Abstract
Background
Diabetic kidney disease (DKD) is defined by the functional, structural, and clinical abnormalities of the kidney that are caused by diabetes.
Summary
One-third of both type 1 diabetes and type 2 diabetes patients suffer from DKD, which is the leading cause of end-stage renal disease, and is also associated with cardiovascular disease and high public health care costs. Serum glucose level and lipid level are key factors in the pathogenesis of DKD and are modifiable. The goal of this review is to provide an update on the roles of glucose and lipid metabolism in DKD and their crosstalk at the molecular level. We will further discuss the recent advances regarding metabolic nuclear receptors in glucose-lipid crosstalk, which may provide new potential therapeutic targets for DKD.
Key Message
AMPK, SREBP-1, and some metabolic hormone receptors including liver X receptors, farnesoid X receptors, and peroxisome proliferator-activated receptors mediate the crosstalk of hyperglycemia and dyslipidemia in diabetic kidney disease and might be potential treatment candidates.
Keywords: Diabetic kidney disease, Dyslipidemia, Hyperglycemia, Nuclear receptors, Renal lipid metabolism
Introduction
Diabetic kidney disease (DKD), which also known as diabetic nephropathy, is defined as diabetes with albuminuria (ratio of urine albumin to creatinine ≥30 mg/g) or an impaired glomerular filtration rate (GFR) (<60 mL/min/1.73 m2), or both [1]. DKD is the most common complication of diabetes mellitus, and is a leading cause of end-stage renal disease, necessitating dialysis or transplantation. The number of patients with DKD continues to increase despite improved management of diabetes [2, 3]. DKD is characterized by a reduced GFR, glomerular hypertrophy, albuminuria, glomerular basement membrane thickening, mesangial expansion, podocyte loss, glomerular sclerosis, and interstitial fibrosis [4]. The mechanisms responsible for the development and progression of DKD remain largely unknown. In spite of glycemic control and antagonists of the renin-aldosterone system, DKD still progresses in most patients. Apart from genetics, inadequate metabolic control over many years might lead to DKD via epigenetic modification as a cumulative result. Among these metabolic dysregulations, hyperglycemia and dyslipidemia are two key metabolic disorders that have a close association with DKD.
Dyslipidemia is associated with DKD and has been shown to play crucial roles in the development and progression of DKD [5]. Treating uncontrolled glucose levels can directly improve lipid levels, especially those of triglycerides (TG). The most used antidiabetic drug, metformin, exerts a strong glucose-lowering effect, and it can improve the lipid profile at the same time, suggesting there is crosstalk between hyperglycemia and dyslipidemia. However, several glucose-lowering agents do not have a beneficial effect on the lipid profile and even cause weight gain while improving glucose levels. Therefore, it is important to figure out what the background mechanism linking glucose and lipid metabolism is.
The conception “metabolic memory” means the phenomenon where periods of good/bad metabolic control can have long-lasting effects on the development of complications in diabetes even after abolishment of good/bad control in diabetic patients [6]. Clinical observational studies revealed that this metabolic memory could even last more than 25 years, suggesting a long-term environmental programming effect during DKD development [6]. Metabolic nuclear receptors, defined as a group of nuclear hormone receptor transcription factors, play key roles in regulating a variety of metabolic processes such as adipogenesis, glucose metabolism, lipid metabolism, energy metabolism, insulin sensitivity, and so on [7]. Well-known metabolic nuclear receptors include peroxisome proliferator-activated receptors (PPARs), liver X receptors (LXRs), and farnesoid X receptors (FXRs). Dysregulation of theses metabolic nuclear receptors is closely related to metabolic syndrome, and thus plays an important role in the development of DKD.
In this review, we will review the recent progress in elucidating the roles of hyperglycemia and dyslipidemia in DKD and also discuss the roles of metabolic nuclear receptors in glucose-lipid crosstalk.
Hyperglycemia and DKD
Chronic hyperglycemia plays a major role in the pathogenesis of DKD-related complications. The human kidney is involved in the regulation of glucose homeostasis and abnormalities through the release, uptake, and reabsorption of glucose. The liver and kidney are the only organs in humans that have abundant glucose-6-phosphatase levels and therefore are able to perform gluconeogenesis. Under the fast condition, the liver can produce glucose through glycogenolysis; the kidney, however, cannot release glucose through glycogenolysis, for it contains very limited amounts of glycogen [8].
One important function of the kidney is the reabsorption of glucose from glomerular filtrate. Glucose is freely filtered by the glomeruli. Approximately 180 L of plasma and around 180 g of glucose are filtered by the kidney every day. Under normal conditions, more than 99% of the filtered glucose is reabsorbed into the circulation, and the urine is free of glucose [9]. Sodium-glucose cotransporters (SGLTs) in the proximal convoluted tubule are the transporters responsible for kidney glucose reabsorption. There are six identified members of the SGLT family (SGLT1–6) [10]. In the kidney, the transport of glucose is mediated primarily by SGLT1 and SGLT2. Of these two transporters, SGLT1 plays important roles in glucose absorption/reabsorption and is mainly localized on the luminal side of the small intestinal epithelial cells and segment 3 (S3) of the renal proximal tubules. SGLT2 is a high-capacity, low-affinity glucose transporter that is predominantly located in the brush border membrane of the S1 and S2 segments of the renal proximal tubule and is responsible for reabsorption of more than 90% of the filtered glucose in the kidney [11]. The proximal tubule cells of the kidney actively contribute to glucose homeostasis by reabsorbing glucose through the transporter SGLT2.
In patients with diabetes, the blood glucose levels increase and exceed the transport maximum (Tm) glucose level at a threshold of approximately 200 mg/dL. It is reported that in diabetes, expression of the SGLT2 transporter gene is upregulated and the renal threshold increased, leading to increased glucose reabsorption from glomerular filtrate, thereby reducing urinary glucose excretion and worsening the hyperglycemic condition. In db/db mice, genes involved in glucose metabolism such as SGLT2, phosphoenolpyruvate carboxykinase (Pck1), and glucose-6-phosphatase were selectively hypomethylated in the proximal tubules compared with control db/m mice [12]. An aberrant increase in DNA methylation of the PPARγ coactivator-1α (PGC-1α) gene promoter is observed in the skeletal muscles of diabetic patients.
In the kidney, hyperglycemia causes kidney injury by several mechanisms, including: (a) increased expression of transforming growth factor-β (TGF-β) and overproduction of the mesangial matrix; (b) abnormal activation of protein kinase C, which causes mesangial expansion via TGF-β, vascular endothelial growth factor (VEGF), reactive oxygen species (ROS), and angiotensin II; (c) production of ROS; (d) activation of mitogen-activated protein kinase pathways [13]; (e) PPARs; and (f) overproduction of advanced glycation end products.
Dyslipidemia and DKD
Kimmelstiel and Wilson [14] first described the presence of lipid deposits in tubular epithelial cells (TECs) of diabetic kidneys in 1936. After that, a vast amount of evidence has shown that increased lipid accumulation in TECs would lead to lipotoxicity, which contributes to fibrosis [15]. Now there is growing evidence for the indispensable role of dyslipidemia, which is characterized by high plasma TG-rich lipoproteins, low plasma high-density lipoprotein cholesterol (HDL-C), and increased concentrations of small-sized and modified low-density lipoprotein particles (ox-LDL), in the progression of DKD patients [16, 17]. Dyslipidemia in patients with type 2 diabetes (T2D) is a reversible risk factor for the progression of kidney disease. It is thus important to understand the roles of lipid disorder in the progression of DKD.
DKD Is Usually Accompanied by Lipid Abnormalities
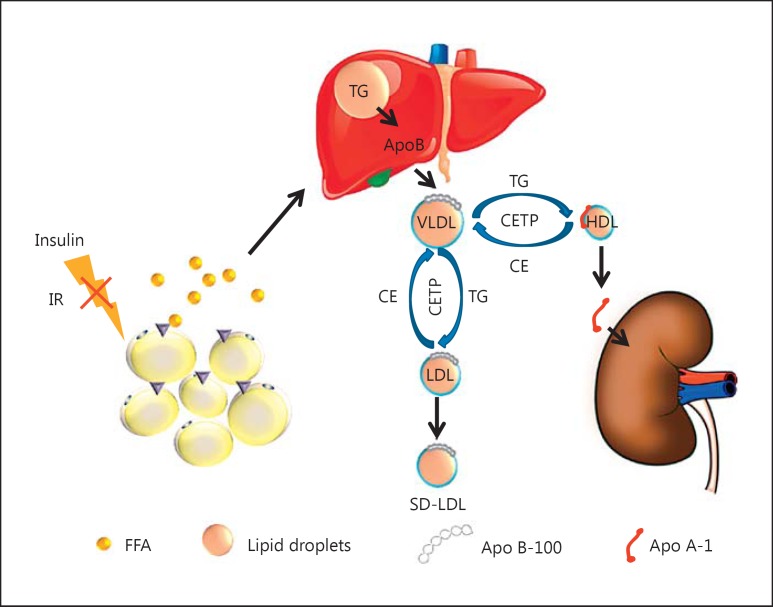
Hyperglycemia is intrinsically linked to insulin resistance, which facilitates hyperlipidemia and contributes to renal disease pathogenesis in diabetic animals [18]. Excess carbohydrates can be converted by lipogenesis into free fatty acids (FAs) and TG by activation of acetyl-CoA carboxylase (ACC), FA synthase (FAS), or stearoyl-CoA desaturase-1. DKD is characterized by tubular epithelial lipid accumulation [14] and decreased FA oxidation (FAO). Heavy lipid deposition and increased lipid droplet accumulation are observed in DKD patients [19]. Insulin resistance increases FA lipolysis and release from adipose tissue into the circulation by activation of hormone-sensitive lipase, which is normally inhibited by insulin. As a consequence, very low-density lipoprotein (VLDL) synthesis and secretion from the liver are also increased. Increased levels of VLDL TG in the presence of cholesteryl ester transfer protein can promote the transfer of TG into LDL or HDL in exchange for LDL cholesteryl ester. The TG-rich LDL can undergo hydrolysis by hepatic lipase or lipoprotein lipase, which leads to a small dense LDL particle. The TG-rich HDL particle loses its normal function and can undergo further hydrolysis, which leads to the dissociation of Apo A-I from the HDL particle and low levels of HDL (Fig. 1). It has also been demonstrated that the LDL-C level is increased and a high level of LDL-C has been found to predict the development of microalbuminuria in type 1 diabetes (T1D). Toxic lipids such as lysophosphatidylcholine, ceramides, and free cholesterol may also accumulate in the tissues under insulin resistance. These results suggest that insulin resistance can cause both hyperglycemia and hyperlipidemia, but the crosstalk between lipid and glucose metabolism remains unclear.
Fig. 1.
Insulin resistance plays a central role in dyslipidemia. In adipose tissue, insulin inhibits the hormone-sensitive lipase. Thus, insulin has an antilipolytic action. Insulin resistance (IR) increases lipolysis, enhancing free fatty acid (FFA) release from the adipose tissue to the circulation, which increases very-low-density lipoprotein (VLDL) production in the liver. In the presence of increased VLDL in the plasma in diabetic kidney disease patients, VLDL triglycerides (TG) can be exchanged for high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol under action by cholesteryl ester (CE) transfer protein (CETP), resulting in TG-rich, cholesterol-depleted HDL and LDL particles. The TG-rich HDL can undergo further hydrolysis, which leads to the dissociation and clearance of Apo A-1. The TG-rich LDL can be converted to small dense LDL (SD-LDL) after hydrolysis.
The Lipid Abnormalities Contribute to DKD
Dyslipidemia is observed in most DKD patients. The “lipid nephrotoxicity hypothesis” was first advocated by Moorhead et al. [20] to describe the effect of dyslipidemia on decreased kidney function in 1982, and updated by Ruan et al. [21] in 2009. As a consequence, dyslipidemia causes podocyte apoptosis and enhances macrophage infiltration and excessive extracellular matrix production in the glomeruli under diabetic conditions, contributing to the development of DKD. Furthermore, lipids can be accumulated in the kidney and directly cause kidney injury by increasing uptake via lipoprotein receptors/transporters, increasing lipogenesis or reducing efflux and consumption (oxidation). High cholesterol absorption and low cholesterol efflux in the kidney have been shown in different models of T1D [20] and T2D [21].
Enhanced lipogenic gene activation contributes to glomerular and tubular lipid deposits. Sterol regulatory element-binding proteins (SREBPs) are key regulators of lipid synthesis that play a central role in renal lipid accumulation in diabetic nephropathy [22]. SREBP1 and FAS expression is markedly increased in streptozotocin-induced diabetic nephropathic rat kidneys, resulting in TG and cholesterol accumulation in the kidney [22, 23]. Induction of SREBP-1 may be related to increase in TGF-β1 and accumulation of extracellular matrix [24]. Treatment of diabetic rats with insulin prevented the increased SREBP-1 and TG accumulation. SREBP-1a transgenic mice exhibited an increased renal TG content, increased expression of TGF-β1 and mesangial expansion, glomerulosclerosis, and proteinuria [22]. Knockdown of SREBP-1 ameliorated the diabetic kidney injury and protected from cell dysfunction [22].
Evidence is emerging that reduced FAO is associated with lipid accumulation in DKD. An imbalance between circulating and cytosolic FA levels results in excessive intracellular accumulation of FAs. Carnitine palmitoyltransferase 1 is the rate-limiting enzyme in FAO. The PPARs and PGC-1α are the key transcription factors that regulate the expression of proteins involved in FA uptake and oxidation. Activation of PPARα by fibrates reduces TG and elevates HDL levels, showing potential to reduce diabetic nephropathy [25, 26]. Pretreatment with low-dose fenofibrate prevents the early onset of nephropathy in diabetic rats but is not effective in established diabetic rat nephropathy [27]. Acyl-CoA oxidase and liver-type FA-binding protein were reduced during DKD. PPAR activity is a potential cause of metabolic syndrome-related disorders. It has been proved that FAO is the key contributor to intracellular ATP levels in TECs [28]. FAO could be the key inciting factor for fibrosis development, and lipid accumulation occurs as a secondary consequence. Thus, reduced FAO might be a new mechanism in DKD.
CD36 is a transmembrane protein of the class B scavenger receptor family [25] functioning as an FA translocase which is expressed in several cell types including macrophages, microvascular endothelial cells, platelets, adipocytes, podocytes, and tubular cells. Long-chain FAs, particularly palmitate and stearate, enter cells mostly through CD36. CD36 expression is upregulated by hyperglycemia in DKD patients [26, 27] and is associated with increased uptake of ox-LDL and with tubular epithelial apoptosis, which in turn is associated with tubular degeneration and progression of DKD [27]. CD36 mediates TEC apoptosis and proinflammatory and profibrotic responses. TEC-specific overexpression of CD36 transgenic mice showed an increase in lipid accumulation in TECs. However, no higher susceptibility to diabetic kidney injury was observed. mRNA of the lipid droplet surface protein adipose differentiation-related protein (ADRP) was specifically upregulated 5.4-fold in 16-week-old db/db mouse kidneys [24], suggesting that the lipid droplet protein is involved in lipid accumulation in DKD kidneys. Podocytes are crucial for maintaining a normally functional glomerular filtration barrier. Lipotoxicity and lipid accumulation cause podocyte injury and apoptosis in DKD patients, which is a feature of DKD, and podocytopenia is an independent predictor of DKD progression [29, 30, 31, 32]. There are several mechanisms which contribute to podocyte loss in diabetes. The reduction in podocyte numbers contributes to the progression of DKD. Lipid droplet accumulation localized within the podocyte foot processes of patients with DKD was observed by Herman-Edelstein et al. [19]. This accumulation was associated with a downregulation of genes that encode proteins involved in cholesterol efflux (ABCA1, ABCG1, and APOE), an upregulation of LDL receptors, impaired FAO, and increased expression of angiopoietin-related protein 4 [19]. CD36 protein expression was markedly enhanced in podocytes treated with palmitic acid in a dose-dependent manner [33]. However, the exact role of CD36 in podocyte apoptosis in diabetic nephropathy with hyperlipidemia still needs to be elucidated. An in vitro experiment recently showed that high glucose induced VEGF and reduced podocyte viability via enhanced autophagy, which was thought to be a self-protective mechanism [34]. Overall, lipids and lipid-modulating proteins are key determinants of podocyte biology and together contribute to the pathogenesis of glomerular diseases during DKD.
Metabolic Dysregulation in DKD: Glucose-Lipid Crosstalk
Metabolic dysregulation including hyperglycemia and hyperlipidemia initiates DKD. The alterations of glucose and lipid metabolism usually affect each other. It is well known that T2D is a metabolic disorder characterized by both hyperglycemia and dyslipidemia. Glucose-lowering drugs that control hyperglycemia usually also have an effect on lipid metabolism. Metformin lowers glucose while improving the lipid profile [35]. Also, a recent glucagon-like peptide-1 receptor agonist has proven efficacious in the glycemic control of T2D coupled with insulin-independent metabolic benefits for dyslipidemia [36]. The above-mentioned phenomena suggest that crosstalk between glucose and lipid metabolism exists during DKD. In this part, we will discuss the possible crosstalk between glucose and lipid metabolism from molecular networks and regulation pathways (Fig. 2).
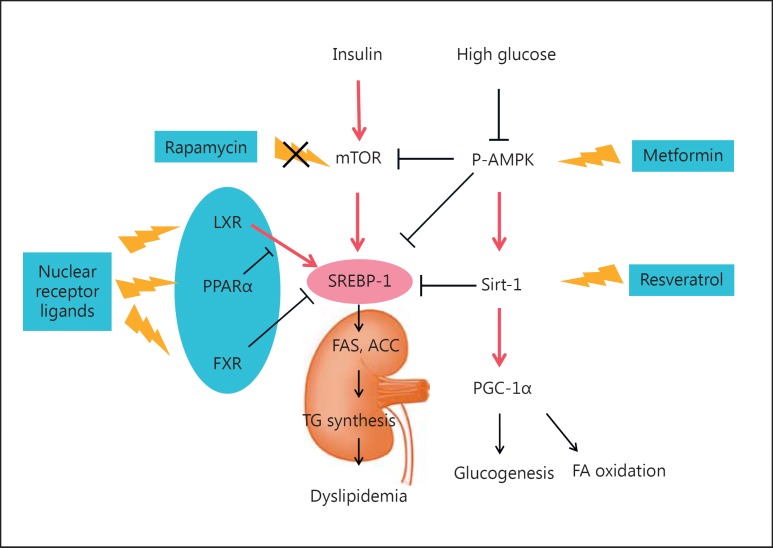
Fig. 2.
Glucose-lipid crosstalk in diabetic kidney disease. (1) SREBP-1 is the master regulator of lipid metabolism and can be upregulated by insulin, glucose, and liver X receptor (LXR). Farnesoid X receptor (FXR) can negatively regulate SREBP-1 expression in the kidney. In diabetic patients, hyperglycemia induces expression of SREBP-1, which enhances lipogenic gene expression and triglyceride (TG) synthesis and leads to excess lipid accumulation in the kidney. (2) Activation of AMP-activated protein kinase (AMPK) suppresses the transcription of SREBP-1 and prevents renal lipotoxicity. Activated AMPK can also participate in the glucose-lipid crosstalk through the p-AMPK/Sirt-1/PGC-1α pathway or through suppression of mTOR. Drugs such as metformin can improve the lipid profile beyond its glucose-lowering effect. FA(S), fatty acid (synthase); ACC, acetyl-CoA carboxylase.
AMP-Activated Protein Kinase-Related Pathway
AMP-activated protein kinase (AMPK) is an energy sensor that is activated by decreases in the cellular energy state, as reflected in the AMP/ATP ratio [37]. Metformin can phosphorylate and activate AMPK through liver kinase B1. Activation of AMPK can suppress the transcription of several key lipogenic regulators in the liver, such as SREBP-1, ACC, and HMG-CoA reductase [38, 39]. First, downregulation of SREBP-1 leads to a reduction in TG synthesis. Second, the inhibition of ACC enzyme activity also causes a reduction in FA and VLDL synthesis from the liver. Third, the suppression of HMG-CoA reduces its capabilities of cholesterol synthesis. Metformin is reported to reduce TG by about 10% and LDL-C by 10–15%, and to increase HDL-C up to 7%. Therefore, metformin, through AMPK, improves the lipid profile beyond its glucose-lowering effect. The AMPK/silent information regulator T1 (SIRT1)/PGC-1α axis also participates in the crosstalk between lipid and glucose metabolism. Resveratrol is a natural plant polyphenol which activates SIRT1, and was reported to prevent renal lipotoxicity and to inhibit mesangial cell glycotoxicity in T2D mainly through the AMPK/SIRT1/PGC-1α axis in a db/db mouse model [40]. Activation of SIRT1 promotes gluconeogenesis and FAO through PGC-1α [41, 42].
SREBP-1 Serves as a Mediator for the Crosstalk between Glucose and Lipid Metabolism
In models of DKD, an increased renal expression of SREBP has been observed [22, 24, 32]. SREBPs are transcription factors well-known for their important roles in lipid homeostasis; the three isoforms are SREBP-1a, -1c, and -2. The inactive/precursor form of SREBP resides in the endoplasmic reticulum membrane. When activated, SREBP cleavage-activating protein binds and escorts SREBP from the endoplasmic reticulum to the Golgi complex and undergoes sequential proteolytic processing. Site 1 protease and site 2 protease are two proteases that could release the mature form of SREBP. Then, the active N-terminal domain of SREBP1 is translocated to the nucleus and the transcript regulates downstream target genes [43]. Transcriptional activation of SREBP-1 can also be upregulated by insulin [44], glucose [45], and LXR [46]. High-glucose-induced SREBP-1 was mediated by AMPK in T1D rats [47]. Uttarwar et al. [48] showed that in primary rat mesangial cells, high-glucose (30 mm) treatment within 1 h could transcriptionally activate SREBP-1, which works through EGFR-PI3K-Akt signaling. Their data further showed that SREBP-1 could bind to the promoter region and directly regulates TGF-β1 by glucose stimulation, mediating profibrogenic responses. In cultured renal tubular cells, high-glucose media could induce expression of SREBP-1 mRNA and protein levels, resulting in an enhanced cellular TG content through induction of FAS and ACC [22, 49]. The relationship between SREBP-1 and an activated PI3K/Akt pathway has been confirmed in diabetic rats and in in vitro-cultured HKC cells [50]. Recently, mTOR was revealed to be involved in diabetic renal lipogenesis and could be a potential target for the treatment of DKD through regulation of SREBP-1 [51]. How mTORC1 regulates SREBP is still not fully understood, but it might be by controlling the nuclear entry of lipin-1, a phosphatidic acid phosphatase [52].
Metabolic Nuclear Receptors
Metabolic nuclear receptors consist of a group of nuclear hormone receptor transcription factors that play central roles in regulating metabolic balance. The metabolic nuclear receptors, including PPARs, LXRs, and FXRs, play important roles in regulating the crosstalk between glucose and lipid metabolism during DKD. Apart from the above-mentioned nuclear receptors, the other nuclear receptors, such as estrogen receptors, might also be involved in DKD, based on the observation that DKD progresses faster in males than in females [53].
Peroxisome Proliferator-Activated Receptors
PPARs, including PPARα (NR1C1), PPARβ/δ (NR1C2), and PPARγ (NR1C3), are key transcription factors in regulating glucose and lipid metabolism. PPARα is highly expressed in the liver, kidney, and skeletal muscles, and is closely correlated with FAO [54]. In the kidney, PPARα is abundantly expressed in the proximal tubules and medullary thick ascending limbs, with lower expression levels in the glomeruli [55]. PPARα binds to a specific peroxisome proliferator response element, thereby forming a heterodimer with RXRα, and controls a set of genes essential for FA β-oxidation and has an important role in renal metabolic control [56]. PPARα gene deficiency markedly accelerated nephropathy in diabetic mice [57]. A PPARα agonist such as fenofibrate improves insulin sensitivity, glucose control, and DKD [25, 58]. The mechanism of fenofibrate's renoprotective effect on DKD may be related to increased phosphorylation of AMPK, activation of PGC-1α, and amelioration of lipotoxicity in the kidney [59]. While improving hyperglycemia, fenofibrate treatment increased HDL-C with little change in VLDL in db/db mice.
PPARγ is the master regulator of differentiation and energy storage by adipocytes and plays an important role in improving insulin sensitivity, which made it a key target for the treatment of T2D. PPARγ ligands regulate systemic lipid and glucose homeostasis. The most representative PPAR ligands, thiazolidinediones, show a strong ability both to lower glucose levels and to improve lipid profiles in T2D. In the kidney, PPARγ is most abundant in the collecting duct [60]. Activation of PPARγ modulates the transcription of a number of insulin-responsive genes involved in the control of glucose and lipid metabolism. PPARα and PPARγ modulate energy utilization in the kidney by regulating FAO [61]. Activation of PPARα can stimulate FA β-oxidation, which can reduce the lipid content, and exerts renoprotective effects.
Farnesoid X Receptor (NR1H4)
FXR is a bile acid-activated nuclear receptor that is highly expressed in the liver, intestine, adrenal glands, and kidney. FXR plays a key role in bile acid, lipid, and glucose homeostasis. FXR is abundantly expressed in the kidney and negatively modulates renal SREBP-1 expression. The FXR agonist obeticholic acid or GW4064 could decrease kidney TG levels, suggesting that FXR might play a pivotal role in diabetic renal injury [31]. FXR deficiency caused increased kidney neutral lipid accumulation and accelerated type 1 diabetic nephropathy partly through enhanced SREBPs [62]. In contrast, treatment with the FXR agonist INT-747 significantly decreased total plasma cholesterol and LDL-C levels, and improved renal injury in a diabetic mouse model by modulating renal lipid metabolism and macrophage infiltration and by negatively modulating the renal expression of SREBPs [62].
Liver X Receptors (NR1H3, NR1H2)
LXRs include two isoforms, namely, LXRα and LXRβ. LXRα is highly expressed in lipid-metabolizing organs such as the liver, kidney, intestine, and adipose tissue [63], while LXRβ is more ubiquitously expressed [64]. The LXR agonist T0901317 could increase cholesterol efflux via activating ABCA1. Macrophage-specific activation of LXR markedly ameliorated hyperlipidemic-hyperglycemic nephropathy through the upregulation of genes related to cholesterol efflux and by suppressing glycated or acetylated LDL-induced cytokines and ROS [65]. However, applications of the LXR agonist have now been limited due to steatotic side effects.
Therapeutic Strategy for DKD
Diabetes treatment for patients with DKD is challenging. The progressive decline in renal function impairs the clearance and metabolism of antidiabetic drugs and insulin. Currently, there are no efficient therapies for slowing the progression of DKD. Evidence has shown that metabolic control could reduce the risk or slow the progression of DKD [66]. The landmark DCCT (Diabetes Control and Complications Trial)/EDIC (Epidemiology of Diabetes Interventions and Complications) research group showed a tight relationship between glucose control and complications and the treatment of T1D [67, 68]. For most patients with T2D, metformin is recommended as an initial therapy [66]. As glycemic control worsens, other antidiabetic drugs with different mechanisms of action are added to metformin. The use of metformin in DKD is more complicated according to different degrees.
SGLT2 Inhibitors: Pros and Cons
The concept of using SGLT inhibitors as T2D targets is not new [11]. Phlorizin is a nonspecific SGLT1/2 inhibitor; already in 1835 it was found to increase glycosuria and reduce hyperglycemia and to normalize insulin sensitivity in T2D mice [69]. However, the nonselectivity for SGLT1 stopped this drug from being developed for clinical use, since SGLT1 also localizes at the intestinal brush border, which is responsible for absorption of dietary glucose [10]. A bunch of highly specific SGLT2 inhibitors are currently being developed by several drug companies. The benefits of SGLT2 inhibitors compared with other glucose-lowering drugs include the following: (1) the action of SGLT2 inhibitors should be independent of insulin action or pancreatic β-cell function; (2) they ameliorate hyperglycemia without causing weight gain or hypoglycemia, which is a common side effect of other T2D drugs [70, 71]; and (3) they lower blood pressure. Treatment with SGLT2 inhibitors has demonstrated improvements in HbA1c, reductions in body weight, and modest lowering of blood pressure. Empagliflozin is a potent and well-studied SGLT-2 inhibitor that was approved by the FDA in 2014 [72]. A recent study showed that empagliflozin could be combined with metformin to provide dual medications in a once-daily tablet to improve long-term HgA1c when compared to metformin alone. Luseogliflozin is a highly selective and potent SGLT2 inhibitor [73] that has been approved for use as monotherapy or in combination with other antidiabetic drugs [74]. A single dose of luseogliflozin significantly increased 24-h urinary glucose excretion and significantly decreased fasting blood glucose and 2-h postprandial plasma glucose in patients with normal-to-moderately reduced renal function but not severely reduced renal function. The ability of the SGLT2 inhibitor to reduce plasma glucose levels is associated with the GFR [75].
Hyperlipidemia: A New Therapeutic Target for DKD?
Clinical studies have demonstrated that lipid-lowering therapy (with statins and fibrates) shows a protective effect on renal function by improving albuminuria and the estimated GFR [76]. Statins, which target cholesterol synthesis, may improve the estimated GFR through downregulation of small GTP-binding proteins and its downstream Rho/Rho-kinase proteins. Statins may decrease albuminuria, but they cannot stop the progression of DKD, indicating that other pathways besides cholesterol synthesis contribute to cholesterol accumulation in the kidneys of DKD patients. Moreover, the effects of statins vary with the stage of chronic kidney disease (CKD). Moderate- to high-quality evidence indicates that statins reduce all-cause mortality, cardiovascular mortality, and cardiovascular events. Thus, other therapeutic strategies for reducing cholesterol accumulation in peripheral organs, especially the kidney, need further investigation. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a serine protease that is produced predominantly in the liver. Through binding to hepatic LDL receptors and promoting their degradation, PCSK9 downregulates plasma levels of LDL-C [77, 78]. PCSK9 is elevated in CKD and proteinuria. Alirocumab [79] and evolocumab [80] are fully human monoclonal antibodies against PCSK9. They reduce LDL-C by up to 65% and have been well tolerated in randomized, placebo-controlled, phase II clinical trials up to 1 year in over 3,000 hypercholesterolemic patients [81, 82, 83, 84, 85]. In a phase III clinical trial, 420 mg of evolocumab every 4 weeks for 52 weeks resulted in a relative reduction in LDL-C levels of 57% [86].
Metabolic Nuclear Receptors: Great Promise?
Now clinical trials and animal studies have taken more interest in the function of nuclear hormone receptors in protection against kidney disease. Fenofibrate is a classic PPARα agonist. It has been demonstrated in multiple animal studies that fenofibrate reduces albuminuria in patients with diabetes [25, 61, 87]. In addition to reducing albuminuria, fenofibrate has been reported to ameliorate hyperglycemia [88, 89]. Acute fibrate-induced creatinine elevation in T2D with relatively preserved renal function may confer longer-term renoprotective effects. Recent studies reported that FXR negatively modulates renal SREBP-1 expression [31, 90] and its activation prevents type 1 diabetic nephropathy [62]. Treatment with the FXR agonist INT-747 improves renal lipid metabolism through negative regulation of SREBP-1 while decreasing proteinuria, glomerulosclerosis, and tubulointerstitial fibrosis [62]. Moreover, activation of FXR by the synthetic agonist GW4064 or by adenovirus-mediated FXR hepatic overexpression improves both hyperglycemia and hyperlipidemia in db/db mice [91]. Thus, FXR indicates that is has therapeutic potential for DKD.
Conclusion
As the number of DKD patients continues to increase worldwide – and, most importantly, the current glucose control strategy is not able to fully prevent DKD development – novel effective and long-lasting therapies for DKD are urgently needed. In this review, we highlighted the molecular crosstalk between dyslipidemia and hyperglycemia in progression of DKD. The roles of AMPK, SREBP-1, and some metabolic hormone receptors including LXR, FXR, and PPARs on the crosstalk between lipid and glucose homeostasis were discussed. Finally, we provided an update on recent drug studies based on due regulation of lipid and glucose in DKD. Lipoprotein may become a new target in CKD patients, especially patients with proteinuric or nephrotic syndrome. Recent advances in the understanding of metabolic nuclear receptors have opened a window for the development of novel drugs. Since SREBP-1 or SREBP-2 inhibitors are still unavailable, metabolic nuclear receptors may be potential candidates for the development of new drugs with which both glucose and lipid disorders can be controlled.
Conflict of Interest Statement
The authors declare no competing interests.
Acknowledgements
This study was supported by grant 2012CB517504 from the Ministry of Science and Technology and grant 81500538 from the National Natural Science Foundation. The project was also funded by the China Postdoctoral Science Foundation (2015M570724), Shenzhen Peacock Program (KQTD20140630100746562), and Shenzhen Research Projects (JCYJ20140509172719310, CXZZ20150601140615135, and JCYJ20160307160819191).
References
- 1.Reidy K, Kang HM, Hostetter T, Susztak K. Molecular mechanisms of diabetic kidney disease. J Clin Invest. 2014;124:2333–2340. doi: 10.1172/JCI72271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg EW, Li Y, Wang J, Burrows NR, Ali MK, Rolka D, et al. Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370:1514–1523. doi: 10.1056/NEJMoa1310799. [DOI] [PubMed] [Google Scholar]
- 3.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. United States Renal Data System 2011 Annual Data Report: atlas of chronic kidney disease and end-stage renal disease in the United States. Am J Kidney Dis. 2012;59((suppl 1)):A7. doi: 10.1053/j.ajkd.2011.11.015. e1-e420. [DOI] [PubMed] [Google Scholar]
- 4.Satchell SC. The glomerular endothelium emerges as a key player in diabetic nephropathy. Kidney Int. 2012;82:949–951. doi: 10.1038/ki.2012.258. [DOI] [PubMed] [Google Scholar]
- 5.Stadler K, Goldberg IJ, Susztak K. The evolving understanding of the contribution of lipid metabolism to diabetic kidney disease. Curr Diab Rep. 2015;15:40. doi: 10.1007/s11892-015-0611-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gosmanov AR, Gosmanova EO. Long-term renal outcomes of patients with type 1 diabetes mellitus and microalbuminuria: an analysis of the DCCT/EDIC cohort. Arch Intern Med. 2011;171:1596. doi: 10.1001/archinternmed.2011.413. author reply 1597. [DOI] [PubMed] [Google Scholar]
- 7.Francis GA, Fayard E, Picard F, Auwerx J. Nuclear receptors and the control of metabolism. Annu Rev Physiol. 2003;65:261–311. doi: 10.1146/annurev.physiol.65.092101.142528. [DOI] [PubMed] [Google Scholar]
- 8.Stumvoll M, Meyer C, Mitrakou A, Nadkarni V, Gerich JE. Renal glucose production and utilization: new aspects in humans. Diabetologia. 1997;40:749–757. doi: 10.1007/s001250050745. [DOI] [PubMed] [Google Scholar]
- 9.Mather A, Pollock C. Glucose handling by the kidney. Kidney Int Suppl. 2011;120:S1–S6. doi: 10.1038/ki.2010.509. [DOI] [PubMed] [Google Scholar]
- 10.Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007;261:32–43. doi: 10.1111/j.1365-2796.2006.01746.x. [DOI] [PubMed] [Google Scholar]
- 11.Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch. 2004;447:510–518. doi: 10.1007/s00424-003-1063-6. [DOI] [PubMed] [Google Scholar]
- 12.Marumo T, Yagi S, Kawarazaki W, Nishimoto M, Ayuzawa N, Watanabe A, et al. Diabetes induces aberrant DNA methylation in the proximal tubules of the kidney. J Am Soc Nephrol. 2015;26:2388–2397. doi: 10.1681/ASN.2014070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. Activation of the receptor for advanced glycation end products triggers a p21ras-dependent mitogen-activated protein kinase pathway regulated by oxidant stress. J Biol Chem. 1997;272:17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- 14.Kimmelstiel P, Wilson C. Intercapillary lesions in the glomeruli of the kidney. Am J Pathol. 1936;12:83–98. 7. [PMC free article] [PubMed] [Google Scholar]
- 15.Murea M, Freedman BI, Parks JS, Antinozzi PA, Elbein SC, Ma L. Lipotoxicity in diabetic nephropathy: the potential role of fatty acid oxidation. Clin J Am Soc Nephrol. 2010;5:2373–2379. doi: 10.2215/CJN.08160910. [DOI] [PubMed] [Google Scholar]
- 16.Hirano T. Lipoprotein abnormalities in diabetic nephropathy. Kidney Int Suppl. 1999;71:S22–S24. doi: 10.1046/j.1523-1755.1999.07106.x. [DOI] [PubMed] [Google Scholar]
- 17.Leiter LA. The prevention of diabetic microvascular complications of diabetes: is there a role for lipid lowering? Diabetes Res Clin Pract. 2005;68((suppl 2)):S3–S14. doi: 10.1016/j.diabres.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 19.Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2014;55:561–572. doi: 10.1194/jlr.P040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moorhead JF, Chan MK, El-Nahas M, Varghese Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet. 1982;2:1309–1311. doi: 10.1016/s0140-6736(82)91513-6. [DOI] [PubMed] [Google Scholar]
- 21.Ruan XZ, Varghese Z, Moorhead JF. An update on the lipid nephrotoxicity hypothesis. Nat Rev Nephrol. 2009;5:713–721. doi: 10.1038/nrneph.2009.184. [DOI] [PubMed] [Google Scholar]
- 22.Sun L, Halaihel N, Zhang W, Rogers T, Levi M. Role of sterol regulatory element-binding protein 1 in regulation of renal lipid metabolism and glomerulosclerosis in diabetes mellitus. J Biol Chem. 2002;277:18919–18927. doi: 10.1074/jbc.M110650200. [DOI] [PubMed] [Google Scholar]
- 23.Ishigaki N, Yamamoto T, Shimizu Y, Kobayashi K, Yatoh S, Sone H, et al. Involvement of glomerular SREBP-1c in diabetic nephropathy. Biochem Biophys Res Commun. 2007;364:502–508. doi: 10.1016/j.bbrc.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 24.Jun H, Song Z, Chen W, Zanhua R, Yonghong S, Shuxia L, et al. In vivo and in vitro effects of SREBP-1 on diabetic renal tubular lipid accumulation and RNAi-mediated gene silencing study. Histochem Cell Biol. 2009;131:327–345. doi: 10.1007/s00418-008-0528-2. [DOI] [PubMed] [Google Scholar]
- 25.Park CW, Zhang Y, Zhang X, Wu J, Chen L, Cha DR, et al. PPARα agonist fenofibrate improves diabetic nephropathy in db/db mice. Kidney Int. 2006;69:1511–1517. doi: 10.1038/sj.ki.5000209. [DOI] [PubMed] [Google Scholar]
- 26.Ansquer JC, Foucher C, Rattier S, Taskinen MR, Steiner G, DAIS Investigators Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in type 2 diabetes: results from the Diabetes Atherosclerosis Intervention Study (DAIS) Am J Kidney Dis. 2005;45:485–493. doi: 10.1053/j.ajkd.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Kadian S, Mahadevan N, Balakumar P. Differential effects of low-dose fenofibrate treatment in diabetic rats with early onset nephropathy and established nephropathy. Eur J Pharmacol. 2013;698:388–396. doi: 10.1016/j.ejphar.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 28.Kang HM, Ahn SH, Choi P, Ko YA, Han SH, Chinga F, et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat Med. 2015;21:37–46. doi: 10.1038/nm.3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pagtalunan ME, Miller PL, Jumping-Eagle S, Nelson RG, Myers BD, Rennke HG, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–348. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M. Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes. 2006;55:2502–2509. doi: 10.2337/db05-0603. [DOI] [PubMed] [Google Scholar]
- 31.Jiang T, Wang XX, Scherzer P, Wilson P, Tallman J, Takahashi H, et al. Farnesoid X receptor modulates renal lipid metabolism, fibrosis, and diabetic nephropathy. Diabetes. 2007;56:2485–2493. doi: 10.2337/db06-1642. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, Jiang T, Li J, Proctor G, McManaman JL, Lucia S, et al. Regulation of renal lipid metabolism, lipid accumulation, and glomerulosclerosis in FVBdb/db mice with type 2 diabetes. Diabetes. 2005;54:2328–2335. doi: 10.2337/diabetes.54.8.2328. [DOI] [PubMed] [Google Scholar]
- 33.Hua W, Huang HZ, Tan LT, Wan JM, Gui HB, Zhao L, et al. CD36 mediated fatty acid-induced podocyte apoptosis via oxidative stress. PLoS One. 2015;10:e0127507. doi: 10.1371/journal.pone.0127507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miaomiao W, Chunhua L, Xiaochen Z, Xiaoniao C, Hongli L, Zhuo Y. Autophagy is involved in regulating VEGF during high-glucose-induced podocyte injury. Mol Biosyst. 2016;12:2202–2212. doi: 10.1039/c6mb00195e. [DOI] [PubMed] [Google Scholar]
- 35.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU) Diabet Med. 2009;26:268–278. doi: 10.1111/j.1464-5491.2009.02666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 38.Wang W, Guo XH, Wu HH, Wang NH, Xu XS. Effect of fenofibrate and metformin on lipotoxicity in OLETF rat kidney (in Chinese) Beijing Da Xue Xue Bao. 2006;38:170–175. [PubMed] [Google Scholar]
- 39.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim MY, Lim JH, Youn HH, Hong YA, Yang KS, Park HS, et al. Resveratrol prevents renal lipotoxicity and inhibits mesangial cell glucotoxicity in a manner dependent on the AMPK-SIRT1-PGC1α axis in db/db mice. Diabetologia. 2013;56:204–217. doi: 10.1007/s00125-012-2747-2. [DOI] [PubMed] [Google Scholar]
- 41.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 42.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Brown MS, Goldstein JL. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc Natl Acad Sci USA. 1999;96:11041–11048. doi: 10.1073/pnas.96.20.11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JB, Sarraf P, Wright M, Yao KM, Mueller E, Solanes G, et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J Clin Invest. 1998;101:1–9. doi: 10.1172/JCI1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hasty AH, Shimano H, Yahagi N, Amemiya-Kudo M, Perrey S, Yoshikawa T, et al. Sterol regulatory element-binding protein-1 is regulated by glucose at the transcriptional level. J Biol Chem. 2000;275:31069–31077. doi: 10.1074/jbc.M003335200. [DOI] [PubMed] [Google Scholar]
- 46.Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, et al. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRα and LXRβ. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soetikno V, Sari FR, Sukumaran V, Lakshmanan AP, Harima M, Suzuki K, et al. Curcumin decreases renal triglyceride accumulation through AMPK-SREBP signaling pathway in streptozotocin-induced type 1 diabetic rats. J Nutr Biochem. 2013;24:796–802. doi: 10.1016/j.jnutbio.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 48.Uttarwar L, Gao B, Ingram AJ, Krepinsky JC. SREBP-1 activation by glucose mediates TGF-β upregulation in mesangial cells. Am J Physiol Renal Physiol. 2012;302:F329–F341. doi: 10.1152/ajprenal.00136.2011. [DOI] [PubMed] [Google Scholar]
- 49.Hao J, Zhu L, Zhao S, Liu S, Liu Q, Duan H. PTEN ameliorates high glucose-induced lipid deposits through regulating SREBP-1/FASN/ACC pathway in renal proximal tubular cells. Exp Cell Res. 2011;317:1629–1639. doi: 10.1016/j.yexcr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Hao J, Liu S, Zhao S, Liu Q, Lv X, Chen H, et al. PI3K/Akt pathway mediates high glucose-induced lipogenesis and extracellular matrix accumulation in HKC cells through regulation of SREBP-1 and TGF-β1. Histochem Cell Biol. 2011;135:173–181. doi: 10.1007/s00418-011-0777-3. [DOI] [PubMed] [Google Scholar]
- 51.Hao J, Zhu L, Li F, Liu Q, Zhao X, Liu S, et al. Phospho-mTOR: a novel target in regulation of renal lipid metabolism abnormality of diabetes. Exp Cell Res. 2013;319:2296–2306. doi: 10.1016/j.yexcr.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 52.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sibley SD, Thomas W, de Boer I, Brunzell JD, Steffes MW. Gender and elevated albumin excretion in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) cohort: role of central obesity. Am J Kidney Dis. 2006;47:223–232. doi: 10.1053/j.ajkd.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 54.Braissant O, Foufelle F, Scotto C, Dauça M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 55.Guan Y, Zhang Y, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. Am J Physiol. 1997;273((pt 2)):F1013–F1022. doi: 10.1152/ajprenal.1997.273.6.F1013. [DOI] [PubMed] [Google Scholar]
- 56.Portilla D. Energy metabolism and cytotoxicity. Semin Nephrol. 2003;23:432–438. doi: 10.1016/s0270-9295(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 57.Park CW, Kim HW, Ko SH, Chung HW, Lim SW, Yang CW, et al. Accelerated diabetic nephropathy in mice lacking the peroxisome proliferator-activated receptor α. Diabetes. 2006;55:885–893. doi: 10.2337/diabetes.55.04.06.db05-1329. [DOI] [PubMed] [Google Scholar]
- 58.Zhou Y, Kong X, Zhao P, Yang H, Chen L, Miao J, et al. Peroxisome proliferator-activated receptor-α is renoprotective in doxorubicin-induced glomerular injury. Kidney Int. 2011;79:1302–1311. doi: 10.1038/ki.2011.17. [DOI] [PubMed] [Google Scholar]
- 59.Hong YA, Lim JH, Kim MY, Kim TW, Kim Y, Yang KS, et al. Fenofibrate improves renal lipotoxicity through activation of AMPK-PGC-1α in db/db mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096147. e96147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guan Y, Hao C, Cha DR, Rao R, Lu W, Kohan DE, et al. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat Med. 2005;11:861–866. doi: 10.1038/nm1278. [DOI] [PubMed] [Google Scholar]
- 61.Wu J, Chen L, Zhang D, Huo M, Zhang X, Pu D, et al. Peroxisome proliferator-activated receptors and renal diseases. Front Biosci (Landmark Ed) 2009;14:995–1009. doi: 10.2741/3291. [DOI] [PubMed] [Google Scholar]
- 62.Wang XX, Jiang T, Shen Y, Caldas Y, Miyazaki-Anzai S, Santamaria H, et al. Diabetic nephropathy is accelerated by farnesoid X receptor deficiency and inhibited by farnesoid X receptor activation in a type 1 diabetes model. Diabetes. 2010;59:2916–2927. doi: 10.2337/db10-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–2838. doi: 10.1101/gad.850400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Repa JJ, Mangelsdorf DJ. The role of orphan nuclear receptors in the regulation of cholesterol homeostasis. Annu Rev Cell Dev Biol. 2000;16:459–481. doi: 10.1146/annurev.cellbio.16.1.459. [DOI] [PubMed] [Google Scholar]
- 65.Kiss E, Kränzlin B, Wagenblass K, Bonrouhi M, Thiery J, Gröne E, et al. Lipid droplet accumulation is associated with an increase in hyperglycemia-induced renal damage: prevention by liver X receptors. Am J Pathol. 2013;182:727–741. doi: 10.1016/j.ajpath.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 66.American Diabetes Association Standards of Medical Care in Diabetes – 2016 Abridged for Primary Care Providers. Clin Diabetes. 2016;34:3–21. doi: 10.2337/diaclin.34.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diabetes Control and Complications Trial Research Group, Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 68.Nathan DM, Bayless M, Cleary P, Genuth S, Gubitosi-Klug R, Lachin JM, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62:3976–3986. doi: 10.2337/db13-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987;79:1510–1515. doi: 10.1172/JCI112981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bays H. Sodium glucose co-transporter type 2 (SGLT2) inhibitors: targeting the kidney to improve glycemic control in diabetes mellitus. Diabetes Ther. 2013;4:195–220. doi: 10.1007/s13300-013-0042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tahrani AA, Barnett AH, Bailey CJ. SGLT inhibitors in management of diabetes. Lancet Diabetes Endocrinol. 2013;1:140–151. doi: 10.1016/S2213-8587(13)70050-0. [DOI] [PubMed] [Google Scholar]
- 72.Neumiller JJ. Empagliflozin: a new sodium-glucose co-transporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. Drugs Context. 2014;3:212262. doi: 10.7573/dic.212262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jinnouchi H, Nozaki K, Watase H, Omiya H, Sakai S, Samukawa Y. Impact of reduced renal function on the glucose-lowering effects of luseogliflozin, a selective SGLT2 inhibitor, assessed by continuous glucose monitoring in Japanese patients with type 2 diabetes mellitus. Adv Ther. 2016;33:460–479. doi: 10.1007/s12325-016-0291-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014;30:1245–1255. doi: 10.1185/03007995.2014.912983. [DOI] [PubMed] [Google Scholar]
- 75.Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27:136–142. doi: 10.1111/j.1464-5491.2009.02894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rosario RF, Prabhakar S. Lipids and diabetic nephropathy. Curr Diab Rep. 2006;6:455–462. doi: 10.1007/s11892-006-0079-7. [DOI] [PubMed] [Google Scholar]
- 77.Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The PCSK9 decade. J Lipid Res. 2012;53:2515–2524. doi: 10.1194/jlr.R026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stein EA, Swergold GD. Potential of proprotein convertase subtilisin/kexin type 9 based therapeutics. Curr Atheroscler Rep. 2013;15:310. doi: 10.1007/s11883-013-0310-3. [DOI] [PubMed] [Google Scholar]
- 79.Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367:1891–1900. doi: 10.1056/NEJMoa1201832. [DOI] [PubMed] [Google Scholar]
- 80.Nissen SE, Dent-Acosta RE, Rosenson RS, Stroes E, Sattar N, Preiss D, et al. Comparison of PCSK9 inhibitor evolocumab versus ezetimibe in statin-intolerant patients: design of the Goal Achievement after Utilizing an Anti-PCSK9 Antibody in Statin-Intolerant Subjects 3 (GAUSS-3) Trial. Clin Cardiol. 2016;39:137–144. doi: 10.1002/clc.22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380:29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- 82.Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308:2497–2506. doi: 10.1001/jama.2012.25790. [DOI] [PubMed] [Google Scholar]
- 83.McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–2353. doi: 10.1016/j.jacc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 84.Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. LAPLACE-TIMI 57 Investigators Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380:2007–2017. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–2417. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- 86.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. DESCARTES Investigators A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 87.Gabbi C, Kong X, Suzuki H, Kim HJ, Gao M, Jia X, et al. Central diabetes insipidus associated with impaired renal aquaporin-1 expression in mice lacking liver X receptor β. Proc Natl Acad Sci USA. 2012;109:3030–3034. doi: 10.1073/pnas.1200588109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xie W, Nie Y, Du L, Zhang Y, Cai G. Preventive effects of fenofibrate on insulin resistance, hyperglycaemia, visceral fat accumulation in NIH mice induced by small-dose streptozotocin and lard. Pharmacol Res. 2007;55:392–399. doi: 10.1016/j.phrs.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 89.Liu J, Lu R, Wang Y, Hu Y, Jia Y, Yang N, et al. PPARα agonist fenofibrate reduced the secreting load of β-cells in hypertriglyceridemia patients with normal glucose tolerance. PPAR Res. 2016;2016:6232036. doi: 10.1155/2016/6232036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang XX, Jiang T, Shen Y, Adorini L, Pruzanski M, Gonzalez FJ, et al. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol. 2009;297:F1587–F1596. doi: 10.1152/ajprenal.00404.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, et al. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci USA. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]