Abstract
Standard primary therapy for chronic graft-versus-host disease (GVHD) is incompletely effective. Based on biologic insights implicating pathogenic B cells, we conducted a phase I trial examining the combination of standard (1 mg/kg/day prednisone) glucocorticoid therapy with ofatumumab, a humanized anti-CD20 monoclonal antibody, for primary chronic GVHD therapy. Patients ages ≥ 18 with National Institutes of Health Consensus moderate-to-severe chronic GVHD newly requiring 1 mg/kg/day prednisone were treated at 3 escalating dose levels (300 mg, 700 mg, and 1000 mg) of i.v. ofatumumab on days 1 and 14 of initial glucocorticoid therapy. Dose-limiting toxicity (DLT) was defined by grade 4 infusion reactions, related grade 4 constitutional symptoms, related grade ≥ 3 organ toxicities, or grade 4 neutropenia lasting > 14 days. A total of 12 patients (median age 54; range, 25 to 72) were treated (dose level 1: n = 3; level 2: n = 3; level 3: n = 6). At enrollment, overall chronic GVHD was moderate (n = 7) or severe (n = 5), with diverse organ involvement (skin: n = 8; mouth: n = 8; eye: n = 8; lung: n = 4; gastrointestinal: n = 3; liver: n = 5; genital: n = 2; joint/fascia: n = 5). Infusion of ofatumumab was well tolerated, and no DLT was observed. From the total number of adverse events (n = 29), possibly related adverse events (n = 4) included grade 1 fatigue, grade 1 transaminitis, and 2 infusion reactions (grades 2 and 3). Infectious complications were expected, and there were no cases of hepatitis B reactivation or progressive multifocal leukoencephalopathy. Ofatumumab in combination with prednisone is safe and a phase II examination of efficacy is ongoing.
Keywords: Chronic graft-versus-host, disease, Primary therapy, Prednisone, Ofatumumab
INTRODUCTION
Chronic graft-versus-host disease (GVHD), a major late complication of allogeneic hematopoietic cell transplantation (HCT), is associated with mortality, disability, infectious complications, prolonged immune suppression, and impaired quality of life [1–8]. Accepted standard primary therapy for chronic GVHD includes ≥ 1 mg/kg/day of prednisone with or without a calcineurin inhibitor [9,10] as combination therapy with other broadly immune suppressive agents has not demonstrated consistent benefit over prednisone alone in controlled trials [11–13]. Only 30% of patients will experience complete resolution of chronic GVHD by 6 to 9 months of primary steroid therapy and another 30% will achieve only a partial response [11–14]. Failure-free survival, defined by absence of second-line immune suppressive treatment, nonrelapse mortality (NRM), and recurrent malignancy after initial systemic treatment for chronic GVHD is only 68% at 6 months and 54% at 12 months [15].
Multiple observations support a role for B lymphocytes in human chronic GVHD pathogenesis: B cell–activating factor (BAFF) and the BAFF/B cell ratio have been associated with risk for subsequent chronic GVHD development [16]. In the setting of established chronic GVHD, investigators have detected anti–H-Y in the setting of sex mismatched HCT [17,18] and activating anti-platelet-derived growth factor receptor (PDGFR) antibodies in the context of cutaneous sclerosis [19]. Active chronic GVHD has been shown to correlate with BAFF levels [20,21], BAFF/B cell ratio [22], as well as various B cell subpopulations [22–27]. Interestingly, several associations have also been detected with chronic GVHD severity [26], phenotype [19,21,26], and response to therapy [21,25,28]. Alongside these observations, anti-CD20 therapy in chronic GVHD has proven successful: rituximab has demonstrated activity as a single-agent in steroid refractory chronic GVHD [29]. As well, 2 independent trials testing varied schedules of rituximab for chronic GVHD prophylaxis after HCT have suggested improvements over expected chronic GVHD rates [30,31].
Ofatumumab is a fully humanized IgG1kappa monoclonal antibody (mAb) targeting a unique epitope on the CD20 molecule expressed on human B cells. As a type I CD20 mAb, like rituximab, potent complement-dependent cytotoxicity is dependent on ability to translocate CD20 into lipid rafts. In contrast to rituximab, it has increased binding affinity to CD20, prolonged dissociation rate, and increased cell kill (including B cells with low-density CD20 expression) due to greater complement-dependent cytotoxicity [32–34]. Compared with rituximab and obinutuzumab, ofatumumab demonstrates greater complement activation, independent of CD20 density, and elicits the greatest monocyte-derived macrophage mediated Ab-dependent cellular phagocytosis [35]. Ofatumumab was approved for advanced chronic lymphocytic leukemia therapy in 2009 and has also demonstrated activity in multiple lymphoid malignancies and autoimmune conditions. Given experimental evidence of increased potency, observations supporting activity in the setting of prior rituximab exposure or rituximab resistance [36–46] and evidence of chronic GVHD development after rituximab prophylaxis [30,31], ofatumumab holds promise as a novel chronic GVHD intervention.
The primary objective of the phase I component of this phase I/II trial was to examine the safety of ofatumumab in combination with 1 mg/kg/day prednisone as primary therapy of chronic GVHD.
METHODS
Overview of Trial Design
The overall trial is a phase I/II trial to examine the safety and efficacy of prednisone and escalating dose of ofatumumab for primary therapy of chronic GVHD (NCT01680965). Here we report the phase I trial results. Phase II accrual is ongoing. Prednisone is uniformly initiated at 1 mg/kg/day and the phase I component examined escalating dose of ofatumumab at dose level cohorts of 300 mg, 700 mg, and 1000 mg given on days 0 and 14 of study. In the phase II component, patients are followed for 24 months (baseline, study therapy day 0 and day 14, and months 1, 3, 6, 12, 18, and 24 after therapy). The primary efficacy endpoint for the phase II trial is 6-month clinician-reported overall response (composite of complete and partial response). Additional endpoints include other therapeutic response metrics, utilization of second-line systemic immune suppressive therapy, additional clinical and patient-reported outcomes, and allied biologic correlative studies.
Included Patients
Adults ages ≥ 18 with chronic GVHD newly requiring systemic glucocorticoid therapy were included. Chronic GVHD diagnosis and severity scoring adhered to the National Institutes of Health (NIH) Consensus Criteria on Diagnosis and Staging of Chronic GVHD [47]. In the phase I component of this trial, only those with overall moderate-to-severe chronic GVHD were eligible. Patients had to begin ofatumumab therapy within 14 days from initiation of 1 mg/kg/day of prednisone therapy for chronic GVHD. The following were study exclusion criteria: relapsed malignancy after HCT, previous systemic glucocorticoid therapy at ≥1 mg/kg/day prednisone or equivalent for chronic GVHD, current hepatic/biliary disease (with exception of that due to chronic GVHD), treatment with experimental non–Food and Drug Administration–approved therapy within 5 terminal half-lives or 4 weeks before enrollment, other solid tumor within past 5 years (except completely resected nonmelanoma skin cancer), prior treatment with any anti-CD20 monoclonal antibody or alemtuzumab within 3 months, uncontrolled infectious complications, significant cerebrovascular disease in past 6 months, human immunodeficiency virus positivity, uncontrolled significant cardiac or other medical conditions, clinically active hepatitis B defined as positive HBsAg or positive HBcAb with detectable hepatitis B viral load, active hepatitis C confirmed by viral load, pregnancy or lactation, women and men unable or unwilling to use adequate contraceptive methods through 1 year after completion of protocol therapy, absolute neutrophil count < 1.0 × 109/L; creatinine > 2 × upper limit of normal (ULN), total bilirubin > 1.5 × ULN, transaminase > 2 × ULN, or alkaline phosphatase > 2.5 × ULN (except for that due to chronic GVHD).
Treatment Plan
Prednisone was started at 1 mg/kg actual body weight per day. The duration of prednisone therapy and tapering schedule were not mandated by the protocol. In the setting of increased chronic GVHD activity, prednisone could be increased with upper limit of 1 to 2 mg/kg/day; doses higher than this were considered a separate line of additional systemic immune suppressive therapy. As no cases had discontinued all systemic immune suppression (both initial GVHD prophylaxis agents and any therapy delivered for acute GVHD) by the time of chronic GVHD onset, these agents were continued alongside the new addition of study therapy (1 mg/kg/day prednisone + ofatumumab). Taper of other systemic agents and use of topical agents (eg, ocular drops, mouth rinses, topical steroid creams) were not regulated by the trial. Ofatumumab was administered by i.v. infusion; preparation and infusion conditions were per standard procedures. Dose level cohorts in this phase I trial included the following: cohort 1, 300 mg; cohort 2, 700 mg; and cohort 3, 1000 mg (all delivered once on day 0 and again on day 14 of therapy). This dose/schedule was modeled after a prior trial conducted in rheumatoid arthritis that demonstrated safety, sustained depletion of peripheral blood CD19+ B cells (persistent for 48 weeks), and efficacy with association between increasing dose and clinical response [48]. A dose level −1 of 100 mg was prespecified in the event excess dose-limiting toxicity (DLT) occurred at dose level 1. Premedication was uniformly delivered within 30 minutes before each infusion: acetaminophen 1000 mg, diphenhydramine 50 mg, and methylprednisolone i.v. 50 mg. Vital signs were monitored every 30 (±5) minutes during infusion or more frequently as needed. The initial infusion rate, timing of sequential infusion rate escalation, and rules surrounding response to observed infusion reactions were enforced. If grade 4 infusion reactions occurred, no further ofatumumab therapy was to be given; lesser grade infusion reactions were managed with supportive care including i.v. fluids, antihistamines, and steroids. Supportive antimicrobial prophylaxis followed institutional standards. Immunoglobulin replacement was delivered per discretion of the treating physician, not mandated per protocol.
Phase I Trial Characteristics
The phase I trial followed a traditional (3 + 3) escalation design. Successive dose level cohorts were enrolled and monitored for DLT. The minimum DLT observation period was 46 days after completion of ofatumumab therapy for the last patient on each dose level. DLT was defined by the occurrence of any of the following within 46 days of the last ofatumumab therapy: grade 4 infusion reactions, grade 4 constitutional symptoms at least possibly due to ofatumumab, ≥ grade 3 organ toxicities at least possibly due to ofatumumab, and grade 4 neutropenia lasting more than 14 days. The following were criteria for patient termination from the study: patient withdrawal, death, relapsed malignancy, and completion of study follow-up. Patients in the maximally tolerated dose (MTD) cohort are carried forward in the phase II component and will complete all planned phase II follow-up (24 months), whereas those at lower non-MTD cohorts complete an abbreviated study follow-up with clinical assessment measures and biologic studies only at the 6- and 12-month time points.
Statistical Methods
The occurrence of DLT attributable to ofatumumab in ≤17% served as the boundary for the MTD of ofatumumab in this phase I trial. Escalation to each higher dose level was permitted when 0 of 3 or no more than 1 of 6 (number treated per level was increased to 6 if 1 of 3 experienced a DLT) in a dose level cohort experienced a DLT. Dose de-escalation was indicated if a greater proportion experienced a DLT at any dose level. A total of 6 patients were enrolled at the MTD cohort.
Response of chronic GVHD to therapy was both reported by change in NIH 0 to 3 chronic GVHD severity (per organ sites and overall), as well as clinician-reported overall responses (complete response [CR], partial response [PR], stable disease [SD], progressive disease [PD]). These clinician-reported responses are reported in the following ways: (1) as reported, (2) with response category changed to PD for addition of a new line of systemic immune suppressive therapy beyond first-line prednisone + ofatumumab, and (3) both to account for new systemic therapy and for new topical agents not present before study enrollment.
Data and Safety Monitoring
A comprehensive data and safety monitoring plan adhered with the standard procedures of the H. Lee Moffitt Cancer Center and Research Institute. The trial was conducted with the approval of the University of South Florida institutional review board. The study principal investigator and allied study team members monitored toxicity, and reported adverse (AE) and serious adverse events (scored per Common Terminology Criteria for Adverse Events version 4.0) to the institutional review board, the Moffitt Protocol Monitoring Committee, and the US Food and Drug Administration. All AEs (grades 1 to 5) were collected within 24 hours of start of ofatumumab infusion, grade 3 to 5 AE were collected within 60 days, and only AE deemed related to the investigational therapy were collected beyond day 61. Additional monitoring was employed for hepatic function, screening for hepatitis B reactivation, development of progressive multifocal leukoencephalopathy (PML), and pregnancy.
RESULTS
Baseline Patient and Chronic GVHD Characteristics
Twelve patients in total were treated on the phase I trial, including 3 on dose level 1 (300 mg), 3 on dose level 2 (700 mg), and 6 total on dose level 3 (1000 mg). A patient flow diagram is presented as Supplemental Figure 1. Patient characteristics are summarized in Table 1. Hematologic malignancies serving as HCT indication were diverse, and none had evidence of relapse/progression at time of study enrollment. HCT were from both sibling and unrelated donors and were predominantly peripheral blood mobilized grafts. Female donor/male recipient gender pairing was infrequent.
Table 1.
Baseline Patient Characteristics
| Variable | Frequency (%) |
|---|---|
| Age, median (range), yr | 54 (25–72) |
| Donor age, median (range), yr | 29 (24–56) |
| Donor/recipient gender | |
| Female/male | 1 |
| Others | 11 |
| Race | |
| White/Caucasian | 12 (100%) |
| Disease category | |
| ALL | 4 (33%) |
| AML | 2 (17%) |
| CLL | 1 (8%) |
| CMML | 1 (8%) |
| IMF | 1 (8%) |
| MCL | 1 (8%) |
| MDS | 2 (17%) |
| Remission status at transplantation | |
| CR | 8 (67%) |
| HI | 2 (17%) |
| PR | 2 (17%) |
| Graft source | |
| BM | 1 (8%) |
| PBSC | 11 (92%) |
| Donor type | |
| Matched sibling | 4 |
| Matched unrelated | 8 |
ALL indicates acute lymphoblastic leukemia; AML, acute myelogenous leukemia; CLL, chronic lymphocytic leukemia; CMML, chronic myelomonocytic leukemia; IMF, idiopathic myelofibrosis; MCL, mantle cell lymphoma; MDS, myelodysplastic syndrome; HI, hematologic improvement; BM, bone marrow; PBSC, peripheral blood mobilized stem cells.
Data presented are n (%) unless otherwise indicated.
Chronic GVHD characteristics at study enrollment are presented in Table 2. All cases had an overall global NIH 0 to 3 score of moderate-to-severe (2 and 3). Skin, mouth, and eye involvement were most common. All cases had multiple organ sites concurrently involved at therapy initiation, the majority had overlap subtype of chronic GVHD, and most had previously manifested acute GVHD. We note that the organ involvement represented here is a reflection of the enrolled patients’ heterogeneous chronic GVHD involvement, not an accurate population-based estimate of frequency of organs involved in incident chronic GVHD. Poor performance status, bilirubin elevation, and thrombocytopenia were infrequent.
Table 2.
Chronic GVHD Characteristics at Initiation of Therapy
| Variable | Frequency (%) | |||
|---|---|---|---|---|
|
|
|
|||
| NIH Consensus 0–3 Scores | NIH 0 | NIH 1 | NIH 2 | NIH 3 |
| Skin | 4 (33%) | 2 (17%) | 5 (42%) | 1 (8%) |
| Mouth | 4 (33%) | 6 (50%) | 2 (17%) | 0 |
| Eye | 4 (33%) | 5 (42%) | 2 (17%) | 1 (8%) |
| Lung | 8 (67%) | 2 (17%) | 2 (17%) | 0 |
| GI | 9 (75%) | 2 (17%) | 1 (8%) | 0 |
| Liver | 7 (58%) | 4 (33%) | 0 | 1 (8%) |
| Genital | 10 (83%) | 2 (17%) | 0 | 0 |
| Joint/fascia | 7 (58%) | 2 (17%) | 2 (17%) | 1 (8%) |
| Overall | 0 | 0 | 7 (58%) | 5 (42%) |
| Chronic GVHD onset type | ||||
| De novo | 3 (25%) | |||
| Interrupted | 7 (58%) | |||
| Progressive | 2 (17%) | |||
| Chronic GVHD subtype | ||||
| Classic | 5 (42%) | |||
| Overlap | 7 (58%) | |||
| HCT-CI (at trial enrollment) [49] | ||||
| <3 | 2 (17%) | |||
| ≥3 | 10 (83%) | |||
| KPS | ||||
| <90 | 4 (33%) | |||
| ≥90 | 8 (67%) | |||
| Platelet count, median (range), k/uL | 164 (92–287) | |||
| Bilirubin, mg/dL | .6 (.2–.9) | |||
| Two-minute walk test, median (range), feet [50] | 480 (140–574) | |||
| Baseline systemic immune suppression | ||||
| Sirolimus | 8 (67%) | |||
| Tacrolimus | 10 (83%) | |||
| Prednisone (range, .14–.5 mg/kg daily dose) | 3 (25%) | |||
| ECP | 1 (8%) | |||
| FAM | 2 (17%) | |||
| Baseline topical immune suppression | ||||
| Beclomethasone (GI) | 1 (8%) | |||
| Budesonide (GI) | 2 (17%) | |||
| Triamcinolone (skin) | 2 (17%) | |||
| Dexamethasone (mouth) | 4 (33%) | |||
| Tacrolimus (skin) | 1 (8%) | |||
GI indicates gastrointestinal; HCT-CI, HCT comorbidity index; KPS, Karnofsky performance status; ECP, extracorporeal photopheresis; FAM, combination therapy with fluticasone, azithromycin, monteleukast.
In keeping with institutional practices, the majority were on primary immune suppression prophylaxis, including tacrolimus and sirolimus at the time of chronic GVHD development and enrollment on trial. Other systemically active immune suppressive agents represented at study enrollment (prednisone, extracorporeal photopheresis) were on taper from prior acute GVHD therapy. Two cases with bronchiolitis obliterans were on combined fluticasone, azithromycin, and monteleukast therapy. Baseline topical agents are listed in Table 2.
Study Therapy: Ofatumumab Infusion and Prednisone Taper
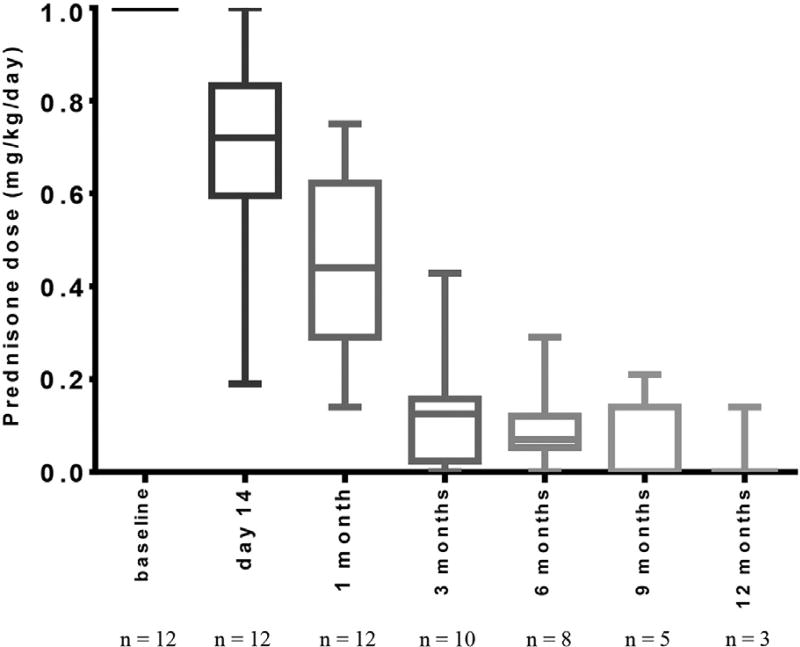
Ofatumumab infusion was well tolerated. The median time required for completion of baseline and day 14 infusions were 5 (range, 4 to 10) and 4 (range, 4 to 9) hours, respectively. Two infusion reactions (grades 2 and 3) occurred and resolved with supportive care, and all patients completed the full planned study therapy. Prednisone was uniformly started at 1 mg/kg/day and then tapered according to the treating clinician’s discretion. As demonstrated in Figure 1, the actual prednisone taper was rapid. The median prednisone dose was .4 (range, .1 to .8) mg/kg/day at 1 month, .1 (range, 0 to .4) mg/kg/day at 3 months, and .07 (range, 0 to .3) at 6 months. These data correspond to a large percentage reduction from starting prednisone dose at 3 (median, 89%; range 57% to 100%) and 6 months (median, 93%; range, 71% to 100%). Whereas systemic immune suppression present before chronic GVHD onset and trial enrollment (ie, those agents still present from primary prophylaxis and acute GVHD therapy) was not regulated on this trial, none of these baseline immune suppressive agents (Table 2) were discontinued during the study follow-up.
Figure 1.
Reduction in systemic glucocorticoid therapy during study period. Box plots present median values and whiskers present minimum-maximum range.
Additional systemic therapy beyond prednisone within 6 months was only used in 1 case: mycophenolate mofetil (MMF) was started 28 days after study therapy initiation for rising liver function tests. Three patients were treated with new topical agents after study enrollment: 1 patient newly received cyclosporine ophthalmic emulsion (Restasis) at day 14, topical triamcinolone (skin) at 3.7 months, and oral azathioprine at 14.2 months. A second patient started topical triamcinolone (skin) at 33 days. The final patient started cyclosporine ophthalmic emulsion (Restasis, Allergan, Irvine, CA) at 5.3 months.
Safety: DLT, AE, and Infectious Complications
No cases of grade 4 infusion reactions, related grade 4 constitutional symptoms, related grade ≥ 3 organ toxicities, or grade 4 neutropenia lasting > 14 days were observed, and hence no DLT events occurred throughout the study. Three patients each were treated on dose levels 1 and 2 (300 mg, 700 mg), and 6 patients were treated on dose level 3 (1000 mg), which was therefore defined as the recommended phase II dose. All AE on trial, regardless of attribution to ofatumumab therapy, are listed in Table 3. From 29 total adverse events, 4 were deemed possibly related to ofatumuab, including the above-mentioned 2 infusion reactions, as well as 1 event each of grade 1 fatigue and grade 1 transaminase elevation. Other observed AE were largely expected complications of high-dose prednisone therapy. One patient with severe bronchiolitis obliterans had multiple events, including recurrent pulmonary infections, hospitalizations, dyspnea, and hypoxia, consistent with the natural history of this disease. There were no cases of hepatitis B reactivation or PML. All infections noted on trial are described in Table 4.
Table 3.
Summary of Adverse Events
| Toxicity Category | Description (Toxicity Type Code) | Grade 1 n (%) |
Grade 2 n (%) |
Grade 3 n (%) |
Grade 4 n (%) |
All n (%) | n |
|---|---|---|---|---|---|---|---|
| Eye disorders | Blurred vision | - | 1 (8.3) | - | - | 1 (8.3) | 12 |
| GI disorders | GI disorders-other, specify (gastritis) | 1 (8.3) | - | - | - | 1 (8.3) | 12 |
| General disorders and administration site conditions | Edema limbs | 1 (8.3) | - | - | - | 1 (8.3) | 12 |
| Fatigue | 1 (8.3) | - | - | - | 1 (8.3) | 12 | |
| Infusion-related reaction | - | 1 (8.3) | 1 (8.3) | - | 2 (16.7) | 12 | |
| Infections and infestations | Infections and infestations - other, specify (surgical site infection after surgery) | - | - | 1 (8.3) | - | 1 (8.3) | 12 |
| Lung infection | - | 1 (8.3) | - | 1 (8.3) | 2 (16.7) | 12 | |
| Nail infection | - | 1 (8.3) | - | - | 1 (8.3) | 12 | |
| Skin infection | - | 1 (8.3) | - | - | 1 (8.3) | 12 | |
| Upper respiratory infection | - | 1 (8.3) | 1 (8.3) | - | 2 (16.7) | 12 | |
| Injury, poisoning, and procedural complications | Bruising | 1 (8.3) | - | - | - | 1 (8.3) | 12 |
| Investigations | Aspartate aminotransferase increased | 1 (8.3) | - | - | - | 1 (8.3) | 12 |
| Metabolism and nutrition disorders | Hyperglycemia | - | - | 1 (8.3) | - | 1 (8.3) | 12 |
| Musculoskeletal and connective tissue disorders | Arthralgia | - | 1 (8.3) | - | - | 1 (8.3) | 12 |
| Chest wall pain | - | - | 1 (8.3) | - | 1 (8.3) | 12 | |
| Musculoskeletal and connective tissue disorder - other, specify (hand/foot cramping) | - | 1 (8.3) | - | - | 1 (8.3) | 12 | |
| Muscle weakness lower limb | 1 (8.3) | - | - | - | 1 (8.3) | 12 | |
| Musculoskeletal and connective tissue disorder - other, specify (fall) | - | - | 1 (8.3) | - | 1 (8.3) | 12 | |
| Nervous system disorders | Facial nerve disorder | - | 1 (8.3) | - | - | 1 (8.3) | 12 |
| Peripheral motor neuropathy | - | 1 (8.3) | - | - | 1 (8.3) | 12 | |
| Peripheral sensory neuropathy | - | 1 (8.3) | - | - | 1 (8.3) | 12 | |
| Tremor | - | 1 (8.3) | - | - | 1 (8.3) | 12 | |
| Psychiatric disorders | Insomnia | - | - | 1 (8.3) | - | 1 (8.3) | 12 |
| Respiratory, thoracic, and mediastinal disorders | Dyspnea | 1 (8.3) | - | 1 (8.3) | - | 2 (16.7) | 12 |
| Hypoxia | - | - | 1 (8.3) | - | 1 (8.3) | 12 | |
| Overall (total AE, % grade AE per total number of AE) | All | 7 (24%) | 12 (41%) | 9 (31%) | 1 (3%) | 29 |
Individual AE (per CTCAE version 4.0) observed on trial from time of treatment initiation through complete study follow-up to date. Individual AE are presented with percent of total (n = 12) patients with each AE.
Total AE per AE grade are presented with percent of total number of AE observed. AE deemed possibly related to ofatumumab therapy are in bold (n = 4, or 14% of total AE)
Table 4.
Summary of Infectious Complications on Trial
| Site | Organism | Day | Grade |
|---|---|---|---|
| Nasopharynx | Rhinovirus* | 4 | 3 |
| Rhinovirus | 78 | 1 | |
| Rhinovirus | 34 | 2 | |
| Oral | Candida | 14 | 2 |
| Pulmonary/BAL | CMV* | 3 | |
| Pseudomonas* | 5 | 3 | |
| MAI* | 5 | 3 | |
| MRSA | 83 | 2 | |
| Surgical site | CNS | 27 | 3 |
| Blood | CMV | 20 | 2 |
Day indicates number of days following initiation of study therapy; BAL, bronchoalveolar lavage; CMV, cytomegalovirus; MAI, mycobacterium avium intracellulare; MRSA, methicillin-resistant staphylococcus aureus; CNS, coagulase-negative staphylococcus.
Denotes multiple infectious complications experienced by 1 individual patient with bronchiolitis obliterans syndrome. All other identified infections were single events in individual patients.
Preliminary Assessment of Efficacy and Biologic Activity
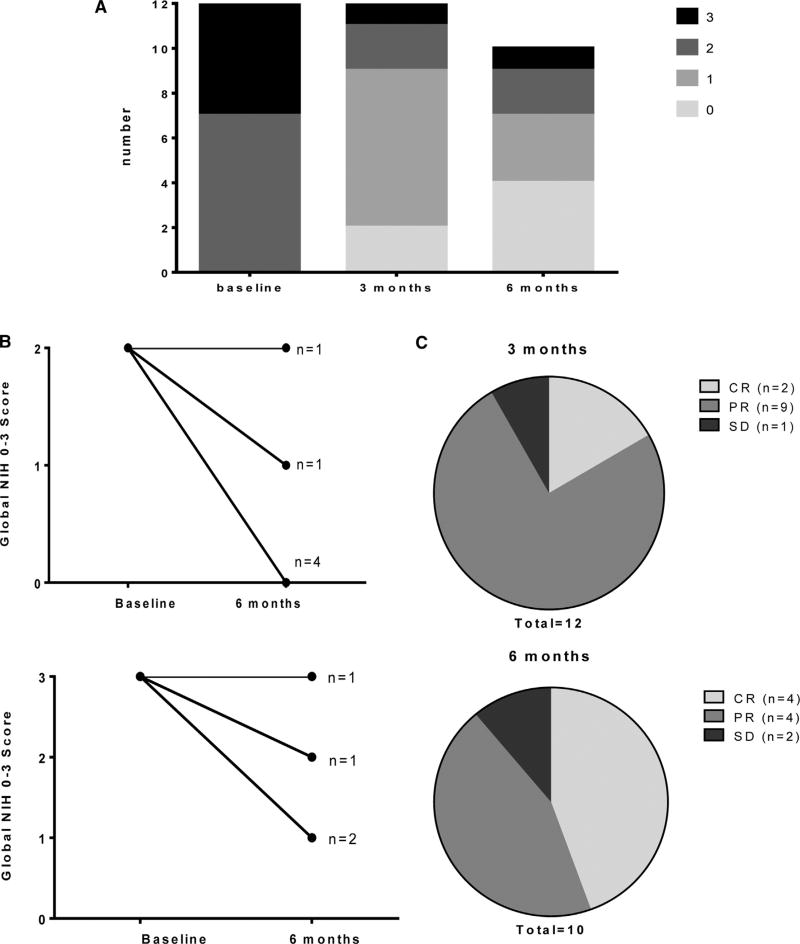
Change in NIH global 0 to 3 scores and clinician-reported response assessments are presented in Figure 2. Responses were seen in moderate and severe chronic GVHD, across individual organ sites (Supplemental Table 1), and in both classic and overlap subtypes of chronic GVHD. Clinician-reported responses for evaluable patients (3-month CR/PR rate = 11 of 12, consisting of CR 2, PR 9, and SD 1; 6-month CR/PR rate = 8 of 10, consisting of CR 4, PR 4, and SD 2) were favorable. Two patients were not eligible for response assessment at 6 months (death: n = 1, inadequate follow-up time: n = 1).
Figure 2.
Change in chronic GVHD severity. (A) Indicates distribution of NIH global 0 to 3 scores at baseline, 3 months, and 6 months. (B) Indicates change in global NIH 0 to 3 score from baseline to 6 months separately for those with initial score 2 (top) versus 3 (bottom). (C) Indicates clinician-reported overall response category at 3 months (top) and 6 (bottom) months.
Response rates accounting for additional systemic immune suppressive therapy are the following: 1 patient received MMF for liver function test (LFT) elevation before 3-month assessment and, thus, would be considered PD. Thus, 3-month CR/PR rate would be 10 of 12 (consisting of CR 2, PR 8, SD 1, and PD 1) and 6-month CR/PR would be 8 of 11 (consisting of CR 4, PR 4, SD 2, and PD 1)—this patient lacks 6-month follow-up currently but would contribute to the total denominator at 6 months and count as failure based on early addition of MMF.
Response rates accounting for both additional systemic and topical agents are the following: accounting for both additional systemic and topical agents added after study enrollment (eliminating response assessment at organ sites for which new topical agents were added, except for permitting assessment of progressive disease), the above response rates would change in the following manner: 3-month response would be unaltered (CR/PR = 10 of 12, consisting of CR 2, PR 8, SD 1, and PD 1), whereas 6-month response (CR/PR = 7 of 11, consisting of CR 4, PR 3, SD 2, and PD 2) would decrease because of the addition of topical Restasis for ocular symptoms not present at baseline (despite response in other multiple organs involved). In the other 2 cases of new topical agents added, these were implemented for existing organ involvement at baseline; silencing response assessment in organs targeted by these topical agents did not alter response category due to responsive disease in other sites and no progression in any sites.
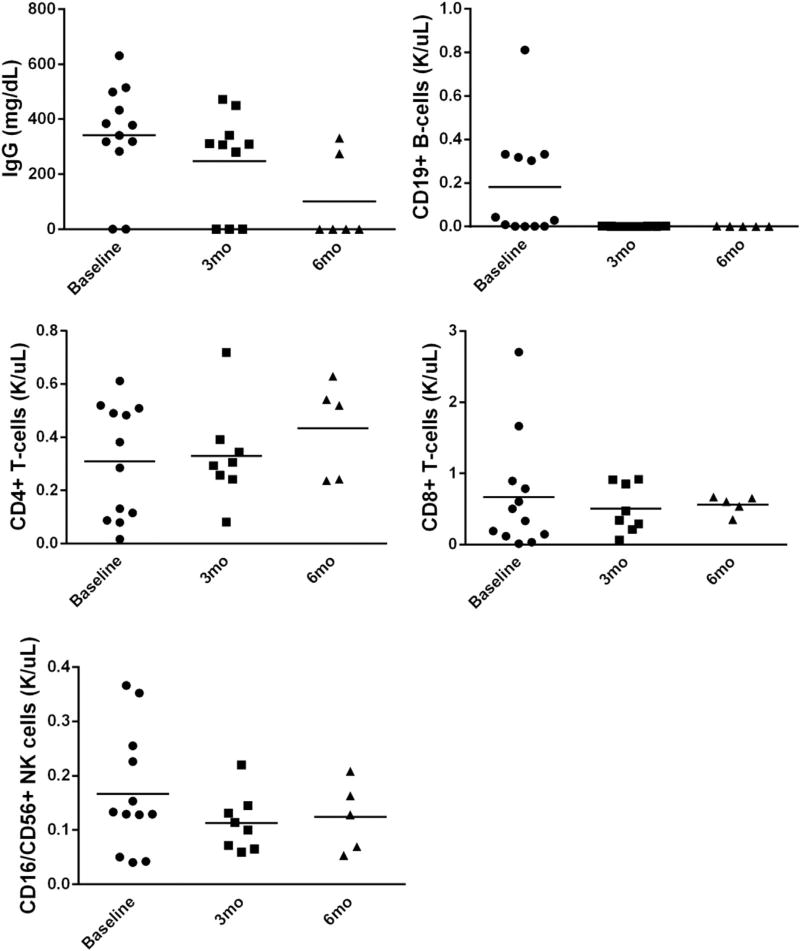
Serial measures of immune globulin and lymphocyte subsets are presented in Figure 3. Therapy was associated with significant reduction in total CD19+ B cells. No significant differences were observed for these parameters according to ofatumumab dose level. No malignancy relapse was seen on trial. The median follow-up time for surviving patients is currently 13 months (range, 6 to 24 months). Two deaths occurred: (severe oxygen-dependent bronchiolitis obliterans (n = 1) complicated by recurrent infections at 11 months after therapy onset, and aspiration pneumonia (n = 1) in a deconditioned patient undergoing rehabilitation at 79 days), and were not deemed related to study therapy.
Figure 3.
Ofatumumab therapy depletes total CD19+ B cells and IgG.
DISCUSSION
Given limited efficacy of currently available chronic GVHD primary treatment strategies, novel approaches are needed. Based on extensive evidence implicating B cells in chronic GVHD pathobiology, we tested ofatumumab, a highly potent humanized anti-CD20 monoclonal antibody, together with standard 1 mg/kg/day prednisone as initial therapy for NIH moderate-to-severe chronic GVHD in a phase I/II trial. The central aim of the phase I component of this trial was to establish the safety of this approach.
The phase I dose escalation was modeled after a prior study in rheumatoid arthritis, as this dose/schedule demonstrated safety, clinical efficacy, and profound sustained B cell depletion [48]. Our data support the safety of this approach in primary therapy of chronic GVHD: no DLT was observed. Thus, escalation proceeded to the planned maximal dose level, which was defined as the recommended phase II dose. All patients completed planned study therapy, and only 2 infusion reactions occurred among the 12 treated. Infusional toxicity of ofatumumab was modest, and the 2 observed reactions were treated to resolution with supportive care. Additionally, the observed AE on trial were largely expected complications of intensive prednisone therapy. Importantly, the additional immune suppression induced by profound B cell depletion in the setting of prednisone therapy raises concern for potential increase in risk. The data, however, provide no signal of excess infectious morbidity, compared with that previously reported in chronic GVHD initial therapy [13], or NRM. Importantly, we also observed no cases of viral hepatitis reactivation or PML. Although the total number on this phase I study limits comparisons to expected NRM seen in much larger chronic GVHD cohorts, the observed survival is encouraging, particularly given the higher risk characteristics (exclusively moderate-to-severe chronic GVHD [1], predominance of overlap subtype [51], presence of lung involvement [52], increased chronic GVHD HCT comorbidity index [53] of the study patients.
Although the phase I component of this trial is not adequately powered to address efficacy, we note that therapy was associated with encouraging response rates at 3 and 6 months, both according to clinician-assessed overall response categories and according to change in NIH 0 to 3 chronic GHVD severity scores. Only 1 patient required an additional line of systemic immune suppressive therapy beyond prednisone/ofatumumab within 6 months of follow-up, and use of additional topical agents was limited. Although specific comparisons are limited, responses were seen in both moderate and severe chronic GVHD, across varied sites of organ involvement, and in both classic and overlap subtype. As a surrogate of effective chronic GVHD control, major reduction in prednisone dose was achieved by 3 and 6 months of therapy. Further conclusions regarding efficacy will be made after completion of the phase II component of the trial.
We acknowledge the existing studies examining the impact of rituximab-based interventions in refractory chronic GVHD therapy [29], primary chronic GVHD therapy together with prednisone [54], and also as chronic GVHD prophylaxis [30,31]. These and allied correlative science have laid an important foundation for B cell depletion strategies in chronic GVHD interventional trials. However, the following speak to the need for ongoing progress: rituximab appears to offer incomplete protection from chronic GVHD as evidenced by chronic GVHD in that setting [30], failure of initial combined therapy or escape associated with resurgence of H-Y antibodies has been noted [54], and therapy in more advanced chronic GVHD—although certainly active—is incompletely effective [29,55]. These shortcomings, taken together with clinical observations and mechanistic insight suggesting greater potency of ofatumumab, support the rationale to study ofatumumab in chronic GVHD therapy. Mature results from the phase II trial, with particular attention to chronic GVHD characteristics and detailed response assessment, will facilitate an assessment of comparative efficacy.
We note the following limitations: whereas the phase I trial established the MTD and the observed safety profile is reassuring, additional and longer-term safety data will be generated through the phase II component of this trial. Additionally, more complete efficacy assessment, including detailed examination of competing proposed response assessments and association with subsequent outcomes including treatment failure and failure-free survival, will only be possible in the phase II setting. As well, we note that taper of prednisone and thresholds for invoking either new topical or systemic immune suppressive agents on trial can’t be mandated, and, thus, are subject to individual clinician’s judgment; this reality complicates analysis but is not a challenge unique to this trial. Finally, we note that the current report of immune populations and immunoglobulins provides only initial biologic insight; more detailed studies and predictive biomarker analyses are planned for the complete phase II trial.
In summary, the addition of ofatumumab at 1000 mg at days 0 and 14 of initial 1 mg/kg/day prednisone therapy for NIH moderate-to-severe chronic GVHD is safe and associated with favorable clinical responses and reduction in prednisone.
Supplementary Material
Acknowledgments
Financial disclosure: This investigator-initiated trial was conducted with support from GlaxoSmithKline. The principal investigator’s effort was also in part supported by the American Cancer Society (MRSG-11-149-01-LIB, PI: J.P). This work has been supported in part by the Biostatistics Core Facility at the H. Lee Moffitt Cancer Center & Research Institute, a National Cancer Institute–designated Comprehensive Cancer Center (P30-CA076292).
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Authorship contributions: J.P. produced study design, conducted the trial, analyzed data, and wrote the manuscript. J.K. contributed to study design, performed statistical analysis, and contributed to writing of the manuscript. B.C.B., M.A., E.A., H.F.F., T.F., M.A.K-D., F.L.L., A.M., T.N., L.O-B., L.P., M.R., and C.A. contributed to study design, data analysis, and writing of the manuscript.
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.bbmt.2015.03.014
References
- 1.Arai S, Jagasia M, Storer B, et al. Global and organ-specific chronic graft-versus-host disease severity according to the 2005 NIH Consensus Criteria. Blood. 2011;118:4242–4249. doi: 10.1182/blood-2011-03-344390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–414. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Kim HT, Ho VT, et al. Quality of life associated with acute and chronic graft-versus-host disease. Bone Marrow Transplant. 2006;38:305–310. doi: 10.1038/sj.bmt.1705434. [DOI] [PubMed] [Google Scholar]
- 4.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 5.Fraser CJ, Bhatia S, Ness K, et al. Impact of chronic graft-versus-host disease on the health status of hematopoietic cell transplantation survivors: a report from the Bone Marrow Transplant Survivor Study. Blood. 2006;108:2867–2873. doi: 10.1182/blood-2006-02-003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stewart BL, Storer B, Storek J, et al. Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood. 2004;104:3501–3506. doi: 10.1182/blood-2004-01-0200. [DOI] [PubMed] [Google Scholar]
- 7.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009;114:7–19. doi: 10.1182/blood-2008-10-182592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pidala J, Kurland B, Chai X, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011;117:4651–4657. doi: 10.1182/blood-2010-11-319509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SJ, Flowers ME. Recognizing and managing chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2008:134–141. doi: 10.1182/asheducation-2008.1.134. [DOI] [PubMed] [Google Scholar]
- 10.Koc S, Leisenring W, Flowers ME, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100:48–51. doi: 10.1182/blood.v100.1.48. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan KM, Witherspoon RP, Storb R, et al. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. 1988;72:546–554. [PubMed] [Google Scholar]
- 12.Arora M, Wagner JE, Davies SM, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001;7:265–273. doi: 10.1053/bbmt.2001.v7.pm11400948. [DOI] [PubMed] [Google Scholar]
- 13.Martin PJ, Storer BE, Rowley SD, et al. Evaluation of mycophenolate mofetil for initial treatment of chronic graft-versus-host disease. Blood. 2009;113:5074–5082. doi: 10.1182/blood-2009-02-202937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilman AL, Schultz KR, Goldman FD, et al. Randomized trial of hydroxychloroquine for newly diagnosed chronic graft-versus-host disease in children: a Children’s Oncology Group study. Biol Blood Marrow Transplant. 2012;18:84–91. doi: 10.1016/j.bbmt.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inamoto Y, Flowers ME, Sandmaier BM, et al. Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood. 2014;124:1363–1371. doi: 10.1182/blood-2014-03-563544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarantopoulos S, Stevenson KE, Kim HT, et al. High levels of B-cell activating factor in patients with active chronic graft-versus-host disease. Clin Cancer Res. 2007;13:6107–6114. doi: 10.1158/1078-0432.CCR-07-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miklos DB, Kim HT, Zorn E, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svegliati S, Olivieri A, Campelli N, et al. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110:237–241. doi: 10.1182/blood-2007-01-071043. [DOI] [PubMed] [Google Scholar]
- 20.Kitko CL, Levine JE, Storer BE, et al. Plasma CXCL9 elevations correlate with chronic GVHD diagnosis. Blood. 2014;123:786–793. doi: 10.1182/blood-2013-08-520072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii H, Cuvelier G, She K, et al. Biomarkers in newly diagnosed pediatric-extensive chronic graft-versus-host disease: a report from the Children’s Oncology Group. Blood. 2008;111:3276–3285. doi: 10.1182/blood-2007-08-106286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarantopoulos S, Stevenson KE, Kim HT, et al. Altered B-cell homeostasis and excess BAFF in human chronic graft-versus-host disease. Blood. 2009;113:3865–3874. doi: 10.1182/blood-2008-09-177840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrahamsen IW, Somme S, Heldal D, et al. Immune reconstitution after allogeneic stem cell transplantation: the impact of stem cell source and graft-versus-host disease. Haematologica. 2005;90:86–93. [PubMed] [Google Scholar]
- 24.D’Orsogna LJ, Wright MP, Krueger RG, et al. Allogeneic hematopoietic stem cell transplantation recipients have defects of both switched and IgM memory B cells. Biol Blood Marrow Transplant. 2009;15:795–803. doi: 10.1016/j.bbmt.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Greinix HT, Pohlreich D, Kouba M, et al. Elevated numbers of immature/transitional CD21− B lymphocytes and deficiency of memory CD27+ B cells identify patients with active chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2008;14:208–219. doi: 10.1016/j.bbmt.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 26.Kuzmina Z, Greinix HT, Weigl R, et al. Significant differences in B-cell subpopulations characterize patients with chronic graft-versus-host disease-associated dysgammaglobulinemia. Blood. 2011;117:2265–2274. doi: 10.1182/blood-2010-07-295766. [DOI] [PubMed] [Google Scholar]
- 27.Khoder A, Sarvaria A, Alsuliman A, et al. Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood. 2014;124:2034–2045. doi: 10.1182/blood-2014-04-571125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarantopoulos S, Stevenson KE, Kim HT, et al. Recovery of B-cell homeostasis after rituximab in chronic graft-versus-host disease. Blood. 2011;117:2275–2283. doi: 10.1182/blood-2010-10-307819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-versus-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cutler C, Kim HT, Bindra B, et al. Rituximab prophylaxis prevents corticosteroid-requiring chronic GVHD after allogeneic peripheral blood stem cell transplantation: results of a phase 2 trial. Blood. 2013;122:1510–1517. doi: 10.1182/blood-2013-04-495895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arai S, Sahaf B, Narasimhan B, et al. Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence. Blood. 2012;119:6145–6154. doi: 10.1182/blood-2011-12-395970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood. 2004;104:1793–1800. doi: 10.1182/blood-2004-01-0039. [DOI] [PubMed] [Google Scholar]
- 33.Beum PV, Lindorfer MA, Beurskens F, et al. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol. 2008;181:822–832. doi: 10.4049/jimmunol.181.1.822. [DOI] [PubMed] [Google Scholar]
- 34.Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol. 2006;177:362–371. doi: 10.4049/jimmunol.177.1.362. [DOI] [PubMed] [Google Scholar]
- 35.Rafiq S, Butchar JP, Cheney C, et al. Comparative assessment of clinically utilized CD20-directed antibodies in chronic lymphocytic leukemia cells reveals divergent NK cell, monocyte, and macrophage properties. J Immunol. 2013;190:2702–2711. doi: 10.4049/jimmunol.1202588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barth MJ, Hernandez-Ilizaliturri FJ, Mavis C, et al. Ofatumumab demonstrates activity against rituximab-sensitive and -resistant cell lines, lymphoma xenografts and primary tumour cells from patients with B-cell lymphoma. Br J Haematol. 2012;156:490–498. doi: 10.1111/j.1365-2141.2011.08966.x. [DOI] [PubMed] [Google Scholar]
- 37.Basu B. Ofatumumab for rituximab-resistant nephrotic syndrome. N Engl J Med. 2014;370:1268–1270. doi: 10.1056/NEJMc1308488. [DOI] [PubMed] [Google Scholar]
- 38.Bologna L, Gotti E, Da Roit F, et al. Ofatumumab is more efficient than rituximab in lysing B chronic lymphocytic leukemia cells in whole blood and in combination with chemotherapy. J Immunol. 2013;190:231–239. doi: 10.4049/jimmunol.1202645. [DOI] [PubMed] [Google Scholar]
- 39.Cheson BD. Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. J Clin Oncol. 2010;28:3525–3530. doi: 10.1200/JCO.2010.27.9836. [DOI] [PubMed] [Google Scholar]
- 40.Czuczman MS, Fayad L, Delwail V, et al. Study I. Ofatumumab mono-therapy in rituximab-refractory follicular lymphoma: results from a multicenter study. Blood. 2012;119:3698–3704. doi: 10.1182/blood-2011-09-378323. [DOI] [PubMed] [Google Scholar]
- 41.Matasar MJ, Czuczman MS, Rodriguez MA, et al. Ofatumumab in combination with ICE or DHAP chemotherapy in relapsed or refractory intermediate grade B-cell lymphoma. Blood. 2013;122:499–506. doi: 10.1182/blood-2012-12-472027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nader K, Patel M, Ferber A. Ofatumumab in rituximab-refractory autoimmune hemolytic anemia associated with chronic lymphocytic leukemia: a case report and review of literature. Clin Lymphoma Myeloma Leuk. 2013;13:511–513. doi: 10.1016/j.clml.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 43.Namberger K, Weiss L, Krause B, et al. A case of long-term remission with ofatumumab maintenance therapy in multiply relapsed and rituximab-refractory chronic lymphocytic leukaemia with deletion 17p. Eur J Haematol. 2013;90:349–350. doi: 10.1111/ejh.12065. [DOI] [PubMed] [Google Scholar]
- 44.Shimada K, Tomita A, Saito S, Kiyoi H. Efficacy of ofatumumab against rituximab-resistant B-CLL/SLL cells with low CD20 protein expression. Br J Haematol. 2014;166:455–457. doi: 10.1111/bjh.12857. [DOI] [PubMed] [Google Scholar]
- 45.Small GW, McLeod HL, Richards KL. Analysis of innate and acquired resistance to anti-CD20 antibodies in malignant and nonmalignant B cells. Peer J. 2013;1:e31. doi: 10.7717/peerj.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wierda WG, Padmanabhan S, Chan GW, et al. Ofatumumab is active in patients with fludarabine-refractory CLL irrespective of prior rituximab: results from the phase 2 international study. Blood. 2011;118:5126–5129. doi: 10.1182/blood-2011-04-348656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945–956. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Ostergaard M, Baslund B, Rigby W, et al. Ofatumumab, a human anti-CD20 monoclonal antibody, for treatment of rheumatoid arthritis with an inadequate response to one or more disease-modifying anti-rheumatic drugs: results of a randomized, double-blind, placebo-controlled, phase I/II study. Arthritis Rheum. 2010;62:2227–2238. doi: 10.1002/art.27524. [DOI] [PubMed] [Google Scholar]
- 49.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pidala J, Chai X, Martin P, et al. Hand grip strength and 2-minute walk test in chronic graft-versus-host disease assessment: analysis from the Chronic GVHD Consortium. Biol Blood Marrow Transplant. 2013;19:967–972. doi: 10.1016/j.bbmt.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pidala J, Vogelsang G, Martin P, et al. Overlap subtype of chronic graft-versus-host disease is associated with an adverse prognosis, functional impairment, and inferior patient-reported outcomes: a Chronic Graft-versus-Host Disease Consortium study. Haematologica. 2012;97:451–458. doi: 10.3324/haematol.2011.055186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer J, Williams K, Inamoto Y, et al. Pulmonary symptoms measured by the national institutes of health lung score predict overall survival, nonrelapse mortality, and patient-reported outcomes in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:337–344. doi: 10.1016/j.bbmt.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wood WA, Chai X, Weisdorf D, et al. Comorbidity burden in patients with chronic GVHD. Bone Marrow Transplant. 2013;48:1429–1436. doi: 10.1038/bmt.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahaf B, Arai S, Otani J, et al. American Society for Blood and Marrow Transplantation Meeting. Salt Lake City; Utah: 2013. Rituximab provides steroid-sparing therapy in new-onset chronic graft-versus-host disease. [Google Scholar]
- 55.Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, et al. Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant. 2009;15:1005–1013. doi: 10.1016/j.bbmt.2009.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.