infarct sizes, we used MR imaging at 4, 10, and 28 days after MCAo. In this cohort, we did not observe a reduction in infarct volumes assed by MR imaging. Infarct sizes in SAR145-treated animals were similar to our previous results (44 ± 8%, 38 ± 8% and 37 ± 8% of the contralateral hemisphere; n = 9 for all time points). Infarct sizes in vehicle-treated animals were smaller than in previous vehicle groups (34 ± 14%, 27 ± 11% and 28 ± 12%; n = 7 for day 4 and n = 6 for all other time points). Infarct sizes did not differ significantly between treatment groups (linear mixed model with repeated measures, between subject factor “group” F(1, 15) = 2.737, p = .118).
Discussion
In this study, we characterized neuroprotective effects of the novel orally bioavailable PPARδ agonist SAR145. Published data on PPARδ agonists in models of ischemic stroke are sparse. Previous studies suggested substantial neuroprotective properties with a reduction in infarct size of up to 41%.8,10,12 Contrary to our hypothesis, we could not demonstrate a statistically significant short-term or long-term neuroprotective effect of SAR145 treatment in this study.
To improve translation from preclinical findings into clinical studies, the STAIR guidelines for preclinical neuroprotective drug testing were introduced in 1999.20,21,28 Following these guidelines, we conducted four independent experiments. First, we studied dose-effect response to SAR145 treatment. Second, we assessed the window of opportunity for SAR145 administration after onset of ischemia. Third, we tested SAR145 in PPARδ-ko animals to show specificity of any observed treatment effect to PPARδ activation. Fourth, we tested for the long-term functional outcome, as long-term functional recovery is a major end point in clinical trials.4,29
To date, this is the first study testing an orally administrable PPARδ agonist in a murine model of cerebral ischemia as opposed to intracerebroventricular10,12 or intraperitoneal administration.8 We opted to test an oral administration, as routine and repeated intrathecal administration may have limited clinical feasibility. Mass spectroscopy analysis of brain tissue after oral treatment with SAR145 showed concentrations of SAR145 in the brain parenchyma well above the EC50 values of SAR145 at murine PPARδ. Additional pharmacodynamics data by the supplier show a fully agonistic effect of SAR145 for PPARδ with an at least 150-fold selectivity compared to other PPARs. Therefore, we conclude that SAR145 is a potent and selective PPARδ agonist and that it is present in the brain in sufficient concentrations after oral treatment.
We were also first to study PPARδ agonists as a neuroprotective strategy in cerebral ischemia with delayed treatment time points, with multiple dosing schemes and in short and long-term observation. Descriptively, animals treated with 10 mg/kg SAR145 at the time of reperfusion showed a dose and time point dependent modest reduction in lesion size. This effect was observed in three independent groups in the dose-effect response experiment (Figure 2(a)), the window of opportunity experiment (Figure 2(b)) and the experiment comparing ko to wt animals (Figure 4). Pooling these identically treated groups showed a significant reduction in infarct size by 19% of SAR145 (n = 29) compared to vehicle-treated (n = 40) animals (two-tailed t-test for independent samples, p = .011). As the pooled analysis has not been planned a priori in the protocol, this analysis can only have an exploratory character to give an estimate about the observed effect size. Formal statistical testing of the individual experiments per study protocol, however, could not demonstrate a neuroprotective effect of SAR145 treatment. The dose-effect response experiments suggested similar effects for 10 mg/kg and 30 mg/kg. Assuming a logarithmic pharmacodynamic model for SAR145, this would be expected. Higher (100 mg/kg) and lower doses (1 mg/kg) of SAR145 showed lesion sizes comparable to vehicle-treated animals. In the experiment assessing the window of opportunity, we observed comparable effects for animals treated at the time of reperfusion and 3 h after reperfusion (Figure 2). For later time points, there was no beneficial effect of SAR145 treatment observed. Ko animals exhibited a significantly larger mortality after cerebral ischemia compared to wt animals. This suggests an involvement of PPARδ and its activation in the post-ischemic pathophysiology. We did not observe a significant difference in lesion size between ko and wt animals. This, however, might not reflect the actual increase in infarct size in PPARδ-ko mice, as mortality in ko animals was high and animals killed prematurely showed significantly larger infarct sizes than animals surviving 72 h. To evaluate long-term outcome, animals were treated with 10 mg/kg SAR145 3 h after reperfusion and followed up for 28 days. A delayed treatment paradigm was chosen to better model the clinical setting of cerebral ischemia. In long-term observation, we did not see statistically significant differences in lesion size or behaviour between treatment groups. Mortality between SAR145 and vehicle groups differed substantially (10% vs. 40%, respectively). Again, this difference might have influenced the measured mean infarct size.
The present study used a total of 227 wt and 40 ko mice. Sample size calculation was based on an effect size of 30% to achieve a power of .8 at the .05 criterion level. The efficacy of tPA, the only drug proven to show an effect both in animals and humans, is around 30% in studies modeling stroke.30 This effect size is therefore commonly used as the clinically relevant threshold to guide sample size calculation.31 Our study design did not allow us to reject the null hypotheses for most of the conducted experiments. Using the described protocols, the probability of missing an effect of treatment with SAR145 to be 30% or greater (at an .05 level for significance) is therefore below 0.2. Our posthoc performed pooled analysis suggested a reduction in lesion size of 19%. Assuming an effect of 19% to be the “true effect,” a sample size twice as large would be needed to obtain a power of 0.8 in our study. Similarly, functional outcome assessment was planned for an effect comparable to the initially assumed reduction in lesion size. The inherently high inter and intra subject variability in the utilized functional assessments would request exceedingly high animal numbers per group to detect a functional benefit of a much smaller magnitude.32 Our methods therefore could not have reliably detected an effect substantially smaller than previously reported. Assumption of treatment effects of at least 30% was based on previously reported strong neuroprotective effects of PPARδ agonist treatment in models of cerebral ischemia. The first report of PPARδ agonists as a neuroprotective treatment after cerebral ischemia by Iwashita et al.12 in 2007 tested the intracerebroventricular administered compounds L-165041 and GW501516 24 h before MCAo. A reduction in infarct volumes after 24 h of 18% and 36%, respectively, was observed. Using the same dose of GW501516 and an almost identical treatment regime, Yin et al.10 reported similar findings of an infarct volume reduction of 41% after 24 h. The latest study on PPARδ agonists in models of cerebral ischemia by Chao et al.8 tested GW0742 administered 30 min before MCAo by intraperitoneal administration finding a reduction of 44% after 24 h.
The most salient differences of our study to previous studies are the compound, the route and timing of administration and the experimental design. The compound SAR145 has been shown to be a selective PPARδ agonist by the supplier and to be orally bioavailable. Using mass spectroscopy, we confirmed bioavailability of SAR145 in the target tissue using our mode of oral administration. This study, however, does not include data on pharmacokinetics in mice after MCAo and with long-term treatment. Mass spectroscopic brain compound concentrations in this study were limited to MCAo naïve mice after seven days of oral treatment. A reduced brain penetration of the compound after MCAo or after long term treatment could have contributed to a reduced effect size in this study. However, based on pathophysiological considerations, we deem it unlikely that compound penetration into the brain is reduced after MCAo or in an extended period of oral treatment. We will therefore set aside concerns regarding the pharmacokinetic and pharmacodynamics properties of the compound. We consider at least two other paths of reasoning for the divergence in the effect size of our study to previous studies.
The time point and route of administration
To date, there are no reports describing the neuroprotective effect of PPARδ activation at the time of ischemia induction or after. Studies by Pialat et al.11 showed a difference in infarct sizes of PPARδ-ko and wt animals as early as 30 min after induction of cerebral ischemia, indicating an involvement of PPARδ even in the very early phase of infarct formation. Differences in vasculature of ko and wt animals have not been found and are therefore unlikely to explain this difference in infarct size.33 PPARδ agonists might have to be present at the time of ischemia onset to have strong neuroprotective effects. The present study tested an oral route of administration. In contrast to an intravenous or even intraarterial administration, time to peak might be delayed for oral administration. Oral administration is a very realistic test scenario for a new drug, but may at least partially account for the absent therapeutic effects in our study. To study the effect of PPARδ activation on the very early phase of infarct formation after reperfusion, experiments using an intra-arterial administration strategy of a PPARδ agonist would be interesting. Depending on the time point of administration, this strategy could also be used to mimic the clinical situation of a mechanical thrombectomy and immediate intra-arterial PPARδ agonist treatment after MCA reperfusion. This interesting alternative treatment strategy, however, is beyond the scope of the present study.
Bias and experimental quality
It has been shown repeatedly that study quality has an effect on reported effect size in studies of cerebral ischemia.34–36 Effect sizes in studies using randomization as a measure to reduce bias differ substantially from studies not using randomization. For the neuroprotective candidate substance, NXY-059 effect size was 20% in studies using randomization vs. 52% in studies without randomization.35 In two large clinical stroke trials, NXY-059 failed to show a neuroprotective effect. No previous study using PPARδ agonists in models of cerebral ischemia reported randomization or blinded conduction of experiments.8,10,12 Only one study has reported blinded outcome analyses.8 None of the previous studies fully complied to the ARRIVE guidelines for reporting in vivo experiments. Unreported attrition of animals has been shown to be a possible source of bias and lead to overestimation of effect size.37
A combination of the two factors discussed above is deemed most likely to explain the difference in effect size observed in our study compared to previous ones. Opposed to previous studies, the present study did not address immunological endpoints but focused on lesion volume, survival and functional outcomes. Given the overall neutral study results, impacts of such effects on neuroprotection in our study must have been much smaller than previously reported.
We conclude that oral treatment with the selective PPARδ agonist SAR145 at the time of reperfusion or later is unlikely to have the previously assumed effect size of 30% or larger. We cannot rule out an effect of SAR145 treatment below the a priori assumed effect size. In fact, a reduction of lesion size by 19% is suggested by pooled analysis for treatment at the time of reperfusion. Our data from experiments in ko animals suggest an involvement of PPARδ in infarct formation and post-ischemic mortality. These findings do not preclude that SAR145 or other PPARδ agonists may be useful in a clinical setting of cerebral ischemia. On balance, however, this will be unlikely if treatment is commenced at the time of ischemia or later.
Acknowledgments
We thank Petra Loge (Department of Experimental Neurology, Charité University Medicine Berlin, Berlin, Germany) for continuous support in conducting the experiments and Susanne Mueller (Charité Core Facility 7 T Experimental MRIs, Charité University Medicine Berlin, Berlin, Germany) for support with MRI studies.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by grants from the DFG [SFB TRR 43]; the BMBF [Center for Stroke Research Berlin]. The PPARδ agonist SAR145 was kindly provided by Sanofi.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
SK, ME, FB, CH and AK contributed in designing and planning the experiments as well as obtaining official licences to conduct this study. FB signed the MTA with Sanofi. FB supplied the PPARδ-ko animals. SK conducted all experiments and outcome analyses. All authors contributed in drafting and revising the manuscript. All authors approved the manuscript for publication.
References
- 1.O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol 2006; 59: 467–477. [DOI] [PubMed] [Google Scholar]
- 2.Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci 2003; 26: 248–254. [DOI] [PubMed] [Google Scholar]
- 3.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med 2011; 17: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. [DOI] [PubMed] [Google Scholar]
- 5.European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25: 457–507. [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 7.Ouk T, Gautier S, Pétrault M, et al. Effects of the PPAR-α agonist fenofibrate on acute and short-term consequences of brain ischemia. J Cerebr Blood Flow Metab 2014; 34: 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao X, Xiong C, Dong W, et al. Activation of peroxisome proliferator-activated receptor β/δ attenuates acute ischemic stroke on middle cerebral ischemia occlusion in rats. J Stroke Cerebrovasc 2014; 23: 1396–1402. [DOI] [PubMed] [Google Scholar]
- 9.Schnegg CI, Robbins ME. Neuroprotective mechanisms of PPARδ: modulation of oxidative stress and inflammatory processes. PPAR Res 2011; 2011: 373560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yin K-J, Deng Z, Hamblin M, et al. Peroxisome proliferator-activated receptor delta regulation of miR-15a in ischemia-induced cerebral vascular endothelial injury. J Neurosci 2010; 30: 6398–6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pialat J-B, Cho T-H, Beuf O, et al. MRI monitoring of focal cerebral ischemia in peroxisome proliferator-activated receptor (PPAR)-deficient mice. NMR Biomed 2007; 20: 335–342. [DOI] [PubMed] [Google Scholar]
- 12.Iwashita A, Muramatsu Y, Yamazaki T, et al. Neuroprotective efficacy of the peroxisome proliferator-activated receptor-selective agonists in vitro and in vivo. J Pharmacol Exp Ther 2007; 320: 1087–1096. [DOI] [PubMed] [Google Scholar]
- 13.Kota BP, Huang T, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacol Rec 2005; 51: 85–94. [DOI] [PubMed] [Google Scholar]
- 14.Culman J, Zhao Y, Gohlke P, et al. PPAR-gamma: therapeutic target for ischemic stroke. Trends Pharmacol Sci 2007; 28: 244–249. [DOI] [PubMed] [Google Scholar]
- 15.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peters JM, Lee SS, Li W, et al. Growth, adipose, brain, and skin alterations resulting from targeted disruption of the mouse peroxisome proliferator-activated receptor beta(delta). Mol Cell Biol 2000; 20: 5119–5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dirnagl U. Group MOTM-S. Standard operating procedures (SOP) in experimental stroke research: SOP for middle cerebral artery occlusion in the mouse. Nature Preced 2012. DOI: 10.1038/npre.2012.3492.3. [Google Scholar]
- 18.Engel O, Kolodziej S, Dirnagl U, et al. Modeling stroke in mice – middle cerebral artery occlusion with the filament model. J Vis Exp 2011; 47: 2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royl G, Balkaya M, Lehmann S, et al. Effects of the PDE5-inhibitor vardenafil in a mouse stroke model. Brain Res 2009; 1265: 148–157. [DOI] [PubMed] [Google Scholar]
- 20.STAIR STAIR. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999; 30: 2752–2758. [DOI] [PubMed] [Google Scholar]
- 21.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swanson RA, Morton MT, Tsao-Wu G, et al. A semiautomated method for measuring brain infarct volume. J Cerebr Blood Flow Metab 1990; 10: 290–293. [DOI] [PubMed] [Google Scholar]
- 23.Yildirim F, Ji S, Kronenberg G, et al. Histone Acetylation and CREB Binding Protein Are Required for Neuronal Resistance against Ischemic Injury. PLoS One 2014; 9: e95465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Balkaya M, Kröber J, Gertz K, et al. Characterization of long-term functional outcome in a murine model of mild brain ischemia. J Neurosci Meth 2013; 213: 179–187. [DOI] [PubMed] [Google Scholar]
- 25.Royl G, Balkaya M, Lehmann S, et al. Effects of the PDE5-inhibitor vardenafil in a mouse stroke model. Brain Res 2009; 1265: 148–157. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Schallert T, Zhang ZG, et al. A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Meth 2002; 117: 207–214. [DOI] [PubMed] [Google Scholar]
- 27.Schlattmann P, Dirnagl U. Statistics in experimental cerebrovascular research: comparison of more than two groups with a continuous outcome variable. J Cereb Blood Flow Metab 2010; 30: 1558–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saver JL, Albers GW, Dunn B, et al. Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for Extended Window Acute Stroke Therapy Trials. Stroke 2009; 40: 2594–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion–diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol 2009; 8: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sena ES, Briscoe CL, Howells DW, et al. Factors affecting the apparent efficacy and safety of tissue plasminogen activator in thrombotic occlusion models of stroke: systematic review and meta-analysis. J Cereb Blood Flow Metab 2010; 30: 1905–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howells DW, Sena ES, Macleod MR. Bringing rigour to translational medicine. Nat Rev Neurol 2013; 10: 1–7. [DOI] [PubMed] [Google Scholar]
- 32.Hetze S, Römer C, Teufelhart C, et al. Gait analysis as a method for assessing neurological outcome in a mouse model of stroke. J Neurosci Meth 2012; 206: 7–14. [DOI] [PubMed] [Google Scholar]
- 33.Arsenijevic D, de Bilbao F, Plamondon J, et al. Increased infarct size and lack of hyperphagic response after focal cerebral ischemia in peroxisome proliferator-activated receptor β-deficient mice. J Cereb Blood Flow Metab 2005; 26: 433–445. [DOI] [PubMed] [Google Scholar]
- 34.Macleod MR, O'Collins T, Horky LL, et al. Systematic review and metaanalysis of the efficacy of FK506 in experimental stroke. J Cereb Blood Flow Metab 2005; 25: 713–721. [DOI] [PubMed] [Google Scholar]
- 35.Macleod MR, van der Worp HB, Sena ES, et al. Evidence for the efficacy of NXY-059 in experimental focal cerebral ischaemia is confounded by study quality. Stroke 2008; 39: 2824–2829. [DOI] [PubMed] [Google Scholar]
- 36.Macleod MR, Fisher M, O'Collins V, et al. Good laboratory practice: preventing introduction of bias at the bench. Stroke 2009; 40: e50–2. [DOI] [PubMed] [Google Scholar]
- 37.Holman C, Piper SK, Grittner U, et al. Where Have All the Rodents Gone? The effects of attrition in experimental research on cancer and stroke. PLoS Biol 2016; 14: e1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]