Abstract
Importance
Within ten years after breast cancer diagnosis, 5% of patients develop contralateral primary breast cancer (CBC). Randomized trials have found that tamoxifen and aromatase inhibitors (AIs) reduce CBC risk. However, little is known about the magnitude and duration of protective effects within the context of “real world” clinical management settings, where varying durations and gaps in treatment are common.
Objective
To determine the influence of adjuvant tamoxifen and AIs on CBC risk within the general community setting.
Design
Retrospective cohort study of CBC risk among breast cancer patients diagnosed with a first primary unilateral invasive breast cancer at Kaiser Permanente Northwest or Colorado from 1990–2008 and followed through 2011.
Setting
General community health care plan.
Participants
7,541 breast cancer patients, ages 24–85 years, diagnosed with invasive breast cancer, and survived for ≥one year.
Exposures
Adjuvant tamoxifen and AIs assessed using prescription records.
Main Outcome Measure
Incident contralateral breast cancer.
Results
Over a median(range) of 6.3(1.0–20.9) years, 248 women developed CBC. CBC risk decreased significantly with increasing tamoxifen duration. In current users, the relative risk (RR) per year of use was 0.76 (95%CI:0.64–0.89), with a 66% reduction for 4 years of use (RR=0.34, 95%CI:0.29–0.40). Risk reductions were slightly smaller for past users, but still significant 5+ years after stopping (RR/year use=0.85, 95%CI:0.71–0.995). AI use without tamoxifen was also associated with reduced CBC risk (RR=0.48, 95%CI:0.22–0.97). Risk reductions were most apparent among women whose primary and CBCs were ER-positive.
Conclusions and Relevance
We found that tamoxifen reduced CBC risk during treatment and after cessation, with risk progressively decreasing as tamoxifen duration increased. Tamoxifen use for 4+ years was estimated to result in the prevention of three CBCs per 100 women by 10 years following an ER-positive first breast cancer, an absolute risk reduction which is consistent with findings from clinical trials. If adjuvant endocrine therapy is indicated for breast cancer treatment, our findings, in concert with trial data, suggest that women should be encouraged to complete the full course.
Keywords: Breast Neoplasms, Second Primary, Tamoxifen, Aromatase Inhibitors, Cohort Studies
Introduction
Increasing incidence of estrogen receptor (ER)-positive breast cancer in the U.S. in concert with the aging population and improved survival have resulted in an increased number of women at risk of developing second primary contralateral breast cancer (CBC).1 Ten-year cumulative incidence of CBC is approximately 5%.2 Randomized clinical trials have consistently demonstrated that tamoxifen reduces risk of primary cancer recurrence, improves survival, and reduces CBC risk .3,4 An Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) pooled analysis of 20 trials examining long-term effects of five years of adjuvant tamoxifen versus no tamoxifen found an absolute reduction in CBC at 15 years of 3.2% among women whose initial diagnoses were ER-positive; tamoxifen did not significantly alter CBC incidence among women with ER-negative primary tumors.3 Amassing trial data also indicate lower CBC risk with aromatase inhibitors (AIs).5,6
Despite strong evidence from trials, little is known about the magnitude and duration of protective effects within real world treatment scenarios, where varying durations and gaps in treatment are common. Observational studies have reported marked reductions in CBC risk with use of tamoxifen or other unspecified endocrine therapies;7–17 however, most have not directly examined therapy duration and persistence of effects.7–12,14–17 Further, observational studies have offered conflicting evidence as to whether associations differ according to ER status of the CBC,9,12 and none has reported on the influence of AIs on CBC risk.
We undertook an investigation of the influence of adjuvant tamoxifen and AIs on CBC risk in a cohort of women diagnosed with invasive breast cancer within the Kaiser Permanente health plan. Kaiser offers several advantages for studying these associations, including the long-term systematic follow-up of a large number of breast cancer patients with electronic prescription records, facilitating characterization of persistence of effects of varying treatment durations while accounting for treatment gaps.
Methods
Study Population and Data Collection
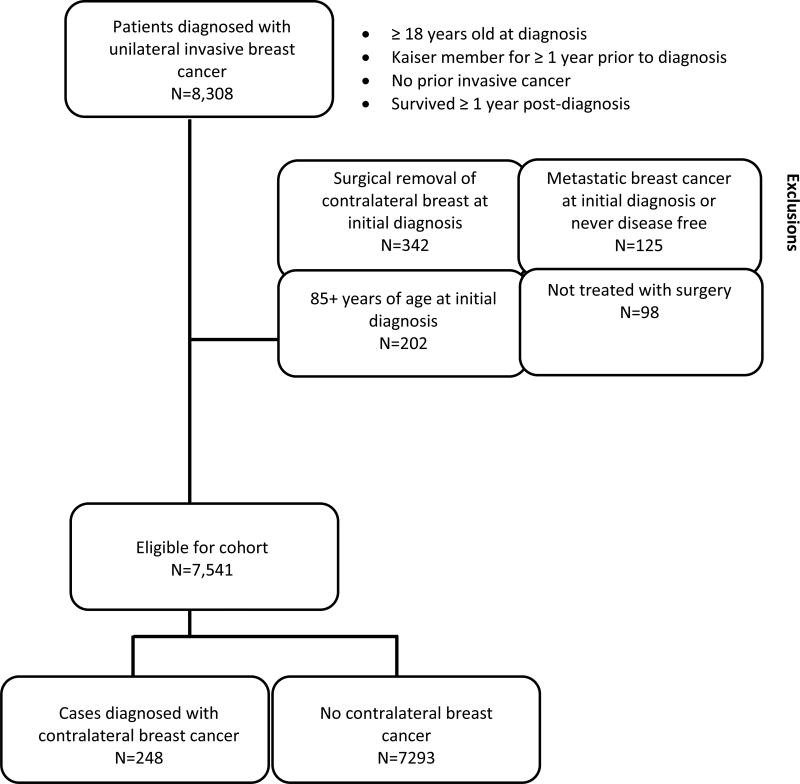
We developed a retrospective cohort of patients (n=8,308), diagnosed with a first primary unilateral invasive breast cancer at Kaiser Permanente (KP) Northwest (n=4,665, 1990–2008) or Colorado (n=3,643, 1994–2008), who survived and remained at CBC risk for ≥one year (Figure 1). We excluded 342 patients with contralateral breast removal at initial surgery, 125 initially diagnosed with metastatic disease, 98 not treated with breast surgery, and 202 diagnosed at ages 85+ (Figure 1), resulting in an analytic cohort of 7,541 patients.
Figure 1.
Inclusion and exclusion criteria for study participants. Women diagnosed with invasive breast cancer at Kaiser Permanente (KP) Northwest (1990–2008) or Colorado (1994–2008) were followed until the earliest of the following events: contralateral breast cancer diagnosis, other second cancer diagnosis, exited the KP plan, death, last tumor-registry follow-up, or end of study date (December 31, 2010 for KP Northwest or 2011 for KP Colorado).
This study was approved by the NCI Special Studies Institutional Review Board (IRB) and KP Northwest and Colorado IRBs. A waiver of written informed consent was granted based on the minimal risk of this linkage-based research.
Exposure and Outcome Assessment
Treatment with tamoxifen, AIs, other hormonal agents, chemotherapeutic agents, and radiotherapy was obtained from electronic KP prescription and medical records. Treatment courses were defined separately for tamoxifen and AIs and consisted of therapy without a gap in prescription coverage of ≥90 days. Women were considered users of adjuvant endocrine therapy if they had accumulated ≥90 days of therapy during any one treatment course following the first primary cancer diagnosis. Due to the relatively recent introduction of AIs in this cohort, we assessed ever use, but could not yet evaluate their recency or duration. Current tamoxifen use was defined as tamoxifen use within the past 30 days whereas former use was defined as ≥30 days. Time since last tamoxifen use was calculated from the end of the last tamoxifen course during the follow-up period; 15% of patients treated with tamoxifen had ≥2 courses.
Patient and clinical characteristics, including race, height, weight (available after 1996 in NW and after 2000 in CO), and tumor characteristics, were obtained from electronic medical records. Measured height and weight were used to calculate BMI (kg/m2). Second cancer diagnoses and vital status were determined from the KP tumor registry.
Statistical Analysis
We used multivariable Poisson regression models to estimate relative risks (RRs) and 95% confidence intervals (CIs) for CBC risk associated with endocrine therapy. Study entry was one year after diagnosis of the first breast cancer, and person-years accrued until the earliest of the following events: CBC diagnosis, other second cancer diagnosis, death, last tumor-registry follow-up, exit from KP plan, or end of study (KP Northwest:12/31/2010; Colorado:2011).
Tamoxifen and AIs were treated as time-dependent exposures. We evaluated risk in relation to a combination of tamoxifen duration in years (continuous variable, log linear trend) and time since last use (i.e., current use and former use with <3 years, 3-<5 years and 5+ years since last use); the reference group was defined as those who had never used tamoxifen or used tamoxifen for <90 days. Among former tamoxifen users, we also assessed risk in relation to categories of tamoxifen duration of 90 days-<1 year, 1-<4 years, and 4+ years, using cut points based on the cohort distribution of cumulative tamoxifen exposure. Sensitivity analyses evaluating CBC risk associated with the first tamoxifen course yielded similar results and are not shown. Poisson regression models were stratified by covariates that we had identified a priori, including study site, age and AJCC stage at diagnosis, ER status of initial tumor, time since initial breast cancer in yearly categories up to 15 years or more, and diagnosis year. We also adjusted models simultaneously for AI use (with or without tamoxifen), chemotherapy with an alkylating agent, and radiotherapy. As BMI was not associated with tamoxifen duration, it was not used in the adjustments.
In subgroup analyses, we evaluated associations by ER status and age (<50 vs. 50+ years, a proxy for menopausal status) at diagnosis of the initial breast cancer. We also examined risk according to ER status of the CBC.
Among women surviving 5+ years after a diagnosis of ER-positive breast cancer, we estimated cumulative incidence of CBC according to tamoxifen duration (no tamoxifen, tamoxifen duration <4 years (the median duration), and duration 4+ years), accounting for death and diagnosis of other second cancers as competing risks.18
P-values of <0.05 were considered statistically significant. All tests of significance were two-tailed. Analyses were performed using Epicure software (v2.1, Seattle, WA).19
Results
Patient characteristics associated with CBC and adjuvant tamoxifen therapy
The median(range) age at initial breast cancer diagnosis was 60.6(24.9–84.9) years. Women were predominantly white (93%)(Supplementary Table 1). Over a median(range) of 6.3(1.0–20.9) years of follow-up, 248 women developed CBC (45 in-situ, 203 invasive). Compared to women who did not develop CBC, CBC cases had similar initial breast cancer stage, grade, histology, and ER status. Nearly 70% of CBC cases and non-cases were prescribed radiotherapy and approximately one-third were prescribed chemotherapy with an alkylating agent for initial treatment. However, during study follow-up, CBC cases were less likely to have been treated with endocrine therapy as compared with non-cases (51% vs. 65%).
Compared with non-users, tamoxifen users were slightly more likely to be white (95% vs. 91%) and <50 years of age at initial diagnosis (22% vs. 19%)(Supplementary Table 2). Tamoxifen users were more likely to have initial diagnoses that were stage II, moderately differentiated grade, and ER-positive. In addition, tamoxifen use was less common among women whose primary breast cancer had been diagnosed more recently.
Relationships between adjuvant endocrine therapy and CBC risk
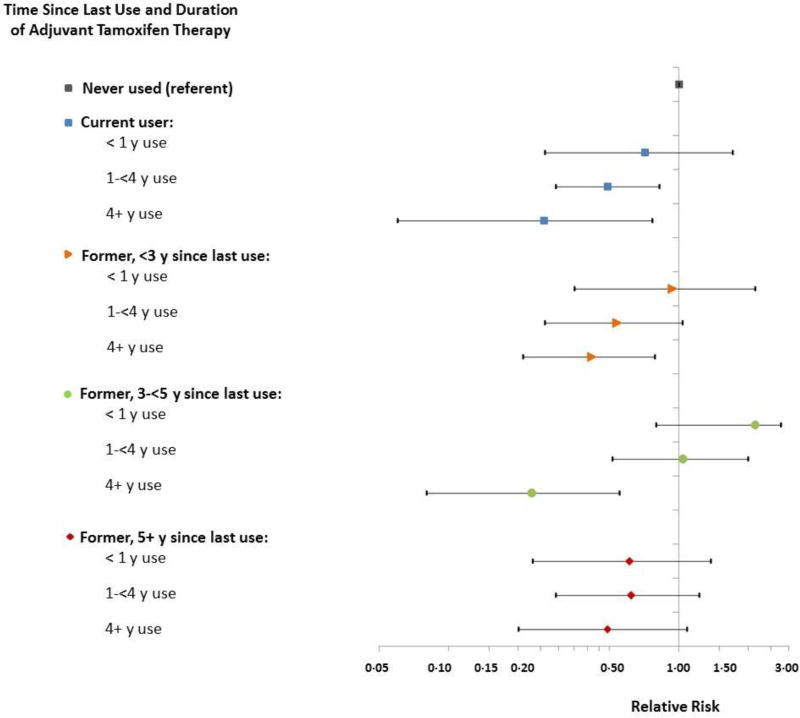
Fifty-two percent of patients used tamoxifen with a median(range) duration of use of 3.3(0.25–16.2) years. CBC risk decreased with increasing tamoxifen duration among current and former users (Figure 2, Supplementary Table 3). In current users, the RR per year of tamoxifen use was 0.76(95%CI:0.64–0.89) (Table 1), resulting in an estimated 66% RR reduction for 4 years of use as compared with non-users (RR=0.34, 95%CI:0.29–0.40). The risk reductions were slightly smaller for past users, but still significant 5+ years after stopping (RR/year use=0.85, 95%CI:0.71–0.995).
Figure 2.
Relative risks (RR) and 95% confidence intervals (CI) for contralateral breast cancer associated with adjuvant tamoxifen by time since last use and duration of therapy, Kaiser Permanente Northwest and Colorado. Poisson models were stratified by study site (NW/CO), age at diagnosis (<40, 40–49, 50–59, 60–69, 70+ years), stage at diagnosis (I, II, III, unknown stage), ER status of the initial tumor (positive, negative, unknown/indeterminate), time since initial breast cancer in yearly categories up to 15 years or more, and, to account for potential cohort effects, year of diagnosis (1990–94, 1995–99, 2000–04, 2005–2008). We also adjusted models simultaneously for aromatase inhibitor use (with or without tamoxifen), chemotherapy with an alkylating agent, and radiotherapy. Numbers of contralateral breast cancer cases, RRs and 95% CIs may be found in Supplementary Table 2.
Table 1.
Multivariate* relative risks (RR) and 95% confidence intervals (CI) for contralateral breast cancer associated with adjuvant endocrine therapy
| All BC patients and risk of any CBCⱡ (N=7,541) |
Initial BC ER-positive# and risk of any CBCⱡ |
Initial BC ER-positive# and risk of ER-positive CBC |
Initial BC ER-positive# and risk of ER-negative CBC |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | No. CBC |
Person- years |
RR/y use† |
95% CI | No. CBC |
Person- years |
RR/y use† |
95% CI | No. CBC |
RR/y use† |
95% CI | No. CBC |
RR/y use† |
95% CI |
| Tamoxifen duration (years) and time since | ||||||||||||||
| last use: | ||||||||||||||
| Never used or used for <90 days | 131 | 20760 | REF | 83 | 12852 | REF | 64 | REF | 10 | REF | ||||
| Current user | 33 | 11199 | 0.76 | 0.64 to 0.89 | 31 | 10385 | 0.77 | 0.64 to 0.90 | 17 | 0.68 | 0.54 to 0.84 | 8 | 1.00 | 0.69 to 1.40 |
| Former user; 30 days-<3y since last use | 32 | 7551 | 0.84 | 0.74 to 0.95 | 28 | 6971 | 0.83 | 0.73 to 0.94 | 16 | 0.79 | 0.67 to 0.91 | 8 | 1.00 | 0.73 to 1.36 |
| Former user; 3-<5y since last use | 25 | 3386 | 0.87 | 0.75 to 0.997 | 22 | 3127 | 0.86 | 0.74 to 0.999 | 17 | 0.83 | 0.70 to 0.98 | 2 | 0.90 | 0.49 to 1.49 |
| Former user; 5+y since last use | 27 | 5078 | 0.85 | 0.71 to 0.995 | 27 | 4650 | 0.86 | 0.73 to 1.02 | 24 | 0.86 | 0.71 to 1.02 | 3 | 0.88 | 0.52 to 1.43 |
| AI use: | ||||||||||||||
| Any AI use, without tamoxifen | 10 | 3531 | 0.48 | 0.22 to 0.97 | 10 | 3446 | 0.50 | 0.22 to 1.01 | 6 | 0.44 | 0.16 to 1.05 | 4 | 1.84 | 0.42 to 7.20 |
| Any AI use, with tamoxifen | 16 | 3526 | 0.86 | 0.46 to 1.51 | 15 | 3385 | 0.83 | 0.43 to 1.49 | 10 | 0.78 | 0.35 to 1.58 | 5 | 1.47 | 0.40 to 4.72 |
AI, aromatase inhibitor; BC, breast cancer; CI, confidence interval; CBC, contralateral breast cancer; REF, referent group; RR, relative risk.
Poisson models were stratified by study site (NW/CO), age at diagnosis (<40, 40–49, 50–59, 60–69, 70+ years), stage at diagnosis (I, II, III, unknown stage), time since initial breast cancer in yearly categories and 15+ years, and year of diagnosis (1990–94, 1995–99, 2000–04, 2005–2008). We simultaneously adjusted for aromatase inhibitor use (with or without tamoxifen), chemotherapy with an alkylating agent, and radiotherapy.
N=5,951 patients whose initial diagnosis was ER-positive.
Risk of any CBC includes ER-positive, ER-negative, and ER unknown/indeterminate CBC.
RRs from tamoxifen duration trend analyses are estimates of risk per year of tamoxifen use; RRs for AIs are estimates of risk associated with any AI use.
During the course of the study, 1,929 patients (26%) received AIs (typically anastrozole or less frequently letrozole), with increasing use in more recent calendar periods. AIs taken with (n=963) and without (n=966) tamoxifen were used for a median(range) duration of 2.24(0.25–10.23) and 2.94(0.25–9.46) years, respectively. AI use without tamoxifen was associated with reduced CBC risk (AI users compared with non-users: RR=0.48, 95%CI:0.22–0.97, n=10 CBC, Table 1). We did not have sufficient statistical power to evaluate these risks in more detail.
Subgroup analyses by ER status of the primary and contralateral breast cancers
Among women whose first cancer was ER-positive (n=5,951), findings were consistent with the overall cohort (Table 1). Likewise, when considering ER status of the subsequent CBC, risk of ER-positive CBC decreased significantly with increasing years of tamoxifen duration among current users (RR/year use=0.68, 95%CI:0.54–0.84) and among former users with <5 years since last use as compared with non-users (RR/year use in those with <3 years since last use=0.79, 95%CI:0.67–0.91; RR/year use in those with 3-<5 years since last use=0.83, 95%CI:0.70–0.98). Among women whose primary cancer was ER-positive, 31 were subsequently diagnosed with ER-negative CBC. Though based on small numbers, there was little indication that tamoxifen reduced ER-negative CBC risk, when evaluated using tamoxifen duration (Table 1) or when tamoxifen users were compared to non-users (RR=1.06, 95%CI:0.41–3.10).
Among women whose first primary cancer was ER-negative (n=1,236), 14.6% used endocrine therapy, for which no clear effects were observed (data not shown). Twenty-five of the CBCs were ER-positive and 13 were ER-negative. Among patients with a first ER-negative cancer, tamoxifen was not associated with reduced risk even of ER-positive CBC (RR=1.97, 95%CI:0.50–6.39; n=4 CBC); however, the numbers of CBCs diagnosed within treatment groups were small.
Subgroup analyses by age of first breast cancer diagnosis
In separate analyses by age at initial diagnosis, findings among women who were 50+ years at initial diagnosis (Supplementary Table 4) were similar to those observed in the overall cohort. Among women whose first breast cancer was diagnosed at age <50 years, risk reductions were similar for current and former users with <5 years since last use but based on only 53 CBC cases and not statistically significant.
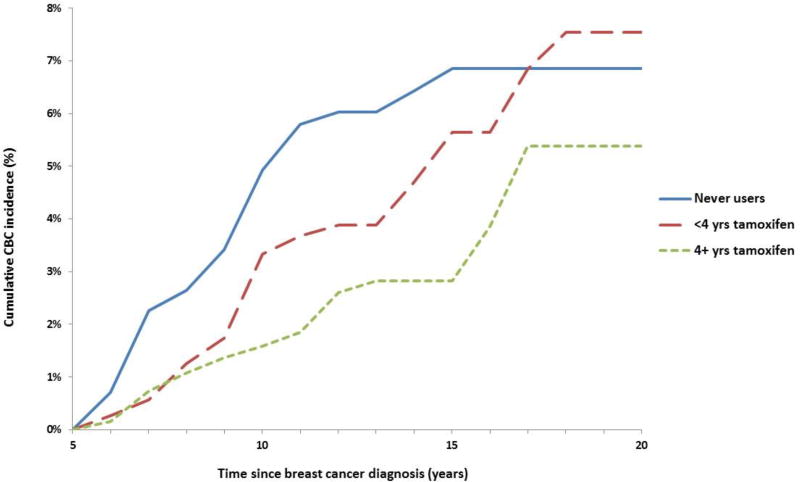
Cumulative incidence of CBC
Figure 3 shows the estimated cumulative incidence of CBC adjusted for competing risks by tamoxifen use and duration among 3,732 women who survived 5+ years after a diagnosis of ER-positive first breast cancer. Among these patients, 67% used tamoxifen with a median(range) duration of use of 4.19(0.25–16.18) years. Patients who did not use tamoxifen had a 10-year cumulative incidence of CBC=4.9% (95%CI:3.3–6.5), whereas incidence was lower among those taking tamoxifen for <4 years (3.3%, 95%CI:2.1–4.6) and was lowest among those taking tamoxifen for 4+ years (1.6%, 95%CI:0.9–2.3). Among these 5+ year survivors, tamoxifen use for 4+ years was estimated to prevent three CBCs per 100 women at 10 years following the first diagnosis. At 15 years post-diagnosis, use of tamoxifen for 4+ years was estimated to prevent 4 CBCs per 100 women.
Figure 3.
Cumulative incidence of contralateral breast cancer adjusted for competing risks by tamoxifen use and duration among 3,732 women who survived 5+ years after being diagnosed with an ER-positive first breast cancer.
Discussion
This retrospective analysis of over 7,500 U.S. patients with invasive breast carcinoma treated in a general community health care plan suggests that adjuvant tamoxifen and AIs significantly reduce CBC risk. We have supplemented clinical trial data by testing associations in an unselected group of patients, utilizing prescription records to accurately quantify the risk reduction in CBC per year of adjuvant tamoxifen use, and estimating the persistence of therapeutic effects in analyses accounting for gaps in therapy and multiple treatment courses. We found that tamoxifen protects against CBC while women are undergoing treatment, and after treatment has ceased. Among former users, the risk reduction was most apparent for those receiving tamoxifen for 4+ years, with protective effects persisting during the first five years after stopping. These associations were stronger among women whose first primary cancer was ER-positive.
The risk reductions in CBC associated with 4+ years of tamoxifen in this observational cohort are consistent with those found in clinical trials of women randomized to five years of tamoxifen.20,21 Whereas the standard “intention-to-treat” analysis in trials only evaluates the prescribed treatment period, we directly evaluated cumulative tamoxifen exposure over a wide range of durations (0.25–16.2 years) and observed progressive reductions in CBC risk with increasing duration. A systematic ASCO review concluded that women randomized to take tamoxifen therapy either indefinitely or for 10 years as compared with five years had lower CBC risk.22 Less than 5% of patients in our cohort received cumulative tamoxifen greater than five years; however, our findings are still an extension beyond prior observational cohorts, which only evaluated risk estimates for tamoxifen lasting ≥1 year.8,9,13
As we have shown, persistence of protective effects of adjuvant tamoxifen on CBC risk likely depends on therapy duration, an issue not sufficiently addressed in prior observational studies.7–12,14–17 In the WECARE case-control study, significant protective effects of tamoxifen on CBC persisted for five years post-diagnosis; therapy duration was not directly examined.7 Two nested case-control studies found that taking tamoxifen for ≥12 months was associated with decreased CBC risk, but persistence of the effect was not reported.8,9 Cohort studies have demonstrated substantial reductions in CBC risk with tamoxifen or other unspecified endocrine therapies.10–17 In the single cohort study to have evaluated the persistence of effects in relation to tamoxifen duration, no clear patterns emerged.13 This Danish study found that current, but not former, adjuvant tamoxifen was associated with reduced CBC risk.13 The lack of effect among former users could be due to shorter tamoxifen duration (median:1–2.5 years)13 relative to our U.S. patient population.
In contrast, findings from our observational study are remarkably consistent with a recent EBCTCG pooled analysis examining long-term effects of adjuvant tamoxifen.3 Similar to EBCTCG’s report of an absolute reduction in CBC at 15 years of 3.2%,3 we saw absolute reductions in CBC of 3.3% at 10 years and 4% at 15 years for 4+ years of tamoxifen versus no tamoxifen among ER-positive patients surviving 5+ years. Also consistent with EBCTCG’s pooled analysis,3 we found that tamoxifen did not appear to lower CBC incidence among women with ER-negative primary tumors, although our numbers were too small for precise evaluation. The agreement of our study with EBCTCG’s report regarding the effectiveness of tamoxifen strengthens evidence for a long-lasting reduction in CBC risk both within and outside of the clinical trial setting.
Just as tamoxifen has been shown to be most effective among women whose initial diagnosis is ER-positive, in our study as well as others,9,12,23 tamoxifen was particularly effective in reducing risk of ER-positive CBC. We and others12 have also shown that tamoxifen does not seem to significantly influence risk of ER-negative CBC; however, these findings conflict with those from a prior nested case-control study, which reported a 4.4-fold elevation in risk of ER-negative CBC (52 cases) associated with 5+ years of adjuvant tamoxifen.9 Although we had limited statistical power to evaluate risk of ER-negative CBC, confidence intervals for tamoxifen-associated ER-negative CBC risk were not consistent with an increased risk of the magnitude reported previously.
For decades, five years of tamoxifen was the standard adjuvant therapy for patients with ER-positive tumors.24 More recently, AIs have provided an alternate therapeutic option for ER-positive postmenopausal patients.6,25 In the 10-year analysis of the ATAC trial, women randomized to receive anastrozole versus tamoxifen experienced significantly lower CBC risk.26 The relatively recent introduction of AIs into clinical practice in our cohort precluded assessments of drug classes and persistence of effects of varying AI durations on CBC risk. This will be the subject of future investigations.
In the U.S., most observational studies evaluating the influence of tamoxifen on CBC risk have been designed as cross-sectional7 or nested8,9 case-control studies. A major strength of our retrospective cohort study is that it was conducted within a large, prepaid health plan that provided all aspects of care, including cancer treatment and provision of prescription medications. Furthermore, the cohort design allowed for absolute risk estimation, which is essential for clinical decision-making.
Despite these advantages, our study had several limitations. As our study observational, patients who used endocrine therapy for long durations may differ in some manner as compared to never or short-term users. While we adjusted for multiple patient and clinical characteristics, the possibility of residual or unmeasured confounding exists. ASCO guidelines recommend that pre- or perimenopausal women who have hormone receptor-positive breast cancer be offered adjuvant tamoxifen.22 We could not fully evaluate this issue as we lacked menopausal status. Using age <50 years as a proxy, we found that tamoxifen was weakly associated with reduced CBC risk only among current and former users with <5 years since last use. Current clinical guidelines recommend that postmenopausal women be offered the choice of continuing tamoxifen or switching to an AI for 10 years of adjuvant endocrine therapy.22 Thus, despite the older age of our cohort, our findings demonstrating significant reductions in CBC risk with adjuvant tamoxifen (and AI) therapy are still relevant within the context of contemporary clinical guidelines.
Early reports of reduced CBC risk in adjuvant tamoxifen trials motivated its evaluation as a preventive agent and drew attention to the importance of the contralateral breast as a risk marker.27,28 Thus, our findings may have implications not only for women undergoing adjuvant therapy but also for breast cancer prevention. In a recent meta-analysis of tamoxifen prevention trials, breast cancer incidence was significantly reduced within the five-year active treatment period and the first five years after stopping.29 Our analysis of patients treated with adjuvant tamoxifen during a 20-year follow-up period might provide some clues as to the persistence of protection in the chemopreventive setting.
As the decision to elect contralateral prophylactic mastectomy increases in popularity, despite evidence suggesting no survival benefit over breast conservation,5,30 data from the general community setting on the effects of adjuvant endocrine therapy on CBC risk as well as on recurrence and death will be important. Non-adherence to adjuvant endocrine therapy has been attributed to multiple factors, including side effects and financial constraints.31–33 Although tamoxifen has been available in a generic form since 2002, generic AIs were not available until 2010. Compared with brand-name users, AI-generic users have been shown to have increased adherence.33 Improved adherence to endocrine therapy has also been associated with patient-centered healthcare services.31 Thus, this is an opportune time for clinicians to engage in shared, informed decision-making with patients regarding the best treatment course. If adjuvant endocrine therapy is indicated for breast cancer treatment, our findings, in concert with trial data, suggest that women should be encouraged to complete the full course.
Supplementary Material
Acknowledgments
GLG and AB had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors thank Brenda Rush and Kathy Pearson at KP Northwest and Maya Palakal at NCI for research assistance and Nikki Carrol at KP Colorado for data support. We also thank Jeremy Miller and Laura Bowen at Information Management Systems for data support and analysis and Dale Preston, Ph.D., Hirosoft International, for statistical assistance.
Funding
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Division of Cancer Epidemiology and Genetics (Bethesda, MD, USA). The funding organization had no role in the study design, collection, analysis, or interpretation of the data, or in the writing of the report.
Abbreviations
- AI
aromatase inhibitor
- AJCC
American Joint Committee on Cancer
- ASCO
American Society of Clinical Oncology
- BMI
body mass index
- CBC
contralateral breast cancer
- CI
confidence interval
- EBCTCG
Early Breast Cancer Trialists’ Collaborative Group
- ER
estrogen receptor
- IRB
Institutional Review Board
- KP
Kaiser Permanente
- NCI
National Cancer Institute
- RR
relative risk
Footnotes
Conflict of Interest: None
References
- 1.Rosenberg PS, Barker KA, Anderson WF. Estrogen Receptor Status and the Future Burden of Invasive and In Situ Breast Cancers in the United States. Journal of the National Cancer Institute. 2015;107(9) doi: 10.1093/jnci/djv159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curtis RE, Ron E, Hankey BF, Hoover RN. New Malignancies Following Breast Cancer. In: Curtis RE, Freedman DMER, et al., editors. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973–2000. NIH Publ. No. 05-5302. Bethesda, MD: National Cancer Institute; 2006. [Google Scholar]
- 3.Early Breast Cancer Trialists' Collaborative G. Davies C, Godwin J, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuzick J, Baum M. Tamoxifen and contralateral breast cancer. Lancet. 1985;2(8449):282. doi: 10.1016/s0140-6736(85)90338-1. [DOI] [PubMed] [Google Scholar]
- 5.Davies KR, Cantor SB, Brewster AM. Better contralateral breast cancer risk estimation and alternative options to contralateral prophylactic mastectomy. International journal of women's health. 2015;7:181–187. doi: 10.2147/IJWH.S52380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goss PE, Ingle JN, Pritchard KI, et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med. 2016;0(0) doi: 10.1056/NEJMoa1604700. null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertelsen L, Bernstein L, Olsen JH, et al. Effect of systemic adjuvant treatment on risk for contralateral breast cancer in the Women's Environment, Cancer and Radiation Epidemiology Study. Journal of the National Cancer Institute. 2008;100(1):32–40. doi: 10.1093/jnci/djm267. [DOI] [PubMed] [Google Scholar]
- 8.Cook LS, Weiss NS, Schwartz SM, et al. Population-based study of tamoxifen therapy and subsequent ovarian, endometrial, and breast cancers. Journal of the National Cancer Institute. 1995;87(18):1359–1364. doi: 10.1093/jnci/87.18.1359. [DOI] [PubMed] [Google Scholar]
- 9.Li CI, Daling JR, Porter PL, Tang M-TC, Malone KE. Adjuvant Hormonal Therapy for Breast Cancer and Risk of Hormone Receptor–Specific Subtypes of Contralateral Breast Cancer. Cancer Research. 2009;69(17):6865–6870. doi: 10.1158/0008-5472.CAN-09-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newcomb PA, Solomon C, White E. Tamoxifen and risk of large bowel cancer in women with breast cancer. Breast Cancer Res Treat. 1999;53(3):271–277. doi: 10.1023/a:1006117220284. [DOI] [PubMed] [Google Scholar]
- 11.Schaapveld M, Visser O, Louwman WJ, et al. The impact of adjuvant therapy on contralateral breast cancer risk and the prognostic significance of contralateral breast cancer: a population based study in the Netherlands. Breast Cancer Res Treat. 2008;110(1):189–197. doi: 10.1007/s10549-007-9709-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bouchardy C, Benhamou S, Fioretta G, et al. Risk of second breast cancer according to estrogen receptor status and family history. Breast Cancer Res Treat. 2011;127(1):233–241. doi: 10.1007/s10549-010-1137-z. [DOI] [PubMed] [Google Scholar]
- 13.Mellemkjaer L, Steding-Jessen M, Frederiksen K, et al. Risk of contralateral breast cancer after tamoxifen use among Danish women. Annals of epidemiology. 2014;24(11):843–848. doi: 10.1016/j.annepidem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Aihara T, Tanaka S, Sagara Y, et al. Incidence of contralateral breast cancer in Japanese patients with unilateral minimum-risk primary breast cancer, and the benefits of endocrine therapy and radiotherapy. Breast cancer (Tokyo, Japan) 2014;21(3):284–291. doi: 10.1007/s12282-012-0396-4. [DOI] [PubMed] [Google Scholar]
- 15.Kuo WH, Yen AM, Lee PH, et al. Incidence and risk factors associated with bilateral breast cancer in area with early age diagnosis but low incidence of primary breast cancer: analysis of 10-year longitudinal cohort in Taiwan. Breast cancer research and treatment. 2006;99(2):221–228. doi: 10.1007/s10549-006-9194-z. [DOI] [PubMed] [Google Scholar]
- 16.Mason BH, Holdaway IM, Benton NM, Benson-Cooper DM, Hadden WE, Kay RG. Detection of contralateral breast cancer by mammography in women with previous breast cancer and the impact of endocrine therapy. The New Zealand medical journal. 1993;106(949):23–25. [PubMed] [Google Scholar]
- 17.Xing B, Huang X, Wang Y. The effect of long-term tamoxifen therapy on the occurrence of contralateral primary breast cancer. Zhonghua zhong liu za zhi [Chinese journal of oncology] 1996;18(2):143–145. [PubMed] [Google Scholar]
- 18.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in medicine. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.EPICURE [software]. Release [computer program]. Version 2.0. Seattle, WA: HiroSoft International Corp; 1996. [Google Scholar]
- 20.Nordenskjold B, Rosell J, Rutqvist LE, et al. Coronary heart disease mortality after 5 years of adjuvant tamoxifen therapy: results from a randomized trial. Journal of the National Cancer Institute. 2005;97(21):1609–1610. doi: 10.1093/jnci/dji342. [DOI] [PubMed] [Google Scholar]
- 21.Hackshaw A, Roughton M, Forsyth S, et al. Long-term benefits of 5 years of tamoxifen: 10-year follow-up of a large randomized trial in women at least 50 years of age with early breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(13):1657–1663. doi: 10.1200/JCO.2010.32.2933. [DOI] [PubMed] [Google Scholar]
- 22.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(21):2255–2269. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arpino G, Weiss HL, Clark GM, Hilsenbeck SG, Osborne CK. Hormone receptor status of a contralateral breast cancer is independent of the receptor status of the first primary in patients not receiving adjuvant tamoxifen. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23(21):4687–4694. doi: 10.1200/JCO.2005.04.076. [DOI] [PubMed] [Google Scholar]
- 24.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(23):3784–3796. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dowsett M, Cuzick J, Ingle J, et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(3):509–518. doi: 10.1200/JCO.2009.23.1274. [DOI] [PubMed] [Google Scholar]
- 26.Cuzick J, Sestak I, Baum M, et al. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. The Lancet. Oncology. 2010;11(12):1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 27.Fisher B, Redmond C. New perspective on cancer of the contralateral breast: a marker for assessing tamoxifen as a preventive agent. Journal of the National Cancer Institute. 1991;83(18):1278–1280. doi: 10.1093/jnci/83.18.1278. [DOI] [PubMed] [Google Scholar]
- 28.Johnston SRD. Endocrine treatment for ductal carcinoma in situ: balancing risks and benefits. The Lancet. 2016;387(10021):819–821. doi: 10.1016/S0140-6736(15)01219-2. [DOI] [PubMed] [Google Scholar]
- 29.Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing Use of Contralateral Prophylactic Mastectomy Despite no Improvement in Long-term Survival for Invasive Breast Cancer. Ann Surg. 2016 doi: 10.1097/SLA.0000000000001698. [DOI] [PubMed] [Google Scholar]
- 31.Sawesi S, Carpenter JS, Jones J. Reasons for nonadherence to tamoxifen and aromatase inhibitors for the treatment of breast cancer: a literature review. Clin J Oncol Nurs. 2014;18(3):E50–57. doi: 10.1188/14.CJON.E50-E57. [DOI] [PubMed] [Google Scholar]
- 32.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(4):602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 33.Hershman DL, Tsui J, Meyer J, et al. The change from brand-name to generic aromatase inhibitors and hormone therapy adherence for early-stage breast cancer. Journal of the National Cancer Institute. 2014;106(11) doi: 10.1093/jnci/dju319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.