Abstract
Introduction
miR-99a-5p, known to play an important role in mammalian target of rapamycin (mTOR) regulation, is downregulated in human bladder cancer. The study aimed to investigate the anticancer activity of miR-99a-5p and the possible mechanism associated with mTOR in bladder cancer cells.
Materials and methods
Vectors expressing miR-99a-5p were transfected into human urinary bladder urothelial carcinoma (5637 and T24) cells. The level of miR-99a-5p was monitored by microRNA (miRNA) quantitative polymerase chain reaction (QPCR). Luciferase reporter assays were performed to verify the direct binding of miR-99a-5p to mTOR transcripts. The mTOR transcripts and protein levels were measured by QPCR and Western blot, respectively. Cell viability of miR-99a-5p-transfected cells was detected by tetrazolium salt (WST-1). Inhibition of mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2) signaling was detected by the phosphorylation of mTOR and AKT using Western blot. The ability of miR-99a-5p to enhance RAD001-induced apoptosis was determined as the expression of cleaved caspase 3 and levels of DNA fragmentation.
Results
Transfection of miR-99a-5p-expressing vector elevated the expression level of miR-99a-5p up to sixfold compared to vector-only controls. The results from luciferase assay verified that miR-99a-5p directly binds to the predicted sequence in the 3′ untranslated region (3′-UTR) of mTOR. The levels of mTOR RNA and protein were decreased in miR-99a-5p-transfected cells. Dual inhibition of mTORC1 and mTORC2 by miR-99a-5p was confirmed by the decreased phosphorylation of mTOR (at Ser2448 and Ser2481), phospho-rpS6 and phospho-4EBP1. The phosphorylation of AKT was significantly inhibited in miR-99a-5p-transfected cells upon RAD001 treatment. Enforced expression of miR-99a-5p potentiated RAD001-induced apoptosis in these cells.
Conclusion
This is the first study showing that miR-99a-5p markedly inhibits the growth of bladder cancer cells via dual inhibition of mTORC1 and mTORC2. Our data demonstrated that forced expression of miR-99a-5p inhibits the feedback of AKT survival pathway and enhances the induction of apoptosis in RAD001-treated bladder cancer cells.
Keywords: apoptosis, bladder cancer, miR-99a-5p, mTOR, RAD001
Introduction
Bladder cancer is the fifth most frequently diagnosed tumor in the US. It was estimated that there would be 74,000 new cases and ~16,000 deaths due to bladder cancer in 2015.1 Approximately 90% of diagnosed bladder cancers are urothelial carcinomas. There are two principal forms of bladder cancer cells, low grade and high grade.2 Bladder cancer patients usually suffer from recurrence within 5 years, and 34% of patients die from metastatic bladder cancer.1 Novel treatments are demanded to improve the therapeutic efficacy against bladder cancer.
Mammalian target of rapamycin (mTOR) is a serine/threonine protein kinase that regulates cell growth, cell proliferation, cell survival, translation and transcription.3 mTOR is the catalytic subunit belonging to two structurally distinct complexes: mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2).4 mTORC1 is composed of mTOR, regulatory-associated protein of mTOR (Raptor), mammalian lethal with SEC13 protein 8 (MLST8) and the noncore components PRAS40 and DEPTOR. mTOC1 functions as a nutrient/redox sensor and controls protein synthesis.5 mTORC2 is composed of mTOR, rapamycin-insensitive companion of mTOR (Rictor), MLST9 and mammalian stress-activated protein kinase interacting protein 1 (mSIN1).6 mTORC2 phosphorylates the AKT at serine residue S473, thus affecting metabolism and survival. As a member of phosphatidylinositol 3 kinase-related kinase protein family, mTOR is considered as a therapeutic target in many cancer types. An mTORC1 inhibitor RAD001 (everolimus) has been shown to have limited effects on bladder cancer models both in vivo and in vitro.7 Furthermore, a Phase II study has shown that use of RAD001 for treating metastatic urothelial cancer failed to achieve favorable outcomes.8 It is suggested that RAD001, as an mTOC1 inhibitor, may increase mTORC2 signaling that activates AKT S473 phosphorylation and promotes cancer cell survival.9 Indeed, dual inhibition of mTORC1/C2 has been demonstrated to control acquired endocrine-resistant breast cancer cells, even under conditions where RAD001 fails.10 Hence, dual inhibition of mTORC1 and mTORC2 is considered as a novel approach against bladder cancer.
MicroRNAs (miRNAs) are small (~22 nt) noncoding RNAs, which act as negative regulators of gene expression through binding to the 3′ untranslated region (3′-UTR) of their targeting mRNAs, leading to inhibition of mRNA translation by blocking the translation machinery or facilitating the degradation of mRNAs.11 In the past decade, increasing studies have revealed that miRNA plays significant roles in various biological processes, including cell differentiation, proliferation, apoptosis and cancer progression.11 Dysregulation of miRNAs has been involved in the tumorigenesis of many cancer types, indicating that miRNAs may act as oncogenes or tumor suppressors.12 Since the function of miRNAs depends on their targeting genes, upregulated miRNAs in tumor may function as oncogenes by inhibiting tumor suppressor genes, while downregulated miRNAs may function as tumor suppressors by negatively regulating oncogenes in cancer. Therefore, the identification of the targeting genes of miRNA is very important to understand the functional roles of miRNAs that are involved in tumorigenesis. The miRNAs are also considered as diagnostic biomarkers for cancer detection or targets of cancer therapy.13,14
The downregulation of miR-99a-5p has been reported in several types of cancer, including lung, ovarian and squamous cell carcinoma; hepatocellular carcinoma (HCC); child adrenocortical tumors; and prostate and bladder cancers.15–21 These findings suggest a potential role of miR-99a-5p as a tumor suppressor. In our previous studies, the expression of miR-99a-5p was downregulated in bladder tumor tissues compared to normal adjacent tissues using miRNA microarray approaches.22 miR-99a-5p has been demonstrated to play an important role in mTOR regulation.23–26 The present study was aimed to investigate the anticancer activity of miR-99a-5p in bladder cancer cells and the possible mechanism associated with mTOR.
Materials and methods
Chemicals
RAD001 (No 07741; Sigma-Aldrich Co., St Louis, MO, USA) was dissolved in dimethyl sulfoxide (DMSO). All other chemicals, unless otherwise stated, were from Sigma-Aldrich Co. Antibodies against mTOR, phospho-mTOR (Ser2448), phospho-mTOR (Ser2481), AKT, phospho-AKT (Ser473), rpS6, phospho-rpS6, 4EBP1 and phospho-4EBP1 were purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against enhanced green fluorescent protein (EGFP) and β-actin were from Sigma-Aldrich Co.
Construction of miRNA expression vector
A miR-99a-5p expression vector (pSM-99a) was constructed by annealing a paired oligonucleotide consisting of matured miR-99a-5p sequences and cloning into a small-RNA expression vector, pSM, as described.27 Vectors expressing miR-28-5p (pSM-28-5p) were constructed as negative control. The sequences of oligonucleotides are listed in Table S1.
Cell culture and transfection
Human immortalized uroepithelial cell line (SV-Huc1) and bladder cancer cell lines, 5637 (HTB-9, grade 2) and T24 (HTB-4, grade 3), were obtained from Bioresource Collection and Research Center (BCRC), Hsinchu, Taiwan. The human embryonic kidney cancer cell, 293T, was a gift from Dr Yu-Chiau Shyu (Institute of Biopharmaceutical Sciences, National Yang-Ming University, Taipei, Taiwan). The authentication of these cell lines was performed using short tandem repeat polymerase chain reaction (STR-PCR) profiling at BCRC and routinely check for mycoplasma contamination using PCR-based method in our laboratory as described.28 The SV-Huc1 cells were cultured in the F12 medium. The 5637 and T24 cells were cultured in the Roswell Park Memorial Institute (RPMI)-1640 medium. 293T cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM). Media were supplemented with 10% fetal bovine serum (FBS), 2 mM GlutaMAX-I, 100 units/mL penicillin and 100 μg/mL streptomycin. Medium, FBS and supplements were purchased from Thermo-Fisher Scientific (Waltham, MA, USA). Cells with 70%–80% confluency were transfected or co-transfected with indicated plasmids in six-well plates or 10 cm dishes using a polymer-based transfection reagent (Transfec 293; GeneDireX, Taipei, Taiwan) according to the manufacturer’s instructions. Transfected cells were selected with 200 μg/mL G418 for 24 h prior to the administration of RAD001.
Detection of miR-99a-5p expression by quantitative polymerase chain reaction (QPCR)
The expression level of miR-99a-5p in SV-Huc1, 5637, T24 and transfected cells was measured using miRNA QPCR as described.27 In brief, total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific), reverse transcribed with NCode™ miRNA First-Strand cDNA synthesis Kit (Thermo-Fisher Scientific). The detection of miR-99a-5p was performed using NCode™ SYBR Green miRNA quantitative reverse transcription PCR (RT-qPCR) kit (Thermo Fisher Scientific). The level of U6 RNA was measured and used to normalize the relative abundance of miR-99a-5p. The cells transfected with pSM or pSM-99a were selected with 200 μg/mL G418 for 24 h prior to the RNA extraction.
Construction of 3′-UTR reporter plasmids and luciferase assays
Synthesized double-strand oligonucleotides containing 19 bases upstream and 11 bases downstream of miR-99a-5p seed sequences on the 3′-UTR of mTOR mRNA were cloned downstream to the luciferase gene in the pmiR-GLO reporter vector (Promega Corporation, Madison, WI, USA). This cloning step generated reporter vector, pmiR-GLO-99a-mTOR-TS (targeting sequence), containing the 3′-UTR of mTOR. We constructed another reporter vector, pmiR-GLO-99a-mTOR-MTS (with mutated targeting sequence), by reversing the seed targeting sequence of mTOR. A similar cloning step using oligonucleotides containing three repeats of anti-sense miR-99a-5p was performed to generate another reporter construct designated as pmiR-GLO-99a-PTS (with antisense miR-99a-5p sequence, positive targeting sequence). The 293T cells were co-transfected with either miR-99a-5p (pSM-99a) or control (pSM) and target reporter plasmids. In all, 24 h after transfection, the firefly and Renilla luciferase activities were detected consecutively using Dual-Luciferase Kit (Promega Corporation). Relative protein levels were expressed as Firefly/Renilla luciferase ratios.
Detection of mTOR expression in transfected cells
Cells were transfected with the indicated miRNA-expressing vector. At 24 h post transfection, transfection medium was replaced by G418 (400 μg/mL) containing medium. At 48 h post transfection, RNA samples were collected and extracted using the TRIzol reagent. The RNA quality and quantities were measured using NanoDrop 2000 (Thermo Fisher Scientific). First-strand cDNA was synthesized from total RNA (2.5 μg) using First Strand cDNA Synthesis Kit (Thermo Fisher Scientific) and oligo dT primers. QPCR was performed in a 20 μL reaction as described.22 Each quantification was conducted in triplicate, and the experiments were done in triplicate using the indicated cell lines. EGFP co-expressed with miR-99a-5p was used as an internal loading control of mRNA expression to normalize the individual gene expression level and to eliminate the possible difference of transfection efficiency. The specific primer pairs used to amplify genes are listed in Table S1. Protein samples were extracted at 96 h post transfection using radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 10 mM Tris, pH 7.2, 0.1% sodium dodecyl sulfate [SDS], 1.0% Triton X-100, 5 mM EDTA, pH 8.0) containing 1× protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Protein concentration was determined by Micro BCA Protein Assay Kit (Thermo Fisher Scientific). Detection of mTOR, phospho-mTOR (Ser2448), rpS6, phospho-rpS6 (Ser235/236), phospho-mTOR (Ser2481), 4EBP1, phospho-4EBP1 (Ser65 and Thr37/46), AKT and phospho-AKT (Ser473) was performed as described.29,30 The expression of EGFP in each transfected sample was also detected to eliminate possible difference in transfection efficiency. The dilution of first antibodies in the immunoblotting was according to the manufacturer’s instructions. Band signals were acquired in the linear range of the scanner using GeneTools software (Syngene, Frederick, MD, USA). The ratio between the mTOR and the β-actin bands was used to quantitate mTOR modulation by miR-99a-5p.
Cell viability assays
Cell viability in pSM-99a-transfected cells with or without the co-transfection of p-miR-GLO-99a-PTS, a reporter vector expressing positive targeting sites for miR-99a-5p to compete with exogenous miR-99a-5p expression, or the treatment of 50 μM RAD001 was measured using tetrazolium salt (WST-1) reagent as described.30
Immunoprecipitations (IPs)
Cells were transfected with indicated miRNA-expressing vector and subjected to protein extraction using 1× Cell Lysis Buffer (Cell Signaling Technology) at 96 h post transfection. Protein concentration was determined as described earlier; subsequently, ~800 μg of total protein was used in IPs by adding Protein A plus G agarose beads (EMD Millipore, Billerica, MA, USA) with rotation for 1 h. The beads were removed by centrifugation, and mTOR antibodies (No OP-97; EMD Millipore) were added to the cleared lysates, which were rotated overnight at 4°C. Protein A plus G agarose beads were added to the supernatant and incubated at 4°C for an additional 3 h. Immunoprecipitated complexes were washed in 1× Cell Lysis Buffer, boiled in SDS-sample buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (10% gels) and analyzed by immunoblotting.
Detection of apoptosis
The induction of apoptosis in pSM-99a-transfected cells with or without 24 h treatment of RAD001 was detected by 1) the expression level of cleaved caspase 3 (c-Cas3) by immunoblotting,30 2) the activation of caspase 3/7 as described,31 and 3) the induction of DNA fragmentation (terminal deoxynucleotidyl transferase dUTP nick end labeling [TUNEL] assay) using a BD Pharmingen™ APO-DIRECT™ Kit (Becton Dickinson [BD], Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. The percentage of TUNEL-positive cells was detected using an Accuri™ C5 flow cytometer, and the data were analyzed using CellQuest Pro software (BD).
Statistics
All experiments were performed at least three times. Statistical significance was determined using Student’s t-test. Differences were considered statistically significant when P-value was <0.05.
Results
Expression of miR-99a-5p in human bladder cancer cells
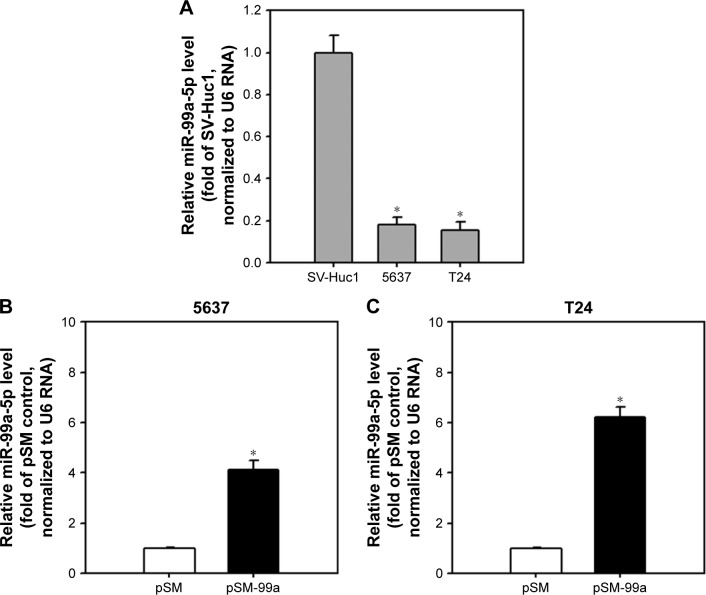
Downregulation of miR-99a-5p has been reported in T24 cells.32 To study the effect of miR-99a-5p on the expression of predicted targeting genes in bladder cancer cells, we increased the expression of miR-99a-5p in two bladder cancer cell lines, including 5637 and T24 cells, by transfecting an miR-99a-5p expression vector, pSM-99a. As expected, the expression level of miR-99a-5p was increased in pSM-99a-transfected cells compared to control (pSM). As shown in Figure 1, the expression of miR-99a-5p transcripts was increased to 4.13±0.38- and 6.22±0.41-fold in 5637 and T24 cells, respectively.
Figure 1.
Elevation of miR-99a-5p expression levels in 5637 and T24 cells transfected with pSM-99a.
Notes: (A) miR-99a-5p was downregulated in 5637 and T24 cells. Cells at 70% confluence were collected, and the expression of miR-99a-5p was detected by miRNA QPCR. (B and C) Transfection of pSM-99a elevated the expression level of miR-99a-5p in 5637 and T24 cells. Transfected cells were selected with 200 μg/mL G418 for 24 h, while co-expressed EGFP was detectable under fluorescent microscopy. Total RNA was extracted and reverse transcribed, and the resulting first cDNA was applied to the subsequent miRNA QPCR. miR-99a-5p expression was normalized by U6 RNA. Data represent three independent experiments and are expressed as fold ± SD relative to the SV-Huc1 or vector only control (pSM). *P<0.05.
Abbreviations: miRNA, microRNA; QPCR, quantitative polymerase chain reaction; EGFP, enhanced green fluorescent protein.
mTOR is a direct target of miR-99a-5p in bladder cancer cells
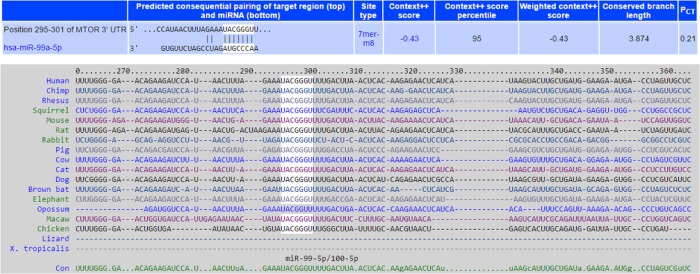
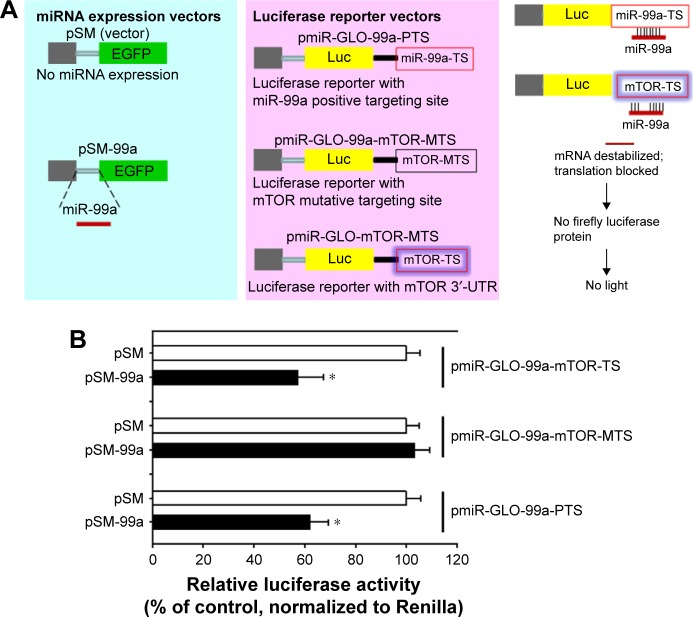
To identify target genes of miR-99a-5p that may contribute to bladder cancer tumorogenesis, we searched miRNA-target databases, including TargetScan and PicTar, and found that mTOR was one of 59 predicted transcripts. Putative and evolutionary conserved miR-99a-5p targeting site was in the 3′-UTR of mTOR mRNA (Figure 2). Indeed, several groups have reported that miR-99a-5p targets to mTOR in various types of human cancers.18,19,21,23,33 To confirm that mTOR is a direct target of miR-99a-5p, we cloned the positive targeting sites (three repeats of reverse and complementary sequences as matured miR-99a-5p, pmiR-GLO-99a-PTS) and 3′-UTR of mTOR harboring miR-99a-5p targeting site (pmiR-GLO-99a-mTOR-TS) or mutated targeting site (with reverse seed sequence of miR-99a-5p, pmiR-GLO-99a-mTOR-MTS) into a reporter plasmid downstream of luciferase reporter, and performed reporter assays. As depicted in Figure 3, the result showed a significant decrease in the relative luciferase activity with miR-99a-5p-positive targeting sites (pmiR-GLO-99a-PTS) compared to pSM control, indicating the expression of functional and matured miR-99a-5p in pSM-99a-transfected cells. Similarly, the relative luciferase activity of reporter containing a wild-type 3′-UTR of mTOR reporter (pmiR-GLO-99a-mTOR-TS) was decreased in pSM-99a-transfected cells compared to pSM control. In contrast, there was no change in the relative luciferase activity of the mutated mTOR 3′-UTR reporter (pmiR-GLO-99a-mTOR-MTS) among groups, indicating that these sequences failed to respond to miR-99a-5p. These data suggested that miR-99a-5p binds directly to the predicted target site located on the 3′-UTR of mTOR mRNA.
Figure 2.
mTOR is a putative target of miR-99a-5p.
Notes: mTOR was predicted as a putative target of miR-99a-5p by searching TargetScan database. The targeting site of miR-99a-5p located in the 3′-UTR of mTOR is listed (upper panel). The 3′-UTR sequences that contain the putative miR-99a-5p targeting site are highly conserved among vertebrate species (lower panel). The miR-99a-5p seed sequences are marked white. Sequences listed are Hsa, human; Ptr, chimpanzee; Cfa, dog; Fca, cat; Rno, rat; and Mmu, mouse.
Abbreviations: mTOR, mammalian target of rapamycin; 3′-UTR, 3′ untranslated region.
Figure 3.
miR-99a-5p directly binding to the 3′-UTR of mTOR.
Notes: (A) Illustration of reporter constructs and design of luciferase reporter assays. (B) miR-99a-5p targets to mTOR 3′-UTR. Luciferase activity in 293T cells transiently co-transfected with pSM-99a and the positive control reporter vectors (pmiR-GLO-99a-PTS) or with 3′-UTR of mTOR reporter vectors (TS and MTS). Data represent mean ± SD values of three independent experiments, each performed in triplicate. *P<0.05.
Abbreviations: 3′-UTR, 3′ untranslated region; mTOR, mammalian target of rapamycin; EGFP, enhanced green fluorescent protein.
mTOR expression was inhibited by miR-99a-5p
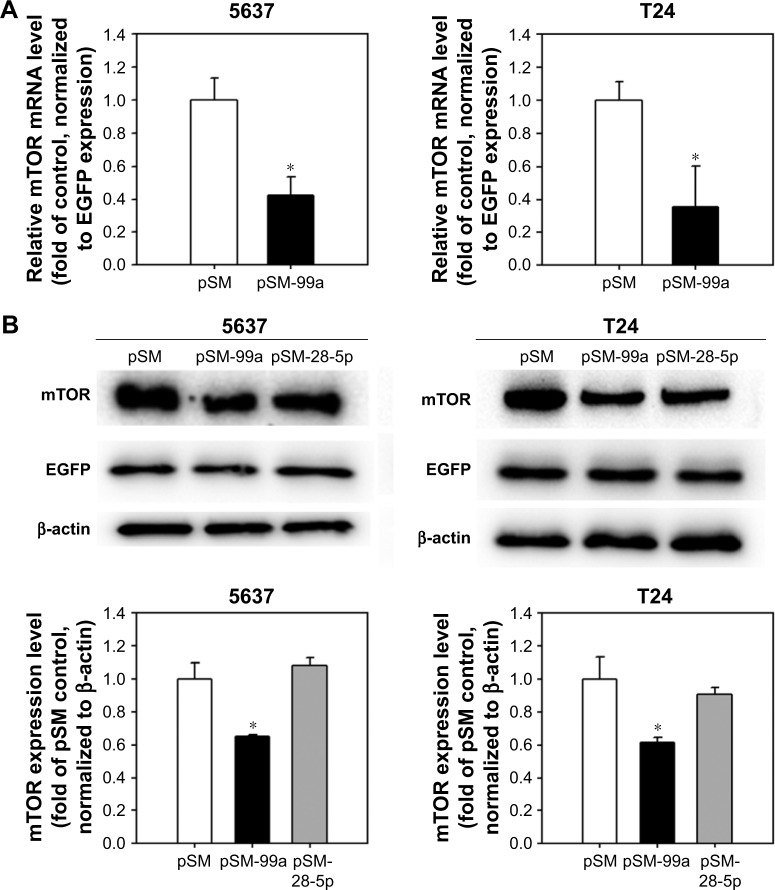
miRNAs negatively regulate their target genes by reducing mRNA stability or blocking protein translation. Our results showed that the expression level of mTOR was regulated by miR-99a-5p. The mTOR mRNA level was detected in cells transfected with the pSM-99a by miRNA QPCR at 48 h post transfection. In pSM-99a-transfected cells, the expression level of mTOR was reduced to 0.42%±0.11% and 0.41%±0.15% in 5637 and T24 cells, respectively, compared to the control (pSM-transfected cells; Figure 4A). Second, we examined the mTOR protein level in response to the elevated miR-99a-5p. As shown in Figure 4B, the mTOR protein level was decreased in miR-99a-5p-transfected cells compared to pSM-transfected control (5637, 0.71%±0.09%; T24, 0.51%±0.05%). Third, we included another miRNA, miR-28-5p, which has no predicted targeting site on the 3′-UTR of mTOR, as a negative control. As expected, transfection of pSM-28-5p resulted in no change in the expression level of mTOR. These results suggest that miR-99a-5p suppresses mTOR mRNA and protein expression in bladder cancer cells.
Figure 4.
miR-99a-5p inhibits mTOR expression.
Notes: (A) mTOR mRNA level in cells transfected with pSM or pSM-99a was detected by QPCR. Data represent mean ± SD values of three independent experiments, each performed in triplicate. *P<0.05. (B) Protein expression level of mTOR in cells transfected with pSM, pSM-99a or pSM-28-5p was detected by Western blot. A representative blot is shown in the upper panel. The EGFP, which co-expressed with miRNA transcripts, was detected, and β-actin was used as a loading control. The relative protein levels of mTOR in each treatment were quantified as shown in the lower panel. *P<0.05.
Abbreviations: mTOR, mammalian target of rapamycin; QPCR, quantitative polymerase chain reaction; EGFP, enhanced green fluorescent protein.
Expression of miR-99a-5p reduces cell viability of bladder cancer cells
To determine whether expression of miR-99a-5p is involved in tumor suppression, we detected the cell viability in pSM-99a-transfected bladder cancer cells. The cell viability decreased significantly in pSM-99a-transfected cells compared to pSM control (5637, 60.11%±2.53%; T24, 51.22%±3.71%; Figure 5). Co-transfection of pSM-99a with pmiR-GLO-99a-PTS, which generates complementary sequences of miR-99a-5p to compete with exogenous miR-99a-5p expression, restored cell viability. These results indicate that miR-99a-5p overexpression inhibits the growth of human bladder cancer cells.
Figure 5.
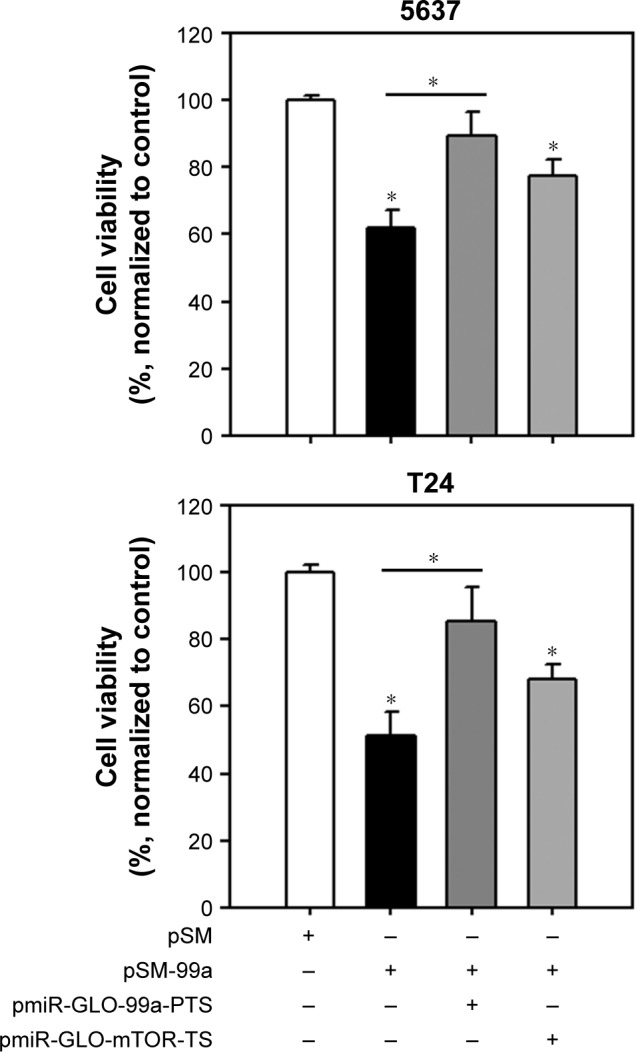
Overexpression of miR-99a-5p reduces cell viability in human bladder cancer cells.
Notes: Cells were transfected with the indicated vectors. At 72 h post transfection, cell viability was measured using tetrazolium salt (WST-1) reagents. pmiR-GLO-99a-PTS or pmiR-GLO-99a-mTOR-TS was co-transfected in the rescue experiments. *P<0.05.
Abbreviations: mTOR, mammalian target of rapamycin; PTS, positive targeting sites; TS, targeting sites.
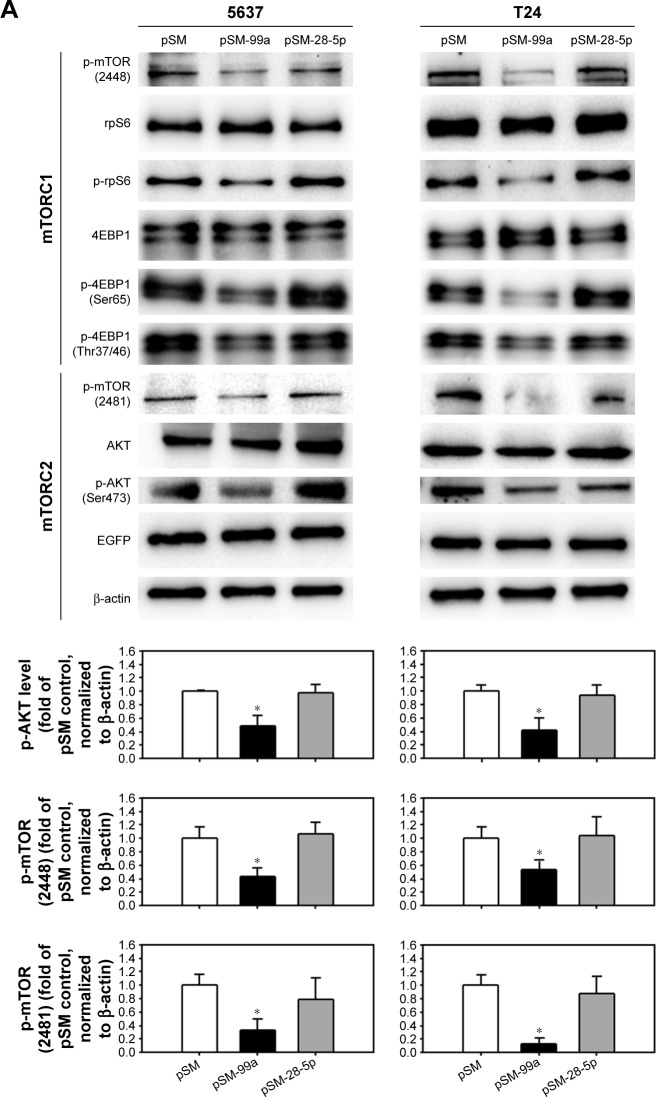
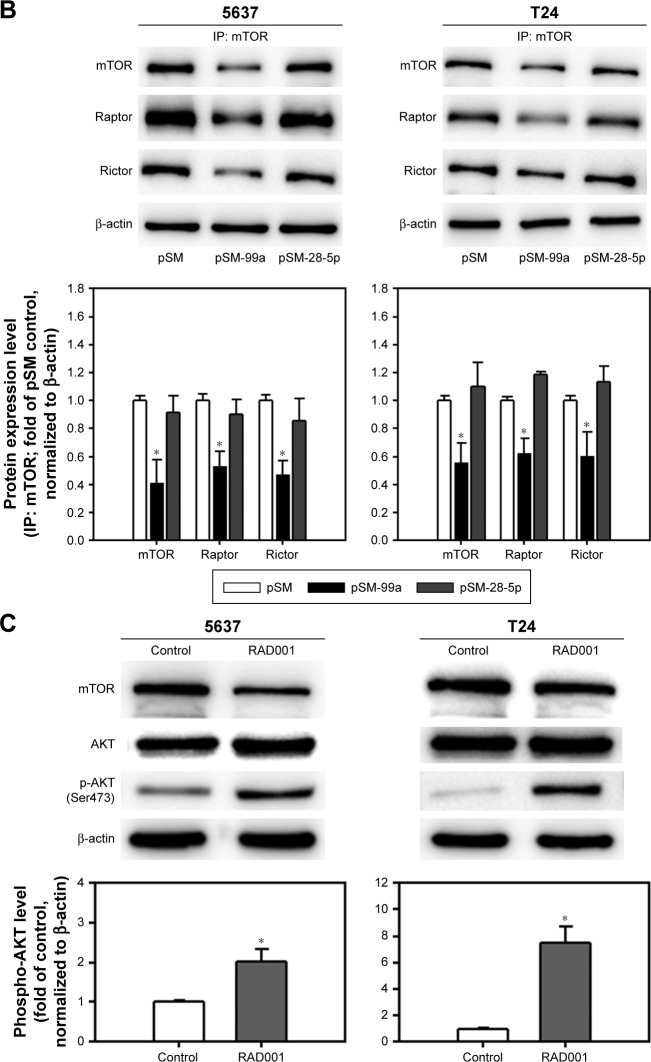
Dual inhibition of mTORC1 and mTORC2 signaling by miR-99a-5p overexpression
We have demonstrated that forced expression of miR-99a-5p suppressed mTOR expression. Since mTOR is the core protein of mTORC1 and mTORC2, it is postulated that mTORC1 and C2 signaling were both inhibited by miR-99a-5p overexpression. As shown in Figure 6A, the phosphorylation of mTOR on residue Ser2448 and Ser2481 was inhibited by the transfection of pSM-99a, while not affected by transfection of pSM-28-5p. Furthermore, the decreased phosphorylation level of S6 ribosomal protein (rpS6) and 4EBP1, which are downstream effectors of mTORC1, suggested that miR-99a-5p exhibits an inhibitory effect on mTORC1 signaling. Treatment of 1 μM RAD001, which inhibited only mTORC1, resulted in the increased level of AKT activation, possibly through the feedback of mTORC2 (Figure 6B). As expected, phospho-AKT was decreased in pSM-99a-transfected cells in response to inhibition of mTORC2 (Figure 6A). To further demonstrate that mTORC1 and mTORC2 are both inhibited in miR-99a-5p-transfected cells, the levels of Raptor and Rictor that co-immunoprecipitate with mTOR were evaluated. As shown in Figure 6C, transfection of pSM-99a suppressed the levels of both Raptor and Rictor that co-immunoprecipitated with mTOR. These results indicated that overexpression of miR-99a-5p suppresses the signaling transduction modulated by mTORC1 and mTORC2 via targeting to mTOR core protein.
Figure 6.
Dual inhibition of mTORC1 and mTORC2 in miR-99a-5p-expressing cells compared to RAD001-treated cells.
Notes: (A) The expression level of phospho-mTOR (Ser2448), rpS6, phospho-rpS6, 4EBP1, phospho-4EBP1 (Ser65), phospho-4EBP1 (Thr37/46), phospho-mTOR (Ser2481), total AKT and phospho-AKT (Ser473) was detected in cells transfected with pSM, pSM-99a or pSM-28-5p. EGFP, which co-expressed with miRNA transcripts in the pSM vector, was detected, and β-actin was used as a loading control. The expression level of phospho-AKT in each treatment was quantified as shown in the lower panel. (B) The protein lysates from cells transfected with pSM, pSM-99a or pSM-28-5p were subjected to IP with anti-mTOR antibody overnight, and then, mTOR immunoprecipitates (IP: mTOR) were subjected to Western blot analysis for mTOR, Raptor and Rictor. (C) The expression level of phospho-AKT (Ser473) in 24 h control (dimethyl sulfoxide[DMSO])-treated) or RAD001-treated cells was determined by Western blot. The expression of β-actin served as a loading control. The relative protein levels in each treatment were quantified as shown in the lower panel. *P<0.05.
Abbreviations: mTORC1, mTOR complex 1; mTORC2, mTOR complex 2; mTOR, mammalian target of rapamycin; EGFP, enhanced green fluorescent protein; IP, immunoprecipitation.
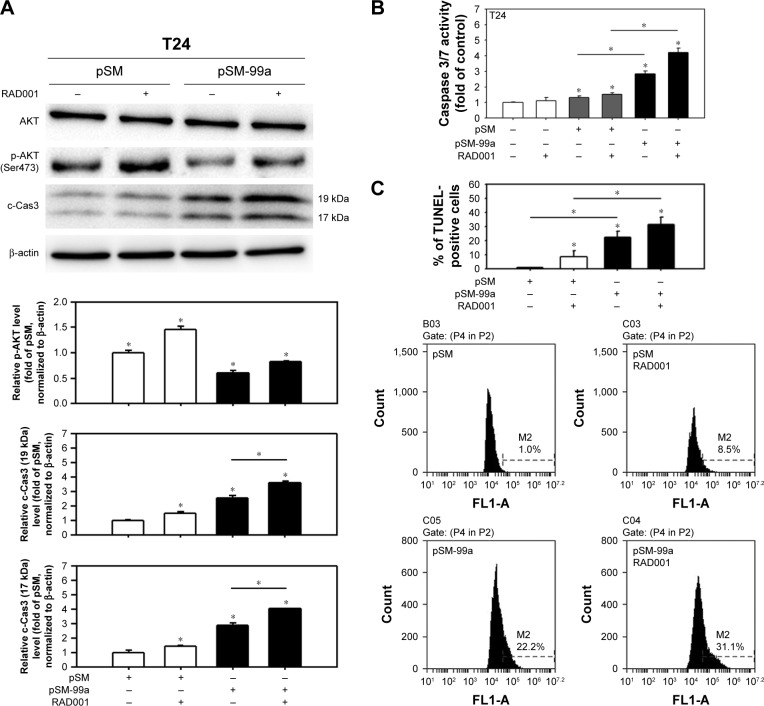
miR-99a-5p enhanced RAD001-induced apoptosis
In our previous study, T24 cells were more resistant to RAD001 treatment than 5637 cells.31 We therefore investigated whether the overexpression of miR-99a-5p potentiates the effects of RAD001 against bladder cancer cells by transfecting T24 cells with miR-99a-5p prior to the treatment of 1 μM RAD001. As shown in Figure 7, the expression level of phospho-AKT reduced significantly in RAD001-treated cells with enforced expression of miR-99a-5p (pSM-99a transfected). Hence, the dual inhibition of mTORC1 and mTORC2 by miR-99a-5p increased the induction of apoptosis in RAD001-treated cells, judging by the increased level of c-Cas3 (Figure 7A), caspase 3/7 activities (Figure 7B), and the level of DNA fragmentation (Figure 7C). These results revealed that miR-99a-5p enhanced the efficacy of RAD001 in human bladder cancer cells.
Figure 7.
Forced expression of miR-99a-5p suppressed RAD001-induced AKT activation and enhanced apoptosis.
Notes: (A) Cells were transfected with pSM or pSM-99a for 48 h and treated with RAD001 for 24 h prior to the extraction of total protein. The expression levels of phospho-AKT (Ser473) and c-Cas3 were detected. The representative blot from three independent experiments with similar results is shown, and β-actin serves as a loading control. The relative protein levels of phospho-AKT and c-Cas3 (19 kDa and 17 kDa) in each treatment were quantified as shown in the lower panel. (B) The caspase 3/7 activity was determined in cells that were transfected with pSM or pSM-99a for 24 h, G418 selection for 24 h and then treated with 1 μM RAD001 for another 24 h. Control cells (no transfection) were cultured for 48 h and then treated with RAD001 for another 24 h. (C) The apoptotic level in cells with the indicated treatment was detected by the TUNEL assay. The representative flow cytometry histograms are present in the lower panel. *P<0.05.
Abbreviations: c-Cas3, cleaved caspase 3; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling.
Discussion
The PI3K/AKT/mTOR regulates key processes involving tumor growth and progression. Our study showed that reactivation of miR-99a-5p, which is downregulated in bladder cancer cells, contributes to the inhibition of bladder cancer growth through dual disruption of mTORC1 and mTORC2 signaling by targeting mTOR. Recent studies have found that PTEN, the PI3K antagonist, is a target for mutation in bladder cancer, indicating that dysregulation of PI3K signaling may play an important role in bladder tumorigenesis.34,35 Indeed, phospho-AKT expression levels were higher in bladder tumors than in normal urothelium.36 However, due to potential toxicity issues associated with PI3K/AKT inhibition, targeting this tumor survival pathway further downstream may reduce the side effects of chemotherapeutic agents designed to inactivate these signals. mTOR, a downstream mediator of PI3K/AKT, becomes a focus target to circumvent this problem. Furthermore, Ingalla et al37 reported that ~50% of primary human bladder tumors display aberrant activation of mTOR, suggesting that mTOR inhibition might be of clinical benefit. In a mouse animal model, intravesical delivery of rapamycin, a well-studied mTORC1 inhibitor, suppresses tumorigenesis of progressive bladder cancer.38 Inhibition of mTOR using RAD001 also demonstrated significant anticancer activity in a xenografts nude mice model.39 However, clinical trials using rapamycin derivatives such as CCI-779 (temsirolimus)40 and RAD0018 seem to be less effective in patients with bladder cancer. As described in the Introduction section, one possibility is that inhibition of mTORC1 (rapamycin, CCI-779 and RAD001) activates mTORC2, which triggers the phosphorylation of pro-survival of AKT pathway. Therefore, researchers had developed several novel molecules inhibiting both mTORC1 and mTORC2, including AZD8055,41 Torin 142 and PP242.43 Nevertheless, chemical inhibitors may still exhibit undesired side effects when the targets are not specific.
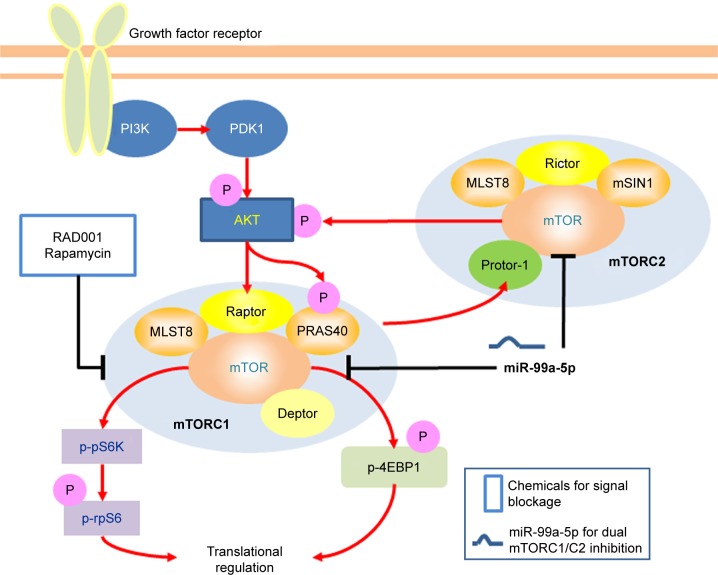
Recent studies have suggested that dysregulation of miRNAs is a common event in human cancers, including bladder cancer.44 Our previous studies demonstrated that miR-99a-5p is one of the dysregulated miRNAs that down-regulated in bladder cancer tissues.22 By comparison of 100 pairs of bladder cancer tissues, the adjacent non-neoplastic tissues and the plasma collected from bladder cancer patients or control patients, Feng et al also found that miR-99a-5p was significantly downregulated in bladder cancer. They reported that the lower expression of miR-99a-5p was correlated with the more aggressive phenotypes of bladder cancer and enforced expression of miR-99a-5p inhibits the cell proliferation of human bladder cancer cells.32 In this study, we showed an up to sixfold enforced expression of miR-99a-5p in transfected human bladder cancer cells using a vector-based approach. The expressed mature miR-99a-5p was demonstrated to be fully functional according to the luciferase reporter assay and targeted to the predicted targeting sequences located in the 3′-UTR of mTOR mRNA. The cell viability was significantly reduced in pSM-99a-transfected cells and was rescued when co-transfected with pmiR-GLO-99a-PTS. As illustrated in Figure 8, the expression level of mTOR was suppressed in miR-99a-5p-trans-fected bladder cancer cells in both mRNA and protein levels, leading to the dual inhibition of mTORC1 activity (decreased level of 4EBP1 and phospho-S6K) and mTORC2 activity (decreased phosphorylation of AKT on Ser473). We also confirmed the activation of AKT in RAD001-treated 5637 and T24 cells, suggesting the existence of mTORC2 feedback loop. Therefore, targeting mTOR expression by enforced miRNA expression seems to be a reasonable strategy for developing novel therapeutic approaches. However, a given miRNA potentially targets to hundreds of mRNAs. Disruption of multiple targets raises the problems of the specificity of miRNAs against specific amplified survival signaling pathways upregulated in cancer cells. On the other hand, enforced miRNAs might also have the potential benefits of suppressing multiple oncogenes that are overexpressed during tumorigenesis. Thus, careful evaluation of miRNA’s targets becomes a critical issue.
Figure 8.
Schematic presentation of a mechanistic model showing miR-99a-5p blocking mTORC1 and mTORC2 signaling.
Note: Targeting mTOR by miR-99a-5p facilitates mTORC1 and mTORC2 blockage, thus without AKT activation.
Abbreviations: mTORC1, mTOR complex 1; mTORC2, mTOR complex 2; mTOR, mammalian target of rapamycin.
Artificially synthesized small interfering RNAs (siRNAs) with target specificity may be considered as another molecular agent that blocks mTOR signaling in bladder cancer cells. However, transfection of siRNA against mTOR was associated with dose-dependent mTOR protein reduction but not proliferation inhibition or apoptosis induction, according to a recent study.45 The limitation of the current study is that our data were collected in cultured human bladder cancer cells in vitro. Further investigation using mouse bladder cancer model to demonstrate that miR-99a-5p inhibits tumor growth or progression but has minimal impact on normal tissues is warranted.
Taken together, this study identified miR-99a-5p as a growth-suppressive miRNA in human bladder cancer cells, at least partly, through repression of mTOR expression in the mRNA and protein levels. Our data provide further evidence that miR-99a-5p inhibits both mTORC1 and mTORC2 activity. The dual inhibition of mTORC1 and mTORC2 enhances the therapeutic effect of RAD001 and therefore it could be considered as an adjunct agent for treating drug-resistant cancer. As miR-99a-5p is downregulated in bladder tumor, the reintroduction of this matured miRNA into tumor tissue may be a therapeutic strategy by suppressing target genes. Although miRNA-based therapeutics are still in their infancy, our data on miR-99a-5p are encouraging and suggest that this miRNA could be a potential target for developing novel treatment of bladder cancer in the future.
Supplementary material
Table S1.
Sequence of oligonucleotides used in this study
| Primer name | Sequence 5′→3′ | Note |
|---|---|---|
| Paired oligonucleotides for the cloning of miRNA expression vectors | ||
| miR-99a_Top | TGCTGAACCCGTAGATCCGATCTTGTGGTTTTGGCCACTGACTGACCACAAGATGATCTACGGGTT | Matured hsa-miR-99a-5p sequence is underlined |
| miR-99a_Bot | CCTGAACCCGTAGATCATCTTGTGGTCAGTCAGTGGCCAAAACCACAAGATCGGATCTACGGGTTC | |
| miR-28-5p_Top | TGCTGAAGGAGCTCACAGTCTATTGAGGTTTTGGCCACTGACTGACCTCAATAGTGTGAGCTCCTT | Matured miR-28-5psequence is underlined |
| miR-28-5p_Bot | CCTGAAGGAGCTCACACTATTGAGGTCAGTCAGTGGCCAAAACCTCAATAGACTGTGAGCTCCTTC | |
| Paired oligonucleotides for the cloning of reporter constructs | ||
| mTOR-TS_Top | CTAGCTGGGGAACAGAAGATCCATAACTTTAGAAATACGGGTTTTGACTTAACG | Seed sequences of miR-99a in mTOR 3′-UTR are boxed |
| mTOR-TS_Bot | TCGACGTTAAGTCAAAACCCGTATTTCTAAAGTTATGGATCTTCTGTTCCCCAG | |
| mTOR-MTS_Top | TCGAGTGGGGAACAGAAGATCCATAACTTTAGAAAATGCCCATTTGACTTAACGC | Mutated seed sequences of mTOR are boxed |
| mTOR-MTS_Bot | GGCCGCGTTAAGTCAAATGGGCATTTTCTAAAGTTATGGATCTTCTGTTCCCCAC | |
| 99a-PTS_Top | CTAGCCACAAGATCGGATCTACGGGTTGATATCCACAAGATCGGATCTACGGGTTATAACACAAGATCGGATCTACGGGTTC | Three miR-99a-5p positive targeting sites are underlined |
| 99a-PTS_Bot | TCGAGAACCCGTAGATCCGATCTTGTGTTATAACCCGTAGATCCGATCTTGTGGATATCAACCCGTAGATCCGATCTTGTGG | |
| Primers used in QPCR detection of mTOR expression | ||
| mTOR_5QP | AGACACCCATCCAACCTGAT | NM_004958; amplicon: 85 bps |
| mTOR_3QP | TCATAGCAACCTCAAAGCAGTC | |
| EGFP_5QP | CGACGGCAACTACAAGAC | |
| EGFP_3QP | TAGTTGTACTCCAGCTTGTGC | |
Abbreviations: Bot, bottom; miRNA, microRNA; mTOR, mammalian target of rapamycin; 3′-UTR, 3′ untranslated region; QPCR, quantitative polymerase chain reaction; EGFP, enhanced green fluorescent protein.
Acknowledgments
The study was supported by Shin-Kong Wu Ho-Su Memorial Hospital (SKH-8302-101-0202 and SKH-8302-102-0202 to J-FL and SKH-8302-102-0201 to TI-SH) and Ministry of Science and Technology (NSC102-2314-B-341-003-MY3 to J-FL), Taipei, Taiwan. We thank Dr Guangwei Du for kindly providing the pSM plasmids and the cell bank at BCRC for providing the STR profiling service and data.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Knowles MA, Hurst CD. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15(1):25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 3.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 4.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124(3):471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 5.Kim DH, Sarbassov DD, Ali SM, et al. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110(2):163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 6.Sarbassov DD, Ali SM, Kim DH, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14(14):1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 7.Vasconcelos-Nobrega C, Pinto-Leite R, Arantes-Rodrigues R, et al. In vivo and in vitro effects of RAD001 on bladder cancer. Urol Oncol. 2013;31(7):1212–1221. doi: 10.1016/j.urolonc.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Milowsky MI, Iyer G, Regazzi AM, et al. Phase II study of everolimus in metastatic urothelial cancer. BJU Int. 2013;112(4):462–470. doi: 10.1111/j.1464-410X.2012.11720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breuleux M, Klopfenstein M, Stephan C, et al. Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol Cancer Ther. 2009;8(4):742–753. doi: 10.1158/1535-7163.MCT-08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jordan NJ, Dutkowski CM, Barrow D, et al. Impact of dual mTORC1/2 mTOR kinase inhibitor AZD8055 on acquired endocrine resistance in breast cancer in vitro. Breast Cancer Res. 2014;16(1):R12. doi: 10.1186/bcr3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5(7):522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 12.Esquela-Kerscher A, Slack FJ. Oncomirs – microRNAs with a role in cancer. Nat Rev Cancer. 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 13.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 14.Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66(15):7390–7394. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 15.Wu D, Zhou Y, Pan H, Zhou J, Fan Y, Qu P. microRNA-99a inhibiting cell proliferation, migration and invasion by targeting fibroblast growth factor receptor 3 in bladder cancer. Oncol Lett. 2014;7(4):1219–1224. doi: 10.3892/ol.2014.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Zhao Z, Liu Y, Mu D. microRNA-99a is downregulated and promotes proliferation, migration and invasion in non-small cell lung cancer A549 and H1299 cells. Oncol Lett. 2015;9(3):1128–1134. doi: 10.3892/ol.2015.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nam EJ, Yoon H, Kim SW, et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin Cancer Res. 2008;14(9):2690–2695. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Chen Z, Tan X, et al. MicroRNA-99a/100 promotes apoptosis by targeting mTOR in human esophageal squamous cell carcinoma. Med Oncol. 2013;30(1):411. doi: 10.1007/s12032-012-0411-9. [DOI] [PubMed] [Google Scholar]
- 19.Li D, Liu X, Lin L, et al. MicroRNA-99a inhibits hepatocellular carcinoma growth and correlates with prognosis of patients with hepatocellular carcinoma. J Biol Chem. 2011;286(42):36677–36685. doi: 10.1074/jbc.M111.270561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doghman M, El Wakil A, Cardinaud B, et al. Regulation of insulin-like growth factor-mammalian target of rapamycin signaling by microRNA in childhood adrenocortical tumors. Cancer Res. 2010;70(11):4666–4675. doi: 10.1158/0008-5472.CAN-09-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun D, Lee YS, Malhotra A, et al. miR-99 family of MicroRNAs suppresses the expression of prostate-specific antigen and prostate cancer cell proliferation. Cancer Res. 2011;71(4):1313–1324. doi: 10.1158/0008-5472.CAN-10-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai T-F, Lin Y-C, Chen H-E, Chou K-Y, Lin J-F, Hwang TIS. Involvement of the insulin-like growth factor I receptor and its downstream antiapoptotic signaling pathway is revealed by dysregulated microRNAs in bladder carcinoma. Urol Sci. 2014;25(2):58–64. [Google Scholar]
- 23.Hu Y, Zhu Q, Tang L. MiR-99a antitumor activity in human breast cancer cells through targeting of mTOR expression. PLoS One. 2014;9(3):e92099. doi: 10.1371/journal.pone.0092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang HG, Luo X, Wu S, Jian B. MiR-99a inhibits cell proliferation and tumorigenesis through targeting mTOR in human anaplastic thyroid cancer. Asian Pac J Cancer Prev. 2015;16(12):4937–4944. doi: 10.7314/apjcp.2015.16.12.4937. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Chang L, Li Z, et al. miR-99a and -99b inhibit cervical cancer cell proliferation and invasion by targeting mTOR signaling pathway. Med Oncol. 2014;31(5):934. doi: 10.1007/s12032-014-0934-3. [DOI] [PubMed] [Google Scholar]
- 26.Yang Z, Han Y, Cheng K, Zhang G, Wang X. miR-99a directly targets the mTOR signalling pathway in breast cancer side population cells. Cell Prolif. 2014;47(6):587–595. doi: 10.1111/cpr.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin CJ, Gong HY, Tseng HC, Wang WL, Wu JL. miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochem Biophys Res Commun. 2008;375(3):315–320. doi: 10.1016/j.bbrc.2008.07.154. [DOI] [PubMed] [Google Scholar]
- 28.Ishikawa Y, Kozakai T, Morita H, Saida K, Oka S, Masuo Y. Rapid detection of mycoplasma contamination in cell cultures using SYBR Green-based real-time polymerase chain reaction. In Vitro Cell Dev Biol Anim. 2006;42(3–4):63–69. doi: 10.1290/0505035.1. [DOI] [PubMed] [Google Scholar]
- 29.Lin JF, Lin YC, Lin YH, et al. Zoledronic acid induces autophagic cell death in human prostate cancer cells. J Urol. 2011;185(4):1490–1496. doi: 10.1016/j.juro.2010.11.045. [DOI] [PubMed] [Google Scholar]
- 30.Lin JF, Tsai TF, Liao PC, et al. Benzyl isothiocyanate induces protective autophagy in human prostate cancer cells via inhibition of mTOR signaling. Carcinogenesis. 2013;34(2):406–414. doi: 10.1093/carcin/bgs359. [DOI] [PubMed] [Google Scholar]
- 31.Lin JF, Lin YC, Yang SC, et al. Autophagy inhibition enhances RAD001-induced cytotoxicity in human bladder cancer cells. Drug Des Devel Ther. 2016;10:1501–1513. doi: 10.2147/DDDT.S95900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y, Kang Y, He Y, et al. microRNA-99a acts as a tumor suppressor and is down-regulated in bladder cancer. BMC Urol. 2014;14(1):50. doi: 10.1186/1471-2490-14-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oneyama C, Ikeda J, Okuzaki D, et al. MicroRNA-mediated downregulation of mTOR/FGFR3 controls tumor growth induced by Src-related oncogenic pathways. Oncogene. 2011;30(32):3489–3501. doi: 10.1038/onc.2011.63. [DOI] [PubMed] [Google Scholar]
- 34.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, et al. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23(6):675–680. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aveyard JS, Skilleter A, Habuchi T, Knowles MA. Somatic mutation of PTEN in bladder carcinoma. Br J Cancer. 1999;80(5–6):904–908. doi: 10.1038/sj.bjc.6690439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calderaro J, Rebouissou S, de Koning L, et al. PI3K/AKT pathway activation in bladder carcinogenesis. Int J Cancer. 2014;134(8):1776–1784. doi: 10.1002/ijc.28518. [DOI] [PubMed] [Google Scholar]
- 37.Ingalla E, Yen L, Funes M, de Vere White R, Sweeney C. Could mTOR be a therapeutic target for bladder cancer? Cancer Res. 2008;68(9 suppl):1510. [Google Scholar]
- 38.Seager CM, Puzio-Kuter AM, Patel T, et al. Intravesical delivery of rapamycin suppresses tumorigenesis in a mouse model of progressive bladder cancer. Cancer Prev Res (Phila) 2009;2(12):1008–1014. doi: 10.1158/1940-6207.CAPR-09-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mansure JJ, Nassim R, Chevalier S, Rocha J, Scarlata E, Kassouf W. Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol Ther. 2009;8(24):2339–2347. doi: 10.4161/cbt.8.24.9987. [DOI] [PubMed] [Google Scholar]
- 40.Gerullis H, Eimer C, Ecke TH, et al. A phase II trial of temsirolimus in second-line metastatic urothelial cancer. Med Oncol. 2012;29(4):2870–2876. doi: 10.1007/s12032-012-0216-x. [DOI] [PubMed] [Google Scholar]
- 41.Chresta CM, Davies BR, Hickson I, et al. AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity. Cancer Res. 2010;70(1):288–298. doi: 10.1158/0008-5472.CAN-09-1751. [DOI] [PubMed] [Google Scholar]
- 42.Thoreen CC, Kang SA, Chang JW, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284(12):8023–8032. doi: 10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng Z, Shi YX, Tsao T, et al. Targeting of mTORC1/2 by the mTOR kinase inhibitor PP242 induces apoptosis in AML cells under conditions mimicking the bone marrow microenvironment. Blood. 2012;120(13):2679–2689. doi: 10.1182/blood-2011-11-393934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han Y, Chen J, Zhao X, et al. MicroRNA expression signatures of bladder cancer revealed by deep sequencing. PLoS One. 2011;6(3):e18286. doi: 10.1371/journal.pone.0018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schedel F, Pries R, Thode B, et al. mTOR inhibitors show promising in vitro activity in bladder cancer and head and neck squamous cell carcinoma. Oncol Rep. 2011;25(3):763–768. doi: 10.3892/or.2011.1146. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Sequence of oligonucleotides used in this study
| Primer name | Sequence 5′→3′ | Note |
|---|---|---|
| Paired oligonucleotides for the cloning of miRNA expression vectors | ||
| miR-99a_Top | TGCTGAACCCGTAGATCCGATCTTGTGGTTTTGGCCACTGACTGACCACAAGATGATCTACGGGTT | Matured hsa-miR-99a-5p sequence is underlined |
| miR-99a_Bot | CCTGAACCCGTAGATCATCTTGTGGTCAGTCAGTGGCCAAAACCACAAGATCGGATCTACGGGTTC | |
| miR-28-5p_Top | TGCTGAAGGAGCTCACAGTCTATTGAGGTTTTGGCCACTGACTGACCTCAATAGTGTGAGCTCCTT | Matured miR-28-5psequence is underlined |
| miR-28-5p_Bot | CCTGAAGGAGCTCACACTATTGAGGTCAGTCAGTGGCCAAAACCTCAATAGACTGTGAGCTCCTTC | |
| Paired oligonucleotides for the cloning of reporter constructs | ||
| mTOR-TS_Top | CTAGCTGGGGAACAGAAGATCCATAACTTTAGAAATACGGGTTTTGACTTAACG | Seed sequences of miR-99a in mTOR 3′-UTR are boxed |
| mTOR-TS_Bot | TCGACGTTAAGTCAAAACCCGTATTTCTAAAGTTATGGATCTTCTGTTCCCCAG | |
| mTOR-MTS_Top | TCGAGTGGGGAACAGAAGATCCATAACTTTAGAAAATGCCCATTTGACTTAACGC | Mutated seed sequences of mTOR are boxed |
| mTOR-MTS_Bot | GGCCGCGTTAAGTCAAATGGGCATTTTCTAAAGTTATGGATCTTCTGTTCCCCAC | |
| 99a-PTS_Top | CTAGCCACAAGATCGGATCTACGGGTTGATATCCACAAGATCGGATCTACGGGTTATAACACAAGATCGGATCTACGGGTTC | Three miR-99a-5p positive targeting sites are underlined |
| 99a-PTS_Bot | TCGAGAACCCGTAGATCCGATCTTGTGTTATAACCCGTAGATCCGATCTTGTGGATATCAACCCGTAGATCCGATCTTGTGG | |
| Primers used in QPCR detection of mTOR expression | ||
| mTOR_5QP | AGACACCCATCCAACCTGAT | NM_004958; amplicon: 85 bps |
| mTOR_3QP | TCATAGCAACCTCAAAGCAGTC | |
| EGFP_5QP | CGACGGCAACTACAAGAC | |
| EGFP_3QP | TAGTTGTACTCCAGCTTGTGC | |
Abbreviations: Bot, bottom; miRNA, microRNA; mTOR, mammalian target of rapamycin; 3′-UTR, 3′ untranslated region; QPCR, quantitative polymerase chain reaction; EGFP, enhanced green fluorescent protein.