Despite advances in our understanding of heritable lipid disorders and the availability of highly effective lipid-lowering drugs, the awareness, detection, and control of familial hypercholesterolemia (FH) remain suboptimal.1 A major reason for the low detection rate in the United States is the lack of a widely accepted screening strategy, despite the recommendations for universal or targeted lipid screening by several expert panels. While the utility of universal lipid screening remains a matter of debate, cascade screening (a form of targeted screening of family members of affected individuals) is acknowledged as the most cost-effective screening strategy for FH. In the Netherlands >26,000 new cases were identified over 2 decades by genotyping family members of FH probands and it is estimated that genetic cascade screening, coupled with statin therapy for diagnosed patients, could save $92 million per year in the European Union.1
Several factors lead to the low rates of cascade screening for FH in the United States. First, no nationwide strategy for the early detection of FH exists. Second, the very low acceptance of genetic testing for FH in the United States is an impediment to unambiguous diagnosis and cascade screening. Third, patients and family members are often concerned about the stigma associated with genetic diagnoses. Fourth, because of the Health Insurance Portability and Accountability Act, disclosing the risk of genetic disease to family members can incur liability, even if this knowledge leads to early detection and treatment. Fifth, practitioners have poor awareness about FH and often are unable to implement recommended therapies and cascade screening. In this commentary we discuss how health information technology, the internet and social media could be harnessed to overcome these barriers (Figure).
Figure. Use of health information technology, internet and social media to increase awareness, detection, and control of familial hypercholesterolemia (FH).
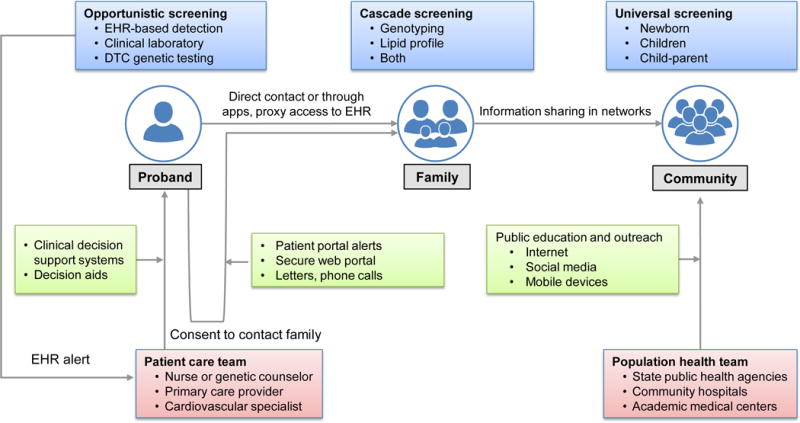
Electronic health record strategies (automated detection by electronic phenotyping algorithms with linkage to clinical decision support, decision aids to promote shared decision making), the web, social media and mobile devices can be leveraged to increase awareness, detection, and control of FH, obtain consent to inform family members and for public education and outreach. The major stakeholders include patient, family and community, patient care and population health teams; and the available modes of screening are: opportunistic, cascade and universal. DTC, direct-to-consumer; EHR, electronic health record.
Lack of a National Cascade Screening Program
Although the Office of Public Health Genomics at the Centers for Disease Control and Prevention has prioritized the detection of prevalent and actionable (ie, Tier 1) genetic disorders such as FH, no formal guidelines or recommendations in the United States exist regarding cascade testing for FH.2 Health Information Exchanges that link medical centers to local and regional public health agencies can facilitate case detection and subsequent cascade screening (Figure). The awareness and acceptance of such programs by the general public could be promoted through the Internet and social media.
Limited Use of Genetic Testing
Genetic testing is useful for confirming diagnosis in the proband and then identifying family members with FH. The acceptance of genetic testing for FH in the United States has been poor, partly because genetic counseling and testing for FH is not always readily available; furthermore, testing is expensive and inconsistently covered by payers. Electronic health record (EHR) based alerts could suggest genetic testing for FH in the appropriate clinical setting. As the cost of genomic sequencing continues to decrease, relatively low-cost screening panels for genetic disorders are becoming available directly to the consumer on the internet, with access to a genetic counselor and the option for follow up testing in family members. An important caveat is that a significant proportion of FH patients do not have an identifiable pathogenic variant and in such cases relatives have to be screened with a lipid profile.
Patient Concerns about Genetic Diagnoses
Patients may be concerned about stigmatization after a genetic diagnosis and potential implications for employment and health insurance coverage. They are often unaware that the Genetic Information Nondiscrimination Act of 2008 prohibits unauthorized disclosure of genetic test results to their employer to prevent discrimination on the basis of that information (such protection, however, does not extend to life or disability insurance). Education of patients and care providers including through links in the electronic health record (EHR), patient portals, internet and social media may promote genetic literacy, increase awareness about the rationale for genetic testing for FH, and lessen the potential for discrimination.
Challenges in Cascade Screening of Family Members
Cascade screening requires balancing privacy of individuals undergoing genetic testing and the need to notify family members of actionable genetic information; testing relatives of a proband is not allowed unless they are registered as patients. Direct contact of family members by health care providers appears to be more effective than contact by the proband, and this approach could be implemented with the proband’s consent.3 If the proband refuses consent and is also unwilling to inform family members, some would argue that the health care provider should directly contact family members.4 There is a need to establish methods for contacting family members that are acceptable to probands and their at-risk family members and to reduce the burden of notification on the patients and care providers. The latter could be facilitated by innovative software applications that allow sharing of relevant health information among family members through mobile devices or by permitting proxy access to the family history section of EHRs. Improved EHR interoperability will enable identification and contact of at-risk relatives, but such data sharing will require the engagement of an informed general public and the medical community.
Low Awareness, Detection, and Control of FH
Increased physician awareness of FH would enable them to target the interview and physical examination to make the diagnosis of FH. In particular, a family history of early-onset CHD or hypercholesterolemia is an important clue to the presence of FH and should be routinely elicited. Health care providers could also ascertain whether a patient meets the diagnostic criteria for FH using available Apps. Automated detection of FH using an electronic phenotyping algorithm (www.phekb.org) can increase the proportion of FH patients who receive the diagnosis.5 Documenting family history as a structured data element in the EHR and using available diagnostic codes for FH will increase the accuracy of such algorithms. Case detection should be linked to clinical decision support to provide guidance to busy practitioners for evaluating and managing FH at the point of care and thereby increasing awareness, detection, and control of FH (Figure).6 In addition, FH-specific decision aids in the EHR may facilitate shared decision-making regarding drug treatments, as well as screening of family members.
In conclusion, advances in genomics have had relatively limited impact on public health, so far. FH is an example of a prevalent and easily treated genetic disorder with persisting gaps in detection and treatment. There is an opportunity to bring precision medicine to public health by establishing partnerships at the federal, state, and local levels among various stakeholders. Health information technology, the internet and social media will be vital to the success of initiatives to reduce the burden of FH.
Footnotes
Disclosures
Dr. Kullo is a consultant for Color Genomics.
References
- 1.Nordestgaard BG, Chapman MJ, Humphries SE, Ginsberg HN, Masana L, Descamps OS, Wiklund O, Hegele RA, Raal FJ, Defesche JC, Wiegman A, Santos RD, Watts GF, Parhofer KG, Hovingh GK, Kovanen PT, Boileau C, Averna M, Boren J, Bruckert E, Catapano AL, Kuivenhoven JA, Pajukanta P, Ray K, Stalenhoef AF, Stroes E, Taskinen MR, Tybjaerg-Hansen A. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus statement of the European Atherosclerosis Society. Eur Heart J. 2013;34:3478–3490a. doi: 10.1093/eurheartj/eht273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowles JW, Rader DJ, Khoury MJ. Cascade screening for familial hypercholesterolemia and the use of genetic testing. JAMA. 2017;318:381–382. doi: 10.1001/jama.2017.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newson AJ, Humphries SE. Cascade testing in familial hypercholesterolaemia: How should family members be contacted? Eur J Hum Genet. 2005;13:401–408. doi: 10.1038/sj.ejhg.5201360. [DOI] [PubMed] [Google Scholar]
- 4.Knoppers BM, Joly Y, Simard J, Durocher F. The emergence of an ethical duty to disclose genetic research results: International perspectives. Eur J Hum Genet. 2006;14:1170–1178. doi: 10.1038/sj.ejhg.5201690. [DOI] [PubMed] [Google Scholar]
- 5.Safarova M, Liu H, Kullo IJ. Rapid identification of familial hypercholesterolemia from electronic health records: The SEARCH study. J Clin Lipidol. 2016;10:1230–1239. doi: 10.1016/j.jacl.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kullo IJ, Jarvik GP, Manolio TA, Williams MS, Roden DM. Leveraging the electronic health record to implement genomic medicine. Genet Med. 2013;15:270–271. doi: 10.1038/gim.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]


