Abstract
Skin has gained substantial attention as a vaccine target organ due to its immunological properties, which include a high density of professional antigen presenting cells (APCs). Previous studies have demonstrated the effectiveness of this vaccination route not only in animal models but also in adults. Young children represent a population group that is at high risk from influenza infection. As a result, this group could benefit significantly from influenza vaccine delivery approaches through the skin and the improved immune response it can induce. In this study, we compared the immune responses in young BALB/c mice upon skin delivery of influenza vaccine with vaccination by the conventional intramuscular route. Young mice that received 5μg of H1N1 A/Ca/07/09 influenza subunit vaccine using MN demonstrated an improved serum antibody response (IgG1 and IgG2a) when compared to the young IM group, accompanied by higher numbers of influenza-specific antibody secreting cells (ASCs) in the bone marrow. In addition, we observed increased activation of follicular helper T cells and formation of germinal centers in the regional lymph nodes in the MN immunized group, rapid clearance of the virus from their lungs as well as complete survival, compared with partial protection observed in the IM-vaccinated group. Our results support the hypothesis that influenza vaccine delivery through the skin would be beneficial for protecting the high-risk young population from influenza infection.
Keywords: Influenza, Vaccine, Skin immunization
1. Introduction
Influenza virus is one of the most common respiratory pathogens and one of the most frequent causes of upper respiratory tract infection [1]. When infection occurs in adults, the course of the illness is often mild and self-limited, without the need for medical care or antiviral drugs in most cases, and the expected recovery time is less than two weeks [2,3]. There are however groups at high risk from influenza infection, in which the disease severity is much greater than that observed in adults. These include the elderly over 65 [4,5], persons with underlying chronic diseases [6,7], infants and children 5 years of age and younger who have not been previously exposed to the virus [8,9], and pregnant women [10,11]. Influenza infection among these groups is often followed by secondary complications such as sinus infection, bronchitis, pneumonia as well as failure of other organs such as the heart and the kidneys, occasionally resulting in death [12,13]. Infants and children 5 years of age and younger represent one of the most susceptible groups and have increased mortality rates [2,14].
Annual vaccination is the most effective method of protection against influenza infection [15]. Despite recommendations for vaccination targeted to high-risk groups and their contacts, the CDC estimates that 36,000 deaths and 1.7 million hospitalizations can be attributed to seasonal influenza infection annually in the U.S. [16,17], with highest disease severity and mortality among high-risk groups [18,19]. While influenza vaccines can be highly effective in adults, there are reduced benefits in young children leaving them vulnerable to infection [20,21]. Live attenuated influenza vaccine (LAIV) is not approved for children under 2 due to safety concerns [22,23]. Several studies have demonstrated the limited ability of inactivated influenza vaccine to induce strong humoral and cellular immune responses in young children, potentially because of the immaturity of their immune system as well as lack of prior exposure to influenza viral antigens [24–26]. As a result of reduced protection, the CDC issued new guidelines recommending 2 or more doses of seasonal influenza vaccine for this age group. This approach, however, can be associated with compliance and timeline issues which limit the desired results. These factors support the need for new vaccine formulations and alternative vaccination routes that will increase compliance, and improve protective immunity, in this high-risk population [27,28].
Several studies have demonstrated that delivery of influenza vaccine through the skin of adult mice, using different types of microneedle patches (MN), leads to enhanced immune responses and improved duration of protective immunity when compared to conventional intramuscular delivery (IM) [29–31]. Because of these promising results, and other studies [32,33] that demonstrated improved immune responses in humans after intradermal delivery of influenza antigen using reduced doses, the skin has gained attention as an attractive target for vaccine delivery.
Here we investigate delivery of a single dose of subunit influenza vaccine through the skin with microneedle patches in 2 week old mice and compare the results to those observed in young mice immunized through the conventional intramuscular route. The results support the advantages of skin vaccination using influenza vaccines not only for adults but also for infants and young children, highlighting new vaccination strategies that will improve protective immunity.
2. Materials and methods
2.1. Microneedle fabrication and coating
As previously described [29] metal microneedles were fabricated by laser etching stainless steel sheets (McMaster-Carr, Atlanta, GA). Each microneedle measured 700μm tall, with a cross sectional area of 170μm by 55μm at the base and tapering to a sharp tip, with five microneedles per row. The size of microneedles was selected based on the depth of skin antigen presenting cell populations that are targeted for vaccine delivery and optimal immune response [34]. Microneedles were dip-coated as described [31].
2.2. Cells and viruses
MDCK cells were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) (Mediatech, Herndon, VA) containing 10% fetal bovine serum (Hyclone, Rockford, IL). Influenza virus stocks (A/California/07/2009) were prepared, purified and inactivated as previously described [30]. Hemagglutination (HA) activity was determined using turkey red blood cells (LAMPIRE, Pipersville, PA). Mouse-adapted virus was prepared by serial passage 8 times in lungs of BALB/c mice. The LD50 was calculated by the method of Reed&Muench, and viral titer was determined by plaque assay [29,30].
2.3. Immunizations and characterization of immune responses
Female BALB/c mice (Charles River, Wilmington, MA) (20 mice per group, 2 weeks old) received one dose (5μg) of a licensed subunit A/California/07/2009 vaccine (obtained from Novartis Pharmaceuticals) through the skin using antigen-coated MN arrays or IM injection as described [31]. The sites of immunization for both MN and IM delivery were the upper gluteal quadrants of both legs and near the dorsal side. Animals were bled retro-orbitally 2 and 4 weeks post-immunization under systemic anesthesia. Anti-Ca/07/09 specific IgG, IgG1, and IgG2a antibody levels were determined quantitatively by enzyme-linked immunosorbent assay (ELISA) as described [35]. The 96-well plates were coated with 2μg of subunit A/Ca/07/09 vaccine. Purified mouse IgG, IgG1, and IgG2a and goat anti-mouse-HRP for ELISA were purchased from Southern Biotechnology Associates (Birmingham, AL). Optical density was read at 490 nm. We determined the hemagglutination inhibition (HAI) titers based on the WHO protocol as described previously [35]. Heat-inactivated sera were treated with receptor-destroying neuraminidase (RDE) (Roche Diagnostics, Indianapolis, IN) overnight at 37°C, heat inactivated for 30 min at 56°C and incubated with packed turkey RBC for 1 h at 4°C to remove any cryoglobulins interfering with the agglutination reaction. They were serially diluted and pre-incubated at room temperature with 4 HA Units/50μl of A/Ca/07/09 virus for 30 min. An equal volume of 0.5% turkey red blood cells was then added to each well for 30 min incubation at room temperature. The HAI titer was read as the reciprocal of the highest dilution of serum that conferred inhibition of hemagglutination. The values were expressed as the geometric mean ± standard deviation.
Virus microneutralization assay was performed as described previously [CDC Influenza manual Rev 06FEB98] with modifications [31]. Briefly, 50 ml of virus diluent containing 100 xTCID50 of each virus was incubated with 50 ml of serially diluted heat-inactivated sera at 37°C for 1 h, and then mixed with 100 ml of freshly trypsinized low-passage MDCK cell suspension (ATCC # CRL-2936) containing 50,000 cells per well in 96-well tissue plates. The controls wells contained cells alone (negative control) and cells infected with virus (positive control). Following an 18–22 h incubation period the cells were fixed with acetone and the infected wells were detected by ELISA. Mouse monoclonal biotinylated anti-NP antibody MAB8257B (Millipore, Temecula, CA), and HRP-streptavidin conjugate (BD # 51-9000209, BD Biosciences, San Jose, CA), were used in the ELISA. Color was developed using OPD substrate (#002003 Invitrogen, Frederick, MD) and optical density was measured at 490 nm in plate reader (model 680 BioRad, Hercules, CA). The highest serum dilution that generated 50% specific signal was considered to be the neutralization titer; 50% specific signal = (OD490 virus control - OD490 cell control)/2 + OD490 cell control.
One month post-immunization, mice (n = 5) were challenged with 5xLD50 of mouse-adapted H1N1 A/California/07/2009 virus and were monitored for 14 days for signs of morbidity (body weight changes, fever and hunched posture) and mortality. Weight loss exceeding 25% was used as the experimental end point, at which mice were euthanized according to IACUC guidelines. The mouse-adapted virus was produced by 8 serial passages in mouse lungs. One month post immunization and four days post-challenge of an independent cohort, lungs were collected to determine levels of viral replication, and bone marrow was collected to determine influenza-specific IgG, IgG1 and IgG2a antibody-secreting cells (ASC) by ELISPOT [29]. Lung viral titers were measured per gram of tissue from lunch homogenates prepared in DMEM serum-free medium as previously described [35]. For ELISPOT assay, 96-well plates were coated overnight at 4°C with subunit A/Ca/07/09 vaccine at a final concentration of 2μg/ml. The plates were washed three times with RPMI medium and blocked for 2 h with 10% fetal calf serum prior to sample addition. Single cell suspensions from bone marrow collected at 4 days after challenge (0.5–1 × 106/well) in cRPMI medium were plated directly on coated blocked plates and were incubated at 37°C for 16 h. Virus-specific ASC were detected as spots after incubation with goat anti-mouse IgG horseradish peroxidase (Southern Biotechnology) and developed with stable diaminobenzidine (Research Genetics). Samples were enumerated in an ELISPOT reader (Cellular Technology, Shaker Heights, OH) and the results shown as the number of ASC per 106 cells [35].
Regional (inguinal) lymph nodes and spleens were collected 7 days and 14 days post-vaccination for the evaluation of activated follicular T helper cells and germinal center formation by FACS. Single cell suspensions were incubated with Fc-block (2.4G2) on ice for 15 min before staining with PNA-FITC, Fas-PE, PD-1-PE-Texas Red, CXCR5-APC, CD44-APC-e780, CD19-e450, CD4-BV510, CD3-BV650, and CD8-BV785 antibodies for 30 min on ice. Antibodies were purchased from BD Biosciences, eBiosciences, and BioLegend. Cells were washed and fixed in 1% paraformaldehyde for 30 min at room temperature prior to analysis. Samples were acquired on a LSR II flow cytometer (BD Biosciences) and data were analyzed with FlowJo software (Tree Star). Serum samples and lung homogenates were individually processed to determine humoral immune responses and neutralization titers respectively. All animal studies had approval of Emory’s Institutional Animal Care and Use Committee.
2.4. Statistics
The statistical significance of differences was calculated by two-tailed unpaired Student’s t-test and one-way ANOVA (one-way analysis of variance including Bonferroni’s multiple comparison test) or two-way ANOVA. Values of p ≤ 0.05 were considered significant. Unless otherwise stated, independent experiments were run at least in triplicate.
3. Results
3.1. Humoral immune responses after MN or IM delivery of influenza subunit vaccine
In order to assess the effectiveness of influenza vaccination in young populations, we first measured the levels of functional antibody titers against the hemagglutinin antigen (HA) of influenza virus induced after the skin delivery with MNs or intramuscularly immunization. Although no significant responses were measured as soon as day 14, young mice that received influenza subunit vaccine through the skin using MN patches demonstrated significantly higher HAI titers at day 28 post vaccination when compared to IM-immunized young mice (Fig. 1A). The HAI titers in young mice that were immunized through the skin were similar to the HAI titers measured in control immunized adult mice vaccinated with either IM or MN routes. In addition, these results were further supported by significantly higher microneutralization titers measured in serum samples from MN immunized young mice 28 days post-vaccination (supplementary Fig. 1). These results indicate that delivery of subunit influenza vaccine through the skin using MN patches in young mice induces higher levels of functional antibodies when compared to IM immunization.
Fig. 1. Comparison of humoral immune responses induced by skin vs. intramuscular immunization.
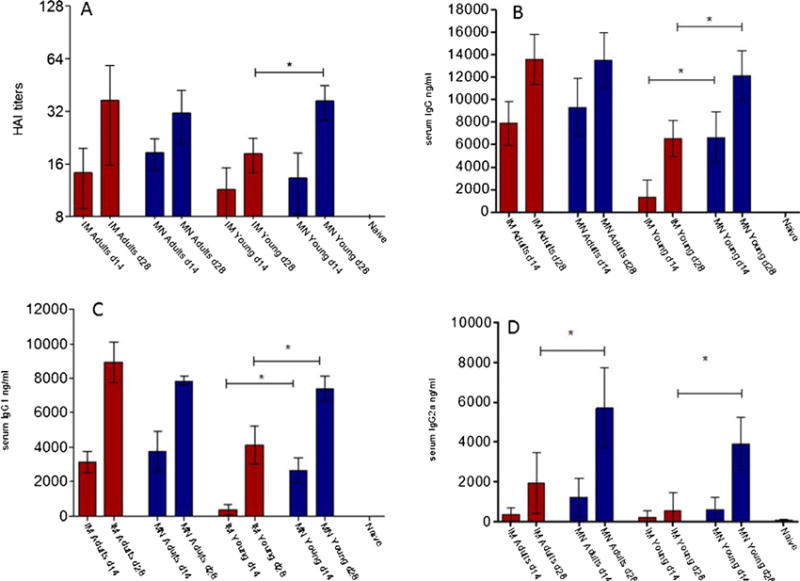
Serum samples from 2 week old mice were collected at 2 and 4 weeks post immunization and analyzed for the levels of functional antibody titers against A/California/07/09 by HAI (A), total serum IgG titers (B), and the IgG isotypes, IgG1 (C) and IgG2a (D) by quantitative ELISA. MN adult: adult microneedle immunized group, IM adult: adult intramuscularly immunized group MN young: young microneedle immunized group, IM young: young intramuscularly immunized group. ELISA antibody results represent the mean ± SD. The HAI titers were read as the reciprocal of the highest dilution of serum that conferred inhibition of hemagglutination. *p < 0.05, **p < 0.005, ***p < 0.0001.
We furthermore determined the levels of influenza-specific circulating IgG antibodies in sera of young mice vaccinated by either MN or IM routes. At two and four weeks post-vaccination, young mice that were immunized via the skin exhibited significantly higher total anti-influenza specific antibody titers when compared to mice immunized IM (Fig. 1B). We also measured significantly higher levels of both IgG1 and IgG2a isotypes in young mice vaccinated by MN which were 2 or 3-fold higher respectively when compared to young mice that were immunized via the IM route (Fig. 1C and D). A significant finding is the low levels of IgG2a antibodies measured in IM vaccinated mice, which could indicate the lack of development of this arm of the immune system in young mice after IM immunization. Several studies have demonstrated the distinct contribution and important roles these isotypes play during protection. We have previously demonstrated that the profile of the immune responses is affected by the route of immunization [29,30]. In this study, young mice that received the subunit vaccine through the MN route exhibited similar levels of IgG2a antibodies as found in adult mice immunized by MN. Although no significant difference in immune response was observed in IM vs. MN vaccinated adult mice 28 days post-vaccination, we have previously demonstrated the effectiveness of MN delivery of influenza vaccine to induce improve immune responses that are long lasting [29]. Overall, the immune response in young mice after MN delivery was similar to that seen in adults after IM or MN vaccination. These results indicate that MN delivery of influenza subunit vaccine in young mice induces higher anti-influenza specific antibodies when compared to the titers observed in young mice immunized by the IM route, and a more closely balanced isotype antibody response.
3.2. Protection against lethal influenza infection
In order to compare the protective capacity of the immune response induced in young mice after delivery of influenza subunit vaccine by MN or IM delivery, we challenged mice from both groups with 5xLD50 of mouse adapted H1N1 A/Ca/07/09 virus by intranasal inoculation with 50μl of the virus. After challenge, all mice were followed for 14 days for signs of morbidity (body weight changes, fever and hunched posture) and mortality. As seen in Fig. 2A, young mice immunized by the IM route exhibited a higher body weight loss when compared to the body weight changes in young mice immunized via the skin. Additionally, young mice immunized IM were only partially protected after lethal challenge, with a mortality rate of 40%. In contrast, young mice that were immunized via the skin with MN patches demonstrated 100% survival (Fig. 2B). The improved protection observed in the young MN immunized mice is reflected by reduced virus titers measured in the lungs of these mice one month post vaccination and 4 days post challenge. In young mice that were immunized by the IM route, we observed high virus titers with a 1 log decrease when compared to unimmunized infected animals (Fig. 2C). In contrast, young MN-immunized mice exhibited a 4 log decrease in lung virus titers when compared to the unimmunized group. Although delivery of influenza vaccine using MN patches conferred completed survival, mice from both MN and IM immunized groups exhibited signs of disease with more intense symptoms in the IM group. This is not surprising since both MN and IM immunization represent systemic routes of vaccine delivery not capable of inducing sterilizing immunity, which can be induced by intranasal vaccination [36]. These results demonstrate the induction of more effective protective immune responses after MN delivery of subunit influenza vaccine in young mice, and a rapid clearance of the virus from their lungs, leading to complete survival.
Fig. 2. Protective efficacy of immunization against H1N1 A/California/07/2009 lethal infection and analysis of lung viral titers.
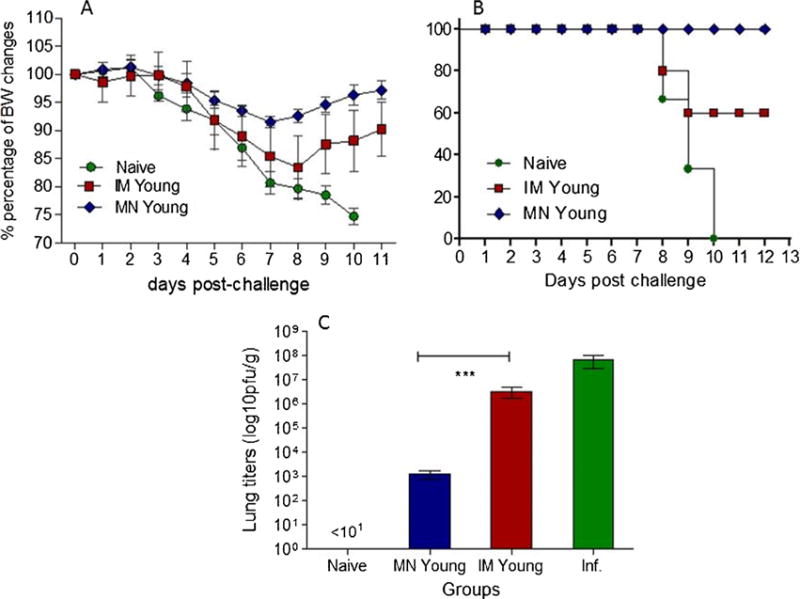
Body weight changes (A) and survival rates (B) were recorded after lethal challenge with 5xLD50 of A/California/07/2009 virus in young MN and IM immunized mice 2 weeks post immunization and naïve mice. Lung viral titers (C) were determined by plaque assay. INF: infected mice. Data represent the mean ± SD, *p < 0.05, **p < 0.005, ***p < 0.0001.
3.3. Evaluation of long-lived bone marrow plasma cells
To further investigate the improved immune responses when compared to IM immunization, we measured the levels of long-lived influenza virus-specific IgG plasma cells in the bone marrow and IgG1 and IgG2a isotypes at 4 weeks after a single vaccination. Young mice that received the vaccine through the skin via MNs exhibited a two-fold higher number of IgG ASCs in the bone marrow when compared to the numbers measured in the IM group (Fig. 3A). Furthermore, we observed significantly higher numbers of IgG1 (p < 0.005) ASCs in the MN group when compared to IM, and threefold higher levels of IgG2a ASCs in the young mice immunized via MN in comparison to IM immunized mice (Fig. 3B and C). The levels of ASCs in the young mice immunized with MN patches were similar to levels measured in MN vaccinated adult mice controls. The increased numbers of influenza virus-specific long-lived plasma cells in the bone marrows of young mice vaccinated by MNs correlate with the higher serum antibody levels observed in these mice, and also correspond with the isotype difference observed between MN and IM vaccinated young mice. These differences reflect the improved humoral immune responses in young MN mice and the differences in protection observed among these groups after lethal influenza challenge.
Fig. 3. Comparison of bone marrow plasma cell levels after IM or MN immunization.
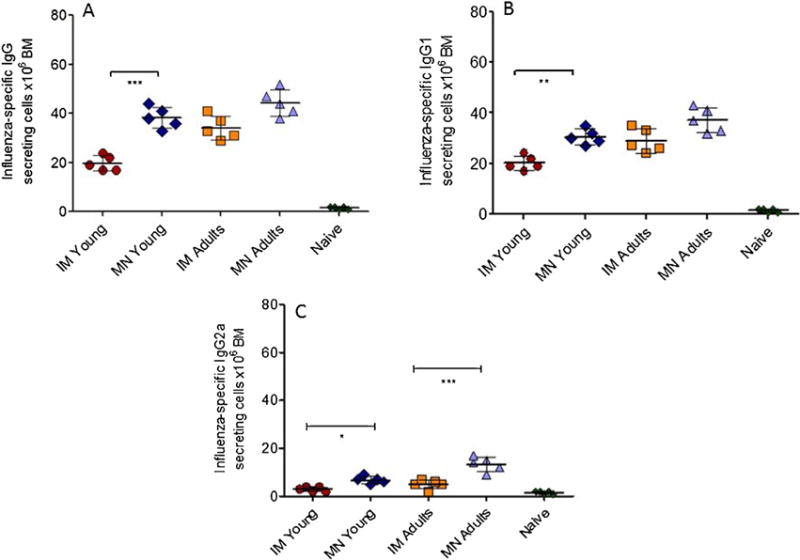
The numbers of influenza-specific IgG (A) and IgG1 (B) and IgG2a (C) plasma cells in the bone marrow were determined by ELISPOT 4 weeks after a single immunization. The cells were cultured in the presence of 40μg/ml subunit A/California/07/2009 vaccine and 16 h later the numbers of plasma cells in the bone marrow from young MN and IM vaccinated mice, adult MN and IM vaccinated mice and unvaccinated mice were determined. Plasma cell numbers of vaccinated mice were considered positive if the numbers of spots were higher than the sum of naïve infected group spots + 3xSDev. N: naïve mice. Data represent the mean ± SD, *p < 0.05, **p < 0.005, ***p < 0.0001.
3.4. Cellular immune responses in the lungs and spleens of vaccinated young mice
The levels of CD4+ and CD8+ IFN-γ T-cells in the lungs and spleens of young mice vaccinated by MN or IM were also evaluated. No significant increase was measured in either of the two T-cell populations in the lungs of both MN and IM young vaccinated groups (supplementary Fig. 2A and B). Similarly, characterization of CD4+ and CD8+ T-cells in the spleens of young mice did not reveal significant cellular immune responses (supplementary Fig. 2C and D). Both MN and IM young mice vaccinated with influenza subunit vaccine demonstrated reduced numbers of CD4+ and CD8+ T-cells when compared to the levels measured in adult MN vaccinated mice (data not shown). These results indicate that in young mice, delivery of subunit influenza vaccine induces a limited activation of the cellular response and that the protection observed after challenge is mediated by humoral responses.
3.5. Evaluation of activated follicular B helper T (Tfh) cells and germinal center formation
Several studies have demonstrated the fundamental role of TFH cells for the generation of adaptive immunity, immunological memory and the formation of germinal centers (GCs). We measured a two-fold increase in the numbers of double-positive CD4+ and CD44high T cells in the inguinal lymph nodes of young mice immunized via the MN route when compared to the numbers observed in the IM immunized group (Fig. 4B). GCs are formations within secondary lymphoid organs in which the B cell response against an antigen occurs. We detected elevated numbers of double positive FAS and PNA CD19 positive lymphocytes (activated GCs) in regional lymph nodes of young mice immunized via MN patches and a 3-fold increase when compared to the numbers measured in young mice immunized via the IM route (Fig. 4A). These results indicate that delivery of influenza subunit vaccine via the skin with MN patches enhances TFH cell levels and increases formation of GCs, leading to an improved antigen-specific B cell response.
Fig. 4. Analysis of activated follicular B helper T cells and germinal center formation.
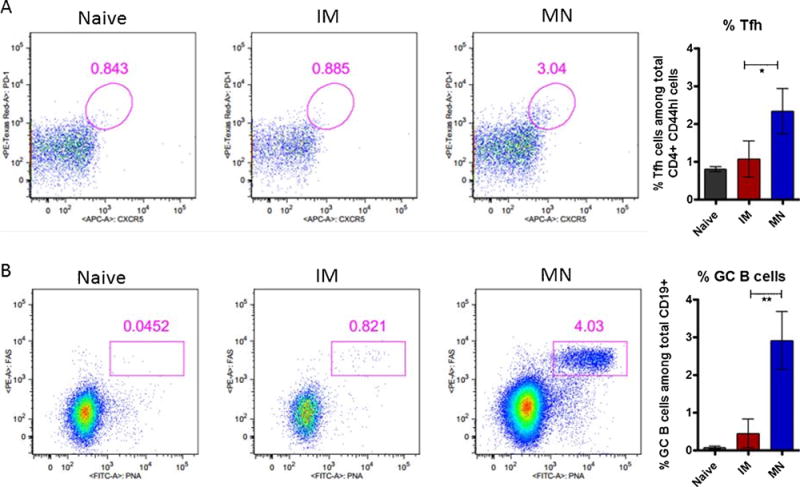
The numbers of GCs (A) and Tfh cells (B) from the inguinal lymph nodes were determined by FACS analysis 7 days post immunization in 2-week-old mice vaccinated with microneedles or intramuscularly. Data represent the mean ± SD, *p < 0.05, **p < 0.005, ***p < 0.0001.
4. Discussion
Skin is the largest immunological organ of the human body [37]. It is composed of two primary layers, the epidermis and dermis [38]. In both layers, populations of specialized APCs with unique phenotypic and functional roles are present: Langerhans cells (LCs) are abundant in the epidermis and dermal dendritic cells (dDCs) are enriched in the dermis [39–42]. These two populations can be distinguished based on their surface markers: LCs express CD207 and CD205 and differential levels of CD11b, while dDCs express CD11b and CD205, but not CD207 [43,44]. Additionally, these two cell populations are characterized by differences in chemokine receptor expression and cytokine release [45].
This is the first study, to our knowledge, that demonstrates improved immune responses and improved protection in young mice after delivery of an influenza vaccine through the skin using MN patches. It has been estimated that each year, influenza infection in the U.S. is responsible for more than 20,000 childhood hospitalizations and more than 100 pediatric deaths. Despite the increased risk from infection, vaccination rates remain low with an average rate of 40% annually. These data strongly support the need for new, more effective influenza vaccines or vaccination routes that can induce improved immune responses and protection, and increased vaccine compliance and vaccination coverage in this high risk group. Multiple factors could be responsible for reduced T cell activation, reduced activation of GCs and an overall reduction in adaptive immune responses [29]. The skin represents an ideal target organ for vaccine delivery because it contains a large network of professional APCs that will take up antigen and migrate to the proximal lymph nodes to activate T and B cells, initiating the adaptive immune responses. Previous studies from our group and other have demonstrated the immunological advantages after skin delivery of influenza vaccine in adults [29,30]. In the present study, we demonstrate improved humoral immune responses after skin delivery of a single dose of influenza subunit vaccine using microneedle patches in young mice, when compared to intramuscular immunization. These improved humoral responses observed in young mice correlated with higher numbers of antibody-secreting cells in their bone marrow. Germinal centers are formations within secondary lymphoid organs where the process of B cell differentiation, proliferation, maturation, clone selection and class switch is occurring [46]. The processes taking place inside germinal centers will define the quality of the B cell response to a specific antigen [47]. In this process, B cells constantly migrate between the light and dark zones within germinal centers during which the selection of long-lived B cells with high affinity for the antigen will occur [48]. The higher number of activated GCs measured in the inguinal lymph nodes of young mice 7 days post skin vaccination with influenza subunit vaccine correlates with the increased numbers of ASCs and improved humoral immune responses observed in this group, when compared to the intramuscularly immunized young mice, and may be responsible for the more rapid virus clearance and complete survival seen after lethal challenge. TFH cells are essential for the regulation of B cell differentiation into plasma cells and memory B cells thus playing a fundamental role in the generation of immunological memory [49,50]. TFH cells play a critical role in mediating the selection of B cells and regulating their survival. These selected B cells will further differentiate into high affinity antibody secreting cells (ASCs) or memory B cells that will be rapidly re-activated upon encounter with a similar antigen [51]. We observed increased numbers of TFH cells in the proximal lymph nodes of young mice immunized via microneedles, which are probably the result of better antigen processing and presentation by antigen-presenting cells residing in the dermis and epidermis.
The significant differences observed in responses to the two routes of immunization used in young mice validate the use of this model, and support previous results showing the benefits of skin delivery of influenza vaccine using microneedle patches [29,31]. In addition to immunological advantages, microneedle skin vaccination offers logistical advantages, such as ease of administration, rapid transport and delivery, lack of a requirement for highly trained personnel to administer the vaccine as needed for intramuscular injection, and little or no bio-hazardous waste [30]. In contrast, the currently used intramuscular vaccination approach delivers the vaccine to the muscle, an area with reduced numbers of APCs to take up and process the antigen, leading to reduced immune responses [29,52]. Furthermore, recent studies among volunteers have demonstrated a preference for this route of vaccine delivery over the conventional IM route [53]. Several factors might contribute to this preference, such as needle phobia, lack of pain, and the potential for self-administration. These factors could also play a crucial role in improving influenza vaccine compliance and coverage among the population [53]. Although in our study the MN patches only contained one influenza strain, it is highly likely that MN delivery of the trivalent or quadrivalent vaccine will have also enhance the immune response. Previous studies that compared the magnitude of the immune response when delivering a single influenza strain, or multiple influenza strains, failed to show any signs of antigenic competition [54]. In addition, the large number of skin APCs and the fast rate of repopulation within 24 h [45] minimize the possibility of differences in the immune response to influenza antigens when delivering either trivalent or quadrivalent influenza vaccine. Taking under consideration the logistical advantages which this technology offers as well as the possibility of administering other pediatric vaccines, we consider this approach to skin immunization to be a very promising vaccination strategy that will improve influenza-related outcomes in all age groups of the population.
Supplementary Material
Acknowledgments
We thank Ms. Erin-Joi Collins for her valuable assistance in the preparation and submission of this manuscript. This study was supported by the National Institutes of Health: HHSN266200700006, U01EB012495, and AI110680. The funders had no role in the preparation and submission of the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.vaccine.2015.01.086.
Footnotes
Authors contribution
DGK conceived, designed, and executed the study, collected and analyzed data, and prepared the article. ESE, SM, PK, JWL, IS, TLD, and JK contributed to and assisted in experiments related to this study. MRP and RWC revised and approved the final version of this manuscript.
Conflict of interest:MRP is an inventor of patents that have been licensed to companies developing microneedle-based products, is a paid advisor to companies developing microneedle-based products and is a founder/shareholder of companies developing microneedle-based products. This potential conflict of interest has been disclosed and is managed by Georgia Tech and Emory University. The other authors have no known conflicts of interest to declare.
References
- 1.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60. [PubMed] [Google Scholar]
- 2.Monto AS. Seasonal influenza and vaccination coverage. Vaccine. 2010;28(Suppl 4):D33–44. doi: 10.1016/j.vaccine.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 3.Foster DA, Talsma A, Furumoto-Dawson A, Ohmit SE, Margulies JR, Arden NH, et al. Influenza vaccine effectiveness in preventing hospitalization for pneumonia in the elderly. Am J Epidemiol. 1992;136:296–307. doi: 10.1093/oxfordjournals.aje.a116495. [DOI] [PubMed] [Google Scholar]
- 4.Baldo V, Baldovin T, Pellegrini M, Angiolelli G, Majori S, Floreani A, et al. Immunogenicity of three different influenza vaccines against homologous and heterologous strains in nursing home elderly residents. Clin Dev Immunol. 2010;2010:517198. doi: 10.1155/2010/517198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glathe H, Bigl S, Grosche A. Comparison of humoral immune responses to trivalent influenza split vaccine in young, middle-aged and elderly people. Vaccine. 1993;11:702–5. doi: 10.1016/0264-410x(93)90252-s. [DOI] [PubMed] [Google Scholar]
- 6.Anar C, Bicmen C, Yapicioglu S, Unsal I, Halilcolar H, Yilmaz U. Evaluation of clinical data and antibody response following influenza vaccination in patients with chronic obstructive pulmonary disease. New Microbiol. 2010;33:117–27. [PubMed] [Google Scholar]
- 7.Bickel M, Wieters I, Khaykin P, Nisius G, Haberl A, Stephan C, et al. Low rate of seroconversion after vaccination with a split virion, adjuvanted pandemic H1N1 influenza vaccine in HIV-1-infected patients. AIDS. 2010;24:F31–5. doi: 10.1097/QAD.0b013e3283398da1. [DOI] [PubMed] [Google Scholar]
- 8.Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA. 2010;303:37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt-Ott R, Schwarz T, Haase R, Sander H, Walther U, Fourneau M, et al. Immunogenicity and reactogenicity of a trivalent influenza split vaccine in previously unvaccinated children aged 6–9 and 10–13 years. Vaccine. 2007;26:32–40. doi: 10.1016/j.vaccine.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 10.Ding H, Santibanez TA, Jamieson DJ, Weinbaum CM, Euler GL, Grohskopf LA, et al. Influenza vaccination coverage among pregnant women – National 2009 H1N1 Flu Survey (NHFS) Am J Obstet Gynecol. 2011;204:S96–106. doi: 10.1016/j.ajog.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Omer SB, Goodman D, Steinhoff MC, Rochat R, Klugman KP, Stoll BJ, et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 2011;8:e1000441. doi: 10.1371/journal.pmed.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121:258–64. doi: 10.1016/j.amjmed.2007.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brydak LB, Roszkowska-Blaim M, Machala M, Leszczynska B, Sieniawska M. Immunological response to influenza vaccination in children with renal failure. Nephrol Dial Transplant. 2001;16:643–4. doi: 10.1093/ndt/16.3.643-a. [DOI] [PubMed] [Google Scholar]
- 14.Andreeva NP, Petrova TI, Golubtsova OI, Kozhevnikova SL, Kostinov MP, Karpocheva SV, et al. Effect of vaccination against pneumococcal infection and influenza in children with asthma. Zh Mikrobiol Epidemiol Immunobiol. 2007;7:4–7. [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Prevention and control of influenza with vaccines: interim recommendations of the Advisory Committee on Immunization Practices (ACIP), 2013. MMWR Morbidity and mortality weekly report. 2013;62:356. [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 17.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 18.Longini IM, Jr, Halloran ME. Strategy for distribution of influenza vaccine to high-risk groups and children. Am J Epidemiol. 2005;161:303–6. doi: 10.1093/aje/kwi053. [DOI] [PubMed] [Google Scholar]
- 19.Whitley RJ, Monto AS. Prevention and treatment of influenza in high-risk groups: children, pregnant women, immunocompromised hosts, and nursing home residents. J Infect Dis. 2006;194(Suppl 2):S133–8. doi: 10.1086/507548. [DOI] [PubMed] [Google Scholar]
- 20.Bhat N, Wright JG, Broder KR, Murray EL, Greenberg ME, Glover MJ, et al. Influenza-associated deaths among children in the United States, 2003–2004. N Engl J Med. 2005;353:2559–67. doi: 10.1056/NEJMoa051721. [DOI] [PubMed] [Google Scholar]
- 21.Heikkinen T, Peltola V. Influenza vaccination of children. Lancet Infect Dis. 2009;9:720–1. doi: 10.1016/S1473-3099(09)70266-X. [DOI] [PubMed] [Google Scholar]
- 22.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, et al. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med. 2007;356:685–96. doi: 10.1056/NEJMoa065368. [DOI] [PubMed] [Google Scholar]
- 23.Rowhani-Rahbar A, Klein NP, Baxter R. Assessing the safety of influenza vaccination in specific populations: children and the elderly. Expert Rev Vaccines. 2012;11:973–84. doi: 10.1586/erv.12.66. [DOI] [PubMed] [Google Scholar]
- 24.Kang EK, Lim JS, Lee JA, Kim DH. Comparison of immune response by virus infection and vaccination to 2009 pandemic influenza A/H1N1 in children. J Korean Med Sci. 2013;28:274–9. doi: 10.3346/jkms.2013.28.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pabst LJ, Chaves SS, Weinbaum C. Trends in compliance with two-dose influenza vaccine recommendations among children aged 6 months through 8 years. Vaccine. 2013 doi: 10.1016/j.vaccine.2013.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He XS, Holmes TH, Zhang C, Mahmood K, Kemble GW, Lewis DB, et al. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J Virol. 2006;80:11756–66. doi: 10.1128/JVI.01460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med. 2010;363:2036–44. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- 28.Monto AS, Ohmit SE. Seasonal influenza vaccines: evolutions and future trends. Expert Rev Vaccines. 2009;8:383–9. doi: 10.1586/erv.09.9. [DOI] [PubMed] [Google Scholar]
- 29.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, Jacob J, Prausnitz MR, Compans RW, et al. Serological memory and long-term protection to novel H1N1 influenza virus after skin vaccination. J Infect Dis. 2011;204:582–91. doi: 10.1093/infdis/jir094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan SP, Koutsonanos DG, Del Pilar Martin M, Lee JW, Zarnitsyn V, Choi SO, et al. Dissolving polymer microneedle patches for influenza vaccination. Nat Med. 2010;16:915–20. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koutsonanos DG, Vassilieva EV, Stavropoulou A, Zarnitsyn VG, Esser ES, Taherbhai MT, et al. Delivery of subunit influenza vaccine to skin with microneedles improves immunogenicity and long-lived protection. Sci Rep. 2012;2:357. doi: 10.1038/srep00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Icardi G, Orsi A, Ceravolo A, Ansaldi F. Current evidence on intradermal influenza vaccines administered by Soluvia licensed micro injection system. Hum Vaccines Immunother. 2012;8:67–75. doi: 10.4161/hv.8.1.18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansaldi F, de Florentiis D, Durando P, Icardi G. Fluzone(®) Intradermal vaccine: a promising new chance to increase the acceptability of influenza vaccination in adults. Expert Rev Vaccines. 2012;11:17–25. doi: 10.1586/erv.11.154. [DOI] [PubMed] [Google Scholar]
- 34.Koutsonanos DG, Compans RW, Skountzou I. Targeting the skin for microneedle delivery of influenza vaccine. Adv Exp Med Biol. 2013;785:121–32. doi: 10.1007/978-1-4614-6217-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koutsonanos DG, del Pilar Martin M, Zarnitsyn VG, Sullivan SP, Compans RW, Prausnitz MR, et al. Transdermal influenza immunization with vaccine-coated microneedle arrays. PloS ONE. 2009;4:e4773. doi: 10.1371/journal.pone.0004773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McElhaney JE. Influenza vaccine responses in older adults. Ageing Res Rev. 2011;10:379–88. doi: 10.1016/j.arr.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jepps OG, Dancik Y, Anissimov YG, Roberts MS. Modeling the human skin barrier – towards a better understanding of dermal absorption. Adv Drug Deliv Rev. 2013;65:152–68. doi: 10.1016/j.addr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Skountzou I, Quan FS, Jacob J, Compans RW, Kang SM. Transcutaneous immunization with inactivated influenza virus induces protective immune responses. Vaccine. 2006;24:6110–9. doi: 10.1016/j.vaccine.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 40.Steinman RM. Dendritic cells and the control of immunity: enhancing the efficiency of antigen presentation. Mt Sinai J Med. 2001;68:160–6. [PubMed] [Google Scholar]
- 41.Schroder JM, Reich K, Kabashima K, Liu FT, Romani N, Metz M, et al. Who is really in control of skin immunity under physiological circumstances – lymphocytes, dendritic cells or keratinocytes. Exp Dermatol. 2006;15:913–29. doi: 10.1111/j.1600-0625.2006.00506_1.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee HK, Iwasaki A. Innate control of adaptive immunity: dendritic cells and beyond. Semin Immunol. 2007;19:48–55. doi: 10.1016/j.smim.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Itano AA, McSorley SJ, Reinhardt RL, Ehst BD, Ingulli E, Rudensky AY, et al. Distinct dendritic cell populations sequentially present antigen to CD4T cells and stimulate different aspects of cell-mediated immunity. Immunity. 2003;19:47–57. doi: 10.1016/s1074-7613(03)00175-4. [DOI] [PubMed] [Google Scholar]
- 44.Valladeau J, Ravel O, Dezutter-Dambuyant C, Moore K, Kleijmeer M, Liu Y, et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity. 2000;12:71–81. doi: 10.1016/s1074-7613(00)80160-0. [DOI] [PubMed] [Google Scholar]
- 45.del Pilar Martin M, Weldon WC, Zarnitsyn VG, Koutsonanos DG, Akbari H, Skountzou I, et al. Local response to microneedle-based influenza immunization in the skin. MBio. 2012;3:e00012–12. doi: 10.1128/mBio.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boyden AW, Frickman AM, Legge KL, Waldschmidt TJ. Primary and long-term B-cell responses in the upper airway and lung after influenza A virus infection. Immunol Res. 2014 doi: 10.1007/s12026-014-8541-0. [DOI] [PubMed] [Google Scholar]
- 47.O E, Ko EJ, Kim MC, Lee YT, Song JM, Kwon YM, et al. Distinct B-cell populations contribute to vaccine antigen specific-antibody production in a transgenic mouse model. Immunology. 2014 doi: 10.1111/imm.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rothaeusler K, Baumgarth N. B-cell fate decisions following influenza virus infection. Eur J Immunol. 2010;40:366–77. doi: 10.1002/eji.200939798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crotty S. Follicular helper CD4T cells (TFH) Annu Rev Immunol. 2011;29:621–63. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 50.Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG. Follicular helper T cells: lineage and location. Immunity. 2009;30:324–35. doi: 10.1016/j.immuni.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takemori T, Kaji T, Takahashi Y, Shimoda M, Rajewsky K. Generation of memory B cells inside and outside germinal centers. Eur J Immunol. 2014;44:1258–64. doi: 10.1002/eji.201343716. [DOI] [PubMed] [Google Scholar]
- 52.Berzofsky JA, Ahlers JD, Belyakov IM. Strategies for designing and optimizing new generation vaccines. Nat Rev. 2001;1:209–19. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 53.Norman JJ, Arya JM, McClain MA, Frew PM, Meltzer MI, Prausnitz MR. Microneedle patches: usability and acceptability for self-vaccination against influenza. Vaccine. 2014;32:1856–62. doi: 10.1016/j.vaccine.2014.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Van der Vliet D, Pepin S, Lambert M, Fauchoux N, Donazzolo Y, Dupuy M, et al. Similar immunogenicity of the A/H1N1 2009 pandemic influenza strain when used as a monovalent or a trivalent vaccine. Hum Vaccin. 2010;6:823–8. doi: 10.4161/hv.6.10.13600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


