Abstract
Purpose
To report an atypical case of unifocal, unilateral pigmented paravenous retinochoroidal atrophy (PPRCA) characterized by thickening and cystic degeneration of the retinal nerve fiber layer (RNFL).
Observations
A 79-year old Asian woman presented with a large area of atrophic, pigmented change along the inferior arcade of her right eye. She denied nyctalopia and any other visual complaints. Visual acuity was 20/40 in both eyes and visual fields were significant for a large absolute peripheral scotoma superiorly in the affected eye corresponding to the atrophic area. Spectral domain optical coherence tomography through the lesion showed loss of choroid except for largest Haller's layer vessels, significant retinal pigment epithelium atrophy with migration and pigment clumping, outer retinal layer loss and RNFL thickening with cystic degeneration. Fundus autofluorescence imaging showed a large area of hypoautofluorescence corresponding to the area of atrophy. Full field electroretinogram demonstrated normal scotopic response and reduced photopic response in the right eye.
Conclusions and importance
PPRCA is typically bilateral and symmetric, affecting primarily the outer retina and choroid. However, in rare cases, this disease can present unilaterally and/or unifocally, with degeneration extending to the inner retinal layers.
Keywords: Pigmented paravenous chorioretinal atrophy, Pigmented paravenous retinochoroidal atrophy, Retinal degeneration, Retinochoroidal atrophy, Paravenous atrophy
1. Introduction
Pigmented paravenous retinochoroidal atrophy (PPRCA) is a rare disease characterized by retinochoroidal atrophy and pigment clumping along the retinal veins.1 It is typically bilateral, symmetric and non-progressive or slowly progressive and is most often discovered incidentally in asymptomatic patients during routine fundus exam. Originally described by Brown in 1937 in a patient with alopecia, it has since appeared in the literature under a number of different names, including but not limited to: retinochoroiditis radiate, pseudoretinitis pigmentosa, chorioretinitis striata, congenital retinal pigementation, retinal melanosis, paravenous retinal degeneration, pigmented paravenous chorioretinal degeneration and pigmented paravenous retinochoroidal atrophy.2 The etiology of PPRCA is unknown, but both primary retinal pigment epithelium (RPE) dysfunction and developmental abnormalities of the retinal vessels have been proposed. RPE dysfunction appears to be the leading theory for the cause of PPRCA owing to its severely atrophied appearance,3 but this explanation fails to account for its characteristic peri-venular arrangement. Only four cases of unilateral PPRCA have been reported in the literature.3, 4, 5 Here we present an extremely rare case of unilateral and unifocal PPRCA.
2. Case report
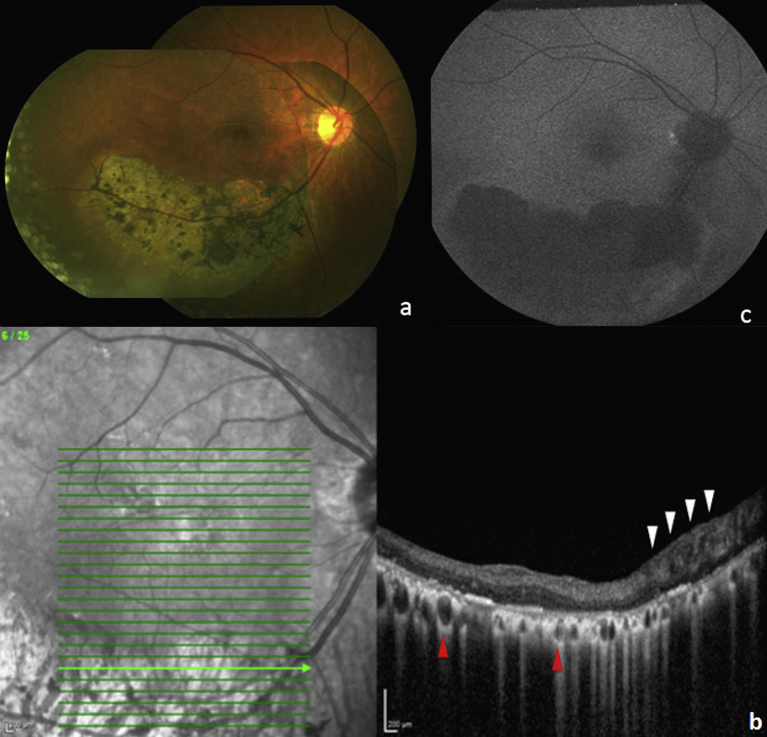
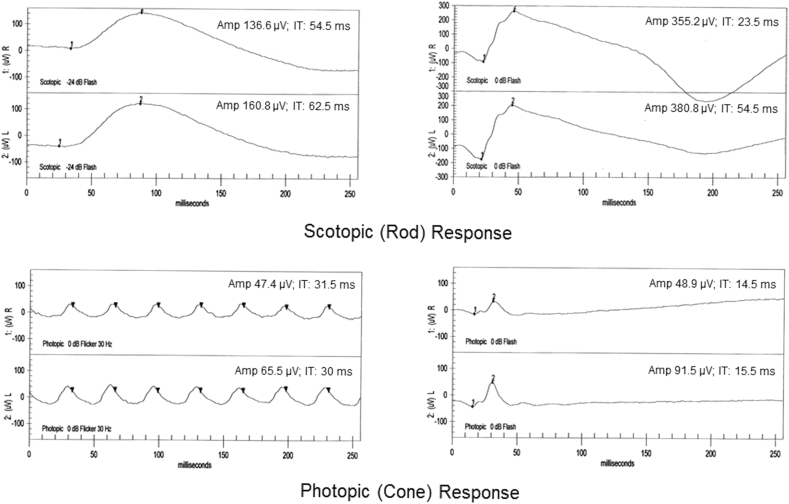
A 79-year old Asian woman presenting for routine eye exam was noted to have large area of retinal atrophy following the inferior arcade in the right eye (Fig. 1a). She described a past ocular history of being diagnosed with “pigment” in her right eye 35 years prior. Records from her past evaluation noted a vision of 20/20 in both eyes with a normal examination except for the presence of a similar sized patch of retinal atrophy at the inferior arcade of the right eye; imaging was not performed at that time. At current presentation, anterior slit lamp examination was normal in both eyes with unchanged fundus examinations except for the additional presence of bilateral epiretinal membranes (ERM's). Best corrected visual acuity was 20/40 in both eyes and visual field testing showed a large peripheral scotoma superiorly in her right eye corresponding to the atrophic retinal area. Spectral domain optical coherence tomography (SD-OCT) through the retinal lesion showed significant RPE atrophy and migration with pigment clumping and loss of the outer retinal layers (Fig. 1b). The choroid appeared atrophic with only large Haller's layer vessels remaining. Of note, thickening and cystic degeneration of the RNFL was observed on SD-OCT of the lesion. Fundus autofluorescence (FAF) imaging showed a large, well-demarcated area of hypoautofluorescence corresponding to the area of atrophy (Fig. 1c). Photopic electroretinogram demonstrated a slight decrease in b-wave amplitude in the right eye (Fig. 2). Past medical history was significant for hypertension, osteoporosis, thyroid disease and bronchiectasis. She denied any visual disturbances or difficulties, any past history of severe infectious or inflammatory illnesses, and any family history of retinal disease or degenerations including retinitis pigmentosa (RP).
Fig. 1.
a) Color Fundus Photograph Montage, OD, showing large well-demarcated area of retinochoroidal atrophy along the inferior arcade with overlying bone-spicule pigmentation; b) Spectral Domain Optical Coherence tomography (SD-OCT) showing thickening and cystic degeneration of retinal nerve fiber layer (RNFL), OD, nasally, with disorganization of all retinal layers (white arrows). Inner retinal layers are preserved and outer retina is lost temporal to the area of cystic RNFL. Note marked penetration of OCT infrared light through vessels in Haller's layer (red arrows). The remainder of the choroid and retinal pigment epithelium (RPE) is atrophic; c) Fundus Autofluorescence, OD, showing uniform, sharply delineated hypoautofluorescence corresponding to area of atrophy.
Fig. 2.
Electroretinogram showing normal scotopic responses for both eyes and decreased photopic b-wave amplitude response in right eye as compared to the left with a difference of 42.6 μV.
3. Discussion
PPRCA is a rare, typically asymptomatic peripheral retinal degeneration characterized by atrophy of the RPE, choroid and outer retinal layers. Although typically quoted as being bilateral, a number of unilateral cases have been described, with lesions typically arranged in a symmetric pattern about the retinal vessels.3, 4 Unifocal disease, as seen in our patient, is exceedingly rare, with a single report describing unifocal disease in the mother and sister of a patient with bilateral PPRCA.5 PPRCA holds many similarities in appearance to RP, and it has been suggested that the two diseases may lie on a continuum. In a retrospective study of 15 patients with PPRCA, three were found to have PPRCA in one eye with RP in the other,6 and cases of RP and PPRCA presenting in the same family have also been reported.2 However, in contrast to RP, PPRCA typically shows little to no progression and the normal-appearing areas of the retina tend to function normally1 without night blindness9 or significant decrease in scotopic amplitudes.2 This is consistent with our patient's presentation, and we attribute her decrease in vision to the presence of new bilateral ERM's.
The cause of PPRCA remains unknown, but genetic, developmental and inflammatory causes have been hypothesized. Early case reports demonstrated the presence of PPRCA in patients with a history of tubercular spondylitis, congenital syphilis as well as other inflammatory conditions such as Behcet's disease, measles, rubeola and uveitis,2 though only two cases of PPRCA with active inflammation could be located in the literature.7 It is therefore unclear if these cases represented true inflammatory PPRCA or coincidence. A minority of reported cases have exhibited a familial inheritance, with one such case exhibiting an association with the CRB-1 gene,8 making a purely genetic etiology unlikely. Another possible etiology, accounting for its perivenular arrangement and its presence in children, is a developmental abnormality of the retinal vasculature resulting in transient ischemia and thus retinochoroidal degradation.2 Two cases of PPRCA in conjunction with macular coloboma and optic disc drusen further support a developmental etiology.2
Advanced imaging techniques, such as FAF and SD-OCT, have allowed further characterization of PPRCA. On FAF imaging, affected areas have been shown to demonstrate patterns of hypoautofluroescence with a hyperautofluorescent border2 as well as hyperautofluorescence prior to eventual RPE atrophy.2 In our patient's case, the lesion appeared wholly hypoautofluoresent on FAF, consistent with complete RPE atrophy without any active process. SD-OCT imaging in previous studies typically showed severe atrophy of the choroid and RPE, with relative sparing of the inner retinal layers,9 although in one case the inner retinal layers appeared mildly thinned.10 These findings are consistent with our patient with the exception of her thickened, cystic retinal nerve fiber layer (RNFL). To our knowledge, there are no other previous reports of thickening of the inner retinal layers, which would support a retinal vessel-based etiology for PPRCA rather than purely an RPE-based cause.
4. Conclusion
PPRCA can, in rare cases, present unilaterally and/or unifocally, with degeneration extending to the inner retinal layers.
Patient consent
The patient provided written consent for publication of personal identifying information including medical record details and photographs. IRB approval was not required for this case study.
Conflict of interest
None of the authors have any conflict of interest to disclose.
There was no grant funding.
Acknowledgements
We thank Karen Holopigian with her assistance with ERG acquisition and interpretation.
References
- 1.Noble K.G., Carr R.E. Pigmented paravenous chorioretinal atrophy. Am J Ophthalmol. 1983;96:338–344. doi: 10.1016/s0002-9394(14)77825-0. [DOI] [PubMed] [Google Scholar]
- 2.Huang H.B., Zhang Y.X. Pigmented paravenous retinochoroidal atrophy (Review) Exp Ther Med. 2014;7:1439–1445. doi: 10.3892/etm.2014.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheung D.S. Pigmented paravenous chorioretinal atrophy. Am J Ophthalmol. 1984;97:113. doi: 10.1016/0002-9394(84)90465-3. [DOI] [PubMed] [Google Scholar]
- 4.Issa C., Scholl H.P., Helb H.M., Fleckenstein M., Inhetvin-Hutter C., Holz F.G. Unilateral pigmented paravenous retinochoroidal atrophy. Klin Monbl Augenheilkd. 2007;224:791–793. doi: 10.1055/s-2007-963600. (Article in german) [DOI] [PubMed] [Google Scholar]
- 5.Bozkurt N., Bavbek T., Kazokoglu H. Hereditary pigmented paravenous chorioretinal atrophy. Ophthalmic Genet. 1998;19:88–104. doi: 10.1076/opge.19.2.99.2317. [DOI] [PubMed] [Google Scholar]
- 6.Choi J.Y., Sandberg M.A., Berson E.L. Natural course of ocular function in pigmented paravenous retinochoroidal atrophy. Am J Ophthalmol. 2006;141:763–765. doi: 10.1016/j.ajo.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 7.Kukner A.S., Yilmaz T., Celebi S., Aydemir O., Ulas F. Pigmented paravneous retinochoroidal atrophy. Ophthalmologica. 2003;217:436–440. doi: 10.1159/000073076. [DOI] [PubMed] [Google Scholar]
- 8.McKay G.J., Clarke S., Davis J.A., Simpson D.A., Silvestri G. Pigmented paravenous chorioretinal atrophy is associated with a mutation within the crumbs homolog 1 (CRB1) gene. Invest Ophthalmol Vis Sci. 2005;46:322–328. doi: 10.1167/iovs.04-0734. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh B., Goel N., Batta S., Raina U.K. SD-OCT in pigmented paravenous retinochoroidal atrophy. Ophthalmic Surg Lasers Imaging. 2012;43:e41–e43. doi: 10.3928/15428877-20120502-01. [DOI] [PubMed] [Google Scholar]
- 10.Junqueira D.L., Lopes F.S., Biteli L.G., Prata T.S. Pattern of inner retinal layers involvement in pigmented paravenous retinochoroidal atrophy as determined by SD-OCT: case report. Arq Bars Oftalmol. 2013;76:380–382. doi: 10.1590/s0004-27492013000600014. [DOI] [PubMed] [Google Scholar]