Abstract
Over 30 million cancer survivors exist worldwide. Survivors have an earlier onset and higher incidence of chronic comorbidities, including endocrinopathies, cardiac dysfunction, osteoporosis, pulmonary fibrosis, secondary cancers and frailty than the general population; however, the fundamental basis of these changes at the cellular level is unknown. An electronic search was performed on Embase, Medline In-Process & Other Non-Indexed Citations, and the Cochrane Central Register of Controlled Trials. Original articles addressing the cellular biology of ageing and/or the mechanisms of cancer therapies similar to ageing mechanisms were included, and references of these articles were reviewed for further search. We found multiple biological process of ageing at the cellular level and their association with cancer therapies, as well as with clinical effects. The direct effects of various chemotherapies and radiation on telomere length, senescent cells, epigenetic modifications and microRNA were found. We review the effects of cancer therapies on recognised hallmarks of ageing. Long-term comorbidities seen in cancer survivors mimic the phenotypes of ageing and likely result from the interaction between therapeutic exposures and the underlying biology of ageing. Long-term follow-up of cancer survivors and research on prevention strategies should be pursued to increase the length and quality of life among the growing population of cancer survivors.
Keywords: aging, anticancer therapeutics, long term side effects
Introduction
Globally, there are over 30 million long-term survivors of cancer.1 By 2025, an estimated 19 million new cancer cases will be diagnosed each year, the majority of which will produce long-term survivors. Studies among long-term cancer survivors indicate numerous possible clinical complications resulting in considerable morbidity and mortality, related to chemotherapy, radiation therapy (RT) or both.2–6 Because of the elevated risk of chronic disease among cancer survivors, such as frailty syndrome, second cancers, psychosocial comorbidities and endocrinopathies, and because of the lack of standardised system-wide longitudinal medical follow-up, the Institute of Medicine has emphasised research in this area to establish guidance on survivorship care.7
A wealth of observational data on the development of late complications in cancer survivors are available, including our previous reviews on late complications in childhood cancer survivors8 and adults9 who undergo haematopoietic cell transplantation (HCT), but information documenting the pathological basis for development of these effects is sparse. To understand the biology of late effects better and provide a foundation for the development of interventions, it is important to characterise late effects at the cellular level. Cancer survivors, in general, appear to develop age-related diseases and phenotypes sooner than members of the general population. This is likely because damage to normal tissues from cancer therapies diminishes physiological reserve, accelerates processes typically associated with ageing or both. The roles of telomeres, senescent cells, epigenetic modifications and micro RNA (miRNA) have been described in terms of their contributions to the pathobiology of accelerated ageing. However, published data linking clinical phenotypes seen in cancer survivors with processes of accelerated ageing at the cellular level is lacking. We do not wish to suggest that cancer therapies are not valuable or worthwhile; on the contrary, the advances made in cancer treatment have allowed many individuals to live long and healthy lives. However, the purpose of the current paper is to clarify the biological mechanisms by which the ageing-like sequelae cancer therapies occur. To do this, we conducted a comprehensive review of the pathogenesis of the accelerated ageing-like state in survivors of cancer.
Design
A review of Embase, Medline In-Process and Other Non-Indexed Citations and the Cochrane Central Register of Controlled Trials was conducted by an experienced medical librarian resulting in 1259 articles by electronic search in December 2015. Only English language articles were searched. The following medical subject heading terms were used: neoplasms, ageing, age factor, premature, survival, survival analysis, disease-free survival, telomere, telomere shortening, frail elderly, patients, randomised controlled trial, double-blind method, single-blind method, placebos, placebo effect, retrospective study, case report, animals and humans. The data were synthesised based on the publications pertaining specifically to the biological basis of late complications at cellular level. Translational studies on late complications of HCT were also included due to potentially similar mechanisms of tissue and cellular damage by chemotherapy or RT in HCT recipients. We first present data describing the current evidence for accelerated ageing due to cancer therapies and then detail the effects on premature ageing and senescence at cellular level by specific treatments.
Results
Evidence for accelerated ageing in cancer survivors
Ageing is a natural and unavoidable process inherent to life. However, for many living creatures, ageing is associated with a variety of physical changes, hardships and illnesses. On a microscopic level, ageing is a consequence of gradual, lifelong accumulation of molecular and cellular damage and loss of physiological integrity. Hallmarks of ageing include genomic instability, telomere attrition, epigenetic alterations, mitochondrial dysfunction, loss of proteostasis, chronic low-grade inflammation and cellular senescence.10
Although a great success, cure or control of cancer leads to a higher prevalence and earlier onset of multiple ageing-associated health problems; these include abnormal thyroid function, decreased bone mineral density and increased osteoporosis, infertility, compromised tissue regeneration, cardiotoxicity and decreased left ventricular function, pulmonary fibrosis and chronic sterile inflammation.11–13 For example, at age 50, 45.5% of paediatric and adolescent/young adult Hodkgin’s lymphoma survivors have experienced at least one severe cardiovascular condition (defined using a modified common terminology for adverse events grading rubric).13 Cumulative incidence of second malignant neoplasms at 30 years after childhood cancer diagnosis is nearly 8%14; survivors of childhood cancer are 3.3–6 times more likely than age-matched individuals in the general population to develop a second malignancy.14–16 The estimated life expectancy for this population is 30% less than that of the general population.17 The prevalence of any chronic health condition among childhood cancer survivors at least 5 years post-treatment ranges from 66% to 88%.6 Collectively, these epidemiological data suggest that cancer therapies may accelerate the biology of ageing.
Humans age at remarkably different rates, even in the absence of cancer therapies.18 This separation between chronological age and biological age in part reflects an organism’s resilience to intrinsic (eg, reactive oxygen species) and extrinsic (eg, RT) stressors and capacity to restore cellular homeostasis.19 Clinically, the increased vulnerability to stress and inability to restore physiological integrity is referred to as frailty.20 Multiple and varied indices of frailty exist,21 but all consistently show that those deemed frail are at significantly increased risk for adverse health outcomes, including morbidity, disability and death.22 About 10% of individuals 65 years or older in the general population are considered frail, illustrating the link between frailty and ageing.23
Frailty is a trait that is shared by many survivors of HCT as well. The Bone Marrow Transplant Survivor Study (BMTSS), including nearly 1000 survivors of HCT, aged 18–64, found that the risk of frailty among survivors was 10.8-fold higher than in siblings4 and that the prevalence of frailty (8.4%) was comparable to that seen in individuals older than age 65 years.24 Participating survivors and siblings were queried for information about physical health, extent of chronic graft-versus-host-disease (GVHD), sociodemographic factors, ‘health-risk behaviours’ and physical activity level. Rates of frailty were eightfold higher among HCT survivors than among their siblings.4 Among survivors of HCT at least 10 years after transplant, the 15-year cumulative incidence of severe/life threatening/fatal conditions was 41%.25
Frail health is often demonstrated in the population of childhood cancer survivors as well. Improvements in therapy and supportive care for children diagnosed with cancer have increased the overall 5-year survival rate from 20% in the early 1960s26 to around 80% in recent years.27 However, the risk of death of paediatric cancer survivors in 10 years can vary between diagnosis groups by at most 12% even up to 20 years postdiagnosis.28 The Childhood Cancer Survivor Study (CCSS) compared the health status of 9353 adult long-term survivors of childhood cancer diagnosed between 1970 and 1986 to the health status of a randomly selected cohort of the survivors’ siblings.29 Survivors were more likely to report poor general health, poor mental health, activity limitations and functional impairment. Forty-four per cent of survivors reported at least one adverse health status domain. Additionally, 20-year-old survivors of childhood cancer were found to have the same cumulative incidence of severe, life threatening and fatal chronic health conditions as 50-year-old siblings.24 Furthermore, compared with sibling controls, childhood cancer survivors report poor general health with a prevalence ratio (PR) of 2.37 and adverse health status outcome in any domain with a PR of 2.10.30 These studies provide a quantitative description of the phenotype of premature ageing in this population.
The St Jude Lifetime Cohort Study (SJLIFE) compared patients who were treated for childhood cancer at St Jude Children’s Research Hospital between 1962 and 2003, at least 10 years prior, to age-matched controls in the general population.31 Frailty, defined as ≥3 of low lean mass, weakness, exhaustion, low energy expenditure and slow walking speed, was found in 2.7% of male participants and 13.1% of female participants. None of the comparison population fulfilled criteria for frailty. Furthermore, survivors of childhood acute lymphoblastic leukaemia exposed to cranial radiotherapy had reduced cognitive status and memory as well as reduced integrity by neuroimaging in neuroanatomical regions consistent with early onset mild cognitive impairment and dementia.32 The long-term health-related outcome among childhood cancer survivors has been compared between those who were treated with HCT and those who were treated with conventional therapy.33 Those who received HCT demonstrated significantly elevated risk for severe/life-threatening conditions (relative risk of 3). The higher than expected prevalence of frailty among HCT and childhood cancer survivors, their increased risk for adverse health outcomes and their shortened lifespan lend further evidence to the premise that cancer therapies may accelerate the fundamental biology of ageing.
The aforementioned childhood cancer therapies and HCT are vastly different from typical therapies used to treat adult cancers. Dose intensification occurs in the majority of standard regimens used to treat childhood cancer.34 Adult patients with cancer receive far less volumes of chemotherapeutics, in part due to the genetic complexity of childhood cancer tumours and in part due to the ability of children’s bone marrow to quickly recover. HCT conditioning regimens historically relied on dose intensification as well.35 While some regimens now rely on reduced intensity conditioning, most HCT survivors are faced with long-term immunosuppression and unique challenges like GVHD. Despite the relatively mild doses of chemotherapeutics used to treat adults with cancer, these individuals often develop features of accelerated ageing as well.
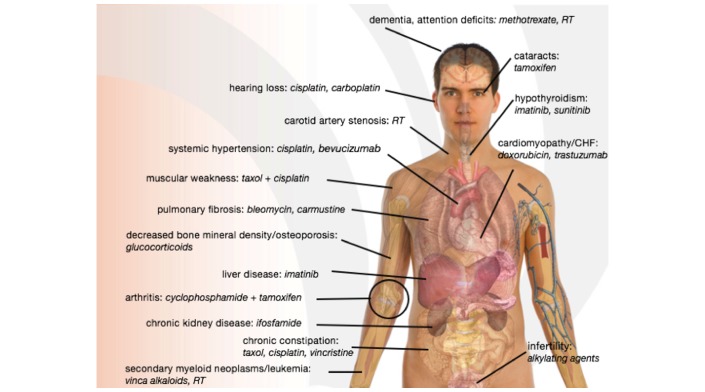
Among adult cancer survivors, various conditions that occur in normal ageing are seen prematurely as well. They include neurocognitive decline, osteoporosis, skin and ocular changes, sexual dysfunction, second cancers and chronic fatigue.36 Muscle dysfunction often occurs as a result of cancer therapy37; prolonged bed rest decreases whole body protein synthesis and leads to wasting of skeletal muscle.38 Adjuvant chronic corticosteroid treatment, which is used in many cancer treatment regimens, can induce cataracts, osteoporosis, proximal myopathy, thinning of the skin, infection and impaired wound healing.39 All of the aforementioned phenotypes occur during normal ageing (see figure 1).
Figure 1.
Diagram depicting age-related effects of respective cancer therapies. CHF, congestive heart failure; RT, radiation therapy.
We have demonstrated with clinical data that cancer survivors develop the health-related manifestations of ageing more quickly than their peers. While ageing prematurely is a better alternative to dying prematurely, a better understanding of what drives this process presents an opportunity for improvement.
Mechanisms of cellular ageing induced by cancer therapies
At the tissue level, the hallmarks of premature ageing can be further categorised into four interlinked processes that contribute to the ageing phenotypes of decreased resilience, geriatric syndromes and predisposition to age-related chronic diseases. These include telomere attrition, cellular senescence, stem and progenitor cell dysfunction, macromolecular (DNA) damage and epigenetic alterations (see tables 1 and 2).
Table 1.
Processes inducing an accelerated ageing-like state
| Process | Phenotypic effects |
| Telomere attrition |
|
| Cellular senescence |
|
| Free radical generation |
|
| Stem cell exhaustion |
|
| Epigenetic alterations |
|
Table 2.
Specific therapies inducing/mitigating the development of an accelerated ageing-like state
| Agent modality | Agent | Cellular effects |
| Radiotherapy |
|
Cellular senescence, changes to DNA repair genes, epigenetic alterations |
| Hormonal |
|
Cellular senescence |
| Tyrosine kinase inhibitors |
|
Cellular senescence Dasatinib→senolytic |
| Anthracyclines |
|
Free radical generation, DNA damage, telomere attrition, cellular senescence, stem cell exhaustion |
| Alkylating agents |
|
DNA damage, cellular senescence, epigenetic alterations |
| Topoisomerase inhibitors |
|
DNA damage, epigenetic alterations |
| Antimetabolites/cytotoxic drugs |
|
Cellular senescence, DNA damage |
| BRAF inhibitor |
|
Cellular senescence |
| Antitumor antibiotics |
|
Cellular senescence, epigenetic alterations |
| Isoquinololine alkaloid |
|
Cellular senescence |
| Bcl-2 inhibitor |
|
Senolytic (apoptosis of senescent cells) |
| HCT (includes conditioning regimen) |
|
Telomere attrition, stem cell exhaustion |
| Telomerase inhibitors |
|
Possible telomere attrition |
| Nucleoside analogue reverse-transcriptase inhibitor |
|
Telomere attrition |
| DNA cross-linking agents |
|
Epigenetic alterations |
| Ribonucleotide reductase inhibitors |
|
Epigenetic alterations |
| Microtubule inhibitors |
|
Epigenetic alterations |
| miRNA |
|
Cellular senescence, telomere attrition |
| GVHD |
|
Telomere attrition |
BRAF, B-Raf proto-oncogene; Bcl-2, B cell lymphoma 2; GVHD, graft-versus-host disease; HCT, haematopoietic cell transplantation; miRNA, micro RNA; N/A, not available.
Telomeres are regions of repetitive nucleotide sequences found on both ends of eukaryotic chromosomes that aid in chromosomal separation during mitosis and ensure that chromosomes are retained and properly inherited in chromosomal separation.40 Telomere shortening occurs with each cycle of cellular division until the cell reaches its ‘Hayflick limit’—a cultured human cell’s capacity to divide for a limited number of cycles before exiting the cell cycle in G1 phase and undergoing cellular senescence.41 Shorter telomeres have been linked to higher mortality rates in a meta-analysis.42 There is also a direct relationship between average telomere length and risk for developing de novo cancers,43 and telomere shortening that occurs during the natural process is thought to be a contributing factor to the increased rates of cancers among the population.44
Ageing humans have an increased burden of senescent cells.45 Senescent cells exhibit somewhat specific characteristics that allow their identification in vitro and in vivo, including senescence-associated β-galactosidase.46 The senescence-associated secretory phenotype (SASP) comprises proinflammatory cytokines, chemokines, growth factors, extracellular matrix proteases and other factors that impact nearby and distant normal cells, leading to local and systemic consequences of senescent cells, such as frailty and insulin resistance.45 47 48
Due to the numerous replicative cycles they undergo, cancer cells have shorter telomeres than cells in normal tissue.49 They can survive high numbers of replications by maintaining telomeres with telomerase, an enzyme expressed in over 90% of immortal cell lines and cancers but undetectable in most normal tissues.50 Inhibiting telomerase therefore limits the growth of human cancer cells.51 The properties of telomerase can be exploited to create targeted cancer therapies including telomerase inhibitors, telomerase-targeted immunotherapies and telomerase-driven virotherapies.52 Many cancer therapies impact telomere length by causing high proliferative demand for haematopoietic reconstitution.53 In addition to increasing replicative demand, some anticancer therapies (eg, cisplatin) directly impair telomerases.54 This telomere-initiated senescence reflects a DNA damage checkpoint response activated by chemotherapy-induced dysfunctional telomeres.
Short telomeres have also been associated with stem cell exhaustion in mice and humans.55 Stem cell lines are exhausted after a certain number of serial transplantations into successive recipients.56 Depletion of haematopoietic reserve is a major mechanism of normal ageing as ageing directly affects stem cells due to exhaustive proliferation.57 Patients receiving HCT from adult donors have shorter telomeres than patients receiving HCT from younger donors, likely due to telomere shortening in ageing.58 Clonal haematopoietic disorders occur more frequently in elderly individuals who are thus are more prone to developing cancer.59 In elderly people who are already predisposed to cancer, any further insult that can affect telomeres or induce senescence can increase the risk of development of new cancers significantly.
Additionally, many chemotherapeutic agents generate free-radical intermediates, which cause direct damage to DNA.60 DNA damage is also induced by alkylating agents, a process thought to introduce secondary malignancies.61 DNA repair enzymes are inhibited by some cancer therapies, such as topoisomerase I and topoisomerase I inhibitors.36
Many epigenetic modifications occur as part of the normal cellular ageing process. Adult identical twins, despite identical genotypes, have different disease susceptibilities, largely attributed to the epigenetic changes that occur as they age.62 Overall, global hypomethylation, CpG island hypermethylation and changes in histone methylation increase with normal ageing.63
The involvement of miRNAs has also been implicated in senescence and ageing.64 The most upregulated miRNAs are found in cells in states of stress-induced senescence, followed by those in replicative senescence, then by those in quiescence.65 It is likely through these primary mechanisms that adjuvant chemotherapy accelerates the development of age-related phenotypes and causes early onset frailty.36
Evidence of ageing at the cellular level due to specific cancer therapies
Tumour cells can be induced by chemotherapy to undergo senescence in both in vivo and in vitro models.66 One way by which this occurs is through allowing γH2AX to accumulate in telomeric DNA in cells undergoing senescence.67 γH2AX accumulation is an indicator for developing age-related disease.45 Various cytotoxic drugs (cyclophosphamide, doxorubicin, azidothymidine, 5-fluorouracil) have been shown to induce senescence directly in tumours via DNA damage.68 Many drugs act similarly, inducing ageing-related biological pathways.66
Telomere shortening from chemotherapy and HCT is dose dependent.69 Wynn et al referred to the effects of HCT on telomeres as equivalent to those of ‘several decades of ageing’.59 In a study of patients being treated for non-Hodgkin’s lymphoma, mean telomere length declined after chemotherapy.70 Following successful treatment for chronic myeloid leukaemia, telomere restriction fragment lengths have been demonstrated to be shorter in myeloid cells of patients than in age-matched healthy subjects.71 Mononuclear cells and granulocytes of children treated for acute lymphoblastic leukaemia and solid tumours also demonstrate telomere shortening.53 The phenomenon of telomere shortening appears to be common among cancer survivors.
The human catalytic subunit of telomerase, hTERT, is overactive in 85%–90% of cancers.72 Many anticancer therapeutic agents exploit the human catalytic subunit of telomerase, hTERT, including small molecule inhibitors, antisense oligonucleotides, immunotherapies, gene therapies and G-quadruplex stabilisers. Preclinical studies with hTERT peptides have led to the creation of vaccines that target telomerase, including GV-1001, GRNVAC1 and Vx-001. Normally, a self-antigen molecule, hTERT can stimulate specific cytotoxic T lymphocytes through major histocompatibility complex presentation, leading to tumour cell lysis.
Many epigenetic modifications occur during normal cellular ageing. Overall, global hypomethylation, CpG island hypermethylation and changes in histone methylation increase with normal ageing.63 While CpG hypermethylation is a component of drug-induced cytotoxicity that is damaging to cancer cells and thus advantageous in the treatment of cancer, it may also be a toxic side effect in normal cells of patients receiving such drugs. DNA hypermethylation at CpG islands is induced in human cells after exposure to each of the following commonly used cancer chemotherapy agents: topoisomerase II inhibitors, antibiotics, microtubule inhibitors, DNA cross-linking agents, hydroxyurea, antimetabolites and methotrexate.73 Clinically relevant drugs reported as DNA hypomethylating agents include azacitidine and decitabine, hydralazine and MG98.74 While these agents may be effective in making cancer cells more susceptible to cytotoxic damage, they may also accelerate ageing changes in normal cells. Furthermore, the same epigenetic alterations that are targeted by some anticancer drugs are likely involved in the development of new secondary cancers.52
The involvement of miRNAs has been implicated in senescence and ageing. Inhibitors for oncogenic miRNAs and limitations of tumour suppressor miRNAs are being developed for potential use in various cancer treatments as correcting abnormal miRNA expression holds potential for overcoming resistance in certain types of cancer cells. miRNA-29 has shown promise in modifying lung cancer cells.75 Expression profiles of miRNAs are different in lung cancer and normal lung. The enforced expression of miRNA-29 in lung cancer restores normal patterns of DNA methylation and induces expression of several tumour suppressor genes. miRNA-451 also holds potential to decrease cancer cells’ resistance to treatment.65 Transfection of breast cancer cells resistant to doxorubicin with this miRNA results in increased sensitivity to chemotherapy.
‘Stem cell exhaustion’ refers to the replicative stress of transplanted haematopoietic stem cells (HSCs) following haematopoietic reconstitution after HCT.59 Stem cells may effectively undergo the normal ageing process at an accelerated rate, leading to premature bone marrow failure. A single serial transplantation causes a large decline in HSC repopulating ability, and transplantation effectively causes an acceleration of the normal ageing process.56 The administration of doxorubicin to c-kit positive human cardiac progenitor cells (hCPCs) leads to the expression of the senescence markers p16INK4a and γH2AX, suggesting that anthracycline cardiomyopathy is caused by depletion of the hCPC pool (stem cell exhaustion).76
After HCT, prevalence of myelodysplasia and secondary malignancies increases, likely induced by conditioning agents used in HCT and excessive proliferative stress following HCT.77 Newer non-myeloablative and reduced intensity preparative regimens for allogeneic HCT induce less toxicity and treatment-related mortality in recipients. While myeloablative conditioning is based on high-dose drug toxicity to haematopoietic stem cells, non-myeloablative conditioning utilises immunosuppression, resulting in a state of mixed chimerism (at least early after transplantation).77 However, telomeres are comparably shortened in recipients of both non-myeloablative and myeloablative conditioning, suggesting that the primary mechanism of telomere shortening is accelerated proliferation rather than the cytotoxic effects of myeloablative conditioning drugs.
Androgens have been used to treat various bone marrow failure syndromes, such as dyskeratosis congenita, since the 1960s. It has been used in patients who cannot undergo HCT. The proposed mechanism of sex hormone therapy is upregulation of telomerase gene expression.78 In a recently published clinical trial, patients with telomeropathies received daily doses of the synthetic sex hormone danazol for 24 months, and about 40% demonstrated a gain in telomere length from baseline.79 Additional study of sex hormones as potential mitigators of telomere attrition in patients at risk for acquired telomeropathy, including those receiving cancer therapies, is warranted. This study points towards potential future strategies with sex hormones to mitigate telomere attrition in patients at risk for telomeropathy, including those receiving cancer therapies.
As many cancer treatments appear to induce an accelerated ageing-like state, interventions that target fundamental ageing processes may have a role in cancer survivors.45 80–82 Since many cancer therapies induce cellular senescence, among the most promising agents are senolytics, drugs that selectively eliminate senescent cells and SASP inhibitors, which blunt local and systemic effects of the SASP.45 81 83 These agents alleviate frailty, restore progenitor function, reduce insulin resistance, rescue cardiac and vascular dysfunction, decrease adverse effects of RT and reduce osteoporosis in a variety of animal models of ageing and disease. Senolytics are effective when administered intermittently, potentially reducing toxicity, and resistance to these drugs is unlikely to develop as, unlike cancer cells or microbes, senescent cells that do not divide.81 83
Discussion
Despite the negative sequelae that many cancer therapies lead to or accelerate, it must be emphasised that these therapies are indeed valuable and worthwhile to countless patients with cancer. Ageing is a natural and in some ways desirable phenomenon; however, we believe that a decline in health that mimics age-related illness is a negative consequence experienced by many recipients of cancer treatment. Recognising this phenomenon and understanding the mechanisms underlying it offer an opportunity for the development of alternative therapies and ameliorating medications. We believe that cancer survivors deserve long-term follow-up for mitigation of the late effects.
Conclusion
Our expert review of the associations of cancer treatments with accelerated ageing reveals that multiple ageing pathways contribute to late complications in cancer survivors. A variety of chemotherapeutic agents have been implicated in the pathogenesis of senescence and acquired telomeropathies, which culminate in morbidity and mortality due to frailty phenotypes and ageing-associated diseases. Our search also identified a paucity of long-term cancer survivorship studies evaluating ageing parameters (telomeres, p16INK4a+ senescent cells, miRNA, methylomes) in the context of clinical outcomes. Future research to better understand mechanisms of accelerated ageing-like phenotypes is essential for oncology community as well as from a public health and health policy perspective. The ultimate goal of these studies will be to prevent late complications using early interventions including lifestyle changes and medications (eg, androgens, mTOR inhibitors and senolytic drugs).
Acknowledgments
The authors thank Larry J Prokop MLS, Mayo Clinic Libraries, Mayo Clinic, Rochester, Minnesota, USA for conducting the electronic search for the systematic review.
Footnotes
Contributors: MCC-L and SKH wrote the manuscript. All authors contributed substantially to the conception, acquisition, analysis and interpretation of data for the work and approved the final approval of the version to be published.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Unpublished data are not available for this study.
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–E386. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2. Oeffinger KC, Mertens AC, Sklar CA, et al. Childhood Cancer Survivor Study. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006;355:1572–82. 10.1056/NEJMsa060185 [DOI] [PubMed] [Google Scholar]
- 3. Armstrong GT, Liu Q, Yasui Y, et al. Late mortality among 5-year survivors of childhood cancer: a summary from the Childhood Cancer Survivor Study. J Clin Oncol 2009;27:2328–38. 10.1200/JCO.2008.21.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arora M, Sun CL, Ness KK, et al. Physiologic frailty in nonelderly hematopoietic cell transplantation patients: results from the bone marrow transplant survivor study. JAMA Oncol 2016;2:1277-1286 10.1001/jamaoncol.2016.0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Armstrong GT, Chen Y, Yasui Y, et al. Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 2016;374:833–42. 10.1056/NEJMoa1510795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phillips SM, Padgett LS, Leisenring WM, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev 2015;24:653–63. 10.1158/1055-9965.EPI-14-1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. From cancer patient to cancer survivor: lost in transition. : Hewitt MGS, Stovall E, Washington, DC: National Academies Press. [Google Scholar]
- 8. Cupit MC, Duncan C, Savani BN, et al. Childhood to adult transition and long-term follow-up after blood and marrow transplantation. Bone Marrow Transplant 2016;51:176–81. 10.1038/bmt.2015.228 [DOI] [PubMed] [Google Scholar]
- 9. Hashmi S, Carpenter P, Khera N, et al. Lost in transition: the essential need for long-term follow-up clinic for blood and marrow transplantation survivors. Biol Blood Marrow Transplant 2015;21:225–32. 10.1016/j.bbmt.2014.06.035 [DOI] [PubMed] [Google Scholar]
- 10. López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell 2013;153:1194–217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armstrong GT, Kawashima T, Leisenring W, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol 2014;32:1218–27. 10.1200/JCO.2013.51.1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Syrjala KL, Langer SL, Abrams JR, et al. Late effects of hematopoietic cell transplantation among 10-year adult survivors compared with case-matched controls. J Clin Oncol 2005;23:6596–606. 10.1200/JCO.2005.12.674 [DOI] [PubMed] [Google Scholar]
- 13. Bhakta N, Liu Q, Yeo F, et al. Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: an analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 2016;17:1325–34. 10.1016/S1470-2045(16)30215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst 2010;102:1083–95. 10.1093/jnci/djq238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reulen RC, Frobisher C, Winter DL, et al. Long-term risks of subsequent primary neoplasms among survivors of childhood cancer. JAMA 2011;305:2311–9. 10.1001/jama.2011.747 [DOI] [PubMed] [Google Scholar]
- 16. Olsen JH, Möller T, Anderson H, et al. Lifelong cancer incidence in 47,697 patients treated for childhood cancer in the Nordic countries. J Natl Cancer Inst 2009;101:806–13. 10.1093/jnci/djp104 [DOI] [PubMed] [Google Scholar]
- 17. Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J 2011;1:e16 10.1038/bcj.2011.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitnitski AB, Graham JE, Mogilner AJ, et al. Frailty, fitness and late-life mortality in relation to chronological and biological age. BMC Geriatr 2002;2:1 10.1186/1471-2318-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirkwood TB, Austad SN. Why do we age? Nature 2000;408:233–8. 10.1038/35041682 [DOI] [PubMed] [Google Scholar]
- 20. Walston J, Hadley EC, Ferrucci L, et al. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 2006;54:991–1001. 10.1111/j.1532-5415.2006.00745.x [DOI] [PubMed] [Google Scholar]
- 21. Buta BJ, Walston JD, Godino JG, et al. Frailty assessment instruments: systematic characterization of the uses and contexts of highly-cited instruments. Ageing Res Rev 2016;26:53–61. 10.1016/j.arr.2015.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sternberg SA, Wershof Schwartz A, Karunananthan S, et al. The identification of frailty: a systematic literature review. J Am Geriatr Soc 2011;59:2129–38. 10.1111/j.1532-5415.2011.03597.x [DOI] [PubMed] [Google Scholar]
- 23. Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–92. 10.1111/j.1532-5415.2012.04054.x [DOI] [PubMed] [Google Scholar]
- 24. Arora M, Sun C-L, Ness KK, et al. Physiologic frailty among hematopoietic cell transplantation (HCT) survivors suggests accelerated aging and is a predictor for premature mortality: a report from the Bone Marrow Transplant Survivor Study (BMTSS). Blood 2015;126:739.26059948 [Google Scholar]
- 25. Sun CL, Kersey JH, Francisco L, et al. Burden of morbidity in 10+ year survivors of hematopoietic cell transplantation: report from the bone marrow transplantation survivor study. Biol Blood Marrow Transplant 2013;19:1073–80. 10.1016/j.bbmt.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farber S, Diamond LK. Temporary remissions in acute leukemia in children produced by folic acid antagonist, 4-aminopteroyl-glutamic acid. N Engl J Med 1948;238:787–93. 10.1056/NEJM194806032382301 [DOI] [PubMed] [Google Scholar]
- 27. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009;59:225–49. 10.3322/caac.20006 [DOI] [PubMed] [Google Scholar]
- 28. Mertens AC, Yong J, Dietz AC, et al. Conditional survival in pediatric malignancies: analysis of data from the childhood cancer survivor study and the surveillance, epidemiology, and end results program. Cancer 2015;121:1108–17. 10.1002/cncr.29170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA 2003;290:1583–92. 10.1001/jama.290.12.1583 [DOI] [PubMed] [Google Scholar]
- 30. Hudson MM, Oeffinger KC, Jones K, et al. Age-dependent changes in health status in the Childhood Cancer Survivor cohort. J Clin Oncol 2015;33:479–91. 10.1200/JCO.2014.57.4863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ness KK, Krull KR, Jones KE, et al. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: a report from the St Jude Lifetime cohort study. J Clin Oncol 2013;31:4496–503. 10.1200/JCO.2013.52.2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Armstrong GT, Reddick WE, Petersen RC, et al. Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. J Natl Cancer Inst 2013;105:899–907. 10.1093/jnci/djt089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Armenian SH, Sun CL, Kawashima T, et al. Long-term health-related outcomes in survivors of childhood cancer treated with HSCT versus conventional therapy: a report from the Bone Marrow Transplant Survivor Study (BMTSS) and Childhood Cancer Survivor Study (CCSS). Blood 2011;118:1413–20. 10.1182/blood-2011-01-331835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith M, Abrams J, Trimble EL, et al. Dose intensity of chemotherapy for childhood cancers. Oncologist 1996;1:293–304. [PubMed] [Google Scholar]
- 35. Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood 2014;124:344–53. 10.1182/blood-2014-02-514778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses 2006;67:212–5. 10.1016/j.mehy.2006.01.045 [DOI] [PubMed] [Google Scholar]
- 37. Christensen JF, Jones LW, Andersen JL, et al. Muscle dysfunction in cancer patients. Ann Oncol 2014;25:947–58. 10.1093/annonc/mdt551 [DOI] [PubMed] [Google Scholar]
- 38. Ferrando AA, Lane HW, Stuart CA, et al. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol 1996;270(Pt 1):E627–33. [DOI] [PubMed] [Google Scholar]
- 39. Poetker DM, Reh DD. A comprehensive review of the adverse effects of systemic corticosteroids. Otolaryngol Clin North Am 2010;43:753–68. 10.1016/j.otc.2010.04.003 [DOI] [PubMed] [Google Scholar]
- 40. Tchkonia T, Zhu Y, van Deursen J, et al. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013;123:966–72. 10.1172/JCI64098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Counter CM. The roles of telomeres and telomerase in cell life span. Mutat Res 1996;366:45–63. 10.1016/S0165-1110(96)90006-8 [DOI] [PubMed] [Google Scholar]
- 42. Boonekamp JJ, Simons MJ, Hemerik L, et al. Telomere length behaves as biomarker of somatic redundancy rather than biological age. Aging Cell 2013;12:330–2. 10.1111/acel.12050 [DOI] [PubMed] [Google Scholar]
- 43. Wentzensen IM, Mirabello L, Pfeiffer RM, et al. The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 2011;20:1238–50. 10.1158/1055-9965.EPI-11-0005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shay J, Wright W, Werbin H. Loss of telomeric DNA during aging may predispose cells to cancer (review). Int J Oncol 1993;3:559–63. 10.3892/ijo.3.4.559 [DOI] [PubMed] [Google Scholar]
- 45. Dimri GP, Lee X, Basile G, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995;92:9363–7. 10.1073/pnas.92.20.9363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu M, Palmer AK, Ding H, et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 2015;4:e12997 10.7554/eLife.12997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Lange T, Shiue L, Myers RM, et al. Structure and variability of human chromosome ends. Mol Cell Biol 1990;10:518–27. 10.1128/MCB.10.2.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xu M, Tchkonia T, Ding H, et al. JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A 2015;112:E6301–E6310. 10.1073/pnas.1515386112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994;266:2011–5. 10.1126/science.7605428 [DOI] [PubMed] [Google Scholar]
- 50. Hahn WC, Stewart SA, Brooks MW, et al. Inhibition of telomerase limits the growth of human cancer cells. Nat Med 1999;5:1164–70. 10.1038/13495 [DOI] [PubMed] [Google Scholar]
- 51. Buseman CM, Wright WE, Shay JW. Is telomerase a viable target in cancer? Mutat Res 2012;730:90–7. 10.1016/j.mrfmmm.2011.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Franco S, Ozkaynak MF, Sandoval C, et al. Telomere dynamics in childhood leukemia and solid tumors: a follow-up study. Leukemia 2003;17:401–10. 10.1038/sj.leu.2402815 [DOI] [PubMed] [Google Scholar]
- 53. Zhang RG, Zhang RP, Wang XW, et al. Effects of cisplatin on telomerase activity and telomere length in BEL-7404 human hepatoma cells. Cell Res 2002;12:55–62. 10.1038/sj.cr.7290110 [DOI] [PubMed] [Google Scholar]
- 54. Hao LY, Armanios M, Strong MA, et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell 2005;123:1121–31. 10.1016/j.cell.2005.11.020 [DOI] [PubMed] [Google Scholar]
- 55. Harrison DE. Proliferative capacity of erythropoietic stem cell lines and aging: an overview. Mech Ageing Dev 1979;9:409–26. 10.1016/0047-6374(79)90082-4 [DOI] [PubMed] [Google Scholar]
- 56. Beauséjour C. Bone marrow-derived cells: the influence of aging and cellular senescence : Kauser K, Zeiher A-M, Bone marrow-derived progenitors Berlin, Heidelberg: Springer, 2007:67–88. [DOI] [PubMed] [Google Scholar]
- 57. Akiyama M, Hideshima T, Hayashi T, et al. Nuclear factor-kappaB p65 mediates tumor necrosis factor alpha-induced nuclear translocation of telomerase reverse transcriptase protein. Cancer Res 2003;63:18–21. [PubMed] [Google Scholar]
- 58. Wynn RF, Cross MA, Hatton C, et al. Accelerated telomere shortening in young recipients of allogeneic bone-marrow transplants. Lancet 1998;351:178–81. 10.1016/S0140-6736(97)08256-1 [DOI] [PubMed] [Google Scholar]
- 59. Chabner BA, Myers CE, Coleman CN, et al. The clinical pharmacology of antineoplastic agents. N Engl J Med Overseas Ed 1975;292:1107–13. 10.1056/NEJM197505222922107 [DOI] [PubMed] [Google Scholar]
- 60. Levine EG, Bloomfield CD. Leukemias and myelodysplastic syndromes secondary to drug, radiation, and environmental exposure. Semin Oncol 1992;19:47–84. [PubMed] [Google Scholar]
- 61. Fraga MF, Ballestar E, Paz MF, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A 2005;102:10604–9. 10.1073/pnas.0500398102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fraga MF, Agrelo R, Esteller M. Cross-talk between aging and cancer: the epigenetic language. Ann N Y Acad Sci 2007;1100:60–74. 10.1196/annals.1395.005 [DOI] [PubMed] [Google Scholar]
- 63. Wang Y, Scheiber MN, Neumann C, et al. MicroRNA regulation of ionizing radiation-induced premature senescence. Int J Radiat Oncol Biol Phys 2011;81:839–48. 10.1016/j.ijrobp.2010.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li G, Luna C, Qiu J, et al. Alterations in microRNA expression in stress-induced cellular senescence. Mech Ageing Dev 2009;130(11-12):731–41. 10.1016/j.mad.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res 2003;63:2705–15. [PubMed] [Google Scholar]
- 66. Siddiqui MS, François M, Fenech MF, et al. Persistent γH2AX: a promising molecular marker of DNA damage and aging. Mutat Res Rev Mutat Res 2015;766:1–19. 10.1016/j.mrrev.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 67. te Poele RH, Okorokov AL, Jardine L, et al. DNA damage is able to induce senescence in tumor cells in vitro and in vivo. Cancer Res 2002;62:1876–83. [PubMed] [Google Scholar]
- 68. Lobetti-Bodoni C, Ferrero D, Genuardi E, et al. Telomere loss in Philadelphia-negative hematopoiesis after successful treatment of chronic myeloid leukemia: evidence for premature aging of the myeloid compartment. Mech Ageing Dev 2012;133:479–88. 10.1016/j.mad.2012.05.007 [DOI] [PubMed] [Google Scholar]
- 69. Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. The Lancet 2014;384:2027–35. 10.1016/S0140-6736(14)60525-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lee JJ, Nam CE, Cho SH, et al. Telomere length shortening in non-Hodgkin’s lymphoma patients undergoing chemotherapy. Ann Hematol 2003;82:492–5. 10.1007/s00277-003-0691-4 [DOI] [PubMed] [Google Scholar]
- 71. Ruden M, Puri N. Novel anticancer therapeutics targeting telomerase. Cancer Treat Rev 2013;39:444–56. 10.1016/j.ctrv.2012.06.007 [DOI] [PubMed] [Google Scholar]
- 72. Nyce J. Drug-induced DNA hypermethylation and drug resistance in human tumors. Cancer Res 1989;49:5829–36. [PubMed] [Google Scholar]
- 73. Johnson AA, Akman K, Calimport SR, et al. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res 2012;15:483–94. 10.1089/rej.2012.1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Fabbri M, Garzon R, Cimmino A, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A 2007;104:15805–10. 10.1073/pnas.0707628104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Piegari E, De Angelis A, Cappetta D, et al. Doxorubicin induces senescence and impairs function of human cardiac progenitor cells. Basic Res Cardiol 2013;108:334 10.1007/s00395-013-0334-4 [DOI] [PubMed] [Google Scholar]
- 76. Lahav M, Uziel O, Kestenbaum M, et al. Nonmyeloablative conditioning does not prevent telomere shortening after allogeneic stem cell transplantation. Transplantation 2005;80:969–76. 10.1097/01.TP.0000173649.99261.DF [DOI] [PubMed] [Google Scholar]
- 77. Calado RT, Yewdell WT, Wilkerson KL, et al. Sex hormones, acting on the TERT gene, increase telomerase activity in human primary hematopoietic cells. Blood 2009;114:2236–43. 10.1182/blood-2008-09-178871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Townsley DM, Dumitriu B, Liu D, et al. Danazol treatment for telomere diseases. N Engl J Med 2016;374:1922–31. 10.1056/NEJMoa1515319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Justice J, Miller JD, Newman JC, et al. Frameworks for proof-of-concept clinical trials of interventions that target fundamental aging processes. J Gerontol A Biol Sci Med Sci 2016;71:1415–23. 10.1093/gerona/glw126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. LeBrasseur NK, Tchkonia T, Kirkland JL. Cellular senescence and the biology of aging, disease, and frailty. Nestle Nutr Inst Workshop Ser 2015;83:11–18. 10.1159/000382054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kirkland JL, Tchkonia T. Clinical strategies and animal models for developing senolytic agents. Exp Gerontol 2015;68:19–25. 10.1016/j.exger.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhu Y, Armstrong JL, Tchkonia T, et al. Cellular senescence and the senescent secretory phenotype in age-related chronic diseases. Curr Opin Clin Nutr Metab Care 2014;17:324–8. 10.1097/MCO.0000000000000065 [DOI] [PubMed] [Google Scholar]
- 83. Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 1961;25:585–621. 10.1016/0014-4827(61)90192-6 [DOI] [PubMed] [Google Scholar]