Abstract
Purpose
To report atypical presentation of neuronal ceroid lipofuscinoses type 8 (CLN8) to the eye clinic and review clinical features of CLN8.
Observations
Detailed eye exam by slit lamp exam, indirect ophthalmoscopy, fundus photography, optical coherence tomography, visual fields and electroretinogram (ERG). Molecular genetic testing using Next Generation Sequencing panel (NGS) and array Comparative Genomic Hybridization (aCGH).
The siblings in this study presented to the eye clinic with retinitis pigmentosa and cystoid macular edema, and a history of seizures but no severe neurocognitive deficits or regression. Genetic testing identified a c.200C > T (p.A67V) variant in the CLN8 gene and a deletion encompassing the entire gene. Electron microscopy of lymphocytes revealed fingerprint inclusions in both siblings.
Conclusions
and Importance: Pathogenic variants in CLN8 account for the retinitis pigmentosa and seizures in our patients however, currently, they do not have regression or neurocognitive decline. The presentation of NCL can be very diverse and it is important for ophthalmologists to consider this in the differential diagnosis of retinal disorders with seizures or other neurological features. Molecular genetic testing of multiple genes causing isolated and syndromic eye disorders using NGS panels and aCGH along with additional complementary testing may often be required to arrive at a definitive diagnosis.
Keywords: Chromosome microarray, Epilepsy, Lysosomal storage disorder, Neuronal ceroid lipofuscinosis, Next generation sequencing panel, Vision loss
1. Introduction
Neuronal ceroid lipofuscinoses (NCL) are a heterogeneous group of neurodegenerative disorders characterized by lysosomal accumulation of autofluorescent material in multiple tissues. Clinical features include developmental delay or regression, seizures, abnormal movements, impaired vision and premature death1, 2, 3, 4. NCL is classified as infantile, late-infantile, juvenile and adult forms based on age of onset and progression of disease5, 6. The juvenile form (Batten disease) caused by CLN3 is the most common and often present to eye clinic with vision problems6, 7. We report two siblings with a protracted presentation of NCL caused by mutations in CLN8 gene confirmed by electron microscopy (EM).
2. Findings
2.1. Case 1
A 14-year-old Hispanic female presented to eye clinic with decreased central vision for one year. She had well-controlled generalized tonic-clonic seizures since 7 years of age and bilateral postaxial polydactyly, but no history of motor or cognitive decline. However, she had formal psychoeducational evaluation during 3rd grade and was diagnosed as having ADHD. She is currently on treatment with Ritalin. She has completed 10th grade with tutoring in math and chemistry. She also requires extra time to perform written tests due to her ADHD. Family history revealed a 22-year-old brother with RP and seizures, an unaffected brother, and a mother with a history of postaxial polydactyly.
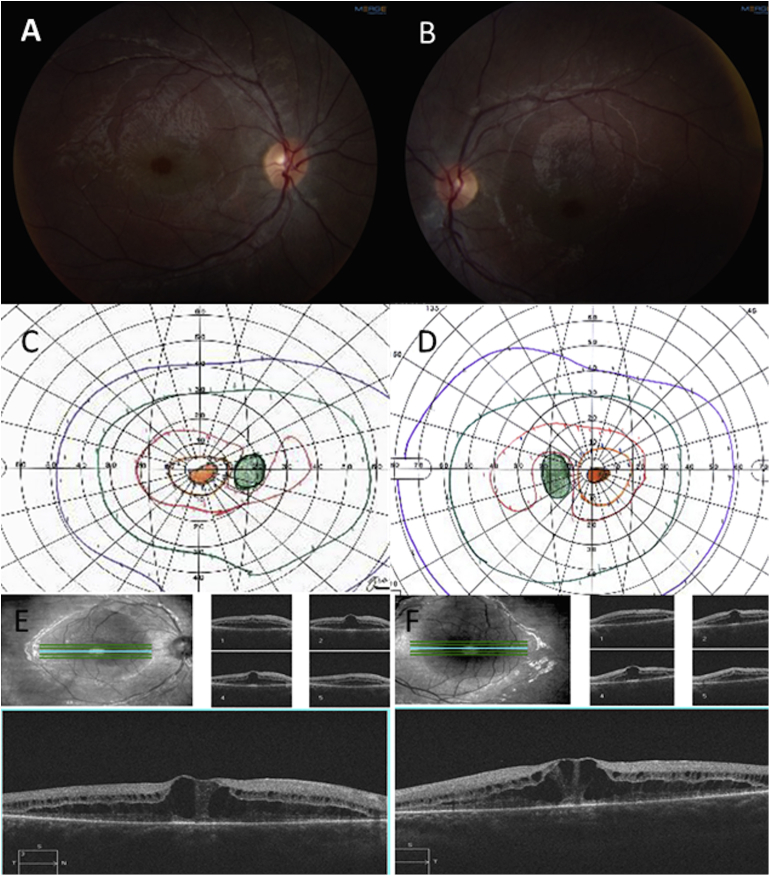
Her visual acuity was 20/40 OU, color vision was normal, fundus exam showed macular cystoid macular edema OU, and spectral domain optical coherence tomography demonstrated macular cystic change and diffuse macular schisis involving the outer nuclear and the outer plexiform layers. The schisis is an unusual finding (Fig. 1).
Fig. 1.
Case 1, Fundus photos showing healthy appearing optic discs, mild arteriolar attenuation and cystoid macular edema. (A, B). Goldman Visual Field-showing bilateral central scotomas (C, D). Optical coherence tomography demonstrating cystoid macular edema and diffuse macular schisis involving the outer nuclear and outer plexiform layers of the retina (E, F).
Full field ERG revealed severe rod dysfunction and milder cone dysfunction. Goldmann visual field revealed large central scotoma bilaterally (Fig. 1). The rest of the physical and neurologic exam as well as MRI of brain were normal.
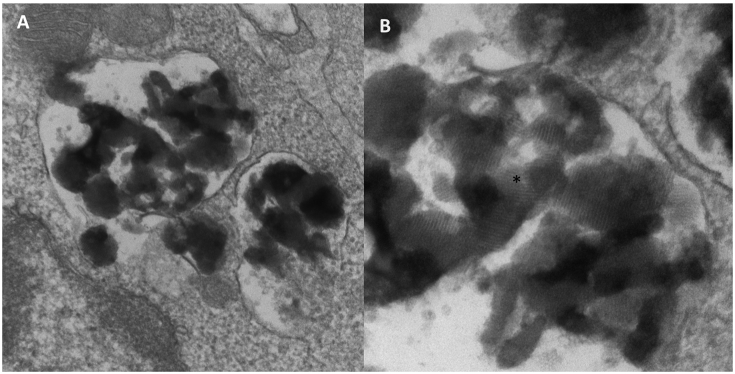
Given the history of seizures and vision loss, she was referred for genetic evaluation. Genetic testing was pursued via a comprehensive NGS Eye Panel including genes causing CLNs. It revealed a hemizygous variant of unknown significance (VUS) c.200C > T (p.A67V) in the CLN8 gene. A search in Exome Aggregation Consortium (ExAC) revealed that this variant is very rare with an allele frequency = 0.00003296 and did not report any homozygotes. Further, deletion/duplication analysis showed a heterozygous deletion in 8p23.3 encompassing the entire CLN8 gene. Parental testing revealed that these variants were in trans; mother carried the deletion and the father carried the c.200C > T (p.A67V) missense VUS. EM of lymphocytes was performed and showed fingerprint like inclusions consistent with NCL (Fig. 2).
Fig. 2.
Case 1, Electron microscopy of lymphocytes from Case 1 showing intracytoplasmic inclusions (A). Fingerprint like inclusions seen in high resolution (B).
2.2. Case 2
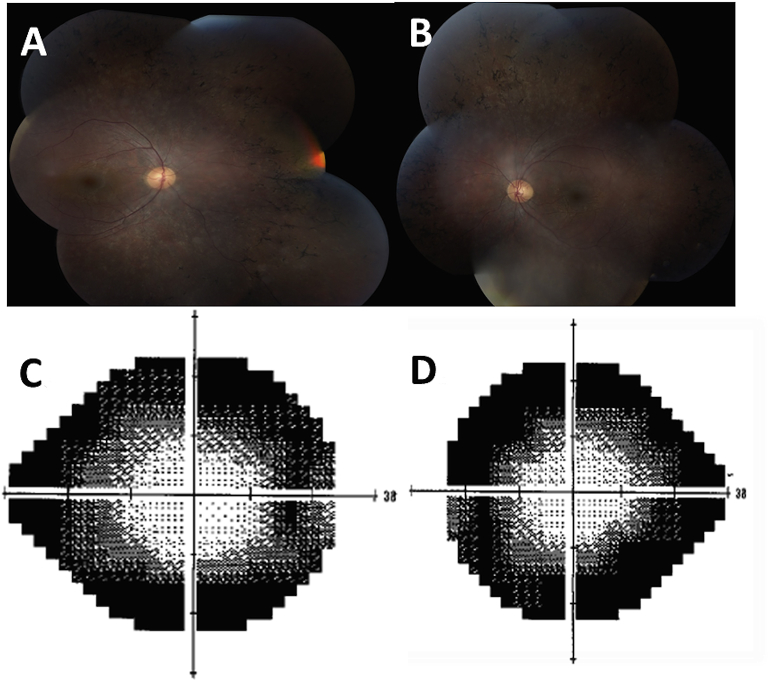
This patient is a 22-year-old Hispanic male, brother of case 1, followed in ophthalmology clinic due to decreased night vision since age 5 years. He developed tonic-clonic seizures, well controlled by medication at 16 years of age. He did not require special help during school nor had a formal psychoeducational testing. He has a master's degree in engineering and is in a full time job living independently. At 22 years of age, his visual acuity was OD 20/25 and OS 20/30. His fundus exam revealed pallor of optic nerves, attenuated vasculature, peripheral bone spicules and diffuse retinal pigment epithelial mottling but no cystoid macular edema or schisis (Fig. 3). His full field ERG showed markedly attenuated rod and cone photoreceptor system function bilaterally. Humphrey's visual fields revealed generalized constriction as well as large peripheral scotomas (Fig. 3). OCT was not performed. At time of initial assessment, he was diagnosed with retinitis pigmentosa, likely autosomal recessive. He was followed annually and had a stable course. Given sister's presentation and molecular results, he had targeted genetic testing that revealed the same VUS, c.200C > T (p.A67V), as well as the heterozygous deletion of the CLN8 gene and positive EM test suggesting that they have the same condition.
Fig. 3.
Case 2, Fundus photos showing pallor of optic nerves, attenuated vasculature, peripheral bone spicules and diffuse retinal pigment epithelial modeling (A, B). Humphrey visual field showing peripheral scotomas (C, D).
3. Discussion
Pathogenic variants in the CLN8 gene cause two distinct allelic diseases, a progressive epilepsy with mental retardation (EPMR, OMIM #610003), and a more severe variant late infantile form (CLN-8, OMIM #600143). EPMR is common in Finnish populations, presents at 5–10 years of age with seizures and cognitive decline but usually no vision loss8.
The variant late infantile form of NCL is common in Turkish and Italian populations9, 10, 11, 12 presenting around 3–7 years of age with motor decline, severe epilepsy and severe vision loss6, 9, 10. We compare the variation in the clinical presentation of our patients with other previously reported individuals with CLN8 in the Supplementary table 6, 7, 11, 12, 13, 14, 15. Other common findings reported include dystonia, ataxia and other pyramidal signs. Life expectancy of variant late infantile CLN8 is between ages 6 and adolescence. However, our patients are currently 15 and 23 years of age and have no developmental delays, cognitive decline or psychomotor symptoms.
Missense variants and haploinsufficiency have been previously reported but with a more severe phenotype than our patients5, 6, 12, 15, 16. It is possible that the novel missense variant, c.200C > T (p.A67V) in the CLN8 gene in our family results in a milder disease. This a very rare allele with a frequency of 0.00003296 in ExAC database17. We also report the finding of macular schisis involving the outer nuclear and plexiform layers for the first time in an individual with CLN8 gene mutations.
Our proband and her mother also had finding of post-axial polydactyly. Non-syndromic post-axial polydactyly is usually inherited in an autosomal dominant manner and has a prevalence of 1.6–10.7 in 100018. We believe this was an isolated finding and that it is not associated with our patients' diagnosis of CLN8. Our patients did not exhibit other signs of Bardet-Biedl syndrome or other ciliopathies. Moreover, the next generation sequencing panel assessed in our patients included many ciliopathy genes and we did not find any pathogenic variants in these genes.
4. Conclusions
In individuals presenting with retinal degenerative conditions, history of additional clinical features such as seizures, polydactyly or developmental delays should prompt evaluation of systemic disorders such as NCL or ciliopathies. The individuals presented here demonstrate extensive variability in clinical presentation, rate of progression and age of onset and have a milder disease. It is possible that they may develop neurocognitive decline at a later date. Testing of multiple genes by NGS panels with reflex to deletion/duplication analysis aid in more accurate diagnoses and additional investigations such as EM of leukocytes help to resolve the uncertainty of VUS. Our patients increase the phenotypic spectrum of neuronal ceroid lipofuscinoses type 8.
Patient consent
The patients provided written consent for publication of personal information including medical record details and photographs.
Acknowledgements
We are grateful to the family for allowing us to present their findings. Emory, Department of Ophthalmology is supported by unrestricted RPB grant P30EY006360.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.ajoc.2016.07.005.
Conflict of interest
Suma P Shankar is medical director for Emory Genetics Laboratory; Christin Collins is Laboratory director for Emory Genetics Laboratory. The following authors have nothing to declare: Rossana Sanchez, Jiong Yan, Sarah Richards, Gary Mierau, Eric Wartchow.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Mole S.E., Williams R.E., Goebel H.H. 2ed. UKL Oxford University Press; Oxford: 2011. The Neuronal Ceroid Lipofuscinoses (Batten Disease) [Google Scholar]
- 2.Santavuori P. Neuronal ceroid-lipofuscinoses in childhood. Brain Dev. 1998;10:80–83. doi: 10.1016/s0387-7604(88)80075-5. [DOI] [PubMed] [Google Scholar]
- 3.Anderson Glenn W., Goebel Hans H., Simonati Alessandro. Human pathology in NCL. Biochimica Biophysica Acta. 2013;1832:1807. doi: 10.1016/j.bbadis.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Kousi M., Lehesjoki A.E., Mole S.E. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum. Mutat. 2012;33:42–63. doi: 10.1002/humu.21624. [DOI] [PubMed] [Google Scholar]
- 5.Jalanko Anu, Braulke Thomas. Neuronal ceroid lipofuscinoses. Biochimica Biophysica Acta. 2009;1793:697–709. doi: 10.1016/j.bbamcr.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Mole SE, Williams RE. Neuronal Ceroid-Lipofuscinoses. 2001 Oct 10 [Updated 2013 Aug 1]. In: Pagon RA, Adam MP, Ardinger HH, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2015. Available from: http://www.ncbi.nlm.nih.gov/books/NBK1428/.
- 7.Williams Ruth E., Aberg Laura, Autti Taina, Goebel Hans H., Kohlschutter Alfried, Lonnqvist Tuula. Diagnosis of the neuronal ceroid lipofuscinoses: an update. Biochimica Biophysica Acta. 2006;1762:865–872. doi: 10.1016/j.bbadis.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Herva R., Tyynela J., Hirvasniemi A., Syrjakallio-Ylitalo M., Haltia M. Northern epilepsy: a novel form of neuronal ceroid lipofuscinoses. Hum. Mutat. 2004;10:215–222. doi: 10.1111/j.1750-3639.2000.tb00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelnick Nathanel, Mahajna Muhammad, Iancu Theodore C., Sharony Reuven, Zeigler Marsha. A novel mutation of the CLN8 gene: is there a Mediterranean phenotype? Pediatr. Neurol. June 2007;36(6):411–413. doi: 10.1016/j.pediatrneurol.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Cannelli N., Cassandrini D., Bertini E. Novel mutations in CLN8 in Italian variant late infantile neuronal ceroid lipofuscinosis: another genetic hit in the Mediterranean. Neurogenetics. 2006;7:111e7. doi: 10.1007/s10048-005-0024-y. [DOI] [PubMed] [Google Scholar]
- 11.M Mahajnah, Zelnik N. Phenotypic heterogeneity in consanguineous patients with a common CLN8 mutation. Pediatr. Neurol. 2012 Oct;47(4):303–305. doi: 10.1016/j.pediatrneurol.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Allen N.M., O'hIci B., Anderson G., Nestor T., Lynch S.A., King M.D. Variant late-infantile neuronal ceroid lipofuscinosis due to a novel heterozygous CLN8 mutation and de novo 8p23.3 deletion. Clin. Genet. 2012;81(6):602–604. doi: 10.1111/j.1399-0004.2011.01777.x. Epub 2011 Dec 28. [DOI] [PubMed] [Google Scholar]
- 13.Jadav R.H., Sinha S., Yasha T.C., Aravinda H., Rao S., Bindu P.S., Satishandra P. Magnetic resonance imaging in neuronal ceroid lipofuscinosis and its subtypes. Neuroradiol. J. 2012;25(6):755–761. doi: 10.1177/197140091202500616. Epub 2012 Dec 20. [DOI] [PubMed] [Google Scholar]
- 14.P1 Striano, Specchio N., Biancheri R., Cannelli N., Simonati A., Cassandrini D., Rossi A., Bruno C., Fusco L., Gaggero R., Vigevano F., Bertini E., Zara F., Santorelli F.M., Striano S. Clinical and electrophysiological features of epilepsy in Italian patients with CLN8 mutations. Epilepsy Behav. 2007;10(1):187–191. doi: 10.1016/j.yebeh.2006.10.009. Epub 2006 Nov 28. [DOI] [PubMed] [Google Scholar]
- 15.NCL resource mutation data base, MRC Laboratory for Molecular Cell Biology, University College London, Gower Street, London. WC1E 6BT. United Kingdom (URL: http://www.ucl.ac.uk/ncl/mutation.shtml).
- 16.Reinhardt K., Grapp M., Schlachter K., Bruck W., Gartner J., Steinfeild R. Novel CLN8 mutations confirm the clinical and ethnic diversity of late infantile neuronal ceroid lipofuscinosis. Clin. Genet. 2010;77(Suppl. 1):79–85. doi: 10.1111/j.1399-0004.2009.01285.x. 7pp. [DOI] [PubMed] [Google Scholar]
- 17.Exome Aggregation Consortium (ExAC), Cambridge, MA (URL: http://exac.broadinstitute.org).
- 18.Malik S. Polydactyly: phenotypes, genetics and classification. Clin. Genet. 2014;85(3):203–212. doi: 10.1111/cge.12276. Epub 2013 Oct 18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.