Abstract
Objective
Obesity pharmacotherapies result in an exponential time course for energy intake whereby large early decreases dissipate over time. This pattern of declining drug efficacy to decrease energy intake results in a weight loss plateau within approximately one year. We aimed to elucidate the physiology underlying the exponential decay of drug effects on energy intake.
Methods
We examined the placebo-subtracted energy intake time courses during long-term obesity pharmacotherapy trials for 14 different drugs or drug combinations within the theoretical framework of a proportional feedback control system regulating human body weight.
Results
Assuming each obesity drug had a relatively constant effect on average energy intake and did not affect other model parameters, our model correctly predicted that long-term placebo-subtracted energy intake was linearly related to early reductions in energy intake according to a pre-specified equation with no free parameters. The simple model explained about 70% of the variance between drug studies with respect to the long-term effects on energy intake, although a significant proportional bias was evident.
Conclusions
The exponential decay over time of obesity pharmacotherapies to suppress energy intake can be interpreted as a relatively constant effect of each drug superimposed on a physiological feedback control system regulating body weight.
Keywords: Pharmacologic Therapy, Energy Intake, Weight Loss
Introduction
In humans, obesity drugs work primarily by decreasing metabolizable energy intake with minor effects on energy expenditure (1). Unfortunately, pharmacotherapy leads to only modest average weight loss that plateaus within about one year. According to mathematical models that accurately account for energy expenditure changes with weight loss (reviewed in (2)), if a drug resulted in a constant reduction of energy intake (dashed curve in Figure 1A) then ongoing weight loss would be predicted to occur for years without reaching a plateau (dashed curve in Figure 1B) (3). However, because the weight loss plateau occurs within one year (solid curve in Figure 1B), mathematical models predict that drug treatment result in an early decline in energy intake followed by a waning of this effect over time (solid curve in Figure 1A).
Figure 1.
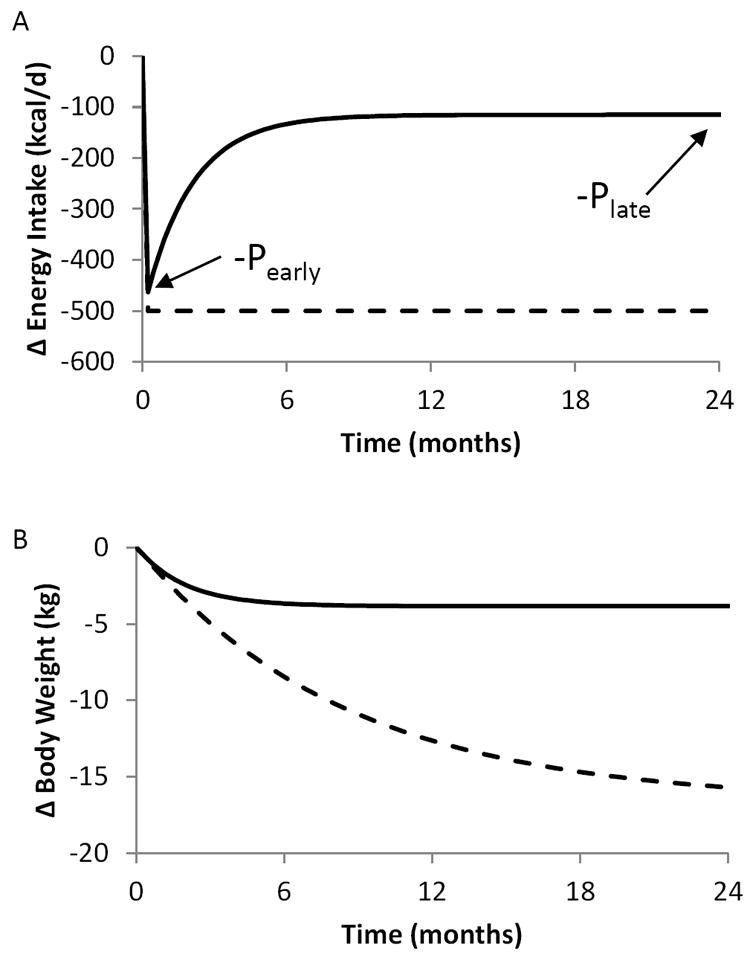
(A) Mathematical model results assuming a constant effect of obesity pharmacotherapy on placebo-subtracted energy intake in the presence (solid curve) or absence (dashed curve) of proportional feedback control of energy intake. In the context of such a feedback control system regulating body weight, a constant drug effect results in an early intake reduction (-Pearly) that exponentially decays to late effect (-Plate) related to Pearly according to the pre-specified model parameters. (B) Corresponding placebo-subtracted body weight changes in the presence (solid curve) or absence (dashed curve) of the proportional feedback control system regulating body weight.
In a previous report (4), we used a validated mathematical method (5) to calculate placebo-subtracted energy intake time courses of 14 different drugs or drug combinations (6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20). The energy intake time courses were all characterized by a universal exponential pattern whereby large early decreases in intake dissipated over time. One possible interpretation of these observations is that all obesity drugs exhibit a decline in their efficacy to suppress energy intake over time.
Here, we show that the exponential waning of early reductions in energy intake during obesity pharmacotherapy can be interpreted within the context of a physiological system employing proportional feedback control of energy intake (21). Under this model, obesity drugs may have a relatively persistent effect, but as weight is lost they fight an ever-increasing battle against physiological feedback processes resisting further weight loss. Under this simple model, the exponential parameters defining early and late changes in energy intake are predicted to be proportional to each other with a pre-specified slope – a theoretical prediction that we demonstrate was evident in the data.
Methods
As previously described (3), the linearized energy balance equation is:
| [1] |
where W and I are the changes from the stable baseline body weight and energy intake, respectively. This model accounts for energy expenditure changes with weight loss and the parameter ρ is the effective energy density associated with the weight change and the parameter ε defines the change in energy expenditure per unit weight change (3).
We assume that each drug intervention affects energy intake at time zero by a fixed amount, Pearly, but that energy intake is controlled by negative feedback control in proportion to the induced weight change with feedback gain k as previously described (21). Therefore, the energy intake time course is given by:
| [2] |
The linearized energy balance equation can therefore be written as:
| [3] |
This differential equation can be solved explicitly and the result is the following exponential time course for weight change, assuming the initial weight change W(0) = 0:
| [4] |
Note that the characteristic time constant is given by τ = ρ /(k + ε) and therefore the time scale to achieve a weight plateau becomes substantially shorter with introduction of the proportional feedback gain k of similar or larger magnitude compared with ε.
Substitution of equation 4 into equation 2 demonstrates that the energy intake changes are also characterized by an exponential time course:
| [5] |
Evaluating equation 5 at the initial time point t=0 results in the early change in energy intake:
| [6] |
Similarly, the late change in energy intake is:
| [7] |
Therefore, the early and late changes in energy intake are predicted to be related by the following proportionality:
| [8] |
The early and late exponential parameters defining the placebo-subtracted energy intake time courses of various obesity pharmacotherapies were previously published (4). The feedback control model predicts that these parameters are linearly correlated with each other according to equation 8. In particular, the predicted slope of the line relating late to early exponential parameters is completely defined by previously specified feedback gain parameters k = 95 kcal/kg/d and ε = 25 kcal/kg/d (21) resulting in a value of 0.21. Each drug is represented by a single parameter: the value of Pearly and the predicted value of Plate for any given drug is defined by equation 8. Importantly, there are no free parameters in the model and we have made the simplifying assumption that the pre-specified model parameters are not altered by the drug treatment.
Statistical analysis was performed using Microsoft Excel 2016.
Results
Figure 2A illustrates the significant positive linear relationship between previously published exponential parameters defining the early (Pearly) and late (Plate) placebo-subtracted energy intake changes using 14 different obesity pharmacotherapies (p<0.0001). Furthermore, the observed slope of the best-fit regression line through the origin (solid line) was 0.24±0.03 and was very close to the theoretical value of 0.21 predicted by the model (dashed line) using previously determined proportional feedback control parameters. The outlier point indicated by the open square in Figure 2A corresponds to the only data point using the drug combination phentermine/fenfluramine that is no longer on the market. Removal of this data point results in even closer agreement between the predicted and observed slope (0.20±0.02) as shown in Figure 2B. Therefore, the predicted linear correlation between the early and late exponential parameters appears to be evident in the data.
Figure 2.
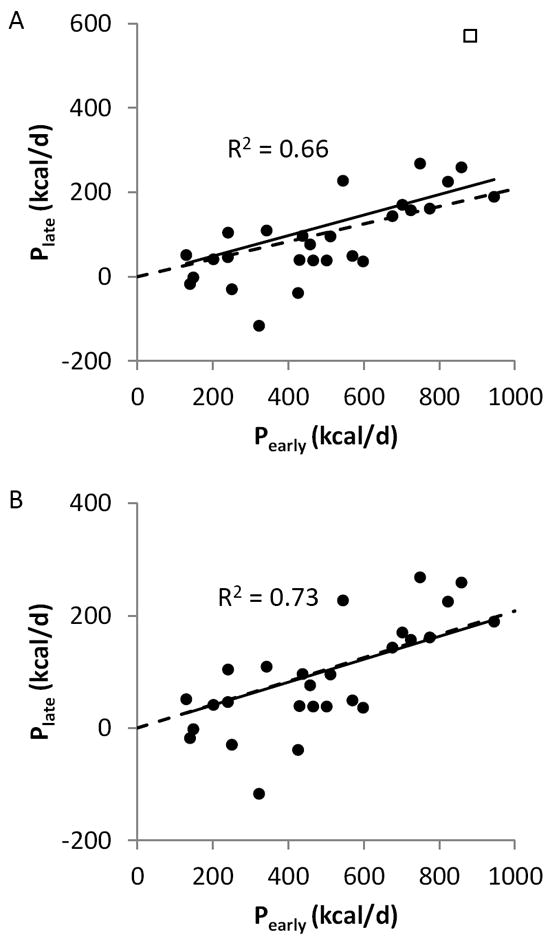
(A) A significant linear relationship was observed between the previously published parameters defining the best-fit exponential time courses characterizing early (Pearly) and late (Plate) effects of 14 different obesity pharmacotherapies on energy intake. The solid line is the best-fit linear regression line through the origin and the dashed line is the predicted linear relationship assuming that each drug has a constant effect without altering any of the pre-specified model parameters. The outlier data point indicated by the open square is for the drug combination Phentermine/Fenfluramine. (B) Removal of the Phentermine/Fenfluramine outlier results in closer agreement between the best-fit regression line (solid) with the predicted slope (dashed).
The predicted values of Plate obtained using equation 8 were not significantly different from the measurements (p = 0.93 using all data and p = 0.34 excluding phentermine/fenfluramine). The rootmean- squared (RMS) error of the Plate predictions was 99 kcal/d for all data and 68 kcal/d excluding phentermine/fenfluramine. The Bland Altman plots in Figure 3 illustrate that the mean bias between the measured and predicted Plate (dashed horizontal line) was 1.6 kcal/d for all data and -12.7 kcal/d excluding phentermine/fenfluramine. However, the positive correlations in the Bland-Altman plots indicate a significant proportional bias regardless of whether the data from phentermine/fenfluramine were included (Figure 3A) (r=0.84, p<0.0001) or excluded (Figure 3B) (r=0.73, p<0.0001). In other words, the drugs that were observed to result in larger long-term decreases energy intake were systematically underestimated using the predicted Plate values from our simple model.
Figure 3.
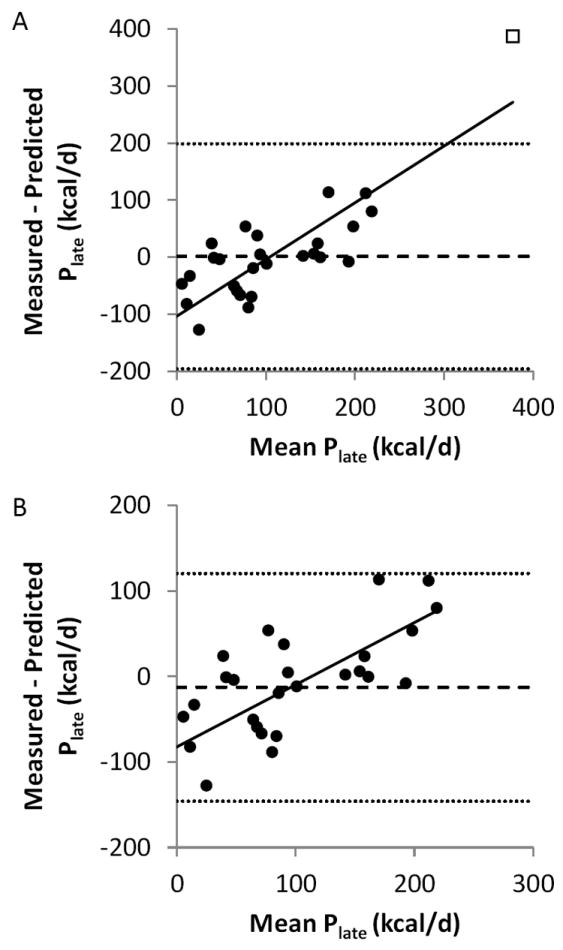
(A) Bland-Altman plot comparing the previously measured parameters defining the long-term effects of obesity pharmacotherapies on energy intake (Plate) with the predicted values assuming that each drug or drug combination each has a constant effect without altering any of the pre-specified model parameters. The mean error is indicated by the dashed horizontal line and the 95% CI limits of agreement are defined by the horizontal dotted lines. The solid best-fit linear regression line indicates a proportional bias in the predictions. (B) Removal of the Phentermine/Fenfluramine outlier results in a similar mean error in the predicted values for Plate (dashed horizontal line) and the 95% CI limits of agreement are more narrow (dotted horizontal lines).
Body weight and energy intake were predicted to follow an exponential time course with a characteristic time scale of τ ~ 75 days assuming that the energy density of body weight change was ρ ~ 9000 kcal/kg (3). From our previous analysis (4), the mean placebo-subtracted characteristic time scale for 14 obesity pharmacotherapies was τ = 115 ± 21 days, which tended to be larger than the predicted value of ~75 days (p=0.067). However, the drug Orlistat resulted in very large values of τ = 400 ± 67 days and excluding Orlistat as potential outlier resulted in a mean characteristic exponential time scale for the remaining 13 drugs of τ = 81 ± 8 days which was not significantly different from the predicted value (p=0.45).
Discussion
Despite the simplicity of our mathematical model and its lack of free parameters, the model correctly predicted the observed positive linear relationship between Pearly and Plate across a variety obesity drugs and doses. Indeed, the slope of the observed best-fit line was accurately predicted using pre-specified model parameters. We know of no other reason why the early and late stage drug effects on energy intake should be linearly related according to the observations, and such a relationship has never been previously postulated. Thus, our results provide substantial support for considering each drug as having a relatively constant effect on appetite within the context of a physiological system regulating body weight via proportional feedback control of energy intake (21).
Furthermore, the characteristic time scale for the exponential pattern of energy intake and body weight, as well as the Plate values for each drug, were relatively accurately predicted by our simple model. However, the simple model explained a modest ~70% of the variability in the long-term reductions in placebo-subtracted energy intake across studies and there was a proportional bias of the model to underestimate the long-term energy intake effects of more potent drugs. It is possible that the remaining variability and proportional bias could be partially explained if the drugs also differentially altered the energy intake feedback control parameter k. Furthermore, some of the variability could also be related to study differences with respect to: run-in diets and weight loss; adjustments of diet during the trial; up and down-titration of the drug; and differential drop-out rates between placebo and drug arms.
We observed two potential outliers where the simple model predictions deviated substantially from the observations. Orlistat exhibited a very long transition time from its early to late stage effects on metabolizable energy intake (11, 18). This may have been due to Orlistat’s unique mechanism to inhibit intestinal fat absorption resulting in side effects that concomitantly reduce fat intake. The drug combination phentermine/fenfluramine exhibited a long-term effect to decrease energy intake that was almost 400 kcal/d greater than predicted by our simple model. While it has been suggested that phentermine may be mildly thermogenic (22), the magnitude of the observed discrepancy suggests that failure of our model to account for this mechanism is an unlikely explanation. Furthermore, the same dose of phentermine in the outlier phentermine/fenfluramine study (19) was used in combination with topiramate in two other studies (6,9) whose results were more in accord with the model predictions. Perhaps the greater than expected long-term effect of the phentermine/fenfluramine combination indicates an important synergistic effect between these compounds that enhances their individual effects over time.
In conclusion, the exponential waning of early decreases in energy intake during obesity pharmacotherapy can be partially explained by a relatively constant effect of each drug to decrease energy intake superimposed on a physiological feedback control system acting to increase appetite in proportion to lost weight (21). While different obesity drugs affect energy intake by different magnitudes, each drug may have a relatively persistent effect over time.
What is already known about this subject
Obesity pharmacotherapies are characterized by a universal exponential pattern whereby large early decreases in energy intake dissipate over time
Drug treatment results in a weight loss plateau within a year due to the exponential waning of drug effects on energy intake
The physiology underlying the exponential decay of obesity drugs on energy intake was unclear
What this study adds
We examine obesity pharmacotherapies within the context of a proportional feedback control system regulating body weight
Our model explains the exponential energy intake time course as the result of relatively constant drug effects superimposed on a proportional feedback control system
Our model correctly predicts that the exponential parameters characterizing early and late effects of obesity drugs on energy intake are linearly related with a slope determined by pre-specified model parameters
References
- 1.Bray GA. Medications for weight reduction. Endocrinology and metabolism clinics of North America. 2008;37:923–942. doi: 10.1016/j.ecl.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Hall KD. Modeling metabolic adaptations and energy regulation in humans. Annu Rev Nutr. 2012;32:35–54. doi: 10.1146/annurev-nutr-071811-150705. [DOI] [PubMed] [Google Scholar]
- 3.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gobel B, Sanghvi A, Hall KD. Quantifying energy intake changes during obesity pharmacotherapy. Obesity (Silver Spring) 2014;22:2105–2108. doi: 10.1002/oby.20813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanghvi A, Redman LA, Martin CK, Ravussin E, Hall KD. Validation of an inexpensive and accurate mathematical method to measure long-term changes in free-living energy intake. Am J Clin Nutr. 2015 doi: 10.3945/ajcn.115.111070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlledrelease phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring, Md) 2012;20:330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. International journal of obesity (2005) 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 9.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 10.Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. The American journal of clinical nutrition. 95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Archives of family medicine. 2000;9:160–167. doi: 10.1001/archfami.9.2.160. [DOI] [PubMed] [Google Scholar]
- 12.Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes care. 2013;36:4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. The New England journal of medicine. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 14.Pfohl M, Luft D, Blomberg I, Schmulling RM. Long-term changes of body weight and cardiovascular risk factors after weight reduction with group therapy and dexfenfluramine. Int J Obes Relat Metab Disord. 1994;18:391–395. [PubMed] [Google Scholar]
- 15.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- 16.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. The New England journal of medicine. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 17.Suplicy H, Boguszewski CL, Dos Santos CM, do Desterro de Figueiredo M, Cunha DR, Radominski R. A comparative study of five centrally acting drugs on the pharmacological treatment of obesity. International journal of obesity (2005) 2013 doi: 10.1038/ijo.2013.225. [DOI] [PubMed] [Google Scholar]
- 18.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 19.Weintraub M, Sundaresan PR, Madan M, Schuster B, Balder A, Lasagna L, et al. Long-term weight control study. I (weeks 0 to 34). The enhancement of behavior modification, caloric restriction, and exercise by fenfluramine plus phentermine versus placebo. Clinical pharmacology and therapeutics. 1992;51:586–594. doi: 10.1038/clpt.1992.69. [DOI] [PubMed] [Google Scholar]
- 20.Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. Jama. 2001;286:1331–1339. doi: 10.1001/jama.286.11.1331. [DOI] [PubMed] [Google Scholar]
- 21.Polidori D, Sanghvi A, Seeley RJ, Hall KD. How Strongly Does Appetite Counter Weight Loss? Quantification of the Feedback Control of Human Energy Intake. Obesity (Silver Spring) 2016;24:2289–2295. doi: 10.1002/oby.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ioannides-Demos LL, Proietto J, McNeil JJ. Pharmacotherapy for obesity. Drugs. 2005;65:1391–1418. doi: 10.2165/00003495-200565100-00006. [DOI] [PubMed] [Google Scholar]


