Abstract
Background/Aim: In chronic kidney disease (CKD), kidneys fail to maintain phosphorus homeostasis in serum. Elevated phosphorus levels in serum have been associated with cardiovascular diseases in CKD patients and in normal individuals. In this study, we evaluated the level of autophagy- and apoptosis-related markers under different concentrations of hyperphosphate in myocardial cells. Methods: Modulation inflicted on the levels of various survival-, autophagy-, and apoptosis-related markers were determined by Western blotting analysis using total protein extract. FITC-annexin V staining was performed to quantify the apoptotic cells in all groups. Results: Hyperphosphate treatments showed to induce autophagy-related proteins beclin-1, ATG7, and LC3 II through the pAMPK-ULK1 pathway in Western blotting analysis. Further, apoptosis-associated proteins such as Bax, Bid, cytochrome c, and c-caspase-9 were also upregulated with hyperphosphate treatment. 3-Methyladenine, an autophagy inhibitor, inhibited apoptosis significantly in FITC-annexin V staining, and the inhibition of Bax, cytochrome c, and c-caspase-3 was shown by Western blotting. Conclusion: The results suggest that hyperphosphate in H9c2 cardiomyoblasts would lead to cellular apoptosis via autophagy, which is mediated by the pAMPK signaling pathway. Our findings revealed the possible mechanism responsible for the heart damage under hyperphosphatemia.
Keywords: Hyperphosphate, Autophagy, Apoptosis, Cardiovascular disease, Cardiorenal syndrome
Introduction
Phosphorus is the second most plentiful mineral present in the form of phosphate in all tissues and is essential for many functions in the human body. Approximately 80–85% of phosphate is present in the skeleton, 10–15% in soft tissues, and only <1% of phosphate circulates in the serum of a healthy adult [1]. Although the required phosphate level is about 580 mg/day, some individuals' daily phosphorus intake exceeds the upper tolerable limit (4,000 mg/day) [2]. Excessive consumption of phosphate disrupts the phosphate homeostasis maintained by kidneys in our body. Subsequently, serum phosphorus level increases significantly, which leads to pathological conditions.
The functions of the heart and kidneys are physiologically interconnected. Dysfunction of one of these organs concurrently affects the function of other [3]. The coexistence of cardiac and renal dysfunction is termed as cardiorenal syndrome which accounts for significant morbidity and mortality [4]. In patients with chronic kidney disease (CKD), serum phosphorus levels increase, even when the intake of phosphate is normal. Clinical and epidemiological studies have proven the association between hyperphosphate level and vascular calcifications (especially in coronary artery disease and arterial stiffening) and ultimately in the pathogenesis of “uremic” cardiovascular disease (CVD) [5].
Elevated phosphate in serum leads to CVD-related mortality. The relationship between hyperphosphatemia and cardiovascular mortality has been reported not only among CKD patients but also among normal individuals [6]. In cell culture, vascular smooth muscle cells and vascular pericytes have been shown to produce bone-forming transcription factors under hyperphosphate condition [7]. Moreover, high-phosphate diet-fed animals showed myocardial hypertrophy and cardiac fibrosis [8]. Elevated phosphate level in serum is a key risk factor for CVD and vascular calcification in CKD patients [9].
Autophagy is an evolutionarily conserved catabolic process by which damaged and disproportionate cellular components are transported to lysosomes and degraded. Autophagy occurs in response to various stimuli, such as nutrient inadequacy and chemical, physiological, and pathological stress [10]. 3-Methyladenine (3-MA), a class III PI3K inhibitor, is widely used to inhibit autophagy [11]. Autophagy has been reported to be associated with hypertrophy and apoptosis. Malfunction of autophagy under normal or pathological conditions would lead to heart failure via pathological cardiac hypertrophy [12]. The mechanism leading to myocyte hypertrophy and apoptosis is less known and thus attracts researchers' attention. Autophagy and apoptosis may be activated via common upstream signals, and under diseased conditions, autophagy and apoptosis may combine [13].
Apoptosis is a tightly regulated and highly organized process, which occurs in multicellular organisms. Apoptosis is mainly activated by a mitochondrion-mediated intrinsic pathway (Bax, Bad, Bid, cytochrome [Cyt] c) and a receptor-mediated extrinsic pathway (FasL/FasR) [14]. Inadequate or extreme level of apoptosis would lead to pathological conditions like cancer, Alzheimer disease, ischemia, etc. Recently, hyperphosphate-mediated cellular apoptosis has been reported in human umbilical vein endothelial cells [15] and in CKD patients [16]. However, the molecular pathway mediating apoptosis in cardiomyoblasts is still elusive. The purpose of the study is to decipher the molecular mechanism behind the hyperphosphate-mediated cardiac complications.
Materials and Methods
Myocardial Cell Culture
Cardiomyoblast cells (H9c2 cells were purchased from BCRC, Taipei, Taiwan, ROC) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% Cosmic Calf Serum (Sigma), 3.7 g/L sodium bicarbonate, 3.5 g/L d-glucose, and antibiotics (100 μg/mL penicillin, 100 μg/mL streptomycin) in 5% CO2 and 85% humidity incubator at 37°C.
Treatment
For treatment, H9c2 cells were incubated with different concentrations of NaH2PO4 (1.4, 1.6, and 1.8 mm) for 24 h. For inhibitor treatment, 60% confluent cells were grown in serum-free medium for 12 h and then treated with inhibitor (3-MA 100 µm) for 1 h prior to PO4 treatment.
Western Blotting
Crude proteins of cultured myocardial (H9c2) cells were isolated using lysis buffer (Roche Molecular Biochemicals, Indianapolis, Ind., USA). The protein concentration in the supernatant was determined by the colorimetric assay (Bio-Rad, Hercules, CA, USA). Samples containing 50 μg of protein were analyzed by Western blot. Protein samples for the Western blotting assay were separated by 12% SDS polyacrylamide gel electrophoresis with a 100 constant voltage of 75 V for 120 min. The proteins were then transferred to Hybond-C membranes (GE Healthcare UK Ltd., Little Chalfont, UK) at 50 V for 3 h. Hybond-C membranes with protein were incubated in 3% bovine serum albumin in tricine buffer 105 solution. To investigate autophagy and apoptosis, primary antibodies, p-Akt (sc-7985, Santa Cruz Biotechnology Inc., USA.), p-mTOR (#2971, Cell Signaling, Danvers, MA, USA), pAMPK 110 (AMP-activated protein kinase) (#2535, Cell Signaling), ULK1 (#4773, Cell Signaling), p62 (#5114, Cell Signaling), beclin-1 (#3738, Cell Signaling), ATG5 (#12994, Cell Signaling), LC3B I/II (#2775, Cell Signaling), Cyt c (sc-13560, Santa Cruz Biotechnology), caspase-3 (sc-7148, Santa Cruz Biotechnology), Bax (sc-526, Santa Cruz Biotechnology), and Bcl2 (sc-7382, Santa Cruz Biotechnology) and their respective secondary antibodies were purchased. β-Actin (Lab Vision Corporation, Fremont, Calif., USA) was used as loading control.
Immunofluorescence Staining
After treatment, cells were washed with 1× PBS thrice. Cells were fixed with 4% formaldehyde for 15 min at room temperature. Then, formaldehyde was removed, and cells were washed with PBS thrice. Permeabilization solution (0.1% sodium citrate in 0.1% triton X 100) was added for 2 min. After washing in PBS, cells were blocked using 5% goat serum for 1 h. Cells were washed with PBS, and the primary antibody (LC3B in 1% goat serum) was added. Cells were incubated along with antibody for 24 h at 4°C. After PBS washes, secondary fluorescent antibody (red) was added for 1 h. Then, cells were washed with PBS thrice; DAPI was added to the cells and incubated for 15 min. Cells were visualized under fluorescence microscope.
qRT-PCR for Bcl2 mRNA Expression
RNA was extracted from control and hyperphosphate-treated H9c2 cells using Quick-RNA miniprep kit (Zymo Research, USA) as per manufacturer's instructions. Extracted RNA was converted to cDNA using Mir-X first strand synthesis kit from Clontech, USA. qRT-PCR was performed using cDNA with a specific primer for Bcl2 (Bcl2 F: 5′GCTACCGTCGTGACTTCGC3′; Bcl2 R: 5′GCTACCGTCGTGACTTCGC3′).
FITC-Annexin V Staining for Apoptosis
FITC-Annexin V Apoptosis Detection kit 1 was purchased from BD Biosciences, USA. In brief, after 24 h of hyperphosphate treatment, cells were collected and washed twice with cold PBS. 1 × 106 cells were resuspended in 100 mL 1× binding buffer. Subsequently, 100 μL of cell suspension was transferred to a 5-mL culture tube, and 5 μL of annexin V and 5 μL of propidium iodide were added. Then, cells were gently vortexed and incubated for 15 min at room temperature in the dark. 1× binding buffer (400 μL) was added to each tube, and the cells were analyzed by flow cytometry within 1 h. Triplicates were used for analysis.
Statistical Analysis
The results of FITC were compared by Student's t test for 3 samples in each group. In all cases, a difference at p value <0.05 was considered as statistically significant.
Results
Hyperphosphate Induces pAMPK-ULK1 Signaling Pathway in H9c2 Cardiomyoblasts
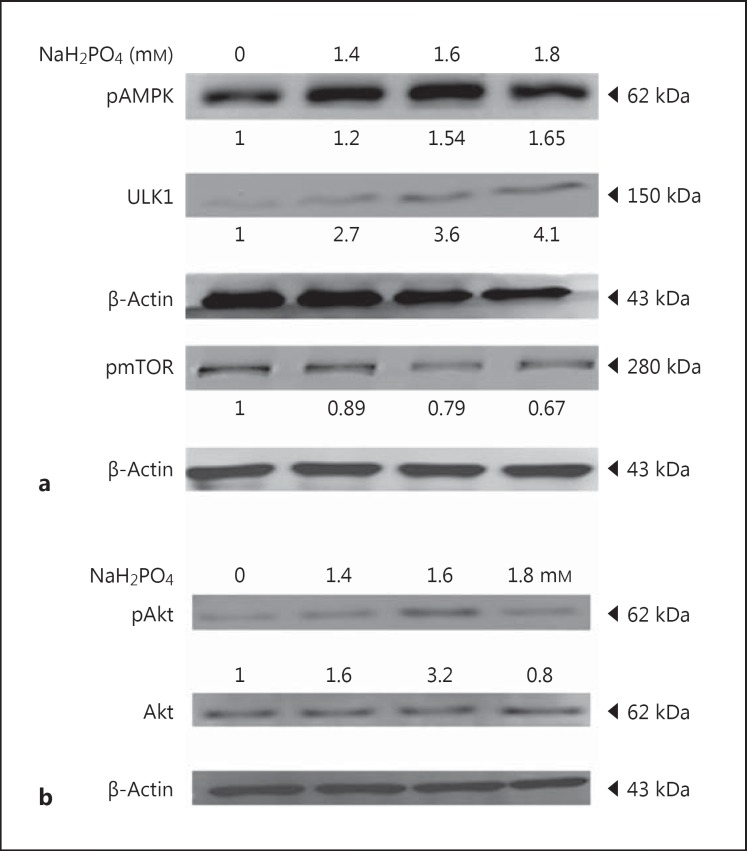
Total proteins extracted from phosphate-treated H9c2 cells (0, 1.4, 1.6, and 1.8 mm concentrations of NaH2PO4/24 h) were analyzed to understand the cellular pathway. The expression of pAMPK was increased (1.65-fold) as the concentration of phosphate increased. The expression of ULK1 was upregulated (4.1-fold), whereas the expression of p-mTOR was decreased (0.67-fold). These results suggest that the expression of ULK1 was mediated by pAMPK not by p-mTOR (Fig. 1a). This suggests that ULK1 (ATG1) is known to mediate autophagy.
Fig. 1.
Effect of NaH2PO4 on autophagy-inducing proteins. a Representative Western blots showing the levels of pAMPK, mTOR, and ULK1 in H9c2 cardiomyoblasts. b Expression levels of Akt and phosphorylated Akt levels in H9c2 cardiomyoblasts administered with NaH2PO4. Quantification was done using Image J, fold difference is given below the blots.
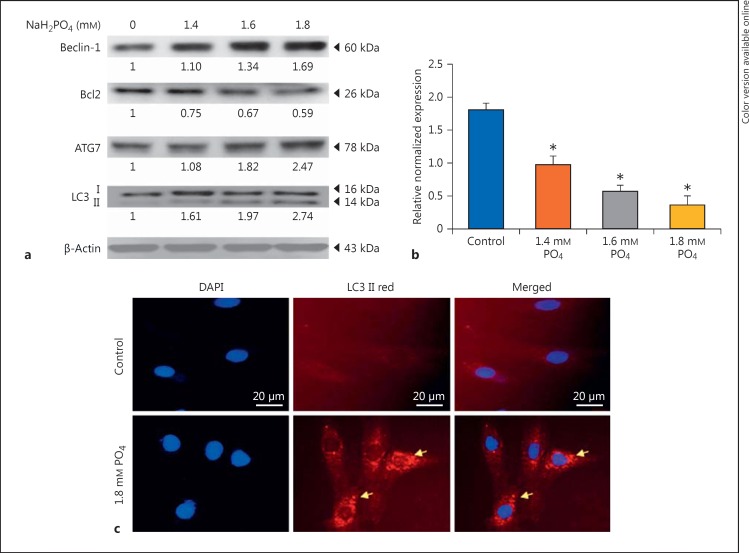
Hyperphosphate Inhibits Bcl2 and Enhances Beclin-1 and ATG7 in H9c2 Cardiomyoblasts to Elevate Autophagy
The expression of survival markers like Akt and pAkt was analyzed. Up to 1.6 mm phosphate, active Akt was increased (3.2-fold), whereas at 1.8 mm concentration, the expression was reduced (0.6-fold) (Fig. 1b). The activation of autophagy-related proteins such as beclin-1, autophagy protein 7 (ATG7), and LC3 II was increased significantly as the concentration of phosphate increased to 1.8 mm, while the expression of Bcl2, a negative regulator of autophagy, was reduced (0.59-fold) at elevated phosphate levels; Bcl2 mRNA expression is also consistent with protein expression level (Fig. 2a, b). These results indicate that hyperphosphate leads to autophagy in cardiomyocytes. Immunofluorescent staining also confirmed the formation of autophagosome (fig. 2c).
Fig. 2.
NaH2PO4 upregulates autophagy markers. a Representative Western blots showing the levels of beclin-1, Bcl2, ATG7, LC3B, in H9c2 cardiomyoblasts administered with NaH2PO4. Quantification was done using Image J, fold difference is given below the blots. b Bcl2 mRNA expression levels. * p < 0.005 denotes significant difference from control. c Immunofluorescent staining showing autophagosome formation in hyperphosphate condition.
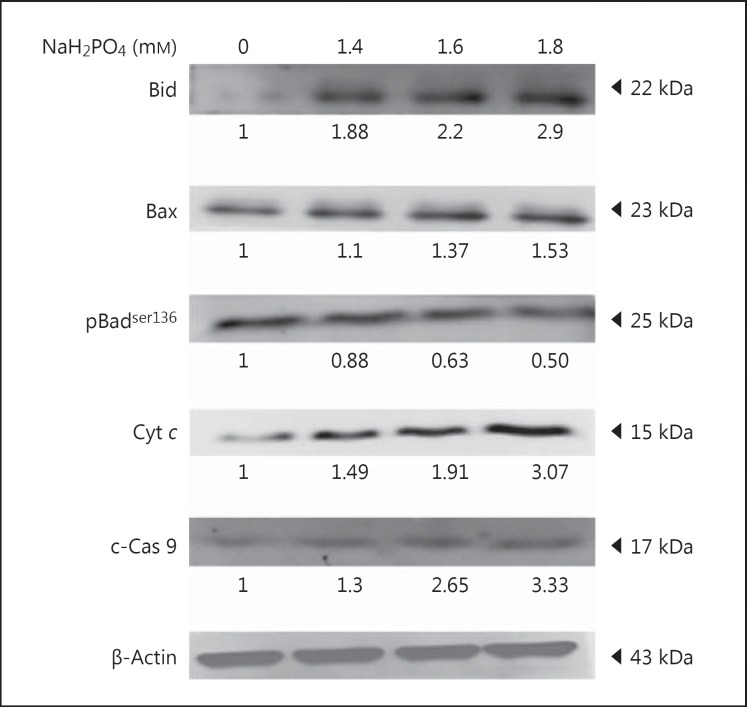
Cellular Apoptosis Induced by Hyperphosphate in H9c2 Cardiomyoblasts
The effect of autophagy on apoptosis was determined using apoptosis-related markers. The upregulation of mitochondrion-mediated, proapoptotic proteins like Bid and Bax was observed, and ultimately Cyt c release (3.07-fold increase) from mitochondria was also observed on hyperphosphate-treated H9c2 cells. Upregulation of cleaved caspase-9 was observed in high-phosphate-treated cells. In contrast, the expression of pBadser136, an antiapoptotic protein was reduced (Fig. 3). H9c2 cells under elevated phosphate showed cellular apoptosis.
Fig. 3.
NaH2PO4 upregulates apoptosis markers. Western blots showing the levels of Bid, Bax, pBadser136, Cyt c, and c-caspase-9 (c-Cas 9) in H9c2 cardiomyoblasts administered with NaH2PO4. Quantification was done using Image J, fold difference is given below the blots.
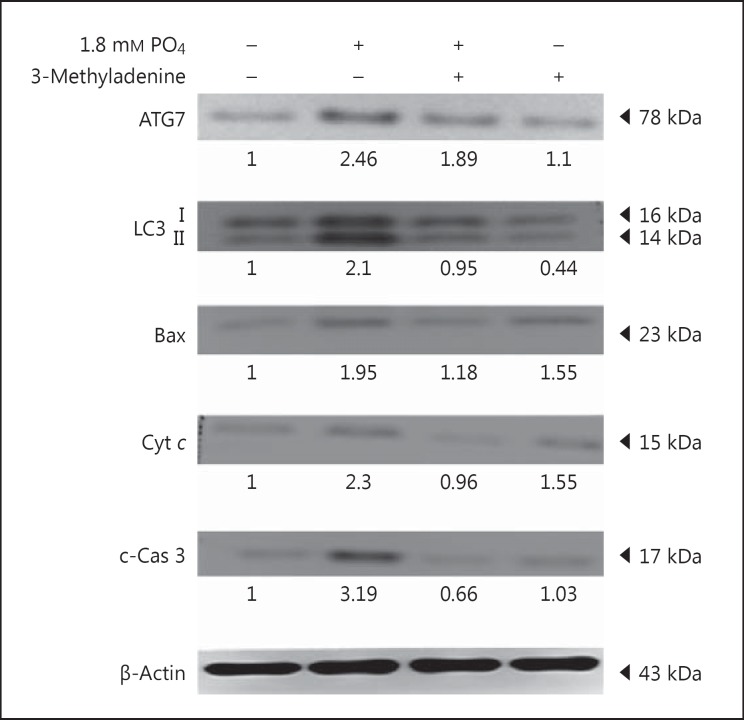
Inhibition of Apoptosis by Autophagy Inhibitor
The effect of autophagy on apoptosis at elevated phosphate levels was investigated. H9c2 cells were pretreated with 100 µm of 3-MA, an autophagy-specific inhibitor. Autophagy-related proteins like ATG7 and LC3 II and apoptosis-related proteins like Bax, Cyt c, and c-caspase-3 were inhibited significantly (fig. 4). This shows that apoptosis under hyperphosphate levels in H9c2 cardiomyocytes was elicited through autophagy.
Fig. 4.
Effect of autophagy inhibitor on autophagy- and apoptosis-related proteins. Western blots showing the levels of ATG7, LC3 II, Bax, Cyt c, and c-caspase-3 (c-Cas 3) in H9c2 cardiomyoblasts administered with NaH2PO4. Quantification was done using Image J, fold difference is given below the blots.
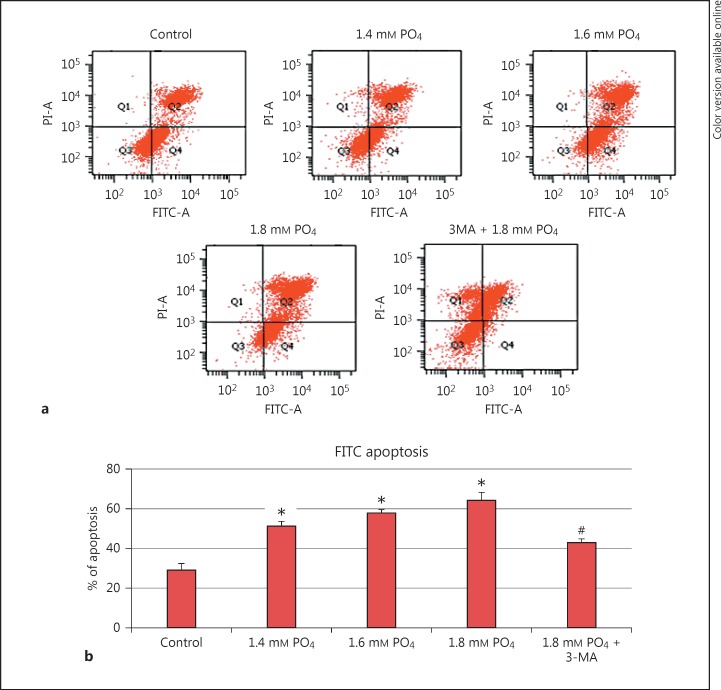
FITC-Annexin V Staining for Apoptosis
FITC-annexin V was used to quantify the apoptotic cells after 3-MA pretreatment and hyperphosphate treatment. The number of apoptotic cells increased as the concentration of phosphate increased. Autophagy inhibitor-pretreated cells showed a significant reduction in apoptosis (Fig. 5). The reduction of apoptosis in autophagy inhibitor-treated cells confirms the relationship between autophagy and apoptosis.
Fig. 5.
Hyperphosphate-induced cellular apoptosis. a FITC-annexin V staining for apoptosis. b Graphical representation of percent of apoptosis in phosphate treated and 3-methyladenine (3-MA) pretreated cells. * p < 0.05 denotes significant difference from control and #p < 0.05 denotes significant difference from 1.8 mm PO4.
Discussion
AMPK is one of the central regulators of the cellular and organismal metabolism, which regulates several transcriptional factors. Moreover, autophagy is promoted by AMPK to maintain energy homeostasis. In the present study, treatment with elevated phosphate has shown to upregulate pAMPK and ULK1, whereas p-mTOR has shown to downregulate them (Fig. 1). Under nutrient-deprived condition, ULK1 (an analogue of yeast ATG1) is known to be directly activated by AMPK in vitro, whereas when nutrient is abundant, mTORC1 activates ULK1 [17]. Inactivation of TORC1 by rapamycin has been reported to stimulate autophagy in the presence of nutrients, suggesting that TOR downregulates autophagy [18]. Elevated phosphate inhibited the expression of p-mTOR in cultured human endothelial cells [19].
Our results are consistent with earlier findings that the expression of pAMPK could trigger autophagy by activating ULK1 in myocardial cells under hyperphosphate condition. Further, increased expression of beclin-1 could possibly initiate autophagy, while Bcl2, a negative regulator of beclin-1, has shown to be reduced both at the mRNA and the protein level (Fig. 2a, b). Bcl2 binds to beclin-1, preventing the assembly of the preautophagosomal structure, which results in the inhibition of autophagy. As the expression level of Bcl2 was decreased, autophagy could continue through the activation of beclin-1; subsequently, ATG7 and LC3 II have also been shown to increase at hyperphosphate levels. Wong et al. [20] reported that the expression of ATG7 is an important mediator of autophagosome formation. Further, cytosolic LC3 I undergoes proteolytic cleavage and LC3 II is formed. Excessive or insufficient level of autophagy has been reported to stimulate pathological cardiac hypertrophy, which leads to heart failure. Hyperphosphate has been reported to induce cellular hypertrophy via ERK signaling pathway in cardiomyoblasts [21]. In human microvascular endothelial cells, hyperphosphate has been found to induce protective autophagy [19].
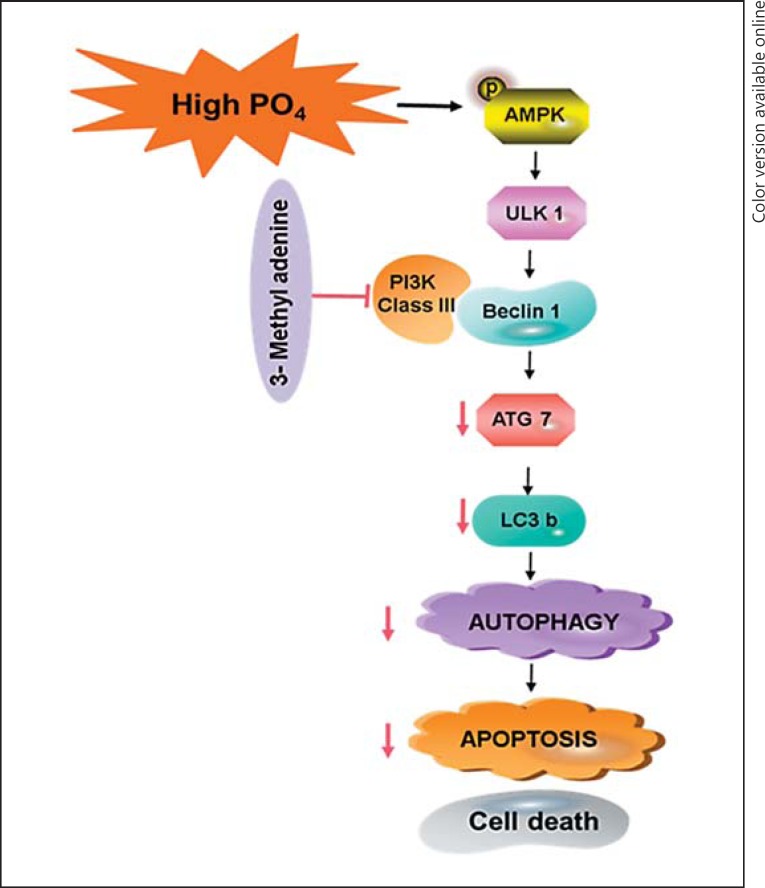
The Bcl2 protein family regulates cellular apoptosis, which is essential for growth and development. A very low level of apoptosis leads to cancer, and excessive apoptosis leads to ischemic conditions [22]. The Bcl2 protein family consists of both prosurvival (Bcl2) proteins which block the release of Cyt c from mitochondria and proapoptotic (Bax, Bak) proteins which stimulate the release of Cyt c. Bad promotes an apoptotic cascade by binding to and inhibiting the actions of Bcl-XL and Bcl2. While the phosphorylated Bad (Ser136) pBadser136 binds with 14-3-3 and reduces the attachment of Bad to Bcl-XL and Bcl2, pBadser136 acts as an antiapoptotic protein [23]. In the present study, the activation of proapoptotic proteins like Bid, Bax, Cyt c, and caspase-9 was observed; simultaneously, downregulation of antiapoptotic proteins like pBadser136 and Bcl2 was also observed. This result suggests that hyperphosphate in cardiomyocytes triggers excessive apoptosis. In CKD patients, hyperphosphatemia was reported to initiate myocardial damage possibly via apoptosis [16]; interestingly, also in the present study, we proved that hyperphosphate induced apoptosis in H9c2 cells, which might lead to cardiac complications. Furthermore, the autophagy inhibitor (3-MA) showed to inhibit apoptosis significantly, which suggests that the cellular apoptosis is autophagy dependent. However, in endothelial cells, inhibition of autophagy has been shown to induce cellular apoptosis under hyperphosphate conditions [19]. Under pathological conditions, apoptosis and autophagy could occur together. H2O2 is known to induce oxidative stress, which induces apoptosis through autophagy [24]. Together, our findings elucidate that hyperphosphate triggers autophagy, which leads to apoptosis in H9c2 cells (Fig. 6).
Fig. 6.
Schematic representation of the mechanism of hyperphosphate-induced autophagy and cellular apoptosis.
Conclusion
The co-occurrence of cardiac apoptosis and autophagy was found in elevated phosphate conditions. Hyperphosphate-mediated autophagy was found to be facilitated by the AMPK pathway. Inhibition of excessive autophagy could prevent cardiac apoptosis in CKD and dialysis patients. Further understanding of autophagy and apoptosis would help in the treatment of “uremic” CVD.
Statement of Ethics
No appropriate ethical approval required.
Disclosure Statement
The authors declare no conflicts of interest.
Funding Sources
The Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW106-TDU-B-212-113004) supported this study financially.
References
- 1.Manghat P, Sodi R, Swaminathan R. Phosphate homeostasis and disorders. Ann Clin Biochem. 2014;51:631–656. doi: 10.1177/0004563214521399. [DOI] [PubMed] [Google Scholar]
- 2.Pizzorno L. Canaries in the phosphate-toxicity coal mines. Integr Med. 2014;13:24–32. [PMC free article] [PubMed] [Google Scholar]
- 3.Chao PC, Hsu CC, Liu WH. Renal protective effects of Porphyra dentate aqueous extract in diabetic mice. Biomedicine. 2014;4:18. doi: 10.7603/s40681-014-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virzi GM, Clementi A, Brocca A, de Cal M, Ronco C. Molecular and genetic mechanisms involved in the pathogenesis of cardiorenal cross talk. Pathobiology. 2016;83:201–210. doi: 10.1159/000444502. [DOI] [PubMed] [Google Scholar]
- 5.Kanbay M, Goldsmith D, Akcay A, Covic A. Phosphate – the silent stealthy cardiorenal culprit in all stages of chronic kidney disease: a systematic review. Blood Purif. 2009;27:220–230. doi: 10.1159/000197562. [DOI] [PubMed] [Google Scholar]
- 6.Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB, Sr, Gaziano JM, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167:879–885. doi: 10.1001/archinte.167.9.879. [DOI] [PubMed] [Google Scholar]
- 7.Giachelli CM. Vascular calcification: in vitro evidence for the role of inorganic phosphate. J Am Soc Nephrology. 2003;14:S300–S304. doi: 10.1097/01.asn.0000081663.52165.66. [DOI] [PubMed] [Google Scholar]
- 8.Amann K, Tornig J, Kugel B, Gross ML, Tyralla K, El-Shakmak A, et al. Hyperphosphatemia aggravates cardiac fibrosis and microvascular disease in experimental uremia. Kidney Int. 2003;63:1296–1301. doi: 10.1046/j.1523-1755.2003.00864.x. [DOI] [PubMed] [Google Scholar]
- 9.Kendrick J, Kestenbaum B, Chonchol M. Phosphate and cardiovascular disease. Adv Chronic Kidney Dis. 2011;18:113–119. doi: 10.1053/j.ackd.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu YT, Tan HL, Shui G, Bauvy C, Huang Q, Wenk MR, et al. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J Biol Chem. 2010;285:10850–10861. doi: 10.1074/jbc.M109.080796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Wang J, Yang X. Functions of autophagy in pathological cardiac hypertrophy. Int J Biol Sci. 2015;11:672–678. doi: 10.7150/ijbs.11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 14.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou R, Kang X, Tang B, Mohan C, Wu TF, Peng A, et al. Ornithine is a key mediator in hyperphosphatemia-mediated human umbilical vein endothelial cell apoptosis: insights gained from metabolomics. Life Sci. 2016;146:73–80. doi: 10.1016/j.lfs.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Qin L, Wu TF, Deng BQ, Sun YR, Hu DY, et al. Elevated cardiac markers in chronic kidney disease as a consequence of hyperphosphatemia-induced cardiac myocyte injury. Med Sci Monitor. 2014;20:2043–2053. doi: 10.12659/MSM.890909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan D, Kim J, Shaw RJ, Guan KL. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011;7:643–644. doi: 10.4161/auto.7.6.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu YJ, Hsu SC, Huang SM, Lee HS, Lin SH, Tsai CS, et al. Hyperphosphatemia induces protective autophagy in endothelial cells through the inhibition of Akt/mTOR signaling. J Vasc Surg. 2015;62:210–221. doi: 10.1016/j.jvs.2014.02.040. [DOI] [PubMed] [Google Scholar]
- 20.Wong CH, Iskandar KB, Yadav SK, Hirpara JL, Loh T, Pervaiz S. Simultaneous induction of non-canonical autophagy and apoptosis in cancer cells by ROS-dependent ERK and JNK activation. PLoS One. 2010;5:e9996. doi: 10.1371/journal.pone.0009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu YL, Huang CC, Chang CC, Chou CY, Lin SY, Wang IK, et al. Hyperphosphate-induced myocardial hypertrophy through the GATA-4/NFAT-3 signaling pathway is attenuated by ERK inhibitor treatment. Cardiorenal Med. 2015;5:79–88. doi: 10.1159/000371454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 23.Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, et al. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- 24.Huang CY, Ting WJ, Huang CY, Yang JY, Lin WT. Resveratrol attenuated hydrogen peroxide-induced myocardial apoptosis by autophagic flux. Food Nutr Res. 2016;60:30511. doi: 10.3402/fnr.v60.30511. [DOI] [PMC free article] [PubMed] [Google Scholar]