Abstract
The functional luminal imaging probe (FLIP) is an FDA approved measurement tool utilized to measure simultaneous pressure and diameter to both diagnose and manage various upper gastrointestinal disorders. Additionally, this tool is also approved to guide therapy during bariatric procedures and specialized esophageal surgery. While it has been commercially available since 2009, as the endolumenal functional lumen imaging probe (EndoFLIP ®), the FLIP has had limited penetrance into clinical settings outside of specialized centers and this is primarily due to a paucity of data supporting its utility in general practice. However, data is accumulating that is providing guidance regarding emerging applications in the evaluation and management of foregut disorders. Thus, the aim of this Clinical Practice Update is to describe the technique and review potential indications in achalasia, eosinophilic esophagitis and GERD.
Keywords: functional lumen imaging probe (FLIP), impedance planimetry, achalasia, gastroesophageal reflux disease, eosinophilic esophagitis
Introduction
The evolution of esophageal function testing has moved rapidly over the last ten years along the continuum of manometric technique and bolus transit assessment. This has involved major improvements in manometry where the current state of the art is high-resolution manometry with esophageal pressure topography1. Along similar lines, the evaluation of bolus transit has also evolved from a singular assessment of bolus transit during videofluoroscopy to a more comprehensive approach where intraluminal impedance monitoring can describe bolus transit in the context of a simultaneous pressure analysis to combine the strengths of both techniques2. However, these advances primarily represent modifications and improvements upon existing technology and the assessment of esophageal function has not moved far beyond an assessment of contractile patterns and bolus movement.
Recently, a new technology has become available, the Functional Lumen Imaging Probe (FLIP), that focuses on measuring mechanical properties of the esophagus as opposed to contractile patterns and bolus transit3. FLIP utilizes high-resolution impedance planimetry during volume controlled distention to measure luminal cross sectional area (CSA) along an axial plane. This is synchronized to a singular pressure measure within a fluid filled bag (Figure 1) and thus, allows for an assessment of luminal geometry and pressure akin to compliance measurement. This technique has been utilized to assess the mechanical properties of the esophageal wall and opening dynamics of the esophagogastric junction in various esophageal diseases. Whether this new information can lead to improvements in clinical outcome is just being explored and there appears to be promising results focused in achalasia and eosinophilic esophagitis (EoE).
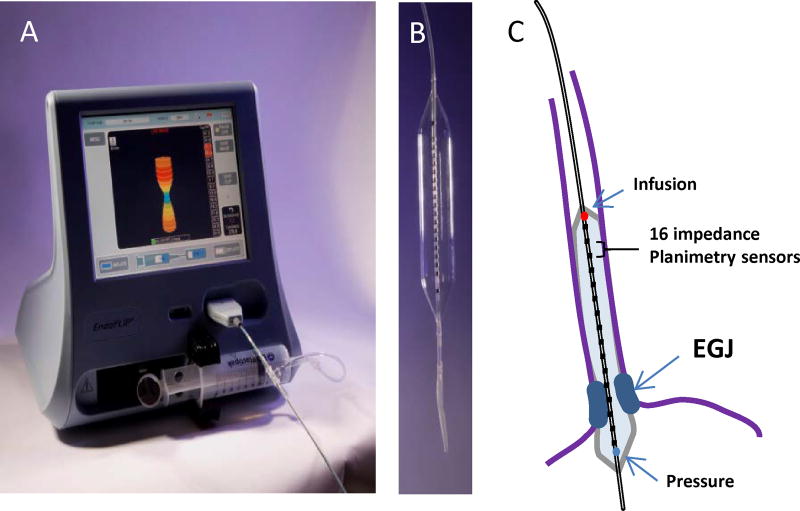
Figure 1. The functional lumen imaging probe (FLIP).
A) The EndoFLIP system (EF-100) with real-time 3-D imaging of the EGJ. The blue color on the screen represents the narrowest portion at the EGJ. B) An 10-cm balloon with 0.5-cm channel spacing housed within an 8-cm length FLIP segment (EF-325). C) Positioning of the 16 cm (EF 322) catheter with the distal portion through the EGJ and 10 recording segments in the body of the esophagus. The paired impedance planimetry rings (black) provide the measure of diameter and cross-sectional area. The pressure sensor (blue circle) is located in the distal aspect of the catheter and the infusion port (red circle) in the proximal aspect of the catheter within the balloon.
The current Clinical Practice Update on FLIP will review both the methodology of the technique and the data supporting its clinical utility in specific esophageal disorders. The review is a summary of expert opinion in the field without a formal systematic review of evidence.
Methodology and Technical Aspects
The FLIP consists of a 240-mm long, 3-mm outer diameter catheter with a balloon mounted on the distal end. The catheter contains 16 paired impedance planimetry electrodes within the balloon spaced at various intervals ranging from 4mm to 1 cm based on the bag configuration and length (Figure 1). An infinitely compliant balloon of varying diameters based on the assessment required is filled with a conductive fluid from an 80 ml syringe that is housed and controlled from the EndoFLIP® system. Excitation electrodes at either end of the balloon emit a continuous low electric current and the voltage is measured across the paired impedance planimetry electrodes by leveraging Ohm’s law to provide a measurement of CSA and volume at intervals based on excitation electrode spacing. A solid-state pressure transducer is located at the distal end of the bag and thus, a simultaneous measures of pressure is made and an assessment of distensibility of the esophageal body and/or EGJ can be performed. Multiple device sizes are commercially available with FLIP measurement segments of 6.0 cm to 16-cm (Table 1)4–7.
Table 1.
Current FDA approved devices and intended use.
| Description | Model Number |
FDA Intended Use (as of June 2016) | Primary Location of Use |
CPT Codes |
|---|---|---|---|---|
| EndoFLIP System | EF-100 | For use in a clinical setting to measure pressure and dimensions in the esophagus, pylorus, and anal sphincters. It is intended to be used as an adjunct to other diagnostic methods as part of a comprehensive evaluation of patients with symptoms consistent with gastrointestinal motility disorders. | 91040 (esophageal measurements) | |
| EndoFLIP 8cm measurement catheter | EF-325N | UES,LES, Pylorus, Anal Sphincters | ||
| EndoFLIP 16cm measurement catheter | EF-322N | Esophagus | ||
| EsoFLIP 6cm –20mm Dilation Catheter | ES-320 | To dilate esophageal strictures due to esophageal surgery,primary gastro-esophageal reflux , radiation therapy | Esophagus | 43220,43229 (both TTS) |
| EsoFLIP 8cm –30mm Dilation Catheter | ES-330 | For use in a clinical setting for dilating the gastro-esophageal junction of a patient with Achalasia | LES | 43214,43233 (both wire guided) |
The FLIP is placed into the esophagus either trans-orally or trans-nasally in a sedated or awake patient. Most protocols are performed with the catheter placed orally during conscious sedation or while the patient is under general anesthesia in the operating room. The FLIP is positioned within the esophagus by identifying the waist-like constriction of the esophagogastric junction on the real-time, 3 dimensional (3-D) geometric display at a low fill volumes (typically 20–30ml) (Figure 1). Alternatively, direct visualization can be utilized by passing the endoscope along-side of the FLIP catheter. Variations in FLIP study methods and protocols exist based on the disease state and the measurement of interest (Table 2): These differences will be covered in the subsequent sections focused on specific esophageal disorders.
Table 2.
Summary of FLIP methods and results of studies evaluating EGJ-distensibility in asymptomatic controls.
| Author, year |
N |
1FLIP length, cm |
IP channel spacing, mm |
2Test setting, sedation |
Placement | Pressure zero |
Fill volume, ml |
3EGJ-DI, mm2/mmHg |
Comparator group |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Beaumont, 200935 | 10 | 6.4 | 4 | awake | oral | gastric | 10–60 | 3.7 +/− 0.9 | Barrett’s, post-ablation |
|
| |||||||||
| Kwiatek, 20104 | 20 | 6.4 | 4 | Endoscopy, Conscious | Oral | Gastric | 10 | 3.2 (2.3 – 6.7) | GERD |
| 20 | 2.2 (1.2 – 6.5) | ||||||||
| 30 | 4.2 (1.7 – 10.4) | ||||||||
| 40 | 7.3 (3.8 – 11.4) | ||||||||
|
| |||||||||
| Kwiatek, 201113 | 15 | 8 | 5 | Endoscopy, Conscious | Oral | NR | 20 | 0.9 (0.3 – 1.4) | EoE |
| 30 | 0.8 (0.4 – 2.8) | ||||||||
|
| |||||||||
| Rohof, 20125 | 15 | 6.4 | 4 | Awake | Nasal | Atm | 50 | 6.3 +/− 0.7 | Achalasia |
|
| |||||||||
| Nathanson, 201236 | 50 | 8 | 5 | Intra-operative, General anesthesia | Oral | Gastric | 30 | 1.1 (0.7 – 1.6) | -- |
| 40 | 1.2 (0.83 – 2.2) | ||||||||
|
| |||||||||
| Tucker, 20137 | 22 | 6.4 | 4 | Endoscopy, Conscious | Oral | NR | 20 | 4.0 (2.4 – 7.5) | GERD |
| 30 | 6.1 (4.3 – 8.0) | ||||||||
|
| |||||||||
| Rieder, 201315 | 4 | 8 | 5 | Awake | Oral | NR | 30 | 2.5 (2.0 – 6.3) | Achalasia |
| 40 | 2.7 (2.4 – 8.3) | ||||||||
|
| |||||||||
| Fukazawa, 201437 | 9 | 8 | 5 | Awake | Nasal | Atm | 20 | 2.9 +/− (0.6) | Intra-subject following mosapride |
| 40 | 7.1 +/− (0.9) | ||||||||
| 50 | 8.2 +/− (0.8) | ||||||||
|
| |||||||||
| Lottrup, 201538 | 10 | 8 | 5 | Awake | Oral | Atm | 20 | 1.9 +/− 0.5 | GERD, hiatal hernia |
| 30 | 2.8 +/− 0.6 | ||||||||
| 40 | 3.8 +/− 0.9 | ||||||||
| 50 | 3.9 +/− 0.7 | ||||||||
|
| |||||||||
| Carlson, 201511 | 10 | 16 | 10 | Endoscopy, Conscious | Oral | Atm | 50 | 7.1 (3.8 – 8.9) | Achalasia |
| 60 | 6.2 (4.6 – 8.1) | ||||||||
|
| |||||||||
| Mikami, 201639 | 9 | 6.4 | 4 | Awake | Nasal | Atm | 20 | 1.8 +/− (0.2) | Intra-subject following metoclopramide |
| 30 | 3.5 +/− (0.6) | ||||||||
| 40 | 4.5 +/− (0.5) | ||||||||
Length of the cylindrical, measurement segment.
Conscious sedation with midazolam and fentanyl or pethidine.
Results extrapolated from figures if not directly reported and represented as median (inter-quartile range) or mean +/− SD (SE).
IP – impedance planimetry. NR – not reported. Atm – atmospheric.
Courtesy of Dustin Carlson
Data analysis
The FLIP is indicated (and FDA approved for use in the United States) for clinical use as a pressure and dimension measurement device, as an adjunctive test in patients with symptoms consistent with esophageal hypersensitivity and to estimate the size of a stoma produced by a gastric band. Thus, clinical applications for FLIP are varied and data analysis can be tailored to target the specific disease of interest.
During FLIP assessment, measurements of 16 CSA and pressure are simultaneously measured using a 10-Hz sampling rate. The 16 CSA measures are recorded at various intervals based on electrode spacing to assess the relationship between luminal CSA and distending (intra-bag) pressure. In its most simple form, FLIP can be used real-time to assess the narrowest area along the 16 recording sites positioned within the anatomic zone of interest while measuring simultaneous pressure. This can be assessed visually using the 3-D display that provides a color-spatial reference and a concomitant number measurement of the diameter (Figure 1). The distensibility index (DI) is the typical measure of sphincter distensibility and is calculated by dividing the median narrowest CSA (within the anatomical zone of interest) by the median intra-bag pressure over a set timeframe (or distension volume)4. The distensibility index and narrowest diameter during distention can be derived from this real-time analysis, however, FLIP measurements are dynamic with both CSA and pressure fluctuation occurring during a stable distension volume due to respiratory and vascular artifacts and the effect of both spontaneous and balloon distension induced esophageal contraction. Thus, various methods using the FLIP Analytics software (Crospon) or other external software methods (e.g. MATLAB, The Math Works, Natick, MA) have been developed to counteract these effects using various filtering techniques and analysis paradigms 8–10.
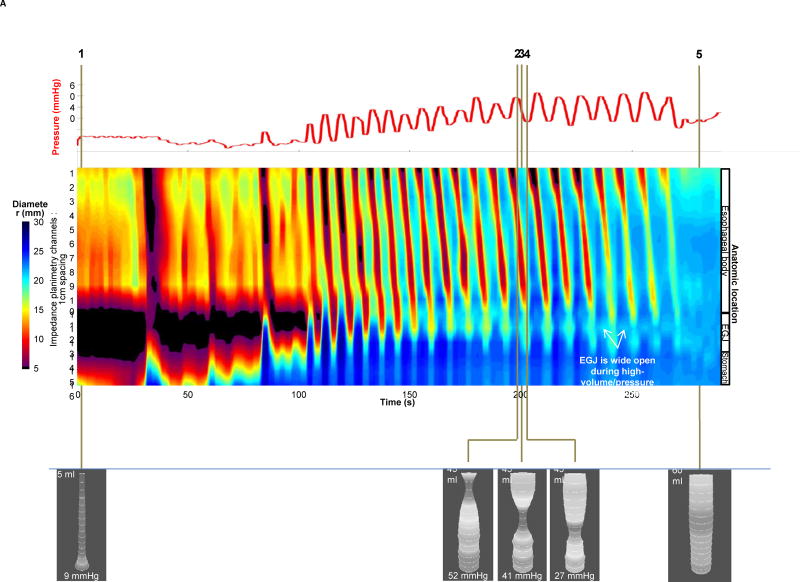
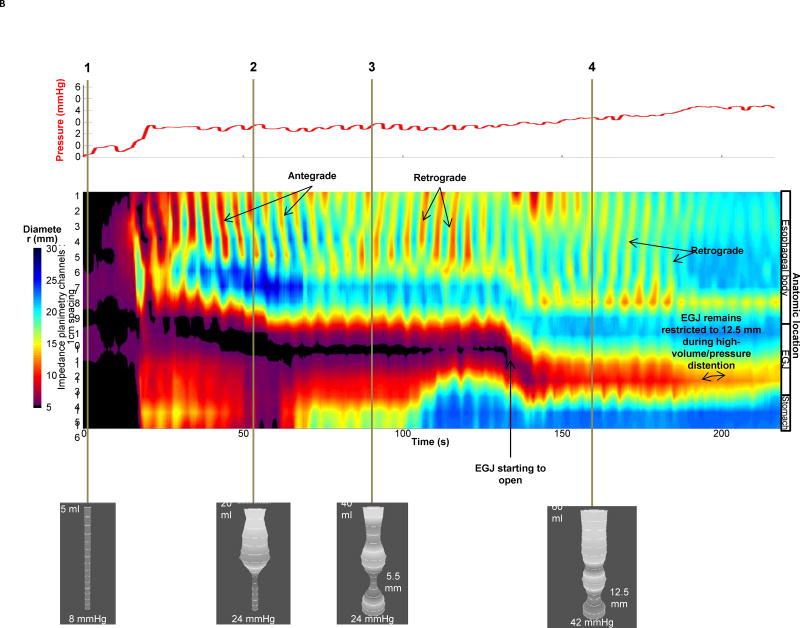
Given the fact that impedance planimetry data is acquired in high-resolution over a space-time domain, the diameter data at each axial location along the catheter can be expressed as a topography plot using interpolation and a color scale to depict diameter size (Figure 2) 8, 9. This is akin to the data presentation utilized in high-resolution manometry and represents a novel method to visualize diameter changes over time with simultaneous pressure during controlled volume distention. The benefits of FLIP topography are the ability to define EGJ distensibility and function while simultaneously obtaining information regarding the contractile activity and distensibility of the esophageal body. This approach has been utilized to better characterize the motor activity in the body of the esophagus in achalasia subtypes where patients were categorized based on three distinct body patterns that consisted of repetitive antegrade contractions (RACs), repetitive retrograde contractions (RRCs) or absence of activity (Figure 2) 11, 12.
Figure 2. FLIP Data Analysis.
Examples of data acquisition and analysis in an asymptomatic control (A) and an achalasia patient (B). The pressure (red) recordings are depicted in the upper panel. Diameter data from each impedance planimerty channel is scaled from 5 to 30 mm and is interpolated and color coded on a hot/cold scale (small diameters are red/large diameters are blue) to generate FLIP topography akin to what is visualized during high-resolution manometry. In the asymptomatic control (2A), real-time 3-D images are depicted to show various points of interest using shape to highlight narrowing. Time point 1 illustrates the baseline with minimal pressure and balloon filling. Time points 2–4 represent propagation of a single antegrade contraction within the sequence of RACs where one can see the narrowing associated with contraction move distally within the 3-D image and the propagating contraction on FLIP topography noted by the red color similar to peristalsis noted on high-resolution manometry. Time point 5 represents a period of quiescence at 60 ml with balloon diameters reaching the limits of the bag at 22mm. In the achalasia patient (2B), time point 1 once again represents baseline with 5 ml filling. As the volume distention occurs with filling from 20 ml to 60 ml, the EGJ is visualized on the 3-D images as a narrowing and low diameter. At time point 3, the EGJ initially opens at an intra-balloon pressure of 24 mmHg and the distensibility index is 1.0 mm2/mmHg. At time point 4 with a 60 ml filling volume, the diameter is 12.5 mm and the pressure is 42 mmHg with a distensibility index of 2.9 which is right at the cut-off value of 2.8 mm2/mmHg for achalasia. The concomitant FLIP topography displays the narrowed zone at the EGJ that appears to remain relatively closed through most of the study and never opens to a level greater than 12.5 mm. In addition, the contractile activity in the body of the esophagus is erratic with both antegrade and retrograde contractions suggesting a motility disorder.
Figure used with permission from the Esophageal Center at Northwestern.
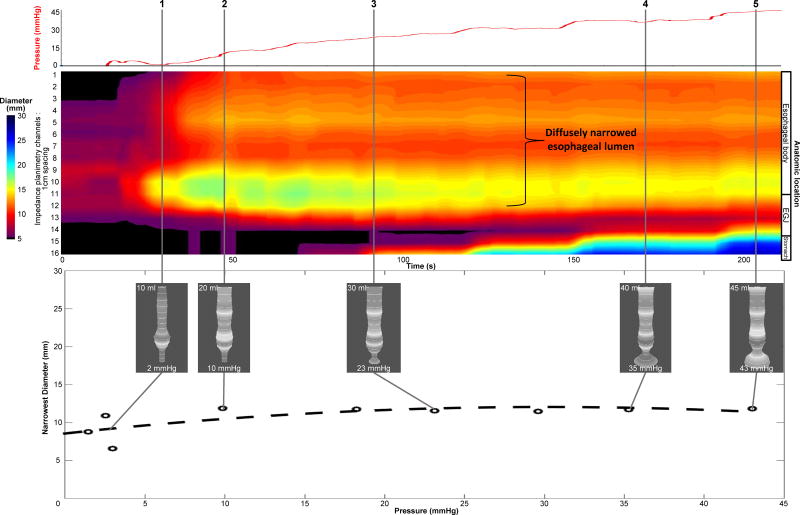
Data analysis techniques have also been modified and refined in the assessment of disease activity in EoE (Figure 3). The distensibility plateau is the metric of primary interest in EoE and represents the point of narrowest diameter (or CSA) along the esophagus that demonstrates resistance to further distention during an escalation in balloon pressure13. This is derived by plotting the narrowest CSA across the esophageal body as a function of pressure and has been shown to be substantially different between asymptomatic controls and EoE patients both with and without stricture. This analysis may also be hampered by catheter movement and esophageal contractility being misinterpreted as a fixed luminal narrowing and thus, various analysis approaches have been used, such as wavelet decomposition (WD), maximal diameter (MD), and FLIP Analytics software filtering. A recent study comparing these three approaches suggests that the MD approach using the maximal diameter attained at each axial location may be the most accurate10. Additionally, FLIP topography can also be utilized to assess areas of narrowing along the body over the entire study to assess the distensibility plateau.
Figure 3.
Example of data acquisition and analysis in eosinophilic esophagitis using filtering schemes and post-acquisition processing to remove the effect of contraction on the assessment of distensibility plateau and restricting diameter. The maximal diameter technique was used to remove the contraction effect and thus, FLIP topography will more accurately display passive lumen geometry. The narrowest diameter and pressure values are plotted in the bottom panel with simultaneous 3-D images from real-time acquisition displayed at time points along the FLIP topography plot. The distensibility plateau is approximately 11 mm (diameter) or 95 mm2 (cross-sectional area). In addition, FLIP topography also supports that the esophagus is diffusely narrowed through the recording segment of the esophagus.
Figure used with permission from the Esophageal Center at Northwestern.
There are limitations in the current analysis techniques as most require post-acquisition processing and specialized programming using MatLAB ™ and are thus, not widely available. However, the manufacturer is developing real-time FLIP topography and analytics of MD in the future software updates.
Achalasia
A hallmark of achalasia is lower esophageal sphincter (LES) dysfunction and achalasia treatments target the resultant EGJ outflow obstruction; thus FLIP appears well suited for evaluation of patients with achalasia.
Studies utilizing FLIP have consistently demonstrated an abnormal reduced EGJ-DI (i.e. more narrowed EGJ diameter at greater distending-pressure) in treatment naïve achalasia patients5, 14, 15. Further, studies assessing achalasia treatment response (e.g. after pneumatic dilation or LES myotomy) have reported lower EGJ-DI in patients with poor symptomatic outcomes (particularly below a threshold of 2.8 – 2.9 mm2/mmHg) than in patients with good symptomatic outcomes 5, 14; they also suggest that EGJ-DI may have a stronger association with symptoms and esophageal retention (per timed-barium esophagram) than manometric measures of LES pressures. FLIP may better characterize EGJ function as the degree of luminal opening is conceptually a more important determinant of bolus flow than LES relaxation. Additionally, evaluation of esophageal contractility (described in more detail below) with achalasia may also provide complementary clinical information to the standard achalasia assessment with esophageal manometry by further refining esophageal body contractile patterns and subtypes (Figure 2B) 11, 12.
Intra-operative use of FLIP during laparoscopic Heller myotomy or per-oral endoscopic myotomy (POEM) also offers the ability to assess the LES myotomy in real-time. Several studies have demonstrated an immediate increase in EGJ-DI following POEM or Heller myotomy (associated with a slight decrease following fundoplasty creation)16–20. Further, intra-operative FLIP may provide a method to ‘tailor’ the myotomy +/− fundoplasty to help improve dysphagia (i.e. avoid too low EGJ distensibility), while limiting GERD (i.e. avoid overly-increasing EGJ-distensibility). A recent multi-center study assessed the association with early clinical outcomes (median follow-up 122 days) and retrospectively-evaluated final intra-operative FLIP measures of 63 patients with achalasia treated with POEM 18. They found a lower EGJ-CSA at a 30-ml fill volume of an 8-cm FLIP (but similar EGJ-CSA at 40-ml) in those with a poor symptomatic outcome (n = 13) than those with a good outcome (n = 50). EGJ-DI was numerically, but not statistically, lower among the poor outcome group at both 30 and 40-ml fill volumes. Another recent study reported that a final intra-operative EGJ-DI (at 40-ml distension volume with an 8-cm FLIP) of 4.5 – 8.5 mm2/mmHg was less likely to have dysphagia or GERD symptoms at > 6 months follow-up after POEM (n = 21) or laparoscopic Heller myotomy (n = 11)21. Though additional study is needed to validate these findings in a prospective manner, FLIP offers significant promise to aid in the treatment of achalasia.
Additionally, esophageal dilatation balloons (incorporating a more rigid balloon material than the measurement FLIP balloons) are available (Table 1). There is no intra-bag pressure sensor included with the dilation balloons and thus, the diameter alone is utilized to target and follow dilation effect22. A recent study demonstrated the feasibility and clinical effectiveness of using the Eso-FLIP™ achalasia hydraulic dilation balloon (maximal diameter of 30-mm)22. As the EGJ can be identified with a waist on the real-time display to position the balloon, this device may allow for achalasia dilation to be performed without the concurrent use of fluoroscopy.
In conclusion, FLIP appears to be useful in the evaluation of achalasia and thus, should be used to provide additive information beyond manometry in the management of achalasia.
Gastroesophageal reflux disease
As EGJ incompetence may contribute to GERD pathophysiology in some patients, it is reasonable to suspect that FLIP may help identify increased EGJ-distensibility (i.e. greater EGJ diameters at lower distending-pressures) in some patients with GERD. Results of studies, however, have been inconsistent. Initially, a study demonstrated greater EGJ-DIs among 20 symptomatic GERD patients than 20 asymptomatic volunteers4. However, another study that compared patients with typical GERD symptoms (n = 18) and 48-hour wireless esophageal pH monitoring with asymptomatic controls (n = 18) found that GERD patients actually had lower EGJ-DI than controls7. Further, EGJ-DI did not differ between patients with normal (n = 9) or abnormal (n = 9) esophageal acid exposure time. Recently, a study evaluating patients with Barrett’s esophagus with hiatal hernia ≥ 2cm (n = 23) reported that FLIP could generally identify hiatal hernia, measure both distensibility of LES and crural diaphragm components of the EGJ, and that the distensibility of the LES in hiatal hernia patients was greater than asymptomatic controls23. Although the diagnostic utility of FLIP in GERD may remain in question, further study may help evaluate physiologic components related to GERD pathophysiology.
As patients with fundoplication appear to have reduced EGJ distensibility compared with controls, 4 FLIP may be a useful tool to aid anti-reflux procedures (such as fundoplication)24. Several studies demonstrated the feasibility of intra-operative FLIP use during fundoplication and a consistent reduction in EGJ-distensibility immediately following fundoplication24, 25. Reduction in EGJ-distensibility following transoral incisionless fundoplication (TIF) has also been reported26, 27. A report of pooled results of intra-operative FLIP during laparoscopic Nissen fundoplication from 4 high-volume centers reported a mean final EGJ Diameter of 6.1 mm(SD 1.4) and EGJ-DI of 1.6 mm2/mmHg (SD 1.1), suggesting these as targets for fundoplication based on historically good outcomes from their centers; however, directly-associated clinical outcomes were not reported24.
In summary, the role of FLIP for physiologic evaluation and management in GERD remains appealing, however, the level of evidence is low and currently FLIP should not be used in routine GERD management. Future outcome studies are needed to substantiate the utility of FLIP in GERD and to develop metrics that predict severity and treatment response after antireflux procedures.
Eosinophilic esophagitis
Eosinophilic esophagitis (EoE) is a chronic, immune-mediated disease of the esophagus characterized by esophageal symptoms (predominantly dysphagia/food impaction) and eosinophilic inflammation28. Chronic inflammation is thought to progress to fibrosis of the esophagus leading to luminal narrowing and a loss in mural compliance29. These remodeling consequences of EoE are the primary determinant of symptoms and are typically assessed by means of endoscopy30. Unfortunately, endoscopic assessment of esophageal narrowing in EoE has substantial limitations in terms of accuracy when compared to fluoroscopic imaging31.
FLIP assessment of the esophageal body in EoE allows for objective and accurate measurement of esophageal narrowing and mechanical properties of the esophageal body Measurement of a narrowest esophageal body CSA and corresponding intra-bag pressure during step-wise volumetric distension with the FLIP positioned in the esophageal body allows identification of the distensibility plateau (Figure 3). The predictive value of this assessment was reported in a study of EoE patients using requirement for dilation therapy and risk of food impaction as endpoints. This study supported that a distensibility plateau < 225 mm2 (diameter ~17mm) was the only independent predictor (i.e. not treatment type or eosinophil count, among others) of future food impaction. Although the severity of endoscopically identified esophageal signs of remodeling (rings) are associated with esophageal distensibility, FLIP offers significantly greater accuracy and precision in estimating the effects of remodeling32.
While FLIP appears to have significant value for the assessment of disease severity and clinically relevant symptom outcomes in EoE, the identification of the narrowest portion of the esophageal body used in the calculation of the distensibility plateau is often technically challenging in the setting of esophageal contractions. Thus post-processing the FLIP data using software programs to filter out the contraction-associated changes in esophageal luminal diameter is necessary 8–10. Software enhancements are being developed that improve the current analysis paradigms and should broaden the clinical utility of FLIP in the evaluation of EoE.
In summary, FLIP appears to be uniquely suited to assess the mechanical properties of the esophageal wall in EoE. Nonetheless, current recommendations are limited by the low level of evidence and lack of generalized availability of the analysis paradigms. Thus, one could consider performing FLIP in routine clinical management of EoE if the analysis expertise is available. Further outcome studies that validate the distensibility plateau threshold and further refinements in software analyses to make this methodology more generalizable will provide important.
Other indications/ Future Directions
Although distention mediated peristalsis observed on FLIP can make assessment of passive mechanical properties slightly more complex, the information within these motor responses may be helpful in evaluating esophageal function. Serially-spaced diameter changes along the axial length of the balloon that are recorded by FLIP can be used to define contractile activity similar to high-resolution manometry11. A recent study comparing data acquired during a FLIP assessment during sedated endoscopy and standard high-resolution manometry suggests that FLIP can accurately diagnose achalasia and may be complementary to manometry in further refining the diagnosis of functional dysphagia (normal endoscopy/normal or borderline manometry)12. Applying this as a tool to assess hypersensitivity in a barostat mode application is also appealing, but currently balloon distention studies are relegated to specialized centers.
The EndoFLIP is also being utilized to assess opening dynamics and mechanical properties of other anatomic zones of interest. Data has been reported in the upper esophageal sphincter33 and there is also great interest in studying the pylorus in normal rhythm gastroparetic patients and anal-rectal function in patients with constipation and incontinence34. Lastly, the technique is still being assessed for its ability to tailor bariatric procedures and currently it is FDA approved for this indication.
Conclusions
The FLIP offers an innovative method that enhances the assessment of esophageal function in various diseases. Although the strongest data appears to be focused on the management of achalasia, emerging evidence supports the clinically relevance of FLIP in the assessment of disease severity and as an outcome measure in EoE intervention trials. Ongoing studies are evaluating the applications of FLIP to GERD interventions targeting the esophagogastric junction as well as other foregut disorders. More work is needed, however, that focuses on optimizing data analysis, standardizing protocols and defining outcome metrics prior to the widespread adoption into general clinical practice.
Best Practice Advice 1: Clinicians should not make a diagnosis or treatment decision based on functional lumen imaging probe (FLIP) assessment alone.
Best Practice Advice 2: FLIP assessment is a complementary tool to assess esophagogastric junction opening dynamics and the stiffness of the esophageal wall.
Best Practice Advice 3: Utilization should follow distinct protocols and analysis paradigms based on the disease state of interest.
Best Practice Advice 4: Clinicians should not utilize FLIP in routine diagnostic assessments of gastroesophageal reflux disease.
Best Practice Advice 5: FLIP should not be used to diagnose eosinophilic esophagitis but may have a role in severity assessment and therapeutic monitoring.
Acknowledgments
Dustin Carlson and Zhiyue Lin for their help with developing Figures 2 and 3.
Funding:
NIDDK
Abbreviations
- CSA
cross-sectional area
- DI
distensibility index
- EoE
eosinophilic esophagitis
- EGJ
esophagogastric junction
- FLIP
functional lumen imaging probe
- EndoFLIP®
Endolumenal Functional Lumen Imaging Probe
- GERD
gastroesophgeal reflux disease
- LES
lower esophageal sphincter
- POEM
per-oral endoscopic myotomy
- TIF
transoral incisionless fundoplication
- UES
upper esophageal sphincter
Footnotes
Conflicts of interest:
John Pandolfino: Medtronic Inc, Sandhill Scientific
Ikuo Hirano: None
Guy Boeckxstaens: None
References
- 1.Kahrilas PJ, Bredenoord AJ, Fox M, et al. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol Motil. 2014 doi: 10.1111/nmo.12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tutuian R, Castell DO. Combined multichannel intraluminal impedance and manometry clarifies esophageal function abnormalities: study in 350 patients. Am J Gastroenterol. 2004;99:1011–9. doi: 10.1111/j.1572-0241.2004.30035.x. [DOI] [PubMed] [Google Scholar]
- 3.McMahon BP, Frokaer JB, Drewes AM, et al. A new measurement of oesophago-gastric junction competence. Neurogastroenterol Motil. 2004;16:543–6. doi: 10.1111/j.1365-2982.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- 4.Kwiatek MA, Kahrilas K, Soper NJ, et al. Esophagogastric junction distensibility after fundoplication assessed with a novel functional luminal imaging probe. J Gastrointest Surg. 2010;14:268–76. doi: 10.1007/s11605-009-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohof WO, Hirsch DP, Kessing BF, et al. Efficacy of Treatment for Patients With Achalasia Depends on the Distensibility of the Esophagogastric Junction. Gastroenterology. 2012 doi: 10.1053/j.gastro.2012.04.048. [DOI] [PubMed] [Google Scholar]
- 6.Verlaan T, Rohof WO, Bredenoord AJ, et al. Effect of peroral endoscopic myotomy on esophagogastric junction physiology in patients with achalasia. Gastrointest Endosc. 2013;78:39–44. doi: 10.1016/j.gie.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Tucker E, Sweis R, Anggiansah A, et al. Measurement of esophago-gastric junction cross-sectional area and distensibility by an endolumenal functional lumen imaging probe for the diagnosis of gastro-esophageal reflux disease. Neurogastroenterol Motil. 2013;25:904–10. doi: 10.1111/nmo.12218. [DOI] [PubMed] [Google Scholar]
- 8.Lin Z, Kahrilas PJ, Xiao Y, et al. Functional luminal imaging probe topography: an improved method for characterizing esophageal distensibility in eosinophilic esophagitis. Therap Adv Gastroenterol. 2013;6:97–107. doi: 10.1177/1756283X12470017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin Z, Nicodeme F, Boris L, et al. Regional variation in distal esophagus distensibility assessed using the functional luminal imaging probe (FLIP) Neurogastroenterol Motil. 2013 doi: 10.1111/nmo.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlson DA, Lin Z, Hirano I, et al. Evaluation of esophageal distensibility in eosinophilic esophagitis: an update and comparison of functional lumen imaging probe analytic methods. Neurogastroenterol Motil. 2016 doi: 10.1111/nmo.12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlson DA, Lin Z, Rogers MC, et al. Utilizing functional lumen imaging probe topography to evaluate esophageal contractility during volumetric distention: a pilot study. Neurogastroenterol Motil. 2015;27:981–9. doi: 10.1111/nmo.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlson DA, Lin Z, Kahrilas PJ, et al. The Functional Lumen Imaging Probe Detects Esophageal Contractility Not Observed With Manometry in Patients With Achalasia. Gastroenterology. 2015;149:1742–51. doi: 10.1053/j.gastro.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwiatek MA, Hirano I, Kahrilas PJ, et al. Mechanical properties of the esophagus in eosinophilic esophagitis. Gastroenterology. 2011;140:82–90. doi: 10.1053/j.gastro.2010.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pandolfino JE, de Ruigh A, Nicodeme F, et al. Distensibility of the esophagogastric junction assessed with the functional lumen imaging probe (FLIP) in achalasia patients. Neurogastroenterol Motil. 2013 doi: 10.1111/nmo.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rieder E, Swanstrom LL, Perretta S, et al. Intraoperative assessment of esophagogastric junction distensibility during per oral endoscopic myotomy (POEM) for esophageal motility disorders. Surg Endosc. 2013;27:400–5. doi: 10.1007/s00464-012-2484-0. [DOI] [PubMed] [Google Scholar]
- 16.Teitelbaum EN, Boris L, Arafat FO, et al. Comparison of esophagogastric junction distensibility changes during POEM and Heller myotomy using intraoperative FLIP. Surg Endosc. 2013 doi: 10.1007/s00464-013-3121-2. [DOI] [PubMed] [Google Scholar]
- 17.Teitelbaum EN, Soper NJ, Pandolfino JE, et al. An extended proximal esophageal myotomy is necessary to normalize EGJ distensibility during Heller myotomy for achalasia, but not POEM. Surg Endosc. 2014 doi: 10.1007/s00464-014-3563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ngamruengphong S, von Rahden BH, Filser J, et al. Intraoperative measurement of esophagogastric junction cross-sectional area by impedance planimetry correlates with clinical outcomes of peroral endoscopic myotomy for achalasia: a multicenter study. Surg Endosc. 2016;30:2886–94. doi: 10.1007/s00464-015-4574-2. [DOI] [PubMed] [Google Scholar]
- 19.Teitelbaum EN, Soper NJ, Pandolfino JE, et al. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc. 2014 doi: 10.1007/s00464-014-3733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teitelbaum EN, Sternbach JM, El Khoury R, et al. The effect of incremental distal gastric myotomy lengths on EGJ distensibility during POEM for achalasia. Surg Endosc. 2016;30:745–50. doi: 10.1007/s00464-015-4269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teitelbaum EN, Soper NJ, Pandolfino JE, et al. Esophagogastric junction distensibility measurements during Heller myotomy and POEM for achalasia predict postoperative symptomatic outcomes. Surg Endosc. 2015;29:522–8. doi: 10.1007/s00464-014-3733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kappelle WF, Bogte A, Siersema PD. Hydraulic dilation with a shape-measuring balloon in idiopathic achalasia: a feasibility study. Endoscopy. 2015;47:1028–34. doi: 10.1055/s-0034-1392481. [DOI] [PubMed] [Google Scholar]
- 23.Lottrup C, McMahon BP, Ejstrud P, et al. Esophagogastric junction distensibility in hiatus hernia. Dis Esophagus. 2015 doi: 10.1111/dote.12344. [DOI] [PubMed] [Google Scholar]
- 24.Perretta S, Dallemagne B, McMahon B, et al. Video. Improving functional esophageal surgery with a "smart" bougie: Endoflip. Surg Endosc. 2011;25:3109. doi: 10.1007/s00464-011-1611-7. [DOI] [PubMed] [Google Scholar]
- 25.Ilczyszyn A, Botha AJ. Feasibility of esophagogastric junction distensibility measurement during Nissen fundoplication. Dis Esophagus. 2014;27:637–44. doi: 10.1111/dote.12130. [DOI] [PubMed] [Google Scholar]
- 26.Hoppo T, McMahon BP, Witteman BP, et al. Functional lumen imaging probe to assess geometric changes in the esophagogastric junction following endolumenal fundoplication. J Gastrointest Surg. 2011;15:1112–20. doi: 10.1007/s11605-011-1562-2. [DOI] [PubMed] [Google Scholar]
- 27.Rinsma NF, Smeets FG, Bruls DW, et al. Effect of transoral incisionless fundoplication on reflux mechanisms. Surg Endosc. 2014;28:941–9. doi: 10.1007/s00464-013-3250-7. [DOI] [PubMed] [Google Scholar]
- 28.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 29.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145:1230–6. e1–2. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Hirano I, Aceves SS. Clinical implications and pathogenesis of esophageal remodeling in eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43:297–316. doi: 10.1016/j.gtc.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gentile N, Katzka D, Ravi K, et al. Oesophageal narrowing is common and frequently under-appreciated at endoscopy in patients with oesophageal eosinophilia. Aliment Pharmacol Ther. 2014;40:1333–40. doi: 10.1111/apt.12977. [DOI] [PubMed] [Google Scholar]
- 32.Chen JW, Pandolfino JE, Lin Z, et al. Severity of endoscopically identified esophageal rings correlates with reduced esophageal distensibility in eosinophilic esophagitis. Endoscopy. 2016 doi: 10.1055/s-0042-107340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Regan J, Walshe M, Rommel N, et al. A new evaluation of the upper esophageal sphincter using the functional lumen imaging probe: a preliminary report. Dis Esophagus. 2013;26:117–23. doi: 10.1111/j.1442-2050.2012.01331.x. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen G, Liao D, Lundby L, et al. Distensibility of the anal canal in patients with idiopathic fecal incontinence: a study with the Functional Lumen Imaging Probe. Neurogastroenterol Motil. 2014;26:255–63. doi: 10.1111/nmo.12258. [DOI] [PubMed] [Google Scholar]
- 35.Beaumont H, Gondrie JJ, McMahon BP, et al. Stepwise radiofrequency ablation of Barrett's esophagus preserves esophageal inner diameter, compliance, and motility. Endoscopy. 2009;41:2–8. doi: 10.1055/s-0028-1103451. [DOI] [PubMed] [Google Scholar]
- 36.Nathanson LK, Brunott N, Cavallucci D. Adult esophagogastric junction distensibility during general anesthesia assessed with an endoscopic functional luminal imaging probe (EndoFLIP(R)) Surg Endosc. 2012;26:1051–5. doi: 10.1007/s00464-011-1996-3. [DOI] [PubMed] [Google Scholar]
- 37.Fukazawa K, Furuta K, Adachi K, et al. Effects of mosapride on esophageal motor activity and esophagogastric junction compliance in healthy volunteers. J Gastroenterol. 2014;49:1307–13. doi: 10.1007/s00535-013-0876-0. [DOI] [PubMed] [Google Scholar]
- 38.Lottrup C, Gregersen H, Liao D, et al. Functional lumen imaging of the gastrointestinal tract. J Gastroenterol. 2015;50:1005–16. doi: 10.1007/s00535-015-1087-7. [DOI] [PubMed] [Google Scholar]
- 39.Mikami H, Ishimura N, Fukazawa K, et al. Effects of Metoclopramide on Esophageal Motor Activity and Esophagogastric Junction Compliance in Healthy Volunteers. J Neurogastroenterol Motil. 2016;22:112–7. doi: 10.5056/jnm15130. [DOI] [PMC free article] [PubMed] [Google Scholar]