Abstract
Development of the brain, including the prefrontal cortex and hippocampus, continues through adolescence. Chronic nicotine exposure during adolescence may contribute to long-term deficits in forebrain-dependent learning. It is unclear if these deficits emerge immediately after exposure and if they can be ameliorated. In this study, C57BL/6J mice were treated with chronic nicotine (6.3 or 12.6 mg/kg/day) over 12 days beginning at adolescence, postnatal day (PND) 38, or adulthood, PND 56–63±3. We investigated the effects of short-term (24 hr) abstinence on trace fear conditioning and found that adult treatment resulted in deficits (6.3 and 12.6 mg/kg/day), but adolescent chronic nicotine treatment had no effect. In contrast, adolescent treatment with chronic nicotine (12.6 mg/kg/day) elicited a long-term (30 days) learning deficit, but adult chronic nicotine treatment did not. Using the elevated plus maze (EPM) we found no long-term changes in anxiety-related behavior after chronic nicotine exposure at either time-point. We investigated if chronic fluoxetine (FLX) could ameliorate adolescent chronic nicotine-associated long-term deficits in trace conditioning. We found that chronic FLX (160 mg/L) in drinking water ameliorated the long-term deficit in trace fear conditioning associated with nicotine exposure during adolescence. Additionally, in the same animals, we examined changes in total BDNF protein in the dorsal hippocampus (DH), ventral hippocampus (VH), and prefrontal cortex (PFC). Chronic FLX increased DH BDNF. Our data indicate nicotine administration during adolescence leads to late onset, long-lasting deficits in hippocampus-dependent learning that chronic FLX treatment ameliorate.
Keywords: adolescence, nicotine, cognition, fluoxetine, learning, addiction
1. Introduction
Adolescence is associated with increased risk taking and impulsivity (Spear, 2000), along with rapid neurocognitive developmental changes (Gogtay et al., 2004). Furthermore, the adolescent brain responds differently to drugs of abuse compared to that of the adult (Faraday, Elliott, & Grunberg, 2001; Portugal, Wilkinson, Turner, Blendy, & Gould, 2012; Reynolds et al., 2015; Slotkin, Card, & Seidler, 2014; Xu et al., 2002). Increased impulsivity and risk taking during adolescence may be adaptive under normal conditions; however, it is also thought to drive experimentation with drugs, which can lead to dependence (Chassin et al., 1990; Counotte et al., 2011). For example, initiation of tobacco use most often occurs during adolescence and individuals that initiate smoking during adolescence show increased tobacco consumption later as adults (Chen and Millar, 1998). A critical feature of adolescent neurodevelopment is the maturation of dopaminergic, serotonergic, and cholinergic systems (An et al., 2008; Gould, Woolf, & Butcher, 1991; Xu, Seidler, Cousins, Slikker Jr., & Slotkin, 2002), and nicotine, the primary psychoactive compound in tobacco, is known to readily act on these neurotransmitter systems (Davis and Gould, 2007a; Dong et al., 2009; Xu et al., 2002). Therefore, during adolescence, individuals are both at risk for initiation of nicotine use and differentially susceptible to the effects of nicotine.
Nicotine can potently alter cognition. For example, acute nicotine enhances associative learning (Gould and Wehner, 1999; Wehner et al., 2004), via high-affinity nicotinic receptors (nAChRs) within the hippocampus and PFC (Kenney et al., 2012; Raybuck and Gould, 2010). In contrast, immediate abstinence (withdrawal) from chronic nicotine elicits deficits in hippocampus-dependent learning in adult mice (Davis et al., 2005; Davis and Gould, 2009; Gould et al., 2012; Raybuck and Gould, 2009). Adolescence appears be a time of unique vulnerability to nicotine-dependent changes in cognition. Importantly, adolescent animals treated with chronic nicotine present with hippocampal learning deficits after 30 days abstinence in adulthood, but adult nicotine-treated animals did not show long-term deficits (Holliday et al., 2016; Portugal et al., 2012).Thus, exposure to nicotine during adolescence can lead to age-dependent long-term alterations in cognition persisting into adulthood.
Chronic nicotine exposure during adolescence elicits long-term deficits in hippocampus-dependent contextual fear conditioning, but not delay cued fear conditioning, a non-hippocampal learning paradigm (Holliday et al., 2016; Portugal et al., 2012). However, it is not known if long-term deficits due to adolescent chronic nicotine are confined to contextual fear learning, or if these deficits present in other hippocampal fear learning tasks. Thus, we decided to examine the effects of prior chronic nicotine exposure on trace fear conditioning, which like delay conditioning also depends on forming a discrete CS-US association. However, unlike delay conditioning in which the CS and US temporally overlap, the CS and US in trace conditioning are temporally separated making the task hippocampus and PFC-dependent and may model aspects of working and declarative memory (Connor and Gould, 2016). Moreover, the PFC and hippocampus undergo maturation during adolescence (Gogtay et al., 2004) and have previously been shown to be altered by adolescent nicotine exposure (Goriounova and Mansvelder, 2012; Holliday et al., 2016; Portugal et al., 2012). Therefore, we hypothesized that chronic treatment during adolescence, but not adulthood would lead to long-term deficits in trace fear conditioning. In addition, because adolescent nicotine exposure can also elicit long-term affective changes (Holliday et al., 2016; Slawecki et al., 2003), we also probed for long-term changes in innate anxiety in the EPM after adolescent or adult chronic nicotine exposure. Finally, immediate abstinence has been shown to result in short-term withdrawal deficits in trace fear conditioning in adult mice (Raybuck and Gould, 2009). However, the effects of short-term abstinence on trace fear conditioning in adolescent animals has not been studied. Therefore, we also investigated whether these deficits emerge immediately after chronic nicotine treatment during adolescence or have a later onset.
Long-term negative effects of adolescent chronic nicotine exposure on cognition support the need to identify potential treatments. For example, treatment with cholinergic agents can reduce the detrimental effects of immediate chronic nicotine withdrawal on cognition in adult animals (Poole et al., 2014; Yildirim et al., 2015). However, pharmacological treatments for the long-term effects of adolescent chronic nicotine exposure on hippocampal learning have not been similarly investigated. Neurobiological evidence suggests that adolescent nicotine exposure can elicit long-lasting dysregulation of the serotonergic system (Slotkin et al., 2016; Xu et al., 2002). Fluoxetine, a selective-serotonin reuptake inhibitor, has been shown to act on neural substrates of learning and ameliorate cognitive deficits associated with chemotherapeutic agents and in a mouse model of Alzheimer’s disease (Jin et al., 2016; Lyons et al., 2011). Adult fluoxetine (FLX) treatment also decreased negative affective outcomes in adulthood due to adolescent nicotine exposure (Iñiguez et al., 2008). Therefore, we hypothesized that chronic FLX treatment would ameliorate long-term trace fear conditioning deficits resulting from adolescent chronic nicotine exposure. In addition, FLX is known to increase brain derived neurotrophic factor (BDNF) gene expression in the PFC and hippocampus (Alme et al., 2007). BDNF is important for synaptic plasticity and learning and memory (Cunha et al., 2010; Korte et al., 1998; Mizuno et al., 2012) and the hippocampus sends BDNF containing projections to the PFC and BDNF within these regions plays an important role in associative learning (Heldt et al., 2007; Hoover and Vertes, 2007; Peters et al., 2010). Because nicotine has been shown to alter BDNF levels, we considered that cognitive deficits due to prior chronic nicotine exposure may be mediated in part by altered levels if BDNF, which could be rescued by treatment with FLX. Thus, we selected the dorsal, ventral and PFC as regions of interest to examine changes in BDNF protein after chronic FLX treatment.
2. Material and Methods
2.1. Subjects
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were used in all experiments. Adolescent mice were PND 31 on day of delivery and adults were PND 49 ± 3. Mice were allowed to acclimate to the vivarium for at least 1 week prior to the start of experiments. Mice were housed 2–4 per cage and provided food ad libitum. A 12 hour light-dark cycle was maintained throughout all studies. All behavioral procedures were approved by the Temple University Institutional Animal Care and Use Committee.
2.2. Drug administration and experimental design
For all experiments, doses of nicotine hydrogen tartrate salt (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% saline and reported as freebase weight. Chronic nicotine (12.6 mg/kg/day and 6.3 mg/kg/day) and saline was administered via subcutaneous osmotic minipumps (Alzet, Cupertino, CA). These doses of chronic nicotine and the rout of administration are based on previous findings showing contextual learning deficits after adult and adolescent chronic nicotine administration (Holliday et al., 2016; Portugal et al., 2012). Additionally, these doses of nicotine are within a range of doses that result in blood plasma nicotine and cotinine levels similar to regular smokers (Cole et al., 2014; Davis et al., 2005; Portugal et al., 2012).
Fluoxetine hydrochloride (Sigma-Aldrich) was administered ad libitum in drinking water (filtered tap water) in opaque light-protected bottles. FLX solutions were changed and refreshed 3 times per week. We administered FLX at 160 mg/L, this concentration was previously shown to increase hippocampal BDNF protein in mice (Bath et al., 2012; Dulawa et al., 2004). Because animals were housed in groups, exact dosage is not known. However, a pilot analysis of consumption patterns indicated that mice consumed ~ 2–3 ml per day of 160 mg/L solution, resulting in estimated 15.25 mg/kg dose over the treatment period. This dose value is similar to that used by Bath and colleagues (2012), who estimated ~16 mg/kg using the same 160 mg/L concentration.
Age-dependent effects of short-term (24hr) and long-term (30 days) abstinence from chronic nicotine
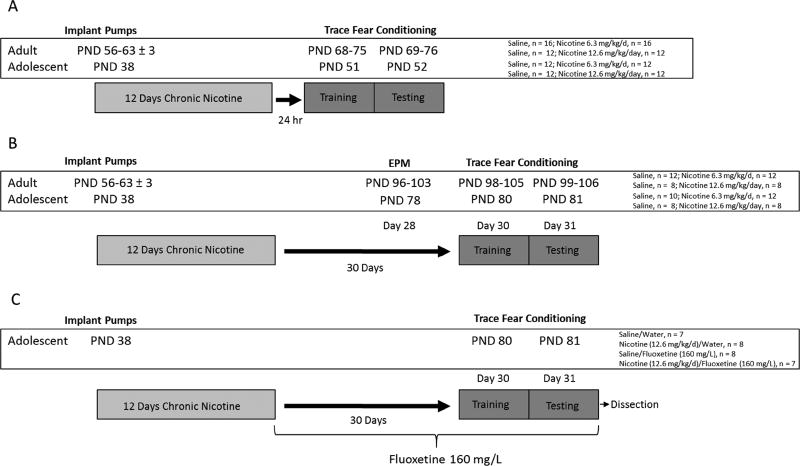
For each dose of chronic nicotine (12.6 and 6.3 mg/kg/day), we used a separate a cohort of mice with aged match saline controls. Mice were treated with chronic nicotine at 12.6 mg/kg/day or 6.3 mg/kg/day for 12 days beginning at adolescence (PND 38) or adulthood (PND 56–63 ± 3). On day 12 of chronic administration, osmotic mini-pumps were surgically removed and animals were returned to the colony room in home cages. To examine age-dependent effects of chronic nicotine exposure on short-term abstinence, mice were trained in trace fear conditioning after 24 hours abstinence and tested 24 hours after training. In a separate set of animals, age-dependent long-term effects of chronic nicotine were tested first in the EPM on day 28 post pump removal, then 2 days later (30 days after pump removal) these same mice were trained in trace fear conditioning and tested 24 hours later (Fig 1).
Figure 1.
Experimental design schematics for (A) short-term abstinence from chronic nicotine, (B) long-term abstinence from chronic nicotine, and (C) FLX treatment during long-term abstinence from adolescent chronic nicotine exposure.
Effects of chronic fluoxetine on long-term (30 day) deficits
To examine the effects of FLX on adolescent chronic nicotine-induced long-term deficits in trace conditioning, mice were treated with 12.6 mg/kg/day chronic nicotine for 12 days beginning at PND 38. On day 12, osmotic pumps were removed and mice were provided FLX 160 mg/L in their drinking water for the duration of the study (Fig 1). Thirty-one days after initiation of FLX treatment and immediately after trace fear conditioning testing mice were euthanized and brain tissue take for BDNF ELISA.
2.3. Behavioral Procedures
2.3.1.Trace Fear Conditioning
Mice were assigned and placed into one of four chambers for training, which occurred within a single session that included 5 trace CS (conditioned stimulus)-US (unconditioned stimulus) trials. Training commenced with a 120 second stimulus free baseline period followed by the first CS-trace-US presentation. All remaining CS-US trials were separated pseudorandomly by a variable intertrial interval (90–120 seconds). CS-US trials consisted of a 30 second white-noise CS (85 dB) and a 30 second stimulus free trace interval followed by a 2 second scrambled footshock US (0.57 mA).
Twenty-four hours after training, mice were tested for conditioned responding (CR) to the CS in a novel context. In a different room from that used for training, mice were placed in chambers with altered visual and tactile cues. For 3 minutes, freezing was observed in the altered context (Pre-CS). After the Pre-CS period, mice were presented with the CS for 3 minutes and assessed for freezing to the CS. Freezing, defined as the lack of movement except for respiration, was measured using a time-sampling method (each animal was observed for 1 second every 10 seconds by an experimenter blind to all drug conditions (Gould and Wehner, 1999). As previously reported (Davis and Gould, 2007b), inter-rater reliability for behavioral testing is >90%.
2.3.2. Elevated Plus Maze
Mice were brought into an area of the testing room separated from the maze by a curtain barrier and allowed to habituate for at least 1 hour prior to testing. Testing of each animal was initiated by placement in the center of the maze. Time spent in closed arms, open arms, and center was recorded over 5 minutes. Each session was video recorded and analyzed using SMART (Pan Lab) tracking software. Open arm data are expressed as percent time (open arm time/total time × 100). The maze was made of opaque grey Plexiglas situated atop a base 62.6 cm above the floor. The maze consisted of opposing open arms (7.6 × 30.6 cm) and opposing closed arms (7.6 × 30.6 × 15.5 cm) and center area (7.6 × 7.6 cm)
2.4. Minipump Surgeries
Prior to surgery, mice were anesthetized with isoflurane (5% induction, 2.5% maintenance), and then implanted with osmotic minipumps (Alzet, Cupertino, CA). Minipumps delivered chronic saline or nicotine (6.3 or 12.6 mg/kg/d) for 12 days and then were removed. Osmotic minipumps were surgically implanted subcutaneously via an incision posterior to the scapulae. The incision site was closed with surgical staples.
2.5. BDNF ELISA
Immediately after testing, mice from the FLX experiment were euthanized via cervical dislocation. After decapitation, brains were rapidly removed and bilateral DH, VH, and PFC tissue was isolated then flash frozen. PFC tissue was isolated by removing the olfactory bulb and making a coronal slice proximal to the middle cerebral artery and then removing striatal tissue (Spijker, 2011). Later, tissue was homogenized on ice via sonication in lysis buffer (RIPA + protease inhibitor cocktail, Cell Signaling). Homogenate was then centrifuged at 12,000 rpm for 30 minute to obtain tissue lysates. Supernatant was taken and stored in −80° C. Total protein levels were assayed using detergent compatible DC protein assay (BIO RAD). Total BDNF protein levels were quantified using BDNF ELISA kit according to manufacturer recommended methods (IBL-America, Minneapolis, MN). Lysates were analyzed in duplicates and raw data were normalized as pg/mg protein. Data are expressed as percent change from Saline/Water control group.
2.6. Data Analysis
The short-term and long-term effect of prior chronic nicotine on trace fear conditioning was analyzed using mixed-design ANOVA to assess learning. In addition, planned comparison independent samples t-tests were performed to examine differences in freezing during baseline period and during CS presenation. Open arm EPM data was analyzed using separate two-way ANOVAs for 12.6 and 6.3 mg/kg/day nicotine doses, significant interactions were followed up with Tukey’s post-hoc simple main effects tests. Data from the nicotine/FLX trace conditioning experiment was analyzed using a one-way ANOVA within each condition (Baseline, Pre-CS, and CS) and because the assumption of homogeneous variance was violated, Games-Howell post-hoc tests performed on all possible comparisons. Brain-derived neurotrophic factor ELISA data was analyzed using one-way ANOVA for each brain region (DH, VH, and PFC) and Dunnet post-hoc tests compared each drug treatment with the Saline/Water control group. Results were considered significant at p ≤ 0.05. Statistics were performed in GraphPad Prism and SPSS 16.0. All data are presented as mean ± SEM.
One animal from the adult treatment 12.6 mg/kg/day 24 hr data was removed due to freezing 2 standard deviations from the mean. The EPM data for 8 animals (6.3 mg/kg/day Adult) was lost due to computer error. Two animals from the adolescent 12.6 mg/kg/day experiment (1 Saline/1 Nicotine) fell off of the EPM and so their data was excluded. Finally, two animals from the FLX data were removed from further analysis due to freezing 2 standard deviations from the mean, their data was also excluded from BDNF ELISA analysis.
3. RESULTS
3.1. Effects of short-term (24 hours) abstinence from adolescent or adult chronic nicotine administration on trace fear conditioning
To investigate the effects of short-term abstinence from chronic nicotine exposure during adolescence or adulthood, separate age-matched cohorts were administered chronic nicotine at 12.6 mg/kg/day, 6.3 mg/kg/day, or saline and trained in trace fear conditioning 24 hr after nicotine cessation (Fig 2). Independent samples t-tests comparing 12.6 or 6.3 mg/kg/day nicotine with the respective age-matched saline group found no differences in baseline freezing levels during training for any experiment, p > 0.05.
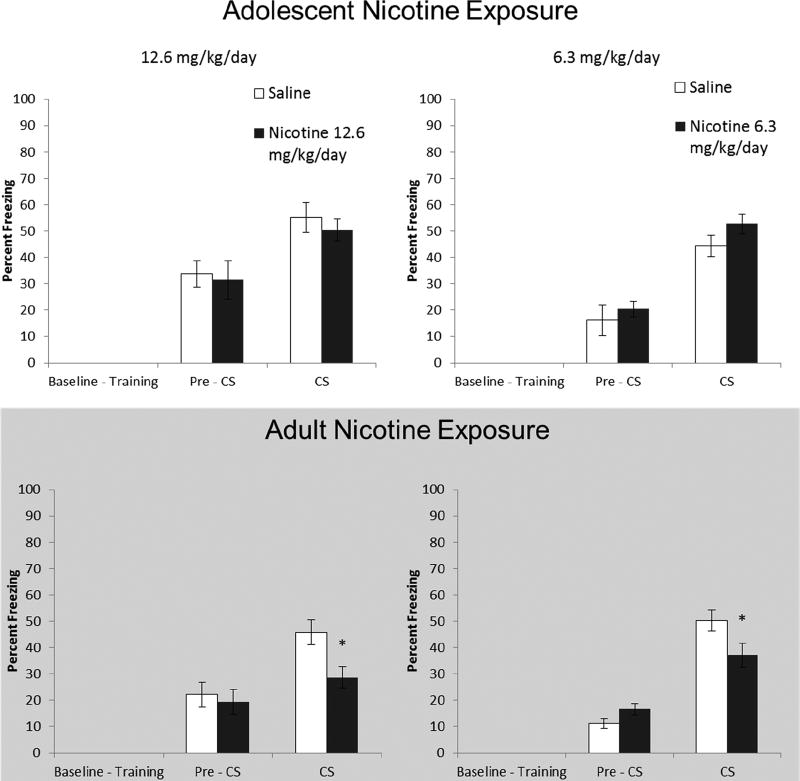
Figure 2.
Effects of short-term (24 hr) abstinence from chronic nicotine administration during adolescence and adulthood on trace fear conditioning. Adult mice treated with 12.6 and 6.3 mg/kg/day chronic nicotine froze significantly less to the CS during testing compared to saline controls. Error bars indicate SEM, (*) indicates a significant between subjects t-test, p < 0.05.
3.1.1. Adolescent Chronic Nicotine Administration
Short-term abstinence had no effect on trace fear conditioning in adolescent mice. For mice exposed to 12.6 mg/kg/day nicotine (n = 12), a mixed model ANOVA, Testing Phase (Pre-CS vs. CS) × Drug (12.6 mg/kg/day vs. Saline), found a main effect of Testing Phase F(1, 22) = 21.92, p < 0.01. Similarly, analysis of the effects of treatment with 6.3 mg/kg/day (n = 12) during adolescence found a main effect of Testing Phase F(1,22) = 65.39, p < 0.01. Planned comparison found no difference in freezing to the CS between any dose and the respective control group, p > 0.05. Thus, mice in all groups showed normal trace fear conditioning (Fig 2).
3.1.2. Adult Chronic Nicotine Administration
Adult nicotine-treated mice had decreased freezing to the CS during trace fear conditioning. In mice exposed to 12.6 mg/kg/day nicotine (n=12), a mixed-model ANOVA revealed a significant main effect of testing phase F(1,22) = 18.71, p < 0.01; but the interaction effect was non-significant F(1,22) = 3.57, p = 0.072. When we examined freezing levels during the CS with a planned independent samples t-test, we found that mice treated with 12.6 mg/kg/day nicotine froze significantly less than controls during presentation of the CS, t(22) = 2.86, p < 0.01. For adult mice treated with 6.3 mg/kg/day (n = 16), a mixed-model ANOVA found a significant interaction F(1,30) = 12.18, p < 0.01. When we tested for difference in freezing during the CS via a planned comparison independent samples t-test, we found that adult 6.3 mg/kg/day nicotine exposure resulted in significantly decreased freezing, t(30) = 2.20, p < 0.05. In sum, chronic nicotine at both doses induced trace conditioning deficits in adult animals after short-term abstinence (Fig 2).
3.2. Effects of long-term (30 days) abstinence from adolescent or adult chronic nicotine administration on trace fear conditioning
To investigate long-term effects of prior chronic nicotine exposure during either adolescence or adulthood, separate cohorts of mice were administered nicotine (12.6 or 6.3 mg/kg/day) or saline for 12 days and trained in trace fear conditioning 30 days after cessation (Fig 3). Independent samples t-tests comparing each dose of nicotine to the respective saline group found no differences in baseline freezing levels during training for any experiment, p > 0.05.
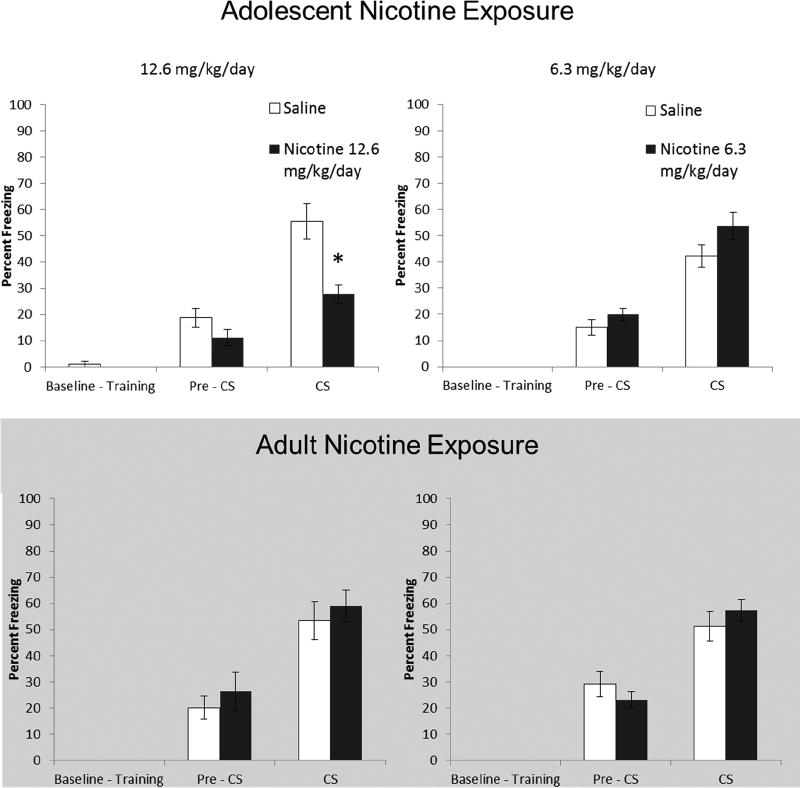
Figure 3.
Effects of long-term (30 days) abstinence from chronic nicotine administration during adolescence and adulthood on trace fear conditioning. Mice treated with 12.6 mg/kg chronic nicotine during adolescence froze significantly less during testing than Saline treated controls. Error bars indicate SEM, (*) indicates a significant between subjects t-test, p < 0.05.
3.2.1. Adolescent Chronic Nicotine Administration
Mice administered 12.6 mg/kg/day (n = 8) during adolescence showed deficits in trace fear conditioning later in adulthood (Fig 3). When examining Pre-CS vs. CS freezing levels, a mixed model ANOVA revealed a significant interaction for mice treated with nicotine (Testing Phase × Drug), F(1, 14) = 7.08, p < 0.05. We examined freezing during the CS using a planned comparison t-test and found that nicotine-treated mice froze significantly less during the CS presentation, t(14) = 3.9, p < 0.01. Analysis of mice treated with 6.3 mg/kg/day (n = 12−10) nicotine during adolescence revealed a main effect of Testing Phase, F(1,20) = 84.46, p < 0.05, while a planned comparison t-test found no difference during CS presentation, p > 0.05. Thus, trace learning was intact in mice treated with 6.3 mg/kg/day chronic nicotine during adolescence.
3.2.2. Adult Chronic Nicotine Administration
Unlike adolescent exposure, adult chronic nicotine did not result in long-term changes in trace fear conditioning. Mixed-model ANOVA found a significant main effect of Testing Phase, F(1, 14) = 30.98, in mice administered 12.6 mg/kg/day (n = 8), p < 0.05. Similarly, mice treated with 6.3 mg/kg/day (n = 12) showed a main effect of Testing Phase, F(1, 22) = 50.97, p < .05. No difference in CS freezing was found between either dose of chronic nicotine and respective age-matched controls, p > 0.05 (Fig 3).
3.3. Age-Dependent effects of long-term abstinence from chronic nicotine administration on the elevated-plus maze
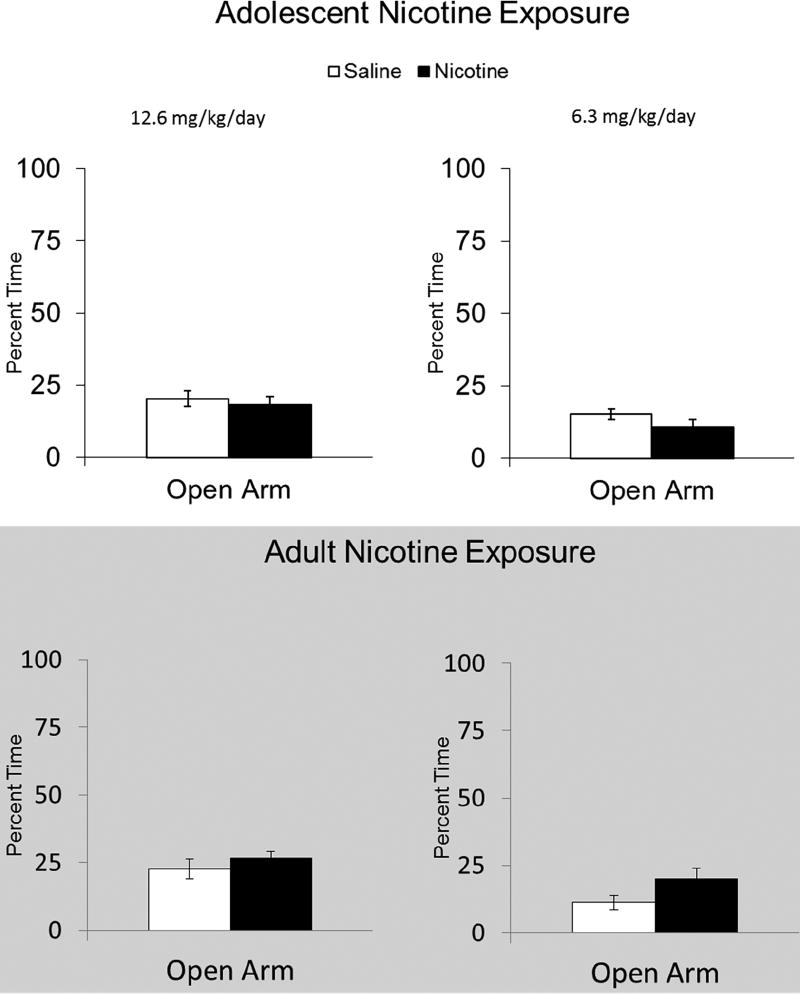
Twenty-eight days after chronic nicotine administration, we investigated changes in anxiety-related behavior in the EPM (Fig 4). Comparison of animals previously treated with 12.6 mg/kg/day nicotine during adolescence and adulthood using a two-way ANOVA, Age (Adolescent vs. Adult) × Drug (Saline vs. Nicotine 12.6 mg/kg/day), found no main effect or interaction, p > 0.05. The same analysis of the effects of prior adolescent and adult 6.3 mg/kg/day chronic nicotine revealed a significant interaction F(1, 30) = 6.34, p < 0.05. However, follow up Tukey’s post-hoc simple main effects found no significant difference in any comparison, p > 0.05. In sum, these data indicate that prior chronic nicotine exposure, during adolescent or adulthood, did not alter behavior EPM compared to age-matched saline controls.
Figure 4.
Age-Dependent effects of long-term abstinence from chronic nicotine on EPM. No effect of prior chronic nicotine exposure was observed for 12.6 or 6.3 mg/kg/day on EPM percent open arm time. Error bars indicate SEM.
3.4. Effect of chronic fluoxetine on adolescent nicotine administration-associated long-term trace fear conditioning deficits
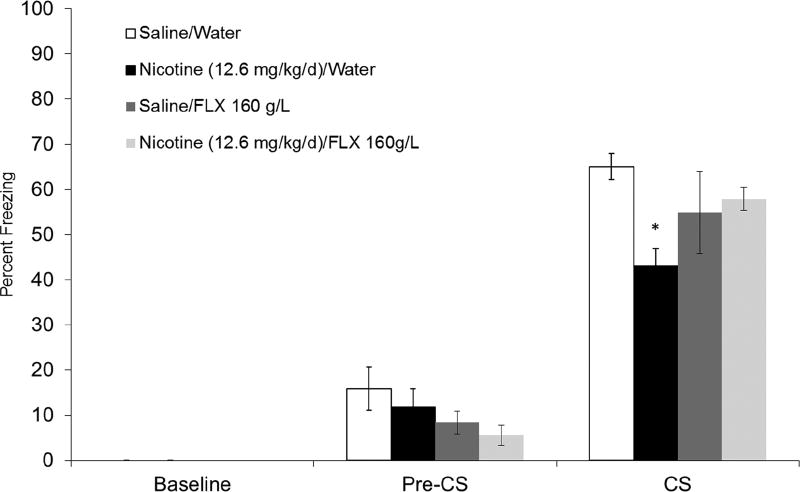
In section 3.2.1, we showed that exposure to 12.6 mg/kg/day chronic nicotine during adolescence resulted in a long-term deficit in trace fear conditioning after 30 days abstinence. Therefore, we sought to investigate if chronic FLX would ameliorate this deficit. To this end, we examined the effects of chronic FLX treatment (160 mg/L) administered in drinking water during abstinence in a new cohort of mice exposed to 12.6 mg/kg/day chronic nicotine for 12 days during adolescence. A one-way ANOVA applied to CS freezing data across all drug conditions, Saline/Water, Nicotine (12.6/mg/kg/day)/Water, Saline/FLX (160 mg/L), and Nicotine (12.6 mg/kg/day/FLX (160 mg/L), n = 7–8, was significant, F(1,30) = 3.13, p <0.05, (Fig 5). Game-Howell post-hoc testing found that mice in the Nicotine/Water group froze significantly less compared to Saline/Water controls and the Nicotine/FLX treatment group, p < 0.05. Furthermore, CS freezing did not differ significantly between Saline/Water controls and Nicotine/FLX, p > 0.05. Finally, one-way ANOVAs indicated no effect of drug condition on freezing during the Pre-CS period, p > 0.05, and no difference was observed in baseline freezing during training, p > 0.05.
Figure 5.
Effect of chronic fluoxetine on adolescent chronic nicotine exposure-induced long-term trace fear conditioning deficits. Mice treated with nicotine froze significantly less to the CS during testing compared to saline controls. In contrast, mice treated with FLX and nicotine froze at levels not significantly different than Water/Saline controls. Error bars indicate SEM, (*) indicates a significant Games-Howell post-hoc test compared to Water/Saline group, p < 0.05.
3.5. Effect of chronic fluoxetine on total BDNF protein
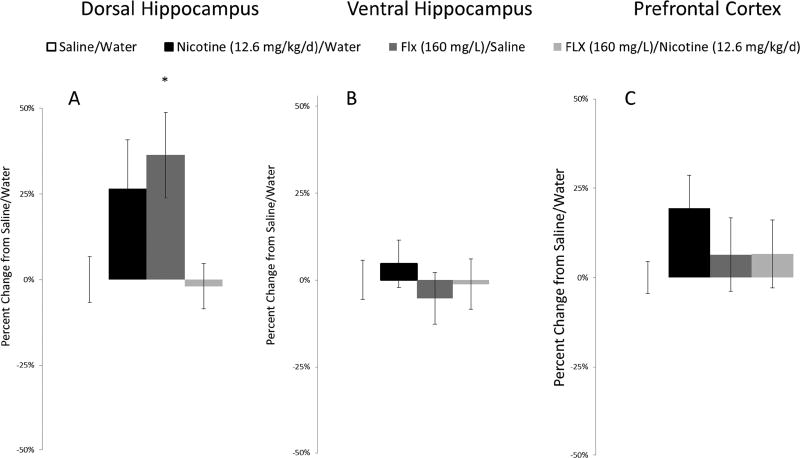
After testing, tissue was take from mice in the FLX experiment and total BDNF protein was assessed with ELISA (DH, 408.8−596.81 pg/mg; VH, 815.8−901.63 pg/mg; PFC, 1105.49−1217.38 pg/mg). For the DH, a one-way ANOVA across all four drug treatment groups (Saline/Water, Nicotine (12.6 mg/kg/day)/Water, Saline/FLX 160 mg/L was significant, F(1,30) = 3.44, p < 0.05 (Fig 6). Dunnet’s post-hoc test found that mice treated with FLX/Saline had increased BDNF protein within the DH compared to Saline/Water controls, p = 0.05. No other treatment group was significantly different from Saline/Water controls, p > 0.05. Finally, one-way ANOVAs revealed no effect of drug treatment within the VH or PFC, p > 0.05.
Figure 6.
Effect of chronic fluoxetine on total BDNF protein. Chronic FLX increased BDNF protein within the DH (A), but no change was seen in the VH (B) or PFC (C). Error bars indicate SEM, (*) indicates a significant Dunnet’s post-hoc test comparison to Saline/Water control group, p ≤ 0.05.
4. Discussion
In the present study, we identified age-dependent effects of chronic nicotine exposure on trace fear conditioning after short and long-term abstinence. We found that mice treated with chronic nicotine (12.6 and 6.3 mg/kg/day) in adulthood presented deficits in trace fear conditioning after short-term, 24 hr, abstinence, but mice exposed to chronic nicotine during adolescence did not. In contrast, we found that chronic nicotine treatment (12.6 mg/kg/day) during adolescence resulted in long-term, 30 days, trace conditioning deficits, which emerged in adulthood. However, this long-term deficit was not recapitulated in mice similarly exposed to chronic nicotine as adults. Thus, while adolescent animals appear to be resilient to short-term trace fear conditioning deficits associated with prior chronic nicotine exposure, they are sensitive to long-term effects of chronic nicotine exposure on learning. Finally, we found that long-term trace fear conditioning deficits in mice exposed to chronic nicotine during adolescence were reversed with chronic FLX treatment, and that FLX increased total BDNF protein within the DH.
Adult exposure to chronic nicotine resulted in short-term trace fear conditioning deficits. These data are in agreement with a previous study that described trace fear conditioning in adult mice trained 24 hr after withdrawal from chronic nicotine (Raybuck and Gould, 2009). This 24 hr short-term abstinence period falls within a temporal window described as immediate spontaneous withdrawal (Davis et al., 2005). Moreover, cognitive deficits resulting from nicotine withdrawal are associated with compensatory changes that occur during the chronic state related to nAChR expression and function (Gould, 2006). In contrast, mice exposed to chronic nicotine during adolescence did not show similar short-term deficits, suggesting that adolescent mice are less sensitive to the immediate effects of chronic nicotine cessation compared to adult subjects. In support, precipitated nicotine withdrawal symptoms are less severe and less aversive in adolescent compared to adult rats (O’Dell et al., 2007, 2004). Similarly, clinical data suggest that adolescents are less sensitive to immediate withdrawal symptoms (Bailey et al., 2009). In adult animals, the time course of nicotine withdrawal-associated cognitive deficits parallels a shift from nAChR upregulation back to baseline expression levels (Gould et al., 2012). Thus, cognitive deficits may be dependent on the shift of cholinergic function occurring during this transition period. One possibility is that the developing cholinergic system of the adolescent compensates for the immediate effects of nicotine cessation leading to differential cognitive outcomes after short-term absence. For example, adolescent rats (P30) were found to have greater and longer-lasting nAChR upregulation in response to nicotine compared to adults (Abreu-Villaca et al., 2003; Trauth et al., 1999). Alternatively, while adult mice showed increased nAChR levels, young adolescent mice (PND 23), which did not show withdrawal deficits in learning, similarly did not show hippocampal nAChR upregulation after immediate withdrawal from chronic nicotine (Portugal et al., 2012), suggesting that nAChR upregulation in the hippocampus is necessary for the withdrawal associated learning deficits. In sum, the adolescent cholinergic system appears to be differentially sensitive to immediate withdrawal, a phenomenon that mirrors the differential behavioral response to nicotine described in this study.
In comparison to the short-term deficits, an inverse age-dependent pattern was observed in trace fear conditioning after long-term abstinence. Specifically, mice exposed to chronic nicotine during adolescence, but not adulthood, showed long-term trace fear conditioning deficits. This findings is parsimonious with previous work showing long-term deficits in hippocampus-dependent contextual learning after adolescent, but not adult chronic nicotine administration (Holliday et al., 2016; Portugal et al., 2012). Importantly, these prior studies also examined delay fear conditioning and found no long-term effect of adolescent treatment. Thus, deficits described here are likely not due to altered fear learning or expression, but rather alterations in complex learning. Moreover, considering that delay fear conditioning is hippocampus and PFC independent (Morgan et al., 1993; Phillips and LeDoux, 1992), our data suggests that adolescent exposure to chronic nicotine can elicit long-term deficits in cued forms of learning that engage forebrain-dependent processes, i.e., trace conditioning (Connor and Gould, 2016; Kim and Jung, 2006). Furthermore, considering that trace conditioning recruits working memory-like processes, our data also fits well with work indicating that adolescent, but not adult, chronic nicotine exposure results in long-term deficits in visuospatial attention, an PFC-dependent task (Counotte et al., 2008).
Our understanding of the mechanisms underlying adolescent-specific long-term cognitive deficits is limited, however, adolescent nicotine exposure has been found to alter neurophysiological and molecular substrates of cognition (Goriounova and Mansvelder, 2012; Portugal et al., 2012). Moreover, adolescent nicotine exposure results in persistent changes within brain regions involved trace conditioning, including the PFC and hippocampus (Goriounova and Mansvelder, 2012; Holliday and Gould, 2017; Slotkin et al., 2016). For example, chronic nicotine exposure results in age-dependent long-term changes in PFC dendritic morphology (Bergstrom et al., 2008) and adolescent exposure elicits long-term decreased mGluR2 function within the PFC (Counotte et al., 2011). In addition, animals exposed to nicotine during adolescence have long-term changes in acetylcholine synthesis (Slotkin and Seidler, 2007) and dendrite arborization within the hippocampus (Holliday et al., 2016). Thus, nicotine-induced modification of developing hippocampal and prefrontal neurocircuitry and neurotransmission is likely involved in long-lasting persistent cognitive deficits.
We did not observe an effect on anxiety-related behavior in the EPM after long-term abstinence from chronic nicotine (12.6 or 6.3 mg/kg/day) treatment during adolescence. Similar null results have been seen after adolescent nicotine exposure (Abreu-Villaça et al., 2007; Holliday et al., 2016). However, the literature suggests that the long-term effects of prior chronic nicotine exposure during adolescence on behavior in the EPM may vary due to differences in age, administration strategy, species, and doses. For example, chronic nicotine treatment for 5 days (P31−36) in rats resulted in decreased open arm time two weeks after cessation (Slawecki et al., 2005). Slawecki and colleagues administered nicotine via adhesive patch, which resulted blood nicotine plasma of 90 ng/ml. This plasma nicotine level is likely higher than that achieved by the doses used in our study. For example, 6.3 mg/kg/day was shown to result in 13 ng/ml nicotine blood plasma levels (Davis et al., 2005). Therefore, the long-term changes in anxiety may be dependent on the blood plasma nicotine level achieved. Another study found decreased open arm time in adult rats that were administered nicotine via twice daily injections during adolescence (Iñiguez et al., 2008). This suggests rout of administration and species may also be important factors in the long-term effects of adolescent nicotine exposure on EPM behavior. Additionally, we found no effect of adult chronic nicotine treatment on behavior in the EPM, which is also similar to other work (Iñiguez et al., 2008), but contrasts with a study that found an anxiolytic effect of adult nicotine exposure 30 days later (i.e., increased open arm time (Holliday et al., 2016)). However, Holliday and colleagues (2016) scored EPM behavior for 10 minutes compared to 5 minutes in the present study, so a direct comparison is difficult. Our EPM data suggest that the observed changes in trace fear conditioning are not likely due to changes in anxiety. Also, examining the field in general highlights that multiple factors can influence EPM data.
The most clinically important finding described herein was that chronic FLX treatment ameliorated the adolescent nicotine long-term deficit in trace fear conditioning. Specifically, we found that mice treated with nicotine (12.6 mg/kg/day) during adolescence showed deficits in trace fear conditioning, but mice that received nicotine followed by FLX 160 mg/L in drinking water throughout abstinence learned normally. This finding is in agreement with previous work showing FLX treatment can ameliorate cognitive deficits (Dong et al., 2004; ELBeltagy et al., 2010; Jin et al., 2016; Li et al., 2009; Lyons et al., 2011). For example, chronic FLX (15 days) ameliorated spatial learning and contextual learning deficits in 3xTgAD mice (Jin et al., 2016), and novel object learning deficits in methotrexate-treated rats (Lyons et al., 2011). Similarly, clinical work shows that FLX can improve memory in patients with mild cognitive impairment (Mowla et al., 2007). Therefore, our results extend this literature and suggest that FLX can also ameliorate adult cognitive deficits associated with adolescent nicotine exposure.
Fluoxetine has been shown to alter synaptic transmission and plasticity (Bath et al., 2012; Rubio et al., 2013; Wang et al., 2008). For example, chronic FLX can upregulate of glutamate receptors (Rubio et al., 2013), enhance basal transmission and long-term potentiation within the dentate gyrus (Bath et al., 2012), and increase postsynaptic excitability within CA1.These effects on synaptic plasticity are likely involved in FLX-associated changes in cognition. For example, chronic FLX ameliorated disruptions in hippocampal LTP in 3xTgAD mice, while also attenuating cognitive deficits (Jin et al., 2016). Fluoxetine exerts effects on plasticity by eliciting changes in gene transcription via epigenetic chromatin remodeling (Vetencourt et al., 2011) and chronic FLX was found to elicit increased histone acetylation within the hippocampus (Wang et al., 2011). Relatedly, FLX has been found to alter cellular morphology including increased synaptic density and spine density within CA1 (Hajszan et al., 2005; Rubio et al., 2013). In contrast, adolescent chronic nicotine exposure was associated with long-term changes in hippocampal cellular morphology, including shortened dendritic length within CA1 (Holliday et al., 2016). Finally, chronic FLX increased hippocampal cell proliferation and maturation of newborn cells (Malberg et al., 2000; Wang et al., 2008, 2011). Such changes in hippocampal neurogenesis may be involved in amelioration of hippocampal cognitive deficits. For example, chronic FLX treatment given toTg2576 mice, which show deficits in neurogenesis and hippocampus-dependent contextual fear conditioning, improved learning. Moreover, trace conditioning is dependent on neurogenesis (Shors et al., 2001), and nicotine can alter DG cell proliferation (Abrous et al., 2002). Thus, future work might investigate long-term changes in hippocampal cell proliferation after adolescent chronic nicotine exposure.
Fluoxetine and nicotine can modulate BDNF, which has been shown to be important for learning and learning-associated synaptic plasticity (Bramham and Messaoudi, 2005; Choi et al., 2012; Kenny et al., 2000; Mizuno et al., 2012). Therefore, after testing, brains were immediately taken to assess total BDFN protein levels within the DH, VH, and PFC. Within the DH, we found increased BDNF protein in the FLX/Saline group. This is consistent prior findings in which chronic FLX increased hippocampal levels of BDNF mRNA and protein (Bath et al., 2012; Engesser-Cesar et al., 2007, 2007). We found no effect of adolescent nicotine exposure on BDNF, suggesting that nicotine-associated deficits might not depend on changes in BDNF protein during adulthood. This null result is compatible with a study that found that mice exposed to cigarette smoke during adolescence (PND 21−30) showed no difference in BDNF mRNA within the hippocampus or PFC later in adulthood (Xiao et al., 2016). Interestingly, we did not see an increase in DH BDNF in the FLX/Nicotine group, which was surprising given that FLX alone increased BDNF and nicotine had no effect. It is worth considering that since we only probed for BDNF after testing, it is unknown if BDNF protein levels are altered immediately after nicotine cessation and what role this may play FLX dependent changes in BDNF protein levels. In a previous report in adult animals, chronic nicotine reduced BDNF protein levels in the dorsal striatum (Ortega et al., 2013). Additionally, acute nicotine decreased and chronic nicotine increased BDNF gene transcripts in the hippocampus (Kenny et al., 2000). Therefore, one possibility is that immediate chronic nicotine-induced changes in BDNF during adolescence mitigated the long-term increase of BDNF due to FLX. Thus, future studies might investigate BDNF protein levels at earlier time-points.
Somewhat surprisingly, we did not observe a change in BDNF within the PFC in FLX treated animals. Previously, chronic FLX has been found to increase BDNF within the PFC (Balu et al., 2008; Molteni et al., 2006). One possible explanation for our diverging result is that changes in BDNF mRNA levels and protein levels do not always correspond. For example, one report indicated FLX induced increased hippocampal mRNA, but no change in BDNF protein (Molteni et al., 2006). Similarly, another study failed to find a change in hippocampal BDNF protein using ELISA after chronic FLX (Altar et al., 2003). It is also possible that altered BDNF protein levels within PFC subfields could be masked by the dissection and homogenization methods used for ELISA protein analysis. Therefore, immunohistochemically investigating changes in PFC BDNF protein might better reveal subtle differences not observed using ELISA. Finally, we found no effect of treatment on BDNF levels within the VH. These data are similar to a conference report also showing increased DH BDNF, but no change in VH BDNF after chronic FLX (Diniz et al., 2014). Therefore, FLX dependent changes in hippocampal BDNF protein may be primarily the result of changes in DH BDNF expression patterns.
It is known that the physiological stress system continues to develop throughout adolescence, leading to increased vulnerability to stressors (Eiland and Romeo, 2013; Romeo and McEwen, 2006). Considering that stress can sensitize responses to nicotine and nicotine activates the stress system (Armario, 2010; Leão et al., 2012), it is important to be aware that differences in shipping age could potentially interact with nicotine exposure in adolescent and adult mice differently. Few studies have directly investigated the long-term effects of adolescent shipping as a stressor and the procedures herein are similar to those used in other studies that investigated the developmental effects of nicotine on long-term changes in learning (Holliday et al., 2016; Portugal et al., 2012). However, one study found that male mice shipped at 6 weeks demonstrate long-term alterations in sexual behavior (Laroche et al., 2009). Therefore, additional studies should investigate the role of shipping stress and other early-life experiences on the effects of adolescent nicotine exposure.
In conclusion, we found that mice exposed to chronic nicotine during adolescence were resilient to short-term cognitive deficits, but sensitive to long-term cognitive deficits. Thus, our data supports the view that adolescent individuals have differential sensitivity to nicotine. Importantly, nicotine abstinence-associated cognitive deficits predict relapse in smokers (Ashare et al., 2014; Falcone et al., 2014; Patterson et al., 2010) and nicotine can ameliorate adolescence chronic nicotine-associated cognitive impairments (Holliday and Gould, 2017). Therefore, exposure to chronic nicotine in adolescence may facilitate nicotine addiction in two ways. First, reduced immediate negative abstinence associated symptoms might lead to continued consumption; Second, long-term cognitive deficits might facilitate the use of nicotine as a means to alleviate these deficits. Further, our data suggests that current treatments for nicotine dependence in adults may be less efficacious in adolescent populations. For example, varenicline and buproprion, treatments for smoking cessation, have been shown to decrease nicotine withdrawal-associated cognitive deficits (Portugal and Gould, 2007; Raybuck et al., 2008). However, if as our data suggests, adolescent individuals experience less immediate negative cognitive consequences during immediate abstinence, these treatments may be less effective. Finally, we found that chronic FLX can ameliorate long-term trace conditioning deficits associated with adolescent chronic nicotine exposure. Interestingly, FLX has not shown to be efficacious in as a nicotine cessation aid in adult populations (Hughes et al., 2014; Saules et al., 2004). Therefore, our data suggest that age may be a critical factor in examining the potential efficacy of FLX as a treatment for chronic nicotine associated cognitive impairments.
Adult treatment with chronic nicotine produced immediate but not long-term deficits in forebrain-dependent learning
Adolescent treatment with chronic nicotine produced long-term but not immediate deficits in forebrain-dependent learning
Fluoxetine ameliorated adolescent chronic nicotine-associated long-term deficits in forebrain-dependent learning
Acknowledgments
This work was supported by NIDA/NIH grant DA017949, Penn State, & the Shibley Endowment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement: The authors have nothing to disclose.
We declare no potential conflict of interest.
References
- Abreu-Villaça Y, Nunes F, Queiroz-Gomes F, do E, Manhães AC, Filgueiras CC. Combined exposure to nicotine and ethanol in adolescent mice differentially affects anxiety levels during exposure, short-Term, and long-term withdrawal. Neuropsychopharmacology. 2007;33:599–610. doi: 10.1038/sj.npp.1301429. [DOI] [PubMed] [Google Scholar]
- Abreu-Villaca Y, Seidler FJ, Qiao D, Tate CA, Cousins MM, Thillai I, Slotkin TA. Short-term adolescent nicotine exposure has immediate and persistent effects on cholinergic systems: Critical periods, patterns of exposure, dose thresholds. Neuropsychopharmacology. 2003;28:1935–1949. doi: 10.1038/sj.npp.1300221. [DOI] [PubMed] [Google Scholar]
- Abrous DN, Adriani W, Montaron M-F, Aurousseau C, Rougon G, Moal ML, Piazza PV. Nicotine Self-Administration Impairs Hippocampal Plasticity. J. Neurosci. 2002;22:3656–3662. doi: 10.1523/JNEUROSCI.22-09-03656.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alme MN, Wibrand K, Dagestad G, Bramham CR. Chronic fluoxetine treatment induces brain region-specific upregulation of genes associated with BDNF-induced long-term potentiation. Neural Plast. 2007;2007:e26496. doi: 10.1155/2007/26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altar CA, Whitehead RE, Chen R, Wörtwein G, Madsen TM. Effects of electroconvulsive seizures and antidepressant drugs on brain-derived neurotrophic factor protein in rat brain. Biol Psychiatry. 2003;54:703–709. doi: 10.1016/S0006-3223(03)00073-8. [DOI] [PubMed] [Google Scholar]
- An JJ, Gharami K, Liao G-Y, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3’ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A. Activation of the hypothalamic-pituitary-adrenal axis by addictive drugs: different pathways, common outcome . Trends Pharmacol Sci. 2010;31:318–325. doi: 10.1016/j.tips.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Falcone M, Lerman C. Cognitive Function During Nicotine Withdrawal: Implications for Nicotine Dependence Treatment. Neuropharmacology. 2014;76 doi: 10.1016/j.neuropharm.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SR, Harrison CT, Jeffery CJ, Ammerman S, Bryson SW, Killen DT, Robinson TN, Schatzberg AF, Killen JD. Withdrawal symptoms over time among adolescents in a smoking cessation intervention: Do symptoms vary by level of nicotine dependence? Addict Behav. 2009;34:1017–1022. doi: 10.1016/j.addbeh.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu DT, Hoshaw BA, Malberg JE, Rosenzweig-Lipson S, Schechter LE, Lucki I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 2008;1211:37–43. doi: 10.1016/j.brainres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath KG, Jing DQ, Dincheva I, Neeb CC, Pattwell SS, Chao MV, Lee FS, Ninan I. BDNF Val66Met Impairs Fluoxetine-Induced Enhancement of Adult Hippocampus Plasticity. Neuropsychopharmacology. 2012;37:1297–1304. doi: 10.1038/npp.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, McDonald CG, French HT, Smith RF. Continuous nicotine administration produces selective, age-dependent structural alteration of pyramidal neurons from prelimbic cortex. Synap. N. Y. N. 2008;62:31–39. doi: 10.1002/syn.20467. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Chassin L, Presson CC, Sherman SJ, Edwards DA. The natural history of cigarette smoking: predicting young-adult smoking outcomes from adolescent smoking patterns. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 1990;9:701–716. doi: 10.1037//0278-6133.9.6.701. [DOI] [PubMed] [Google Scholar]
- Chen J, Millar WJ. Age of smoking initiation: implications for quitting. Health Rep. 1998;9:39–46. (Eng); 39–48(Fre) [PubMed] [Google Scholar]
- Choi DC, Gourley SL, Ressler KJ. Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transl Psychiatry. 2012;2:e205. doi: 10.1038/tp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole RD, Poole RL, Guzman DM, Gould TJ, Parikh V. Contributions of β2 subunit-containing nAChRs to chronic nicotine-induced alterations in cognitive flexibility in mice. Psychopharmacology (Berl.) 2014;232:1207–1217. doi: 10.1007/s00213-014-3754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor DA, Gould TJ. The role of working memory and declarative memory in trace conditioning. Neurobiol. Learn Mem. 2016;134(Part B):193–209. doi: 10.1016/j.nlm.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Smit AB, Pattij T, Spijker S. Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Dev. Cogn Neurosci. 2011;1:430–443. doi: 10.1016/j.dcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer ANM, De Vries TJ, Smit AB, Pattij T. Long-lasting cognitive deficits resulting from adolescent nicotine exposure in rats. Neuropsychopharmacology. 2008;34:299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, Thomas KL. A simple role for BDNF in learning and memory? Front. Mol. Neurosci. 2010;3 doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Hippocampal nAChRs mediate nicotine withdrawal-related learning deficits. Eur. Neuropsychopharmacol. 2009;19:551–561. doi: 10.1016/j.euroneuro.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. β2 subunit-containing nicotinic receptors mediate the enhancing effect of nicotine on trace cued fear conditioning in C57BL/6 mice. Psychopharmacology (Berl.) 2007a;190:343–352. doi: 10.1007/s00213-006-0624-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, Gould TJ. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacol. 2007b;32:2011–9. doi: 10.1038/sj.npp.1301315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JA, James JR, Siegel SJ, Gould TJ. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J. Neurosci. Off. J. Soc. Neurosci. 2005;25:8708–8713. doi: 10.1523/JNEUROSCI.2853-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz CRaF, Antero LS, Resstel LBM, Joca SRL. P.4.C.009 Fluoxetine modulating dorsal and ventral hippocampal BDNF as responsible for facilitating extinction memory retention. Eur. Neuropsychopharmacol. 2014;24:S610–S611. doi: 10.1016/S0924-977X(14)70979-4. [DOI] [Google Scholar]
- Dong H, Goico B, Martin M, Csernansky CA, Bertchume A, Csernansky JG. Modulation of hippocampal cell proliferation, memory, and amyloid plaque deposition in APPsw (Tg2576) mutant mice by isolation stress. Neuroscience. 2004;127:601–609. doi: 10.1016/j.neuroscience.2004.05.040. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhang T, Li W, Doyon W, Dani JA. Route of nicotine administration influences In Vivo dopamine neuron activity: habituation, needle injection, and cannula infusion. J. Mol. Neurosci. 2009;40:164–171. doi: 10.1007/s12031-009-9231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2004;29:1321–1330. doi: 10.1038/sj.npp.l300433. [DOI] [PubMed] [Google Scholar]
- Eiland L, Romeo RD. Stress and the developing adolescent brain. Neuroscience, Stress and the Adolescent Brain. 2013;249:162–171. doi: 10.1016/j.neuroscience.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELBeltagy M, Mustafa S, Umka J, Lyons L, Salman A, Gloria Tu C-Y, Bhalla N, Bennett G, Wigmore PM. Fluoxetine improves the memory deficits caused by the chemotherapy agent 5-fluorouracil. Behav. Brain Res. 2010;208:112–117. doi: 10.1016/j.bbr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C, Anderson AJ, Cotman CW. Wheel running and fluoxetine antidepressant treatment have differential effects in the hippocampus and the spinal cord. Neuroscience. 2007;144:1033–1044. doi: 10.1016/j.neuroscience.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Falcone M, Wileyto EP, Ruparel K, Gerraty RT, LaPrate L, Detre JA, Gur R, Loughead J, Lerman C. Age-related differences in working memory deficits during nicotine withdrawal. Addict. Biol. 2014;19:907–917. doi: 10.1111/adb.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol. Biochem Behav., The Psychopharmacology of Nicotine. 2001;70:475–489. doi: 10.1016/S0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriounova NA, Mansvelder HD. Short- and long-term consequences of nicotine exposure during adolescence for prefrontal cortex neuronal network function. Cold Spring Harb. Perspect. Med. 2012 doi: 10.1101/cshperspect.a012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolf NJ, Butcher LL. Postnatal development of cholinergic neurons in the rat: I. Forebrain. Brain Res Bull. 1991;27:767–789. doi: 10.1016/0361-9230(91)90209-3. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Nicotine and hippocampus-dependent learning. Mol Neurobiol. 2006;34:93–107. doi: 10.1385/MN:34:2:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Portugal GS, André JM, Tadman MP, Marks MJ, Kenney JW, Yildirim E, Adoff M. The duration of nicotine withdrawal-associated deficits in contextual fear conditioning parallels changes in hippocampal high affinity nicotinic acetylcholine receptor upregulation. Neuropharmacology. 2012;62:2118–2125. doi: 10.1016/j.neuropharm.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, Wehner JM. Nicotine enhancement of contextual fear conditioning. Behav. Brain Res. 1999;102:31–39. doi: 10.1016/S0166-4328(98)00157-0. [DOI] [PubMed] [Google Scholar]
- Hajszan T, MacLusky NJ, Leranth C. Short-term treatment with the antidepressant fluoxetine triggers pyramidal dendritic spine synapse formation in rat hippocampus. Eur. J. Neurosci. 2005;21:1299–1303. doi: 10.1111/j.1460-9568.2005.03968.x. [DOI] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007;12:656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday ED, Gould TJ. Chronic nicotine treatment during adolescence attenuates the effects of acute nicotine in adult contextual fear learning. Nicotine Tob Res. 2017;19:87–93. doi: 10.1093/ntr/ntw176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday ED, Nucero P, Kutlu MG, Oliver C, Connelly KL, Gould TJ, Unterwald EM. Long-term effects of chronic nicotine on emotional and cognitive behaviors and hippocampus cell morphology in mice: comparisons of adult and adolescent nicotine exposure. Eur. J. Neurosci. 2016;44:2818–2828. doi: 10.1111/ejn.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Hartmann-Boyce J, Cahill K, Lancaster T. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2014. Antidepressants for smoking cessation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z, Bolaños-Guzmán CA. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2008;34:1609–1624. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Gao L-F, Sun D-S, Wu H, Wang Q, Ke D, Lei H, Wang J-Z, Liu G-R. Long-term ameliorative effects of the antidepressant fluoxetine exposure on cognitive deficits in 3 × TgAD mice. Mol. Neurobiol. 2016:1–12. doi: 10.1007/s12035-016-9952-9. [DOI] [PubMed] [Google Scholar]
- Kenney JW, Raybuck JD, Gould TJ. Nicotinic receptors in the dorsal and ventral hippocampus differentially modulate contextual fear conditioning. Hippocampus. 2012;22:1681–1690. doi: 10.1002/hipo.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, File SE, Rattray M. Acute nicotine decreases, and chronic nicotine increases the expression of brain-derived neurotrophic factor mRNA in rat hippocampus. Mol Brain Res. 2000;85:234–238. doi: 10.1016/S0169-328X(00)00246-1. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neurosci. Biobehav. Rev., The Limbic Brain: Structure and Function. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M, Kang H, Bonhoeffer T, Schuman E. A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology. 1998;37:553–559. doi: 10.1016/s0028-3908(98)00035-5. [DOI] [PubMed] [Google Scholar]
- Laroche J, Gasbarro L, Herman JP, Blaustein JD. Reduced behavioral response to gonadal hormones in mice shipped during the peripubertal/adolescent period. Endocrinology. 2009;150:2351–2358. doi: 10.1210/en.2008-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão RM, Cruz FC, Marin MT, Planeta C, da S. Stress induces behavioral sensitization, increases nicotine-seeking behavior and leads to a decrease of CREB in the nucleus accumbens. Pharmacol. Biochem. Behav. 2012;101:434–442. doi: 10.1016/j.pbb.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Li W-L, Cai H-H, Wang B, Chen L, Zhou Q-G, Luo C-X, Liu N, Ding X-S, Zhu DY. Chronic fluoxetine treatment improves ischemia-induced spatial cognitive deficits through increasing hippocampal neurogenesis after stroke. J. Neurosci Res. 2009;87:112–122. doi: 10.1002/jnr.21829. [DOI] [PubMed] [Google Scholar]
- Lyons L, ElBeltagy M, Umka J, Markwick R, Startin C, Bennett G, Wigmore P. Fluoxetine reverses the memory impairment and reduction in proliferation and survival of hippocampal cells caused by methotrexate chemotherapy. Psychopharmacology (Berl.) 2011;215:105–115. doi: 10.1007/s00213-010-2122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Dempster E, Mill J, Giese KP. Long-lasting regulation of hippocampal BDNF gene transcription after contextual fear conditioning. Genes Brain Behav. 2012;11:651–659. doi: 10.1111/j.1601-183X.2012.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Calabrese F, Bedogni F, Tongiorgi E, Fumagalli F, Racagni G, Riva MA. Chronic treatment with fluoxetine up-regulates cellular BDNF mRNA expression in rat dopaminergic regions. Int. J. Neuropsychopharmacol. 2006;9:307–317. doi: 10.1017/S1461145705005766. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-C. [DOI] [PubMed] [Google Scholar]
- Mowla A, Mosavinasab M, Pani A. Does fluoxetine have any effect on the cognition of patients with mild cognitive impairment?: A double-blind, placebo-controlled, clinical trial. J. Clin Psychopharmacol. 2007;27:67–70. doi: 10.1097/JCP.0b013e31802e0002. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Ghozland S, Markou A, Koob GF. Nicotine withdrawal in adolescent and adult rats. Ann. N. Y. Acad Sci. 2004;1021:167–174. doi: 10.1196/annals.1308.022. [DOI] [PubMed] [Google Scholar]
- O’Dell LE, Torres OV, Natividad LA, Tejeda HA. Adolescent nicotine exposure produces less affective measures of withdrawal relative to adult nicotine exposure in male rats. Neurotoxicol Teratol., Exposures during adolescent development: are neurotoxic risks increased? 2007;29:17–22. doi: 10.1016/j.ntt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega LA, Tracy BA, Gould TJ, Parikh V. Effects of chronic low- and high-dose nicotine on cognitive flexibility in C57BL/6J mice. Behav. Brain Res. 2013;238:134–145. doi: 10.1016/j.bbr.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug Alcohol Depend. 2010;106:61. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav. Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Poole RL, Connor DA, Gould TJ. Donepezil reverses nicotine withdrawal-induced deficits in contextual fear conditioning in C57BL/6J mice. Behav. Neurosci. 2014 doi: 10.1037/bne0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Gould TJ. Bupropion dose-dependently reverses nicotine withdrawal deficits in contextual fear conditioning. Pharmacol. Biochem Behav. 2007;88:179–187. doi: 10.1016/j.pbb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal GS, Wilkinson DS, Turner JR, Blendy JA, Gould TJ. Developmental effects of acute, chronic, and withdrawal from chronic nicotine on fear conditioning. Neurobiol. Learn Mem. 2012;97:482–494. doi: 10.1016/j.nlm.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. The role of nicotinic acetylcholine receptors in the medial prefrontal cortex and hippocampus in trace fear conditioning. Neurobiol. Learn. Mem. 2010;94:353–363. doi: 10.1016/j.nlm.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Gould TJ. Nicotine withdrawal-induced deficits in trace fear conditioning in C57BL/6 mice - a role for high-affinity β2 subunit-containing nicotinic acetylcholine receptors. Eur. J Neurosci. 2009;29:377–387. doi: 10.1111/j.l460-9568.2008.06580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybuck JD, Portugal GS, Lerman C, Gould TJ. Varenicline ameliorates nicotine withdrawal-induced learning deficits in C57BL/6 mice. Behav. Neurosci. 2008;122:1166–1171. doi: 10.1037/a0012601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LM, Makowski CS, Yogendran SV, Kiessling S, Cermakian N, Flores C. Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a dcc-dependent manner. Neuropsychopharmacology. 2015;40:1101–1112. doi: 10.1038/npp.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, McEwen BS. Stress and the adolescent brain. Ann. N. Y. Acad Sci. 2006;1094:202–214. doi: 10.1196/annals.1376.022. [DOI] [PubMed] [Google Scholar]
- Rubio FJ, Ampuero E, Sandoval R, Toledo J, Pancetti F, Wyneken U. Long-term fluoxetine treatment induces input-specific LTP and LTD impairment and structural plasticity in the CA1 hippocampal subfield. Front. Cell. Neurosci. 2013;7 doi: 10.3389/fncel.2013.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saules KK, Schuh LM, Arfken CL, Reed K, Kilbey MM, Schuster CR. Double-blind placebo-controlled trial of fluoxetine in smoking cessation treatment including nicotine patch and cognitive-behavioral group therapy. Am. J Addict. 2004;13:438–446. doi: 10.1080/10550490490512762. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Gilder A, Roth J, Ehlers CL. Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacol. Biochem Behav. 2003;75:355–361. doi: 10.1016/S0091-3057(03)00093-5. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell AK, Khoury AE, Mathe AA, Ehlers CL. Increased CRF-like and NPY-like immunoreactivity in adult rats exposed to nicotine during adolescence: Relation to anxiety-like and depressive-like behavior. Neuropeptides. 2005;39:369–377. doi: 10.1016/j.npep.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Card J, Seidler FJ. Nicotine administration in adolescence reprograms the subsequent response to nicotine treatment and withdrawal in adulthood: Sex-selective effects on cerebrocortical serotonergic function. Brain Res Bull. 2014;102:1–8. doi: 10.1016/j.brainresbull.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. A unique role for striatal serotonergic systems in the withdrawal from adolescent nicotine administration. Neurotoxicol Teratol, Exposures during adolescent development: are neurotoxic risks increased? 2007;29:10–16. doi: 10.1016/j.ntt.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Stadler A, Skavicus S, Seidler FJ. Adolescents and adults differ in the immediate and long-term impact of nicotine administration and withdrawal on cardiac norepinephrine. Brain Res Bull. 2016;122:71–75. doi: 10.1016/j.brainresbull.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav Rev. 2000;24:417–463. doi: 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spijker S. Dissection of rodent brain regions. In: Li KW, editor. Neuroproteomics, Neuromethods. Humana Press; 2011. pp. 13–26. [DOI] [Google Scholar]
- Trauth JA, Seidler FJ, McCook EC, Slotkin TA. Adolescent nicotine exposure causes persistent upregulation of nicotinic cholinergic receptors in rat brain regions. Brain Research. 1999;851:9–19. doi: 10.1016/S0006-8993(99)01994-0. [DOI] [PubMed] [Google Scholar]
- Vetencourt JFM, Tiraboschi E, Spolidoro M, Castrén E, Maffei L. Serotonin triggers a transient epigenetic mechanism that reinstates adult visual cortex plasticity in rats. Eur. J. Neurosci. 2011;33:49–57. doi: 10.1111/j.l460-9568.2010.07488.x. [DOI] [PubMed] [Google Scholar]
- Wang J-W, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J. Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Neumann M, Hansen K, Hong SM, Kim S, Noble-Haeusslein LJ, Liu J. Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury. J. Neurotrauma. 2011;28:259–268. doi: 10.1089/neu.2010.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehner JM, Keller JJ, Keller AB, Picciotto MR, Paylor R, Booker TK, Beaudet A, Heinemann SF, Balogh SA. Role of neuronal nicotinic receptors in the effects of nicotine and ethanol on contextual fear conditioning. Neuroscience. 2004;129:11–24. doi: 10.1016/j.neuroscience.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Xiao L, Kish VL, Benders KM, Wu Z-X. Prenatal and early postnatal exposure to cigarette smoke decreases BDNF/TrkB signaling and increases abnormal behaviors later in life. Int. J. Neuropsychopharmacology. 2016;19 doi: 10.1093/ijnp/pyv117. pyv117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Seidler FJ, Cousins MM, Slikker W, Jr, Slotkin TA. Adolescent nicotine administration alters serotonin receptors and cell signaling mediated through adenylyl cyclase. Brain Res. 2002;951:280–292. doi: 10.1016/S0006-8993(02)03174-8. [DOI] [PubMed] [Google Scholar]
- Yildirim E, Connor DA, Gould TJ. ABT-089, but not ABT-107, ameliorates nicotine withdrawal-induced cognitive deficits in C57BL6/J mice. Behav. Pharmacol. 2015;26:241–248. doi: 10.1097/FBP.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]