Abstract
Introduction
While some studies have reported an association between early exposure to anesthesia and surgery and long-term neurodevelopmental deficit, the clinical phenotype of children exposed to anesthesia is still unknown.
Methods
Data were obtained from the Western Australian Pregnancy Cohort Study (Raine) with neuropsychological tests at age 10 years measuring language, cognition, motor function, and behavior. Latent class analysis (LCA) of the tests was used to divide the cohort into mutually exclusive subclasses of neurodevelopmental deficit. Multivariable polytomous logistic regression was used to evaluate the association between exposure to surgery and anesthesia and each latent class, adjusting for demographic and medical covariates.
Results
In our cohort of 1444 children, LCA identified four subclasses: 1) Normal: few deficits, (n=1135, 78.6%), 2) Language and Cognitive deficits: primarily language, cognitive, and motor deficits (n=96, 6.6%), 3) Behavioral deficits: primarily behavioral deficits, (n= 151, 10.5%) and 4) Severe deficits: deficits in all neuropsychological domains (n=62, 4.3%). Language and Cognitive deficit group children were more likely to have exposure prior to age 3 (adjusted odds ratio [aOR], 2.11; 95% CI, 1.17 – 3.81), while a difference in exposure was not found between Behavioral or Severe deficit children (aOR, 1.00; 95% CI, 0.58 – 1.73, and aOR, 0.85; 95% CI, 0.34 – 2.15 respectively) and Normal children.
Conclusions
Our results suggest that in evaluating children exposed to surgery and anesthesia at an early age, the phenotype of interest may be children with deficits primarily in language and cognition, and not children with broad neurodevelopmental delay or primarily behavioral deficits.
Introduction
In animal models, the exposure of developing brains to N-methyl-D-aspartate antagonists (such as nitrous oxide and ketamine) and γ-amino butyric acid agonists (such as benzodiazepines, propofol, and volatile anesthetics) lead to dose-dependent neuroapoptosis and neurodegenerative changes, with long term cognitive deficits observed in adulthood.(1-10) While an association between early anesthetic exposure and long-term cognitive deficit has been seen in a number of retrospective studies, no association was found in the one published prospective study to date.(11-21) Using data from the Western Australian Pregnancy Cohort (Raine) Study, we previously found that early exposure to surgery and anesthesia was significantly associated with deficit in the domains of language and cognition measured by direct neuropsychological testing.(17) Despite these data, the long-term neurodevelopmental effects of anesthetic exposure in children are still uncertain due to limitations in the published studies. In addition, the clinical phenotype of neurodevelopmental deficit in children exposed to anesthesia at an early age is still unknown, and the assessments most useful for detecting this phenotype remain unclear.(22) The purpose of this present study is to (1) use latent class analysis (LCA) of specific neurodevelopmental outcomes to characterize subgroups of deficit in children, (2) describe differences in demographics, comorbid illness, and types of procedures in each class, and (3) evaluate the association of each subgroup with prior exposure to surgery and anesthesia. We hypothesize that anesthetic and surgical exposure at an early age may be associated with specific subgroups of deficit in children.
Methods
This study was approved by the Columbia University Institutional Review Board and ethical approval for data collection and storage was approved at each age by Ethics Committees at King Edward Memorial Hospital, Princess Margaret Hospital, and the University of Western Australia.
Data Source
Data was obtained from the Raine Study, an established birth cohort consisting of 2,868 children in Perth, Western Australia born from 1989 to 1992. The Raine Study collected detailed demographic and medical data prenatally and at birth from medical records and parental self-report. After birth, all children were assessed at 1, 2, 3, 5, 8, 10, 13, 17, and 20 years of age, with comprehensive neuropsychological testing performed at age 10. During follow-up visits, parents filled out questionnaires describing illnesses and medical problems, which were coded by research staff into International Classification of Diseases, 9th Revision (ICD-9) codes. Coding procedures have been previously described.(23) We classified any child who had a surgical or diagnostic procedure requiring anesthesia before the age of three years as “exposed”, and the rest “unexposed.” An age of less than three years was used for exposure status as it is the time of peak synaptogenesis of various regions of the brain, including the prefrontal cortex.(24,25) Children who missed all three scheduled follow-up visits from one to three years old were deemed “missing” and excluded from further analysis as data on exposure status was not available for them. Demographic information for missing children was previously evaluated.(17) In order to ensure exposure to anesthesia, we reviewed the types of procedures, all of which were performed after leaving the maternity hospital. Children who were found to have diagnostic procedures not requiring anesthesia were placed in the unexposed group.
Neuropsychological Tests
The neuropsychological tests performed at age 10 evaluated a variety of neurodevelopmental domains with trained research staff directly administering five of the six tests. Language was evaluated with the Peabody Picture Vocabulary Test (PPVT), a basic test of receptive vocabulary knowledge, in addition to the more comprehensive Clinical Evaluation of Language Fundamentals (CELF) which is composed of individual scores for Receptive (CELF-R), or language comprehension ability and Expressive (CELF-E), or speaking language ability.(26,27) Cognition was assessed by Colored Progressive Matrices (CPM), which specifically tests abstract reasoning as well as the Symbol Digit Modality Test (SDMT), which assesses visual tracking, attention and motor skill and generates oral (SDMT-O) and written (SDMT-W) scores.(28,29) The McCarron Assessment of Neuromuscular Development (MAND) was used to measure fine and gross motor tasks.(30) Deficit in these neuropsychological tests was defined as scores worse than 1.5 standard deviations below the mean of the entire cohort.(17) Behavioral problems were measured by the Child Behavior Checklist (CBCL). The CBCL is a comprehensive questionnaire completed by the child’s caregiver that evaluates both internalizing problems such as depression and somatic complaints, as well as externalizing problems that involve conflict with others, such as aggressive behavior and rule-breaking. In addition to Internalizing (CBCL-INT) and Externalizing (CBCL-EXT) scores, the CBCL also generates a Total behavior score (CBCL-T) which takes into account internalizing and externalizing scores as well as social, attention, and thought problems.(31) In CBCL scoring, higher scores are considered to be worse, with scores over 60 considered to be a behavioral deficit.
Latent Class Analysis
Among other applications, LCA is a statistical method using maximum likelihood estimation that can define subgroups of patients (latent classes) using observed variables. In this study, the observed variables are the neuropsychological assessments for each child. LCA is based on the concept that the statistical associations among the observed variables are a manifestation of underlying (“latent”) subgroups or classes in the study population. This is a method that has been used to identify other clinical phenotypes and subgroups of cognitive impairment.(32-34) In this study, LCA was used to produce predicted probabilities of each child being in a specific class based on the presence of a deficit in each of 10 different dichotomous neuropsychological outcomes assessed at age 10. Based on these outcomes, each child is assigned to the single, mutually exclusive latent class for which they have the highest predicted probability. Currently as the phenotype of deficit after anesthetic exposure is unclear, LCA was used to identify subclasses of deficit that may be associated with a history of exposure. Maximum likelihood in Mplus, version 7.1 was used to fit latent class models with varying numbers of classes.(35) Determination of the optimal number of classes relied primarily on the Bayesian information criterion (BIC) which balances model fit and parsimony in addition to clinical interpretation of class meaning.(36) When comparing several statistical models, the smallest BIC indicates the optimal fitted model, with a difference in BIC values of >10 suggesting a very strong difference in model fit.(37) The reference group in the latent class regression model was the group having the lowest risk for neurodevelopmental deficit. LCA subgroup classification was performed using only children with data from all 10 neuropsychological measures. After LCA subgroup classification, we then examined differences in demographics, comorbid illness, and types of surgical and diagnostic procedures performed between the LCA groups. Clinical diagnoses were also classified using the Healthcare Cost and Utilization Project Clinical Classifications Software (CCS) and the classes were evaluated for differences in CCS categories.(38) CCS is a medical diagnosis categorization tool that uses ICD-9 codes to determine the presence of specific disease categories. CCS categorization was initially used with all available ICD-9 codes. Subsequently CCS categorization was also performed with only ICD-9 codes that resulted in a hospital visit and could be considered as more serious diagnoses.
Comorbid Illness
The level of comorbid illness was assessed and quantified using the Johns Hopkins ACG Case-Mix System, a method for predicting past and future healthcare utilization and costs.(39) ICD-9 codes were used to calculate Resource Utilization Band (RUB) scores based on the expected levels of resources used by each child. In order to quantify the level of illness up to the time of assessment at age 10, ICD-9 codes from all follow-up visits up to and including age 10 were used to calculate the RUB score. ICD-9 codes for mental, behavioral, and neurodevelopmental disorders were excluded from the calculation of RUB scores as they were directly related to the outcomes of interest. For each child, resource utilization was coded as 0) No diagnoses, 1) Healthy, 2) Low, 3) Moderate, 4) High, and 5) Very High. Children with no diagnoses and verified to have presented for follow-up as well as the healthy category were collapsed in the Low resource utilization category, and children in the High and Very High utilization groups were also combined. Coding of the RUB score was performed with the Johns Hopkins ACG version 10.0.1.
Neurodevelopmental Deficit Analysis in LCA Deficit groups
Regression analyses were used to assess the strength of the association between exposure to surgery and anesthesia and each latent class. A multivariable polytomous logistic regression was used to adjust for demographic, socioeconomic, and baseline perinatal health status factors and comorbid disease. Demographic and socioeconomic covariates included sex, race, income, low birth weight, paternal presence, maternal education, perinatal smoking, and perinatal alcohol use. All covariates were included as categorical variables. The RUB score was included as a discrete covariate in the regression model for comorbidity adjustment. Crude and adjusted odds ratios of association between exposure and deficit were calculated. Regression analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
Results
Characteristics of the Raine cohort
The Raine cohort consists of 2,868 children, of which 260 children had no history of follow-up from ages 1 to 3 and were omitted from the present analysis, as they had no information on anesthesia exposure status. The deleted children were significantly different from the children evaluated in our cohort in most demographic categories, which has previously been discussed.(17) All available neuropsychological outcomes at age 10 were then identified. Of the remaining children, 1444 had all 10 neuropsychological assessments available at age 10 and constituted the study group. In this study group, 188 children had surgical or diagnostic procedures requiring anesthesia before their third birthday, and were classified as “exposed”, while 1256 children did not have a history of a procedure requiring anesthesia and were classified as “unexposed”. The vast majority of the procedures were surgical in nature.
Latent Class Analysis of Neurodevelopmental Deficit
The four-class model was found to fit best (compared to the other 2 through 6 class models) based on the lowest BIC (BIC: 2-class 7649, 3-class 7380, 4-class 7338, 5-class 7350, 6-class 7373) and was also deemed to yield clinically meaningful subgroups. The following clinical descriptions were identified: 1) Normal class, with low likelihood or no deficit in any of the tests, (n=1135, 78.6% of cohort), 2) Behavioral Deficit class with high probabilities for deficits in behavioral outcomes and a low probability for deficits in other outcomes. (n= 151, 10.5% of cohort), 3) Language and Cognitive Deficit class which conversely had high probabilities for language and other cognitive deficits including motor deficits, and low probability for behavioral deficits (n=96, 6.6% of cohort) and 4) Severe Deficit class, with deficits found in all neuropsychological domains (n=62, 4.3% of cohort). (Figure 1)
Figure 1. Proportion of Children in Each of Four Latent Classes with Deficits in Specific Neuropsychological Assessments.
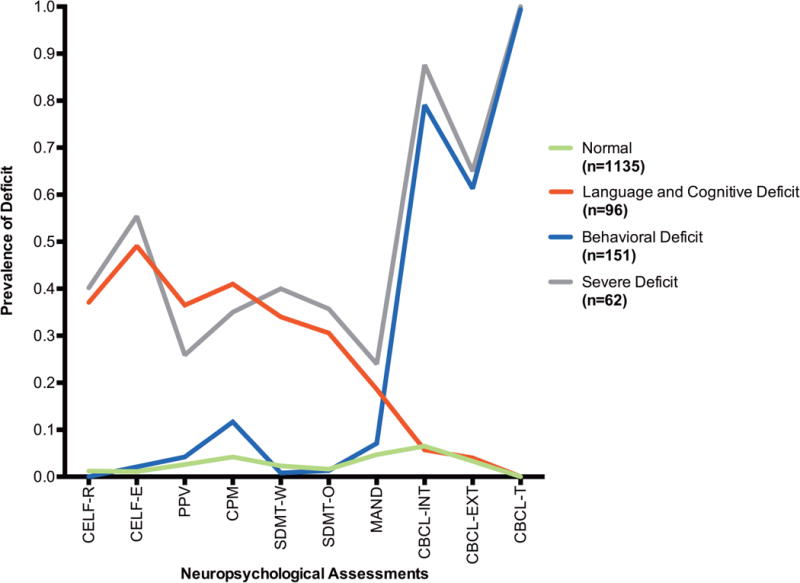
Legend 1:
CELF-R = Clinical Evaluation of Language Fundamentals (CELF) Receptive Language score; CELF-E = CELF Expressive Language score; CPM = Colored Progressive Matrices; SDMT-O = Symbol Digit Modality Test (SDMT) Oral score; SDMT-W = SDMT Written score; MAND = McCarron Assessment of Neuromuscular Development; CBCL-INT = Child Behavior Checklist (CBCL) Internalizing score; CBCL-EXT = CBCL Externalizing score; CBCL-T = CBCL Total score.
Differences Between the Classes
In order to determine if there were specific risk factors for presence in each latent class, we evaluated demographic and clinical differences between the subgroups. In comparing the four different LCA groups, we noted that while the three deficit groups were demographically similar, the Normal group differed substantially from the deficit groups on several demographic variables. (Table 1) The Normal group had an approximately equal number of boys and girls (51% and 49% respectively), while all three-deficit groups were composed of more boys (Language and Cognitive Deficit: 65.6%, Behavioral Deficit: 63.6%, and Severe deficit 58.1%). The Normal group also had higher household income and maternal education levels, as well as a lower incidence of maternal perinatal smoking compared to the deficit groups. The Severe Deficit group was found to have slightly higher rates of extremely low birth weight children and lower maternal education.
Table 1.
Characteristics of Children in Each Latent Class with Complete Outcomes (n=1444)
| Normal (n=1135), n (%) |
Language Deficit (n=96), n (%) |
Behavior Deficit (n=151), n (%) |
Severe Deficit (n=62), n (%) |
|
|---|---|---|---|---|
| Gender | ||||
| Girls | 579 (51) | 33 (34.4) | 55 (36.4) | 26 (41.9) |
| Boys | 556 (49) | 63 (65.6) | 96 (63.6) | 36 (58.1) |
| Birth Weight | ||||
| < 1500g | 18 (1.6) | 0 (0) | 2 (1.3) | 3 (4.8) |
| 1500 – 1999g | 17 (1.5) | 2 (2.1) | 7 (4.6) | 0 (0) |
| 2000 – 2499g | 58 (5.1) | 7 (7.3) | 9 (6) | 5 (8.1) |
| 2500 – 2999g | 181 (15.9) | 18 (18.8) | 23 (15.2) | 6 (9.7) |
| 3000 – 3999g | 745 (65.6) | 58 (60.4) | 97 (64.2) | 43 (69.4) |
| ≥ 4000g | 116 (10.2) | 11 (11.5) | 13 (8.6) | 5 (8.1) |
| Apgar at 5 minutes | ||||
| 0 – 6 | 16 (1.4) | 0 (0) | 2 (1.3) | 2 (3.2) |
| 7 – 10 | 1114 (98.1) | 96 (100) | 148 (98) | 60 (96.8) |
| Unknown | 5 (0.4) | 0 (0) | 1 (0.7) | 0 (0) |
| Race | ||||
| Caucasian | 1007 (88.7) | 84 (87.5) | 135 (89.4) | 55 (88.7) |
| Non-Caucasian | 108 (9.5) | 8 (8.3) | 12 (7.9) | 5 (8.1) |
| Unknown | 20 (1.8) | 4 (4.2) | 4 (2.6) | 2 (3.2) |
| Household Income (AUD) | ||||
| Less than $7000 | 53 (4.7) | 11 (11.5) | 11 (7.3) | 9 (14.5) |
| $7000-$23999 | 290 (25.6) | 40 (41.7) | 65 (43) | 21 (33.9) |
| $24000 – $35999 | 292 (25.7) | 26 (27.1) | 33 (21.9) | 14 (22.6) |
| $36,000 | 433 (38.1) | 10 (10.4) | 32 (21.2) | 11 (17.7) |
| Unknown | 67 (5.9) | 9 (9.4) | 10 (6.6) | 7 (11.3) |
| Father Living at Home | ||||
| Home | 1011 (89.1) | 77 (80.2) | 127 (84.1) | 50 (80.6) |
| Not at home | 97 (8.5) | 15 (15.6) | 20 (13.2) | 10 (16.1) |
| Unknown | 27 (2.4) | 4 (4.2) | 4 (2.6) | 2 (3.2) |
| Maternal Education Beyond High School | ||||
| None | 499 (44) | 58 (60.4) | 84 (55.6) | 46 (74.2) |
| Trade certificate, Professional registration or other | 273 (24.1) | 21 (21.9) | 32 (21.2) | 7 (11.3) |
| College or University degree | 343 (30.2) | 13 (13.5) | 31 (20.5) | 7 (11.3) |
| Unknown | 20 (1.8) | 4 (4.2) | 4 (2.6) | 2 (3.2) |
| Maternal Perinatal Smoking | ||||
| No | 862 (75.9) | 63 (65.6) | 101 (66.9) | 36 (58.1) |
| 1–20 daily | 166 (14.6) | 19 (19.8) | 37 (24.5) | 14 (22.6) |
| 21 or more daily | 17 (1.5) | 5 (5.2) | 2 (1.3) | 3 (4.8) |
| Unknown | 90 (7.9) | 9 (9.4) | 11 (7.3) | 9 (14.5) |
| Maternal Perinatal Alcohol Use | ||||
| Several times per week | 67 (5.9) | 3 (3.1) | 4 (2.6) | 3 (4.8) |
| Once a week | 114 (10) | 9 (9.4) | 11 (7.3) | 2 (3.2) |
| Less than once a week | 256 (22.6) | 20 (20.8) | 33 (21.9) | 8 (12.9) |
| Never | 600 (52.9) | 54 (56.3) | 90 (59.6) | 38 (61.3) |
| Unknown | 90 (7.9) | 10 (10.4) | 13 (8.6) | 11 (17.7) |
|
| ||||
| Surgical Exposure Before Age 3 | ||||
| 0 | 1002 (88.3) | 71 (74) | 129 (85.4) | 54 (87.1) |
| 1 | 133 (11.7) | 25 (26) | 22 (14.6) | 8 (12.9) |
Because of rounding, percentages may not sum to 100
AUD: Australian Dollar
The differences in comorbid illness measured by RUB between the four groups were also evaluated, with distributions of higher RUB scores seen in the Behavioral Deficit and Severe Deficit groups (85.4 and 83.9% with RUB scores ≥3 respectively) compared to the Normal and Language and Cognitive Deficit groups (70.2 and 71.9% with RUB scores ≥3 respectively). (Table 2) Differences in CCS categories were also evaluated with minimal differences in CCS categories using all available ICD-9 diagnosis codes. (Appendix Figure 1) When evaluating CCS category differences between the groups using only ICD-9 codes resulting in a hospital visit, a few differences emerged. (Appendix Figure 2) Children in the Severe Deficit class were found to have a slightly higher incidence of nervous system diseases requiring a hospital visit (9.7% in the Severe Deficit class vs. 3.1 to 6% in the other classes) and children in the Language and Cognitive Deficit as well as the Behavioral Deficit classes had a higher incidence of respiratory diseases (20.8 and 18.5% respectively) compared to the Normal and Severe deficit classes (12.2 and 8.1% respectively).
Table 2.
Distribution of Resource Utilization Band (RUB) Score of Children in Each Latent Class
| Normal Group (n=1135), n (%) |
Language Deficit Group (n=96), n (%) |
Behavior Deficit Group (n=151), n (%) |
Severe Deficit Group (n=62), n (%) |
|
|---|---|---|---|---|
| RUB | ||||
| 0 | 3 (0.3) | 0 (0) | 0 (0) | 0 (0) |
| 1 | 45 (4) | 5 (5.2) | 1 (0.7) | 2 (3.2) |
| 2 | 291 (25.6) | 22 (22.9) | 21 (13.9) | 8 (12.9) |
| 3 | 735 (64.8) | 58 (60.4) | 114 (75.5) | 48 (77.4) |
| 4 | 59 (5.2) | 11 (11.5) | 13 (8.6) | 4 (6.5) |
| 5 | 2 (0.2) | 0 (0) | 2 (1.3) | 0 (0) |
Surgical Exposures
While there were a small number of major cardiac and neurosurgical procedures performed, the vast majority of procedures were minor, with myringotomies, hernias, and circumcisions among the most commonly performed procedures in all four LCA groups. (Appendix Tables 1 – 4) Myringotomies, the most commonly performed procedure, accounted for 25 to 42% of the procedures in the Normal, Language and Cognitive, and Behavioral Deficit class, but only 7% of the Severe LCA group. In the Severe Deficit class, 23% of the procedures were dental procedures. To determine if the prevalence of multiple anesthetic exposures in children in each group differed, of the 1444 children assessed, we further evaluated the 1119 children who had complete follow up from ages 1 to 3.(17) In the normal group, 89 children (79.5%) of those with a surgical exposure had a single exposure while 23 (20.5%) had more than one exposure. In the children with behavioral deficits, of the exposed children, 10 children (58.8%) had a single exposure while 7 (41.2%) had multiple exposures, while in the language and cognitive deficit group, 17 children (85%) had a single exposure while 3 (15%) had multiple exposures. In the severe deficit group, 6 children (85.7%) had a single exposure while 1 (14.3%) had multiple exposures.
Latent Class Association with Surgical Exposure
The percentage of children exposed to anesthesia and surgery before age 3 was 11.7% in the Normal class (133 out of 1135 children), 26% in the Language and Cognitive Deficit Class (25 out of 96 children), 14.6% in the Behavioral Deficit Class (22 out of 151 children), and 12.9% in the Severe Deficit Class (8 out 62 children). In order to determine the clinical implications of early exposure to surgery and anesthesia, we examined the association between exposure before age 3 and latent class deficit group assignment. In the crude model, children in the Language and Cognitive deficit group had an increased odds of prior exposure to surgery and anesthesia of 2.65 times (95% CI, 1.63 – 4.33) than that found in the Normal class of children. However, children in the Behavioral Deficit and Severe Deficit classes (Behavioral Deficit odds ratio [OR],1.29; 95% CI, 0.79 – 2.91 and Severe Deficit OR; 1.16; 95%, CI 0.52 – 2.40) were found to have no increased odds of prior exposure compared to the Normal class of children. (Table 3) After adjusting for sex, race, income, low birth weight, paternal presence, maternal education, perinatal smoking, perinatal alcohol use, and comorbidity, children in the Language and Cognition Deficit class (adjusted odds ratio [aOR], 2.11; 95% CI, 1.17 – 3.81) were still found to have a significantly higher odds of prior exposure to surgery and anesthesia compared to Normal children, while those with Behavioral or Severe deficits (aOR, 1.00; 95% CI, 0.58 – 1.73, and aOR, 0.85; 95% CI, 0.34 – 2.15 respectively) were not more likely to have been exposed than Normal children. Interactions were also evaluated with no significant interaction found between exposure and any covariate.
Table 3.
Crude Odds Ratios (OR) and Adjusted Odds Ratios (aOR) of LCA Subgroup and Exposure to Anesthesia Prior to Age Three
| Association between Anesthesia prior to age 3 and Subclass Category at age 10
| |||
|---|---|---|---|
| Exposure vs. No Exposure
|
|||
| Crude, OR (95% CI) |
Adjusted for Demographics aOR* (95% CI) |
Adjusted for Demographics and RUB aOR** (95% CI) |
|
| Language and Cognitive Deficit vs. Normal | 2.65 (1.63 – 4.33) | 2.40 (1.37 – 4.21) | 2.11 (1.17 – 3.81) |
| Behavioral Deficit vs. Normal | 1.29 (0.79 – 2.91) | 1.19 (0.70 – 2.03) | 1.00 (0.58 – 1.73) |
| Severe Deficit vs. Normal | 1.16 (0.52 – 2.40) | 1.03 (0.42 – 2.53) | 0.85 (0.34 – 2.15) |
Adjusted for sex, race, income, low birth weight (<2500g), paternal presence, maternal education, perinatal smoking (Yes/No), and perinatal alcohol use (Yes/Never).
Adjusted for sex, race, income, low birth weight (<2500g), paternal presence, maternal education, perinatal smoking (Yes/No), and perinatal alcohol use (Yes/Never), and Resource Utilization Band (RUB)
Discussion
Deficits in individual neuropsychological outcomes often do not occur in isolation, but can be correlated with deficits in other outcomes in a way that may be unrecognized. As a result, LCA is useful for identifying clinical phenotypes using the correlations between neuropsychological outcomes. Using an LCA model to evaluate children assessed with neuropsychological tests, we identified four distinct classes, with children grouped into three independent subgroups of deficit and one subgroup with minimal or no deficit. In comparing each deficit class with the Normal class, we found that children with deficits primarily in language and cognition were associated with prior exposure to surgery and anesthesia, while children with primarily behavioral deficits did not show this association. Interestingly, children with language and cognitive deficit coupled with behavioral deficit were also not associated with exposure.
In trying to explain these differences, we evaluated demographic, comorbidity, and other medical diagnosis characteristics to determine if they varied between the LCA subgroups. Overall, while the Normal class of children differed from the three deficit classes, there were minimal differences between the three deficit subgroups themselves. The measured demographic variables are therefore unlikely to completely account for the differences in the association between each LCA subgroup and prior exposure to anesthesia and surgery. While minor differences between subgroups existed in the clinical variables evaluated by RUB scores and CCS diagnosis categories as well as some differences in procedure types in each class, it was difficult to attribute any specific factors as causing the children to be in a particular deficit group.
The association between exposure and language and cognitive deficit, and the lack of an association between behavioral deficit and exposure is consistent with previously published studies.(15,17,18) However, regarding the Severe Deficit group, since language deficits are associated with an increased risk of surgery and anesthesia, it seems counterintuitive that children with concomitant behavioral deficits would be similar to the Normal class of children. One explanation could be that the children in the Severe Deficit class had higher levels of comorbid illness or specific medical diagnoses, which accounted for the increased incidence of deficits independent of exposure, but we were unable to find any obvious potentially causative factor. The Severe deficit class represents a broad-range of neurodevelopmental deficit and if associated with exposure to surgery and anesthesia would suggest pervasive injury across a range of neurodevelopmental domains. Our results suggest that the phenotype for children exposed to surgery and anesthesia is more likely one with specific deficits in higher order brain function and not broad neurodevelopmental injury. The recently published GAS study found no differences in children exposed to awake regional vs. sevoflurane anesthesia for hernia surgery in any neurodevelopmental tests at age two, which included outcomes for language, cognition, motor function, and behavior.(21) While this suggests that sevoflurane has no effect on neurodevelopment at age two, our findings may not be opposed to those results. Our results suggest that language and cognition may be the main differences seen in exposed children. It is therefore not surprising that the GAS study did not find a difference in language and executive function as these domains are difficult to assess in two year olds and assessments at young ages have also been found to lack sensitivity for predicting neurodevelopment later in life.(40,41) This emphasizes the need for studies with neurodevelopmental assessment in older children with early exposure to anesthesia.
There are several limitations in our study. While the data was prospectively collected, this is an analysis of an existing cohort that was not created to answer this question. As such, we were limited by the data we had available, lacked detailed anesthetic information, and had cohort attrition over time. When interpreting our results for external validity, differences in the missing children should be taken into account. While the majority of our neurodevelopmental outcomes were directly assessed, the behavioral outcome, despite being the gold standard in behavioral assessment, relied on parental observation as opposed to a trained observer. Similar to all observational studies, our results are subject to bias from confounding due to underlying differences between the exposed and unexposed children. Despite the fact that we attempted to account for differences in the children, it is unlikely that we were able to completely account for all the effects of comorbid illness. While the association between otitis media and developmental outcomes is disputed, the specific condition of hearing loss in children needing myringotomy should be considered.(42) Deficits may also be associated with specific medical conditions, or procedures. However, due to sample size limitations we were unable to assess procedure or comorbidity specific risk for cognitive deficit. In addition, since the vast majority of children who needed anesthesia also required a surgical procedure, the independent effects of surgery and anesthesia cannot be distinguished. This study however still accomplishes two important goals. The first is to suggest that a phenotype of neurodevelopmental deficit primarily in language and cognition, and not broad neurodevelopmental delay in children may be associated with exposure to anesthesia and surgery, which will guide the assessment of children in future studies. The second is to describe a statistical method for analyzing neurodevelopmental outcomes in children exposed to anesthesia and surgery recognizing that these outcomes are likely to be correlated.
Conclusion
Our results show distinct phenotypes of neurodevelopmental deficit within our cohort, children with language and cognitive deficit, those with behavioral deficit, and those with severe deficit. Children with deficit primarily in language and cognition were associated with prior exposure to anesthesia and surgery, but children with language and cognitive deficit coupled with behavioral deficit were not associated with exposure. While our results are unable to establish a causal link between exposure to anesthesia and neurodevelopmental deficit, they suggest a phenotype of deficit primarily in language and cognition in children who have surgery and anesthesia. While additional studies are needed to validate this phenotype, these results will guide the assessment of children in future prospective studies.
Acknowledgments
We acknowledge the Raine study investigators and staff responsible for the collection of the data presented in this manuscript. Sincere thanks are extended to all study families, as this research could not have been conducted without their participation. We are also grateful to Professor Peter D. Sly, MBBS, FRACP, MD, DSc, Deputy Director, Queensland Children’s Medical Research Institute (Brisbane, Australia) and Jenny Mountain, MClinEpi, Study Manager, Raine Study Team, University of Western Australia (Perth, Australia) for their help in acquiring the data for the manuscript, and Minjae Kim, MD, (New York, NY) for his help reviewing the manuscript.
Funding: This work was supported in part by a grant from SmartTots (San Francisco, CA). The Western Australian Pregnancy Cohort (Raine) Study is funded by project and program grants from the National Health and Medical Research Council of Australia (Canberra, Australia). Core management funding is provided by the Raine Medical Research Foundation, the Telethon Kids Institute, the University of Western Australia (UWA), the UWA Faculty of Medicine, Dentistry and Health Sciences, the Women and Infants Research Foundation, and Curtin University. All institutions providing core management funding reside in Perth, Australia. Dr. Caleb Ing is supported by the Agency for Healthcare Research and Quality (AHRQ) under award number K08HS022941. The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ. Professor Britta von Ungern-Sternberg is partly funded by the Princess Margaret Hospital Foundation and Woolworths Australia (Perth, Australia).
Information for LWW regarding depositing manuscript into PubMed Central: This research was funded by National Institutes of Health grant number K08HS022941.
Appendix Figure 1.
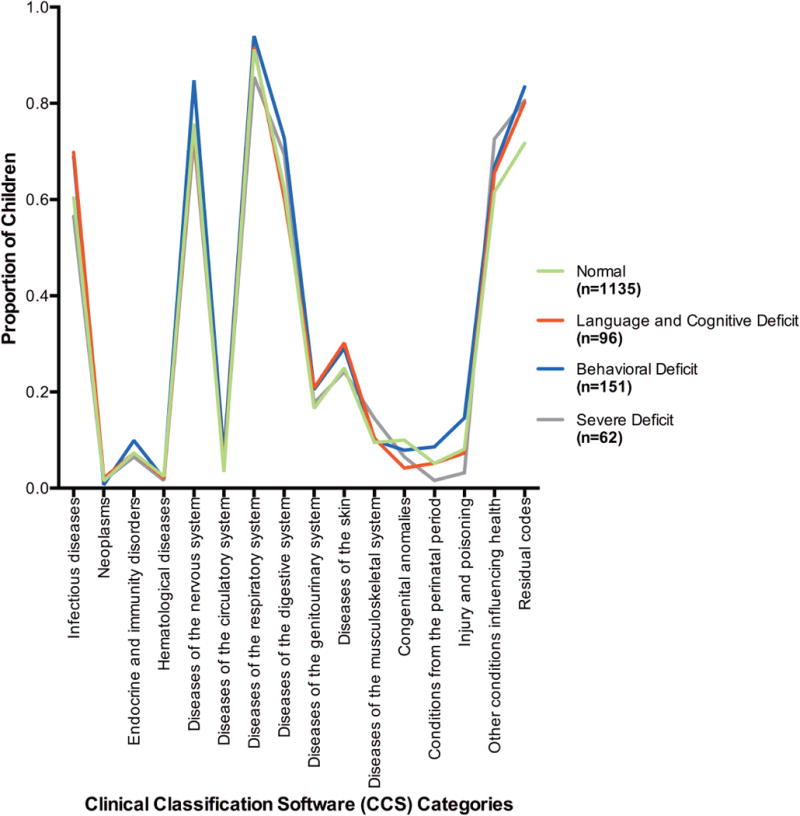
Proportion of Children within Each Latent Class with any ICD-9 Codes in Each Clinical Classification Software (CCS) Category
Appendix Figure 2.
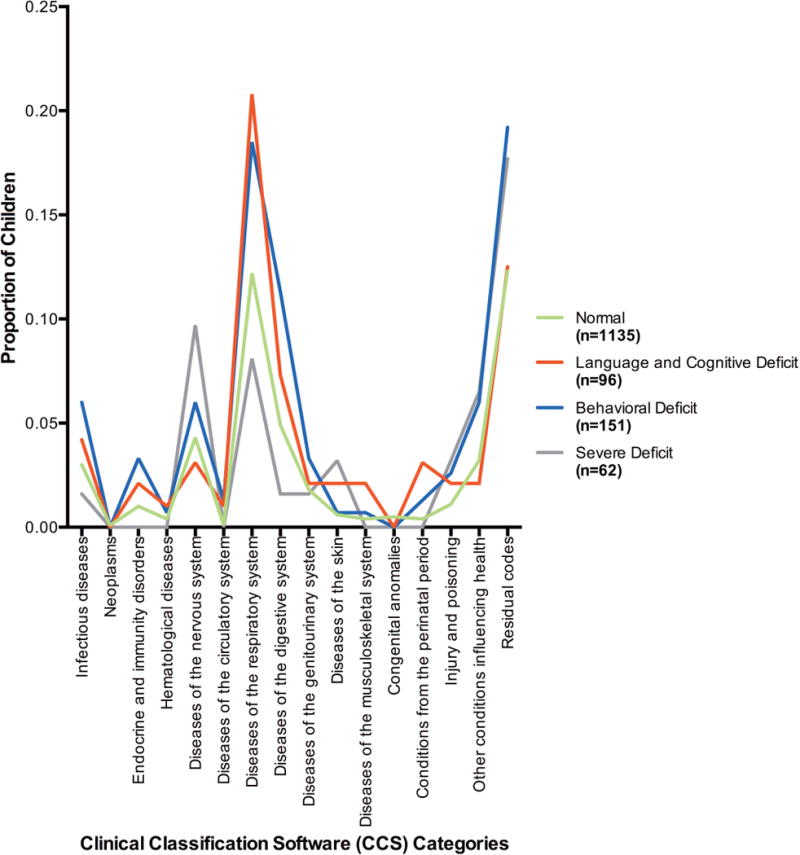
Proportion of Children within Each Latent Class with ICD-9 Codes Requiring a Hospital Visit in Each Clinical Classification Software (CCS) Category
Appendix Table 1.
Procedures Performed Before 3 Years of Age in Normal Class
| Procedure | Frequency Count | Percent of Total |
|---|---|---|
| Myringotomy | 44 | 25 |
| Orchiopexy, hypospadias, circumcision, and other minor urological procedures | 31 | 17.6 |
| Inguinal and umbilical hernia | 20 | 11.4 |
| Tonsillectomy and adenoidectomy | 12 | 6.8 |
| Dental procedure | 11 | 6.3 |
| Procedures on mouth/tongue, nasal airway, and cleft lip and palate repair | 9 | 5.1 |
| Minor skin and nail procedure | 8 | 4.5 |
| Abdominal procedure with laparotomy or laparoscopy | 6 | 3.4 |
| Foot or hand surgery | 6 | 3.4 |
| Laryngoscopy, bronchoscopy, or tracheostomy placement or removal | 4 | 2.3 |
| Minor rectal/anal procedure | 4 | 2.3 |
| Endoscopy or bone marrow biopsy | 3 | 1.7 |
| Kidney and urinary tract procedure | 3 | 1.7 |
| Lymph node excision | 3 | 1.7 |
| Nasolacrimal duct probe | 3 | 1.7 |
| Procedure on orbit, lens, or retina | 3 | 1.7 |
| Computed Tomography scan | 2 | 1.1 |
| Open heart procedure | 2 | 1.1 |
| Cardiac catheterization | 1 | 0.6 |
| Crainiectomy | 1 | 0.6 |
Appendix Table 2.
Procedures Performed Before 3 Years of Age in Language and Cognitive Deficit Class
| Procedure | Frequency Count | Percent of Total |
|---|---|---|
| Myringotomy | 14 | 41.2 |
| Orchiopexy, hypospadias, circumcision, and other minor urological procedures | 4 | 11.8 |
| Tonsillectomy and adenoidectomy | 4 | 11.8 |
| Cardiac catheterization | 2 | 5.9 |
| Inguinal and umbilical hernia | 2 | 5.9 |
| Minor skin and nail procedure | 2 | 5.9 |
| Open heart procedure | 2 | 5.9 |
| Computed Tomography scan | 1 | 2.9 |
| Dental procedure | 1 | 2.9 |
| Nasolacrimal duct probe | 1 | 2.9 |
| PDA closure | 1 | 2.9 |
Appendix Table 3.
Procedures Performed Before 3 Years of Age in Behavioral Deficit Class
| Procedure | Frequency Count | Percent of Total |
|---|---|---|
| Myringotomy | 14 | 42.4 |
| Orchiopexy, hypospadias, circumcision, and other minor urological procedures | 6 | 18.2 |
| Inguinal and umbilical hernia | 4 | 12.1 |
| Dental procedure | 3 | 9.1 |
| Tonsillectomy and adenoidectomy | 3 | 9.1 |
| Minor skin and nail procedure | 1 | 3 |
| Nasolacrimal duct probe | 1 | 3 |
| Procedures on mouth/tongue, nasal airway, and cleft lip and palate repair | 1 | 3 |
Appendix Table 4.
Procedures Performed Before 3 Years of Age in Severe Deficit Class
| Procedure | Frequency Count | Percent of Total |
|---|---|---|
| Dental procedure | 3 | 23.1 |
| Foot or hand surgery | 2 | 15.4 |
| Inguinal and umbilical hernia | 2 | 15.4 |
| Nasolacrimal duct probe | 2 | 15.4 |
| Orchiopexy, hypospadias, circumcision, and other minor urological procedures | 2 | 15.4 |
| Kidney and urinary tract procedure | 1 | 7.7 |
| Myringotomy | 1 | 7.7 |
Contributor Information
Caleb Ing, Department of Anesthesiology, Columbia University College of Physicians and Surgeons, New York, NY.
Melanie M. Wall, Departments of Psychiatry and Biostatistics, Columbia University College of Physicians and Surgeons and Mailman School of Public Health, New York, NY.
Charles J DiMaggio, Departments of Anesthesiology and Epidemiology, Columbia University College of Physicians and Surgeons and Mailman School of Public Health, New York, NY (current affiliation: Department of Surgery, New York University School of Medicine, New York, NY).
Andrew J.O. Whitehouse, Telethon Kids Institute, University of Western Australia, Perth, Australia.
Mary K. Hegarty, Department of Anaesthesia and Pain Management, Princess Margaret Hospital for Children, Perth, Australia.
Ming Sun, Departments of Anesthesiology and Biostatistics, Columbia University College of Physicians and Surgeons and Mailman School of Public Health, New York, NY.
Britta S. von Ungern-Sternberg, School of Medicine and Pharmacology, The University of Western Australia, Perth, Australia and Department of Anaesthesia and Pain Management, Princess Margaret Hospital for Children, Perth, Australia.
Guohua Li, Departments of Anesthesiology and Epidemiology, Columbia University College of Physicians and Surgeons and Mailman School of Public Health, New York, NY.
Lena S. Sun, Departments of Anesthesiology and Pediatrics, Columbia University College of Physicians and Surgeons, New York, NY.
References
- 1.Cattano D, Young C, Straiko MM, Olney JW. Subanesthetic doses of propofol induce neuroapoptosis in the infant mouse brain. Anesth Analg. 2008;106:1712–4. doi: 10.1213/ane.0b013e318172ba0a. [DOI] [PubMed] [Google Scholar]
- 2.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. Journal of Neuroscience. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loepke AW, Soriano SG. An assessment of the effects of general anesthetics on developing brain structure and neurocognitive function. Anesth Analg. 2008;106:1681–707. doi: 10.1213/ane.0b013e318167ad77. [DOI] [PubMed] [Google Scholar]
- 4.Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5:515–21. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- 5.Slikker W, Jr, Zou X, Hotchkiss CE, Divine RL, Sadovova N, Twaddle NC, Doerge DR, Scallet AC, Patterson TA, Hanig JP, Paule MG, Wang C. Ketamine-induced neuronal cell death in the perinatal rhesus monkey. Toxicol Sci. 2007;98:145–58. doi: 10.1093/toxsci/kfm084. [DOI] [PubMed] [Google Scholar]
- 6.Young C, Jevtovic-Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, Olney JW. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br J Pharmacol. 2005;146:189–97. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou X, Liu F, Zhang X, Patterson TA, Callicott R, Liu S, Hanig JP, Paule MG, Slikker W, Jr, Wang C. Inhalation anesthetic-induced neuronal damage in the developing rhesus monkey. Neurotoxicol Teratol. 2011;33:592–7. doi: 10.1016/j.ntt.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Bercker S, Bert B, Bittigau P, Felderhoff-Muser U, Buhrer C, Ikonomidou C, Weise M, Kaisers UX, Kerner T. Neurodegeneration in newborn rats following propofol and sevoflurane anesthesia. Neurotox Res. 2009;16:140–7. doi: 10.1007/s12640-009-9063-8. [DOI] [PubMed] [Google Scholar]
- 9.Paule MG, Li M, Allen RR, Liu F, Zou X, Hotchkiss C, Hanig JP, Patterson TA, Slikker W, Jr, Wang C. Ketamine anesthesia during the first week of life can cause long-lasting cognitive deficits in rhesus monkeys. Neurotoxicol Teratol. 2011;33:220–30. doi: 10.1016/j.ntt.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satomoto M, Satoh Y, Terui K, Miyao H, Takishima K, Ito M, Imaki J. Neonatal exposure to sevoflurane induces abnormal social behaviors and deficits in fear conditioning in mice. Anesthesiology. 2009;110:628–37. doi: 10.1097/ALN.0b013e3181974fa2. [DOI] [PubMed] [Google Scholar]
- 11.Bartels M, Althoff RR, Boomsma DI. Anesthesia and cognitive performance in children: no evidence for a causal relationship. Twin Res Hum Genet. 2009;12:246–53. doi: 10.1375/twin.12.3.246. [DOI] [PubMed] [Google Scholar]
- 12.Block RI, Thomas JJ, Bayman EO, Choi JY, Kimble KK, Todd MM. Are anesthesia and surgery during infancy associated with altered academic performance during childhood? Anesthesiology. 2012;117:494–503. doi: 10.1097/ALN.0b013e3182644684. [DOI] [PubMed] [Google Scholar]
- 13.DiMaggio C, Sun LS, Kakavouli A, Byrne MW, Li G. A retrospective cohort study of the association of anesthesia and hernia repair surgery with behavioral and developmental disorders in young children. J Neurosurg Anesthesiol. 2009;21:286–91. doi: 10.1097/ANA.0b013e3181a71f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiMaggio C, Sun LS, Li G. Early childhood exposure to anesthesia and risk of developmental and behavioral disorders in a sibling birth cohort. Anesth Analg. 2011;113:1143–51. doi: 10.1213/ANE.0b013e3182147f42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flick RP, Katusic SK, Colligan RC, Wilder RT, Voigt RG, Olson MD, Sprung J, Weaver AL, Schroeder DR, Warner DO. Cognitive and behavioral outcomes after early exposure to anesthesia and surgery. Pediatrics. 2011;128:e1053–61. doi: 10.1542/peds.2011-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen TG, Pedersen JK, Henneberg SW, Pedersen DA, Murray JC, Morton NS, Christensen K. Academic performance in adolescence after inguinal hernia repair in infancy: a nationwide cohort study. Anesthesiology. 2011;114:1076–85. doi: 10.1097/ALN.0b013e31820e77a0. [DOI] [PubMed] [Google Scholar]
- 17.Ing C, Dimaggio C, Whitehouse A, Hegarty MK, Brady J, von Ungern-Sternberg BS, Davidson A, Wood AJ, Li G, Sun LS. Long-term Differences in Language and Cognitive Function After Childhood Exposure to Anesthesia. Pediatrics. 2012;130:e476–85. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- 18.Kalkman CJ, Peelen L, Moons KG, Veenhuizen M, Bruens M, Sinnema G, de Jong TP. Behavior and development in children and age at the time of first anesthetic exposure. Anesthesiology. 2009;110:805–12. doi: 10.1097/ALN.0b013e31819c7124. [DOI] [PubMed] [Google Scholar]
- 19.Sprung J, Flick RP, Katusic SK, Colligan RC, Barbaresi WJ, Bojanic K, Welch TL, Olson MD, Hanson AC, Schroeder DR, Wilder RT, Warner DO. Attention-deficit/hyperactivity disorder after early exposure to procedures requiring general anesthesia. Mayo Clin Proc. 2012;87:120–9. doi: 10.1016/j.mayocp.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilder RT, Flick RP, Sprung J, Katusic SK, Barbaresi WJ, Mickelson C, Gleich SJ, Schroeder DR, Weaver AL, Warner DO. Early exposure to anesthesia and learning disabilities in a population-based birth cohort. Anesthesiology. 2009;110:796–804. doi: 10.1097/01.anes.0000344728.34332.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson AJ, Disma N, de Graaff JC, Withington DE, Dorris L, Bell G, Stargatt R, Bellinger DC, Schuster T, Arnup SJ, Hardy P, Hunt RW, Takagi MJ, Giribaldi G, Hartmann PL, Salvo I, Morton NS, von Ungern Sternberg BS, Locatelli BG, Wilton N, Lynn A, Thomas JJ, Polaner D, Bagshaw O, Szmuk P, Absalom AR, Frawley G, Berde C, Ormond GD, Marmor J, McCann ME, consortium GAS Neurodevelopmental outcome at 2 years of age after general anaesthesia and awake-regional anaesthesia in infancy (GAS): an international multicentre, randomised controlled trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00608-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davidson AJ, Becke K, de Graaff J, Giribaldi G, Habre W, Hansen T, Hunt RW, Ing C, Loepke A, McCann ME, Ormond GD, Pini Prato A, Salvo I, Sun L, Vutskits L, Walker S, Disma N. Anesthesia and the developing brain: a way forward for clinical research. Paediatr Anaesth. 2015;25:447–52. doi: 10.1111/pan.12652. [DOI] [PubMed] [Google Scholar]
- 23.Ing CH, DiMaggio CJ, Malacova E, Whitehouse AJ, Hegarty MK, Feng T, Brady JE, von Ungern-Sternberg BS, Davidson AJ, Wall MM, Wood AJ, Li G, Sun LS. Comparative analysis of outcome measures used in examining neurodevelopmental effects of early childhood anesthesia exposure. Anesthesiology. 2014;120:1319–32. doi: 10.1097/ALN.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 24.Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci. 2005;9:104–10. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–78. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 26.Dunn L, Dunn L, Williams K, Wang J. Peabody picture vocabulary test III Circle Pines. MN: American Guidance Services Inc; 1997. [Google Scholar]
- 27.Semel E, Wiig E, Secord W. Clinical Evaluation of Language Fundamentals. 3rd. San Antonio, TX: Psychological Corporation Harcourt Brace Co; 1995. [Google Scholar]
- 28.Raven J, Court J, Raven J. Manual for Raven’s Progressive Matricies and Vocabulary Scales-section 2: Coloured progressive matrices. Oxford: Oxford Psychologists Press; 1990. [Google Scholar]
- 29.Smith A. Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services; 1973. [Google Scholar]
- 30.McCarron LT. MAND McCarron Assessment of Neuromuscular Development: Fine and gross motor abilities. Dallas, TX: Common Market Press; 1997. [Google Scholar]
- 31.Achenbach T. Manual for the Child Behavior Checklist/4-19 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- 32.Davidson JE, Irizarry MC, Bray BC, Wetten S, Galwey N, Gibson R, Borrie M, Delisle R, Feldman HH, Hsiung GY, Fornazzari L, Gauthier S, Guzman D, Loy-English I, Keren R, Kertesz A, George-Hyslop PS, Wherrett J, Monsch AU. An exploration of cognitive subgroups in Alzheimer’s disease. J Int Neuropsychol Soc. 2010;16:233–43. doi: 10.1017/S1355617709991160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Depner M, Fuchs O, Genuneit J, Karvonen AM, Hyvarinen A, Kaulek V, Roduit C, Weber J, Schaub B, Lauener R, Kabesch M, Pfefferle PI, Frey U, Pekkanen J, Dalphin JC, Riedler J, Braun-Fahrlander C, von Mutius E, Ege MJ. Clinical and epidemiologic phenotypes of childhood asthma. Am J Respir Crit Care Med. 2014;189:129–38. doi: 10.1164/rccm.201307-1198OC. [DOI] [PubMed] [Google Scholar]
- 34.Hanfelt JJ, Wuu J, Sollinger AB, Greenaway MC, Lah JJ, Levey AI, Goldstein FC. An exploration of subgroups of mild cognitive impairment based on cognitive, neuropsychiatric and functional features: analysis of data from the National Alzheimer’s Coordinating Center. Am J Geriatr Psychiatry. 2011;19:940–50. doi: 10.1097/JGP.0b013e31820ee9d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muthén LK, Muthén BO. Mplus User’s Guide. Seventh. Los Angeles, CA: Muthén & Muthén; 2012. [Google Scholar]
- 36.Fraley C, Raftery AE. How many clusters? Which clustering method? Answers via model-based cluster analysis. Computer Journal. 1998;41:578–88. [Google Scholar]
- 37.Kass RE, Raftery AE. Bayes Factors. Journal of the American Statistical Association. 1995;90:773–95. [Google Scholar]
- 38.HCUP Clinical Classifications Software (CCS) for ICD-9-CM Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: 2006–20090. [PubMed] [Google Scholar]
- 39.The Johns Hopkins ACG Case Mix System. Version 10.0 Release Notes. PC (DOS/WIN/NT) and Unix Version 10.0 - December 2011. Baltimore, MD: Johns Hopkins Bloomberg School of Public Health; 2010. [Google Scholar]
- 40.Bellinger DC, Newburger JW, Wypij D, Kuban KC, du Plesssis AJ, Rappaport LA. Behaviour at eight years in children with surgically corrected transposition: The Boston Circulatory Arrest Trial. Cardiol Young. 2009;19:86–97. doi: 10.1017/S1047951108003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGrath E, Wypij D, Rappaport LA, Newburger JW, Bellinger DC. Prediction of IQ and achievement at age 8 years from neurodevelopmental status at age 1 year in children with D-transposition of the great arteries. Pediatrics. 2004;114:e572–6. doi: 10.1542/peds.2003-0983-L. [DOI] [PubMed] [Google Scholar]
- 42.Roberts JE, Rosenfeld RM, Zeisel SA. Otitis media and speech and language: a meta-analysis of prospective studies. Pediatrics. 2004;113:e238–48. doi: 10.1542/peds.113.3.e238. [DOI] [PubMed] [Google Scholar]


