Abstract
Purpose
To describe the clinical course of a patient with non-infectious idiopathic unilateral panuveitis and retinal vasculitis treated with subcutaneous repository adrenocorticotropic hormone (ACTH) gel.
Observations
A 33-year-old male presented with blurry vision and floaters in the left eye (OS). The best-corrected visual acuity was 20/20 in the right eye (OD) and 20/50 in OS at the time of initial presentation. Slit-lamp examination revealed mild anterior segment inflammation in OS. There were 1 + vitreous haze and 2 + cells noted in OS. Clinical examination and ancillary imaging assessment including fluorescein angiography revealed retinal vasculitis and optic nerve head inflammation. After infectious etiologies were ruled out, the patient was started on oral corticosteroids and enrolled in a clinical trial employing intravenous tocilizumab therapy. Six months after completion of the tocilizumab trial, the patient demonstrated recurrence of disease. Twice weekly subcutaneous ACTH gel was initiated and the patient demonstrated improvement of retinal vascular inflammation.
Conclusions and importance
Repository subcutaneous ACTH gel formulation may be a safe and viable therapeutic option for patients with non-infectious uveitis and retinal vasculitis. Clinical trials using this formulation in a larger patient cohort with longer monitoring are indicated to evaluate its tolerability and bioactivity.
Keywords: ACTH, Retinal vasculitis, Panuveitis, Non-infectious uveitis, Melanocortins
1. Introduction
Due to the heterogeneity in the clinical features, etiology, pathophysiology, and complications, management of uveitis poses a great challenge for clinicians. Inflammation involving all the compartments of the eye, i.e. panuveitis, may often result in severe visual loss due to associated pathologies such as macular edema, retinal vasculitis, and optic neuritis.1, 2 Active research directed towards effective control of inflammation had led to the discovery of several pathways responsible for inflammatory cascade leading to uveitis. Besides corticosteroids, a number of agents such as sirolimus,3 IL-1 (gevokizumab), IL-6 (tocilizumab) and IL-17 (secukinumab) inhibitors, among others,4 are being explored and revisited to determine their role in the management of uveitis.
One such agent, adrenocorticotropic hormone (ACTH), is part of a group of molecules called melanocortins (α, β, γ-melanocyte stimulating hormone; MSH) that are produced endogenously in the hypothalamo-pituitary axis.5, 6 However, it has been recently demonstrated that melanocortins such as α-MSH can be produced by macrophages at the site of active inflammation in order to counteract the effects of pro-inflammatory cytokines.7 In addition to the local autocrine effects, ACTH and other melanocortins play a role in suppressing inflammation by stimulation of adrenal glands leading to the production of glucocorticoids.8, 9 Evidence suggests that various additional anti-inflammatory mechanisms of actions of ACTH may exist.9
ACTH gel was approved by United States Food and Drug Administration (FDA) for a wide range of inflammatory conditions including uveitis. ACTH gel has been reported to be effective in systemic inflammatory diseases such as nephrotic syndrome, multiple sclerosis, opsoclonus myoclonus, systemic lupus erythematosus, dermatomyositis and polymyositis.10, 11, 12, 13, 14, 15, 16, 17 However, due to the relatively scarce data from clinical trials, ACTH has been rarely employed in uveitis practice. The index case report describes the potential role of subcutaneously administered ACTH gel in the management of a patient with non-infectious panuveitis with significant retinal vasculitis, macular edema, and optic nerve inflammation.
2. Case report
A 33-year-old Caucasian man presented in June 2014 with painless blurring of vision and floaters in the left eye (OS) for the past two years. There was no systemic history of any major illnesses and family history was non-contributory. The patient denied previous history of trauma or surgery. On ocular examination, the Early Treatment Diabetic Retinopathy Study (ETDRS) best-corrected visual acuity (BCVA) was 20/15 in the right eye (OD) and 20/50 in OS. Pupillary reactions were normal. Slit-lamp examination revealed presence of 0.5 + cells and 1 + flare in OS and quiet anterior chamber in OD. There was presence of 2 + vitreous haze and 2 + vitreous cells in OS whereas examination of OD was normal. Intraocular pressure (IOP) was normal in both eyes (OU).
In June 2014, dilated fundus examination revealed no abnormality in OD. In OS, there was mild blurriness of the optic disc margins especially nasally. However, the color and size of the disc appeared normal. The foveal reflex appeared dull. There were focal areas of perivascular sheathing predominantly involving the veins in OS. There were no visible hemorrhages and the caliber and contour of the vessels appeared normal (Fig. 1). Careful examination with indirect ophthalmoscopy revealed presence of snow balls inferiorly in OS. Fluorescein angiography (FA) was performed using the ultra-wide field retinal camera (Optos P200Tx, Optos Inc., Scotland, UK). While there were no perfusion abnormalities noted in OD, FA revealed diffuse, extensive perivenular leakage in the posterior pole extending up to the periphery in OS. In the macular region of OS, there was leakage temporal to the fovea. Subtle perifoveal leakage was also observed. There was early disc hyperfluorescence which persisted in the late phase suggestive of optic nerve head inflammation (Fig. 1). Spectral-domain optical coherence tomography (SD-OCT) revealed presence of macular edema in OS and normal findings in OD (Fig. 2).
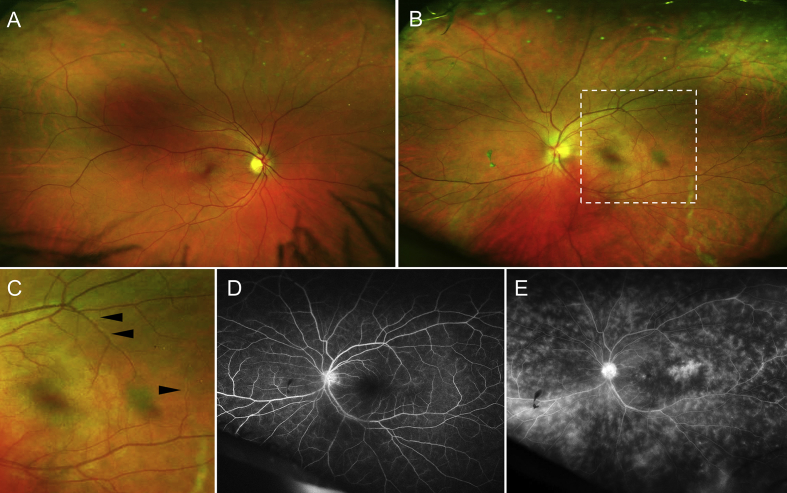
Fig. 1.
Ultra-wide field fundus photography (A–C) and fluorescein angiography (FA) of the patient diagnosed with panuveitis and retinal vasculitis. Fundus examination of the right eye (A) did not reveal any abnormalities. Examination of the left eye (B) reveals blurring of optic disc margins, focal periphlebitis (magnified area demarcated by white dashed square in C), and a dull foveal reflex. FA of the left eye (right eye not shown) in the early phase (D) shows mild early disc hyperfluorescence and subtle leak in the temporal macula. In the late phase (E), there is diffuse perivenular leakage extending up to the periphery, perifoveal leakage, and disc hyperfluorescence.
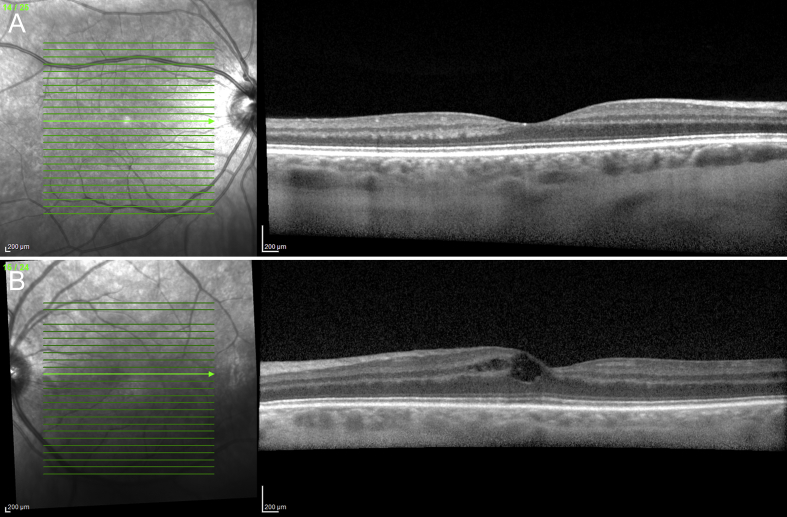
Fig. 2.
Spectral-domain optical coherence tomography of both eyes of the patient shows normal anatomy of the retinal layers in the right eye (A) and cystoid macular edema in the left eye (B). The external limiting membrane and outer retinal layers appear normal.
Laboratory evaluation of the patient revealed elevated erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels. Complete blood counts and peripheral blood smears were normal. The tuberculin skin test was negative. Assays for anti-nuclear cytoplasmic antibody (ANCA), fluorescent treponemal antibody absorption (FTA-ABS) test, Lyme disease serology, anti-nuclear antibody (ANA), anti-double stranded DNA (anti-dsDNA), and serum lysozyme/angiotensin converting enzyme assays were unremarkable. Serology for HIV and titers for toxoplasmosis was negative. Chest radiography was performed and revealed no abnormalities. The patient was diagnosed with unilateral idiopathic panuveitis with retinal vasculitis and optic nerve inflammation and macular edema.
Due to extensive vascular leakage and the elevated serum CRP and ESR, which might suggest presence of systemic-wide involvements, systemic therapies were considered. The patient was started on systemic corticosteroids (oral prednisone 40 mg/day) and topical prednisolone 1% three times a day in OS. Following initiation of oral corticosteroids, there was interval improvement in vitritis and retinal vascular leakage. The BCVA improved to 20/40 in OS. In order to wean the patient off from oral corticosteroids, initiation of immunosuppressive therapy was planned. Thus, in July 2014, he was enrolled in a clinical trial (the STOP-Uveitis Study18) which evaluates two different doses of intravenous tocilizumab (TCZ), an interleukin (IL)-6 inhibitor for non-infectious uveitis. The patient was randomized to receive monthly 8mg/kg dose (mandatory infusions up to 6 months followed by as needed approach) of TCZ beginning July 2014. There was significant improvement in retinal vascular leakage and optic nerve head hyperfluorescence during the course of treatment with tocilizumab. Vitritis and anterior segment inflammation resolved. At month 12 end-of-study (July 2015) visit, the ETDRS BCVA improved to 20/20 in OS with resolution of macular edema (Fig. 3).
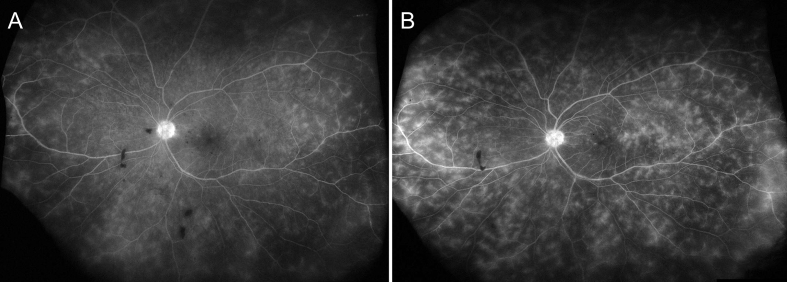
Fig. 3.
Ultra-wide field fluorescein angiography (FA) of the left eye at 12 months (July 2015) after being in the clinical trial evaluating intravenous tocilizumab (A). In comparison with the FA at the time of presentation (Fig. 1D, 1E), there is significant decrease in the amount of vascular leakage and perifoveal leak. Six months after exiting from the tocilizumab study (January 2016), there is recurrence of the retinal vasculitis with significant perivenular leakage and disc hyperfluorescence (B).
Six months after completion of the STOP-Uveitis study (January 2016), the patient presented with mild blurring of vision. Snellen BCVA was recorded as 20/20 in OS. There were 2 + vitreous cells and 1 + vitreous haze, recurrence of significant, diffuse perivenular leakage, and optic nerve hyperfluorescence on FA (Fig. 3, Fig. 4A). The patient was not able to receive additional treatments with tocilizumab because of denial for coverage of tocilizumab for uveitis by his insurance. At this time, the patient was treated with H.P. Acthar® Gel (repository corticotropin injection; Mallinckrodt Pharmaceuticals, St. Louis, MO) (80 units administered subcutaneously twice weekly). Six weeks following treatment with corticotropin (March 2016), there was substantial reduction in retinal vascular leakage. There was an interval decrease in the optic nerve head hyperfluorescence (Fig. 4B) as well. Vitritis resolved and BCVA continued to remain 20/20. IOP was recorded as normal (14 mm Hg). Due to satisfactory clinical response, currently, the patient is being continued on treatment with repository corticotropin gel injections.
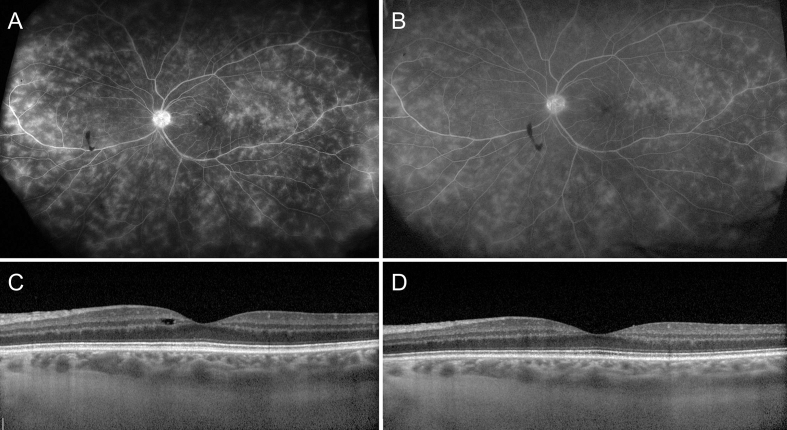
Fig. 4.
Ultra-wide field fluorescein angiography (FA) and spectral-domain optical coherence tomography (SD-OCT) of the left eye prior to initiation of subcutaneous repository corticotropin gel (A and C) and six weeks after therapy (B and D). Prior to subcutaneous corticotropin gel therapy, there is significant active retinal vasculitis with leakage and macular edema on FA and OCT. Within 6 weeks, there is decrease in retinal venular leakage and resolution of disc hyperfluorescence and macular edema on FA and OCT.
3. Discussion
The use of ACTH therapy was approved by the US FDA in 1952 for the management of various ocular inflammatory conditions including posterior uveitis and choroiditis. However, since this agent was administered either intravenously or through an intramuscular route, the amount of drug delivered systemically was relatively high resulting in severe undesirable steroid-related systemic adverse effects.19 Moreover, ACTH was used only as a monotherapy for the more severe forms of uveitis such as sympathetic ophthalmia,20, 21 Behçet's disease,22 and Vogt-Koyanagi-Harada disease,23 among others. Therefore, due to various reasons, ACTH did not gain popularity in the management of uveitis and has been largely ignored in the recent literature.
In 1970, DeVoe, in the thesis for the American Ophthalmological Society, described the use of intravenous ACTH in a patient with sympathetic ophthalmia with adequate control of inflammation but prompt recurrence on cessation of therapy. However, the patient developed multiple features of Cushing's syndrome and osteoporotic fractures necessitating an alternate therapy.21 More recently, in 1973, Aggarwal reported the use of ACTH in a patient with Behçet's disease associated with Bell's palsy. The author described improvement in the course of uveitis and decrease in the recurrence of Bell's palsy following ACTH administration.22
Presently, ACTH is available as H.P. Acthar gel, a highly purified sterile preparation in 16% gelatin to provide a prolonged release after intramuscular or subcutaneous injection. A fixed dose of ACTH gel demonstrates a linear increase in adrenocortical secretion with increasing duration of the infusion. Subcutaneously administered ACTH gel has been shown to bind with all the melanocortin receptors with high affinity resulting in inhibition of leukocyte transmigration, reduction of cytokine synthesis and generation of anti-inflammatory signals locally at the site of inflammation.24 In addition, inhibition of nuclear factor-κB (NF-κB) decreases the expression of various pro-inflammatory cytokines, receptors, adhesion molecules and chemokines reducing the inflammation.25, 26, 27 Experimental studies in mice with experimental autoimmune uveitis have shown that binding of ACTH with melanocortin receptors is associated with recovery from the disease.28, 29 Since uveitis may have a chronic clinical course, therapy with ACTH gel should be individualized. Long-term therapy with ACTH gel may be needed in patients with multiple recurrences. The limitations of the use of ACTH gel in uveitis include high cost of therapy, which may make the agent less available for many patients. ACTH gel is contraindicated in patients with systemic fungal infections, ocular herpes, peptic ulcer, uncontrolled hypertension, recent surgery, congestive cardiac failure, or osteoporosis. The adverse effects of ACTH gel can be similar to systemic corticosteroids and include fluid retention, altered electrolyte balance, increase in blood glucose, systemic hypertension, mood changes, weight gain, Cushingnoid features, among others.30
In the index case, adequate control of inflammation and retinal vasculitis was achieved following the use of intravenous tocilizumab. However, 6 months after the patient completed the clinical trial employing tocilizumab, there was a recurrence of the disease (Fig. 3). At this time, given the young age of the patient and elevated biomarkers for systemic involvements, as well as the possibility of ocular side-effects with local steroid therapy, a systemic agent for the treatment of the recurrence was preferred. Therapy with ACTH gel resulted in rapid and adequate control of retinal vascular leakage, macular edema and optic nerve inflammation (Fig. 4). With fixed interval administration and slow release of ACTH, suppression of hypothalamo-pituitary axis is not expected in our patient. At the end of 6 weeks, there were no safety concerns and no systemic or local (dermal or ocular) adverse events were recorded. Thus, the patient is being continued on ACTH gel therapy with close monitoring for possible steroid-related side-effects.
4. Conclusions
Although rarely employed in ophthalmology practice thus far, subcutaneous repository ACTH gel may have a role in the management of certain uveitic entities such as retinal vasculitis. Short-term follow-up of our patient shows that the onset of drug action is rapid following subcutaneous administration. Potential advantages of this therapy include relatively easier route of administration, no need for daily therapy, and predictable course of action. However, careful monitoring and long-term follow-up are warranted to detect steroid-related adverse events early. The findings in our index patient encourage future clinical trials employing repository corticotropin injection in the management of uveitis.
Patient consent
The consent to publish has been obtained from the participant in writing to report individual patient data.
Funding
No funding or grant support.
Conflict of interest
Dr. Nguyen and Dr. Do serve on the Scientific Advisory Boards for Genentech, Regeneron, and Allergan. Dr. Nguyen also serves on the Scientific Advisory Board for AbbVie, Bausch and Lomb, Mallinckrodt, and Santen.
Dr. Nguyen is the Editor in Chief of American Journal of Ophthalmology Case Reports. Given his role as Editor in Chief, Dr. Nguyen had no involvement in the peer-review of this article and has no access to information regarding its peer-review. Full responsibility for the editorial process for this article was delegated to Dr. Harvey Uy.
The following authors have no financial disclosures: Dr. Agarwal, Dr. Hassan, and Dr. Sepah.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Dick A.D., Tundia N., Sorg R. Risk of ocular complications in patients with noninfectious intermediate uveitis, posterior uveitis, or panuveitis. Ophthalmology. Mar 2016;123:655–662. doi: 10.1016/j.ophtha.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 2.Durrani O.M., Tehrani N.N., Marr J.E., Moradi P., Stavrou P., Murray P.I. Degree, duration, and causes of visual loss in uveitis. Br J Ophthalmol. Sep 2004;88:1159–1162. doi: 10.1136/bjo.2003.037226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal A., Rajagopalan N., Hassan M. Sirolimus for retinal and uveitic diseases. Dev Ophthalmol. 2016;55:276–281. doi: 10.1159/000438951. [DOI] [PubMed] [Google Scholar]
- 4.Maya J.R., Sadiq M.A., Zapata L.J. Emerging therapies for noninfectious uveitis: what may be coming to the clinics. J Ophthalmol. 2014;2014:310329. doi: 10.1155/2014/310329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montero-Melendez T. ACTH: the forgotten therapy. Seminars Immunol. May 2015;27:216–226. doi: 10.1016/j.smim.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Getting S.J. Targeting melanocortin receptors as potential novel therapeutics. Pharmacol Ther. Jul 2006;111:1–15. doi: 10.1016/j.pharmthera.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Star R.A., Rajora N., Huang J., Stock R.C., Catania A., Lipton J.M. Evidence of autocrine modulation of macrophage nitric oxide synthase by alpha-melanocyte-stimulating hormone. Proc Natl Acad Sci U. S. A. Aug 15 1995;92:8016–8020. doi: 10.1073/pnas.92.17.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemson C.M., Yost J., Taylor A.W. The role of alpha-MSH as a modulator of ocular immunobiology exemplifies mechanistic differences between melanocortins and steroids. Ocular Immunol Inflamm. Jan 25 2016:1–11. doi: 10.3109/09273948.2015.1092560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catania A., Lonati C., Sordi A., Carlin A., Leonardi P., Gatti S. The melanocortin system in control of inflammation. Sci World J. 2010;10:1840–1853. doi: 10.1100/tsw.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baram T.Z., Mitchell W.G., Tournay A., Snead O.C., Hanson R.A., Horton E.J. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. Mar 1996;97:375–379. [PMC free article] [PubMed] [Google Scholar]
- 11.Fiechtner J.J., Montroy T. Treatment of moderately to severely active systemic lupus erythematosus with adrenocorticotropic hormone: a single-site, open-label trial. Lupus. Aug 2014;23:905–912. doi: 10.1177/0961203314532562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine T. Treating refractory dermatomyositis or polymyositis with adrenocorticotropic hormone gel: a retrospective case series. Drug Des Dev Ther. 2012;6:133–139. doi: 10.2147/DDDT.S33110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bomback A.S., Canetta P.A., Beck L.H., Jr., Ayalon R., Radhakrishnan J., Appel G.B. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol. 2012;36:58–67. doi: 10.1159/000339287. [DOI] [PubMed] [Google Scholar]
- 14.Mittal T., Dedhia P., Roy-Chaudhury P. Complete remission of post-transplantation recurrence of focal segmental glomerulosclerosis with the use of adrenocorticotrophic hormone gel: case report. Transplant Proc. Sep 2015;47:2219–2222. doi: 10.1016/j.transproceed.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 15.Hladunewich M.A., Cattran D., Beck L.H. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar(R) Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dialysis, Transplant Official Publ Eur Dialysis Transpl Assoc - Eur Ren Assoc. Aug 2014;29:1570–1577. doi: 10.1093/ndt/gfu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simsarian J.P., Saunders C., Smith D.M. Five-day regimen of intramuscular or subcutaneous self-administered adrenocorticotropic hormone gel for acute exacerbations of multiple sclerosis: a prospective, randomized, open-label pilot trial. Drug Des Dev Ther. 2011;5:381–389. doi: 10.2147/DDDT.S19331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., Golubovsky J., Hui-Yuen J. Adrenocorticotropic hormone gel in the treatment of systemic lupus erythematosus: a retrospective study of patients. F1000Research. 2015;4:1103. doi: 10.12688/f1000research.7192.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Study of the Safety . 2012. Tolerability, and Bioactivity of Tocilizumab on Patients with Non-infectious UVEITIS: The STOP-UVEITIS Study (STOP-uveitis)https://clinicaltrials.gov/ct2/show/NCT01717170 (Accessed 6.26.16) [Google Scholar]
- 19.Moutinho H., Basto L.P. Cortisone and ACTH in therapy of uveitis. Rev Port Med Mil. 1953;1:79–83. [PubMed] [Google Scholar]
- 20.Sturman R.M. Sympathetic ophthalmia cured by cortisone and ACTH. Eye, Ear, Nose Throat Mon. Jun 1956;35:372–375. [PubMed] [Google Scholar]
- 21.DeVoe A.G. A ten-year follow-up on a case of sympathetic ophthalmia. Trans Am Ophthalmol Soc. 1970;68:105–112. [PMC free article] [PubMed] [Google Scholar]
- 22.Aggarwal J.L. Behçet's disease with recurrent facial paralysis. Br J Ophthalmol. Sep 1973;57:704–705. doi: 10.1136/bjo.57.9.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nano H.M., Gilabert N. Acute diffuse choroiditis; Harada's disease; treatment with ACTH. La Sem Medica. Nov 24 1955;107:1010–1016. [PubMed] [Google Scholar]
- 24.Brzoska T., Luger T.A., Maaser C., Abels C., Bohm M. Alpha-melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. Aug 2008;29:581–602. doi: 10.1210/er.2007-0027. [DOI] [PubMed] [Google Scholar]
- 25.Manna S.K., Aggarwal B.B. Alpha-melanocyte-stimulating hormone inhibits the nuclear transcription factor NF-kappa B activation induced by various inflammatory agents. J Immunol. Sep 15 1998;161:2873–2880. [PubMed] [Google Scholar]
- 26.Ichiyama T., Campbell I.L., Furukawa S., Catania A., Lipton J.M. Autocrine alpha-melanocyte-stimulating hormone inhibits NF-kappaB activation in human glioma. J Neurosci Res. Dec 1 1999;58:684–689. [PubMed] [Google Scholar]
- 27.Catania A., Gatti S., Colombo G., Lipton J.M. Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev. Mar 2004;56:1–29. doi: 10.1124/pr.56.1.1. [DOI] [PubMed] [Google Scholar]
- 28.Lee D.J., Taylor A.W. Both MC5r and A2Ar are required for protective regulatory immunity in the spleen of post-experimental autoimmune uveitis in mice. J Immunol. Oct 15 2013;191:4103–4111. doi: 10.4049/jimmunol.1300182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee D.J., Taylor A.W. Following EAU recovery there is an associated MC5r-dependent APC induction of regulatory immunity in the spleen. Investigative Ophthalmol Vis Sci. 2011;52:8862–8867. doi: 10.1167/iovs.11-8153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Full Prescribing Information H.P. 2015. Acthar Gel (Repository Corticotropin Injection)www.acthar.com/pdf/acthar-pi.pdf (Accessed 9.15.16) [Google Scholar]