Abstract
Background
Magnesium (Mg2+), the second most abundant cation in the cell, is woven into a multitude of cellular functions. Dysmagnesemia is associated with multiple diseases and, when severe, can be life-threatening.
Summary
This review discusses Mg2+ homeostasis and function with specific focus on renal Mg2+ handling. Intrarenal channels and transporters related to Mg2+ absorption are discussed. Unraveling the rare genetic diseases with manifestations of dysmagnesemia has greatly increased our understanding of the complex and intricate regulatory network in the kidney, specifically, functions of tight junction proteins including claudin-14, -16, -19, and -10; apical ion channels including: TRPM6, Kv1.1, and ROMK; small regulatory proteins including AC3 and ANK3; and basolateral proteins including EGF receptor, γ-subunit (FXYD2) of Na-K-ATPase, Kir4.1, CaSR, CNNM2, and SLC41A. Although our understanding of Mg2+ handling of the kidney has expanded considerably in the last two decades, many questions remain. Future studies are needed to elucidate a multitude of unknown aspects of Mg2+ handling in the kidney.
Key Message
Understanding rare and genetic diseases of Mg2+ dysregulation has expanded our knowledge and furthers the development of strategies for preventing and managing dysmagnesemia.
Keywords: Claudins, TRPM6, Dysmagnesemia, Ion channels, Tight junction proteins
Introduction
Magnesium (Mg2+) is the second most abundant intracellular cation. It carries out multiple and critical functions supporting cellular physiological activities. Sixty percent of the US population, however, show insufficient Mg2+ intake [1], and hypomagnesemia occurs in ∼30% of hospitalized patients [2]. The kidney is the principle organ that regulates Mg2+ homeostasis. In the last two decades, a number of new Mg2+ regulatory mechanisms have been uncovered, mainly through investigating patients with rare genetic alterations as the cause of Mg2+ dysregulation. This review focuses on recent advances in our understanding of Mg2+ regulation in the kidney and dysregulation in patients with specific genetic disorders and poses questions on a number of unresolved issues in this field.
Magnesium Homeostasis and Function
The medicinal function of Mg2+ was recognized as early as 1618, when in Epsom, England, a farmer realized that his bitter salty well water healed scratches. “Epsom salts” (primarily Mg2+ sulfate) can be found today in any big-box store such as Walmart or Costco and are often used as a bath salt and a remedy for relieving many ailments including joint pain, muscle spasms, abdominal pain, constipation, headaches, and more. Over the years, studies have shown that Mg2+ is a vitally important, indeed a critical, intracellular element. It is a cofactor/coactivator for more than 600 intracellular enzymes and an essential component of DNA replication, RNA transcription, amino acid synthesis, and protein formation. It is also critical and participates in DNA repair including nucleotide excision repair, base excision repair, and mismatch repair. Lack of the inner mitochondrial membrane Mg2+ channel, Mrs2, leads to respiratory complex I failure and cell death [3]. Additionally, Mg2+ has anti-inflammatory, immunomodulatory, and crystal-inhibitory properties [4]. In the last two decades, genome-wide association studies have shown that polymorphisms in genes related to Mg2+ homeostasis are associated with multiple diseases or risks for diseases including diabetes in African-American and Spanish-American postmenopausal women [1], nephrolithiasis, and reduced bone mineral density [5]. Recently, TRPM7 gene polymorphism has been linked to breast cancer [6]. In addition, studies have shown that Mg2+ alterations can be associated with neurotransmitter defects [7, 8] and an array of neurological abnormalities [9, 10].
Dietary Mg2+ intake in the general adult population should be in the range of 350–450 mg/day. Fecal excretion of Mg2+ is ∼270 mg/day. Mg2+ is absorbed from the gastrointestinal tract, through paracellular transport in the small intestine and transcellular transport in the colon, into the blood, where ∼25% of the absorbed Mg2+ binds to circulating proteins, primarily albumin. The unbound Mg2+ equilibrates with bone and intracellular Mg2+. Intracellular Mg2+ concentrations are in the range of 10–30 mm, and the free cytosolic Mg2+ concentration is ∼0.5–1.2 mm[11]. Approximately 70–75% of the circulating Mg2+ is unbound and is filtered in the kidneys. The glomeruli filter ∼2,400 mg of Mg2+ daily. More than 95% of the filtered Mg2+ is absorbed, leaving a urinary Mg2+ excretion of ∼100 mg/day. Predictably, Mg2+ is regulated principally by the kidneys.
Magnesium Absorption in the Proximal Convoluted Tubule
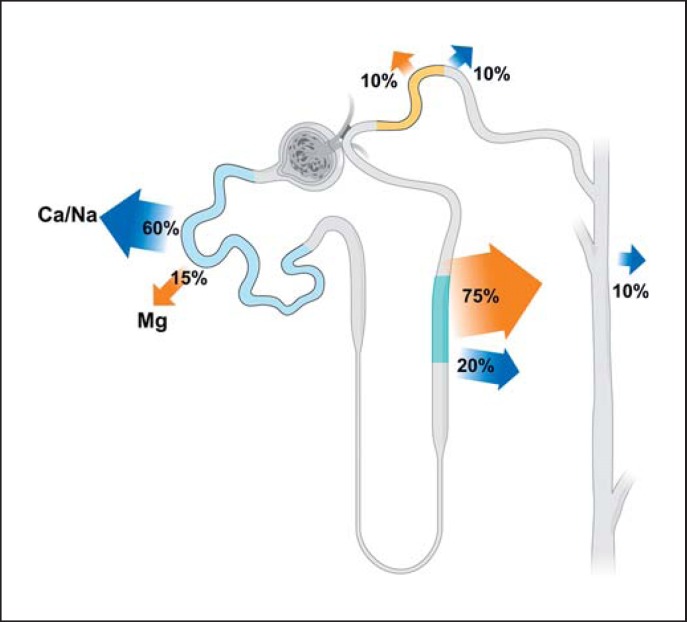
In contrast to the proximal absorption of calcium (Ca2+) and sodium (Na+), a relatively small fraction (15%) of filtered Mg2+ is absorbed in the proximal tubules (Fig. 1) via a paracellular mechanism, although the precise mechanism has not been fully elucidated. In the first portion of the proximal convoluted tubule, Na+, Ca2+, and water are absorbed to a large extent, preceding Mg2+ absorption. When the filtrates reach more distal parts of the proximal tubule, there is an increase in tubular fluid Mg2+ concentration compared to that in the peritubular circulation. When the concentration gradient of Mg2+ between the luminal fluids and the peritubular circulation reaches approximately 1.7–1.9, Mg2+ absorption occurs, and is likely driven by the concentration gradient [12].
Fig. 1.
Renal tubular Mg2+ handling. More than 95% of the filtered Mg2+ is reabsorbed in the kidney, and only approximately 100 mg of Mg2+ is excreted daily. The distal convoluted tubule is the last part where Mg2+ reabsorption occurs. Beyond that section, the kidney tubules are impermeable to Mg2+.
Magnesium Absorption in the Thick Ascending Limb of Henle
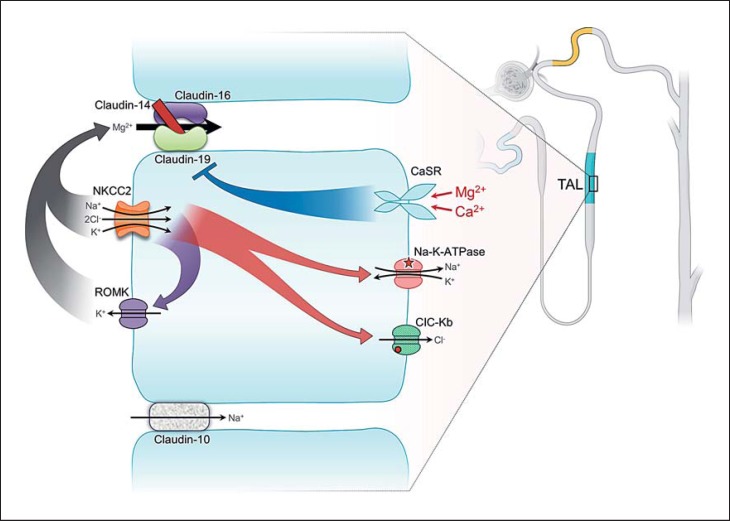
In the thick ascending limb of Henle (TAL), Mg2+ is absorbed paracellularly, facilitated by tight junctional proteins, and the major driving force is the transepithelial voltage gradient. In the initial part of the TAL, the luminal voltage is positive (approx. +8 mV), and the Na+ and Cl− concentrations are relatively high (Fig. 2). The positive voltage is generated primarily through the apical Na-K-2Cl cotransporter (NKCC2)-mediated Na+, K+, and Cl− absorption with parallel K+ excretion to the lumen. The driving force for the NKCC2 is the basolateral Na-K-ATPase. The positive transepithelial voltage gradient propels Mg2+ absorption via paracellular mechanisms. At the distal end of the TAL, where the Na+ concentration diminishes, Mg2+ absorption is further regulated by tight junction proteins between the adjacent tubular epithelial cells, specifically claudin-16, -19, and -14 [13].
Fig. 2.
Mg2+ handling in the thick ascending limb of Henle (TAL). The tight junction proteins claudin-16 and -19 form the Ca2+- and Mg2+-permeable channel. Mg2+ or Ca2+ in the circulation could activate the basolateral calcium-sensing receptor (CaSR), which exerts inhibitory effects on the tight junction claudin complex. Basolateral Na-K-ATPase provides the driving force for the apical Na-K-2Cl cotransporter (NKCC2) and parallel K+ excretion via ROMK, generating a favorable positive luminal voltage to facilitate paracellular Mg2+ and Ca2+ absorption.
Claudins
Claudins (CLDN) are small tetraspan proteins (MW: 20–28 kDa) composing a family with at least 26 members. They are key components in the formation of the tight junction barrier and responsible for regulated paracellular ion flux [13]. In the TAL, paracellular Mg2+ absorption is facilitated by the combined effect of claudin-16 (paracellin-1) and claudin-19, and is inhibited by claudin-14 [14, 15, 16, 17] (Fig. 2). Such arrangements represent a regulatory mechanism for controlled Mg2+ absorption. Studies have shown that the basolateral calcium-sensing receptor (CaSR), when activated, increases the expression of claudin-14, preventing excess Mg2+/Ca2+ absorption, responding to changes of the extracellular Ca2+ or Mg2+ concentration (detailed below).
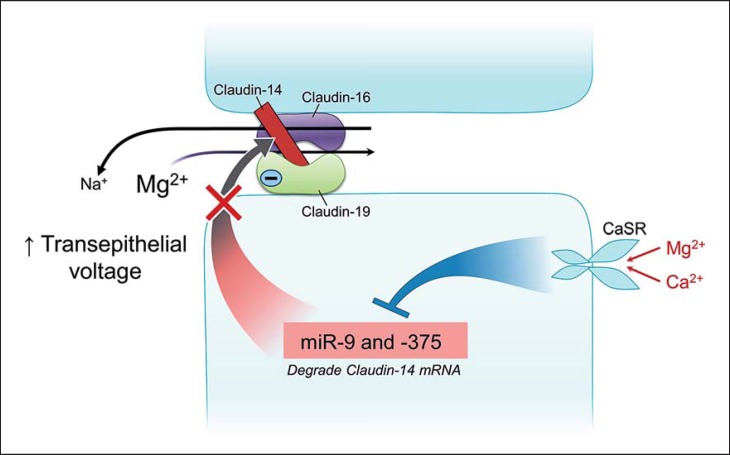
In the last two decades, studies have elucidated underlying mechanisms in more detail. Claudin-16 and -19 colocalize in the TAL to form a cation complex [16]. Claudin-16 by itself is an Na+-permeable channel [18], and claudin-19 is a deterrent protein to the passage of Cl [16]. The Cl deterrence by claudin-19 creates a negative microenvironment in the epithelial junction, creating an attraction for cation (Mg2+/Ca2+) selection. At the end of the TAL, where the luminal Na+ concentration is low and the basolateral Na+ concentration relatively high (approx. 140 mm), through claudin-16 there is a backleak of Na+ from the basolateral aspect to the lumen. Such a backleak enhances the positive luminal voltage, fostering paracellular Mg2+ absorption (Fig. 3).
Fig. 3.
Regulated paracellular Mg2+ absorption in the thick ascending limb of Henle (TAL). Claudin-16 by itself is highly permeable to Na+, and claudin-19 is impermeable to Cl−. Claudin-14 deters the sodium channel permeability of claudin-16. Na+ backleaks into the distal part of the TAL and helps in maintaining a positive luminal voltage. Blockage of this action of claudin-14 compromises the positive luminal voltage and diminishes the driving force for Mg2+ absorption. The deterrence of Cl− passage by claudin-19 creates a negative microdomain charge, creating a selective attraction to luminal Mg2+ and Ca2+. The basolateral calcium-sensing receptor (CaSR), when activated by an elevated concentration of Mg2+ or Ca2+, inhibits miR-9 and miR-375. The inhibition removes the interference of the microRNAs with claudin-14 translation, resulting in an increased claudin-14 translation and claudin-14-mediated inhibition of claudin-16, thus inhibiting Mg2+ and Ca2+ absorption.
Recently, a claudin-10 (CLDN10) knockout mouse model has shown that claudin-10 [19], which is expressed almost exclusively in the tubular junction of the epithelia in the TAL, functions as an Na+-permeable protein. Deletion of claudin-10 would deter Na+ absorption and create an elevated luminal transepithelial voltage to increase Mg2+ absorption. Animals with claudin-10 deletion show elevated serum Mg2+ and Ca2+ concentrations and an impaired capacity of urine Mg2+ and Ca2+ excretion.
Calcium-Sensing Receptor
CaSR, a member of the G protein-coupled receptor superfamily, is a 120-kDa polypeptide containing seven transmembrane domains. CaSR is expressed abundantly in parathyroid glands and in kidney tubules. It also is expressed in multiple organ systems including the cardiovascular, gastrointestinal (specifically the ileum), airway, and central nervous systems [20]. Recent studies have provided compelling evidence that by recognizing and responding to miR-9 and miR-374 (small noncoding RNA molecules) [17], CaSR regulates the expression of claudin-14 in the TAL (Fig. 3), thereby regulating paracellular Mg2+ absorption. Recessive mutations in claudin-14 have been reported as a cause of nonsyndromic recessive deafness (DFNB29) [21] due to failure of ion balance in the organ of Corti [22]; there is no information as to any renal manifestations in affected individuals.
The importance of CaSR in the regulation of Ca2+ and Mg2+ has been demonstrated in mice models of Casr+/− and Casr−/− mutations. The mutant mice phenocopy the manifestations of humans with familial hypocalciuric hypercalcemia (FHH) and neonatal severe hyperparathyroidism (NSHPT). Consistently, the extracellular Mg2+ concentration is moderately elevated in Casr+/− mice, and more severely elevated in Casr−/− mice [23].
Additional Transporters
NKCC, ROMK, ClC-Kb, and barttin all contribute to a positive transepithelial voltage, favoring Mg2+ absorption. ROMK, ClC-Kb, and barttin cross the boundary of TAL and are expressed in the distal convoluted tubule (DCT). They have been described in detail in the context of salt-losing nephropathy (Table 1) [24, 25, 26, 27, 28, 29].
Table 1.
Dysmagnesemia related to salt-losing nephropathy
| Inheritance | Mutant gene | Transporter/protein | Key features | |
|---|---|---|---|---|
| Bartter syndrome I | AR | SLC12A1 | NKCC | Regulated by CaSR, antenatal onset, hypokalemic, hypercalciuric metabolic alkalosis |
| Bartter syndrome II | AR | KCNJ1 | ROMK | Antenatal onset, can be hyperkalemic |
| Bartter syndrome III | AR | ClC-Kb | ClC subunit B | Variable childhood onset; milder form of Bartter syndrome; variable, Gitelman-like presentation |
| Bartter syndrome IV | AR | BSND | Barttin | Sensorineural hearing defect, CKD/ESRD in second or third decade of age |
| Bartter syndrome V | AD | CASR (activating mutation) | CaSR | Neonatal onset, can be variable; urine Mg2+ and Ca2+ loss |
| Gitelman syndrome | AR | SLC12A3 | NCC | Childhood and adolescent onset, hypocalciuric hypokalemic, metabolic alkalosis, urine Mg2+ loss |
AR, autosomal recessive; AD, autosomal dominant; NKCC, Na-K-Cl cotransporter; CaSR, calcium-sensing receptor; NCC, Na-Cl cotransporter; CKD, chronic kidney disease; ESRD, end-stage renal disease.
Dysmagnesemia due to Ion Channel/Transporter Mutations in the TAL
Hypercalciuria and Nephrocalcinosis
CLDN16 and CLDN19 mutations are responsible for familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC) [14, 15]. FHHNC was initially reported in 1997 by Walder et al. [30] in three consanguineous Bedouin kindreds from Israel. CLDN16 mutations were found to be causative. Affected individuals exhibit severe and symptomatic hypomagnesemia in the range of 0.1–0.4 mm, hypercalciuria, and a nephrocalcinosis onset at the age of 2–8 weeks after birth. The symptoms include polyuria/polydipsia, muscular tetany, seizures, nephrolithiasis, and progressive loss of kidney function. Renal failure develops mostly in childhood or in adolescence [31]. In 2006, Konrad et al. [15] reported initial cases of FHHNC due to CLDN19 mutation – but without CLDN16 mutation – in Swiss and Spanish families, with nearly identical presentations. Patients with CLDN19 mutations additionally show ocular abnormalities. Recent studies have shown that both CLDN16 and CLDN19 mutations can give rise to abnormal enamel formations [32, 33].
More information is emerging on the spectrum of mutations, primarily in CLDN16, since approximately 46 mutations have been described to date, as well as the genotype-phenotype correlations. In a study on 25 families with FHHNC in Germany and Eastern European countries, a founder mutation (L151F) in the first extracellular loop of claudin-16 has been identified as being present in about 50% of mutant alleles [31]. Patients with loss-of-function mutations in both alleles in the CLDN16 gene tend to have an early onset of renal failure, whereas those harboring at least one mutant allele with some residual function seem protected from the rapid loss of renal function [34]. Interestingly, a novel homozygous CLDN16 mutation (T233R) has been identified in two families with self-limiting childhood hypercalciuria. The hypercalciuria decreased with age and was not associated with declined renal function. The T233R mutation is the first mutation to be identified in the cytosolic tail of claudin-16, and is predicted to result in an ineffective PDZ domain-binding motif. This inactivation abolishes the association with the tight junction scaffolding protein ZO-1, with subsequent accumulation of the mutant claudin-16 protein in lysosomes [35]. The underlying mechanism for such a mild phenotype in patients with this mutation is unknown. Much less is known about the genotype-phenotype correlations in CLDN19 mutations. Indeed, to date 14 mutations have been described, and no correlation between CLDN19 mutations and the rate of renal functional deterioration has been uncovered [15, 33, 36].
Autosomal Dominant Hypocalcemia and FHH
CASR mutations cause a spectrum of phenotypes. Gain-of-function mutations are causative of autosomal dominant hypocalcemia (ADH). Loss-of-function mutations cause autosomal dominant FHH and autosomal recessive (homozygous or compound heterozygous mutations) NSHPT. Patients with gain-of-function CASR mutations develop varying degrees of hypocalcemia and hypomagnesemia from a stable and often mild form of ADH to a severe form of Bartter syndrome type V (Table 1). Opposite abnormalities of hypercalcemia and hypermagnesemia occur in patients with loss-of-function mutations. Most FHH patients are clinically asymptomatic and exhibit mild hypercalcemia and often hypermagnesemia, while NSHPT patients are characterized by severe hypercalcemia and typically die within 1 year after birth if parathyroidectomy is not provided [37].
Considerable clinical heterogeneity has been observed among patients with heterozygous CASR mutations. A small number of affected patients develop neonatal hyperparathyroidism associated with mild-to-moderate hypercalcemia during infancy. It was formerly assumed that a dominant negative effect and/or paternal inheritance could have modified the clinical presentation. Recent functional studies, however, have found no evidence for either effect [38], suggesting that other environmental or epigenetic factors yet to be identified might have played a role in the various disease presentations. Over 230 CASR mutations have been described, and several mutational hot spots and genotype-phenotype correlations of some degree have been demonstrated. For instance, functional studies of several mutations have shown that mutations in C131W and A843E preferentially alter the Ca2+ response curve, differing from other CASR mutations (IEK47N and P221L) without clinical features of urine Mg2+ wasting and hypomagnesemia [39]. More comprehensive information regarding CASR mutations and clinical manifestations have recently been comprehensively reviewed [40].
Magnesium Absorption in the DCT
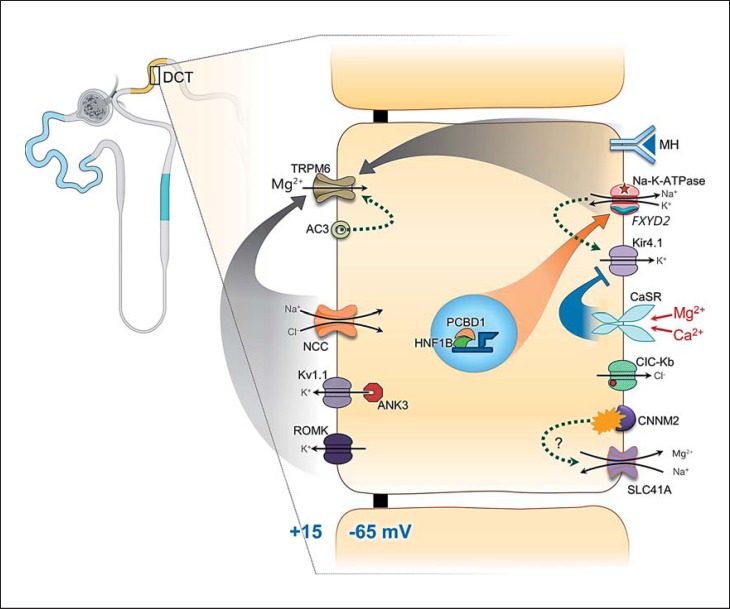
The last approximately 10% of Mg2+ are absorbed in the DCT. Mg2+ absorption in this tubular section is transcellular instead of paracellular. The Mg2+ absorption is tightly regulated through multitudes of channels and transporter proteins. The more stringent regulation is justly called for, since this is the last part of the renal tubules with the capacity for absorbing luminal Mg2+. It fine-tunes and determines the final amount of urine Mg2+ excretion. The chemical gradient of Mg2+ in this section is small (luminal Mg2+ concentration: 0.2–0.7 mm; intracellular free Mg2+: approx. 0.5–1.2 mm). Thus, a positive transapical membrane voltage gradient becomes critical for Mg2+ entry. All known regulatory pathways converge onto influencing the transapical membrane voltage, the principle driving force for Mg2+ entry into the DCT cells via TRPM6 (Fig. 4). The function and regulation of some transporters, e.g., SLC41A and CNNM2, have yet to be fully elucidated.
Fig. 4.
Schematic presentation of magnesium regulation in the distal convoluted tubule. TRPM6 is the major channel for Mg2+ absorption in the distal convoluted tubule. The driving force for TRPM6-mediated Mg2+ intake is a positive apical membrane potential gradient. Na-K-ATPase is critical in the establishment of the transapical membrane gradient. PCBD1 and HNF1B are transcription factors promoting the expression of the γ-subunit of the Na-K-ATPase, encoded by FXYD2. Mutations in HNF1B and PCBD1 interfere with transcriptional expression of the γ-subunit, thus leading to an insufficient transapical membrane potential and diminishing Mg2+ absorption via TRPM6. Channels important for the establishment of the apical membrane potential include transporters for K+ excretion apically, Kv1.1, and ROMK. Calcium-sensing receptor (CaSR) inhibits basolateral K+ exit via Kir4.1. Reduced K+ recycling via Kir4.1 interferes with Na-K-ATPase function. ClC-Kb is a basolateral Cl− exit channel. Its mutation and the mutation of its subunit barttin could both cause defects in Cl− exit in association with Na-Cl cotransporter (NCC) function and the apical membrane potential. CNNM2 is a basolateral Mg2+-Na+ exchanger which may function as a cytosolic Mg2+ sensor. SLC41A encodes an Mg2+ exit channel. The regulation(s) and physiological function(s) of the channels of CNNM2 and SLC41A are yet to be elucidated. MH, magnesiotropic hormones (including EGF and insulin).
TRPM6
Two groups independently identified TRPM6 as the causative gene in hypomagnesemia with secondary hypocalcemia (HSH) in 2002 [41, 42]. TRPM6 is a 234-kDa membrane protein with both its N- and its C-termini residing in the cytosol. It has six transmembrane domains with a channel function between transmembrane domains 5 and 6. It has an approximately 5-fold higher affinity for Mg2+ than for Ca2+[43]. Accordingly, patch clamp analysis showed that TRPM6 is an Mg2+- and Ca-permeable cation channel preferentially transporting Mg2+[43]. TRPM6 proteins form homotetrameric functional complexes as well as heterotetrameric complexes with TRPM7, the closest homolog of TRPM6 [44]. Both TRPM7-dependent and TRPM7-independent TRPM6 activity have been reported [45, 46].
TRPM6 contains multiple interacting regions. For instance, around channel areas, there is a TRP box that interacts with PIP2. Immediately downstream of the channel domain there is a coiled-coiled region that is believed to form dimers with other TRPM monomers. Insulin can react in the Ser-Thr-rich region upstream adjacent to the kinase domain. In the C terminus, there is an α-kinase domain that plays a regulatory role in response to RACK-1 and REA. These regions are all targets of potential signaling interactions [47, 48]. Thus, it comes as no surprise that TRPM6 can be activated by a number of signals including EGF, insulin, estrogen, purinergic signaling, dietary Mg2+ intake, and acid-base alterations. Recent studies have shown that plasma membrane TRPM6 expression can be stimulated by the adenylyl cyclase 3 (AC3)/cAMP/PKA-mediated signaling pathway. AC3 is also colocalized with Na-Cl cotransporter (NCC) in the DCL [49]. In mice with kidney-specific AC3 knockout, urine Mg2+ wasting, hypomagnesemia when on a low-Mg2+ diet, colocalization of AC3 and NCC, and activation of cAMP-PKA with increasing apical membrane expression and channel conductance of TRPM6 have all been demonstrated [49].
Pro-EGF/EGF and EFG Receptor
Pro-EGF is expressed in the renal epithelia, primarily in the DCT [50, 51]. Pro-EGF is proteolytically cleaved, and functional EGF is released. The released EGF activates EGF receptor that is expressed at the basolateral aspect of the renal epithelial cells [52]. Receptor activation triggers a signaling cascade involving Src kinase and Rac1, leading to increased trafficking of TRPM6 from intracellular vesicles to the apical membrane to mediate Mg2+ absorption [53].
Gamma-Subunit of Na-K-ATPase
The γ-subunit of Na-K-ATPase, encoded by FXYD2, is critical for the pump function, as it stabilizes the α-subunit of this pump [54] and increases the pump's affinity for ATP while reducing its affinity for Na+ and K+. In the kidney, two splice forms of FXYD2 genes are expressed: FXYD2a, expressed primarily in the TAL and proximal tubules, and FXYD2b, expressed exclusively in the basolateral membrane of the DCT and collecting duct [55]. A mutation in FXYD2 causes misrouting of the mutant γ-subunit and fails to join the α- and β-subunits to form a complete and fully functional Na-K-ATPase [56]. The impairment of Na-K-ATPase function diminishes the driving force for apical NCC activity and compromises apical membrane voltage generation (partial depolarization of the cell), reducing the driving force for TRPM6-mediated Mg2+ entry. Reduced NCC activity also compromises TRPM6 activation associated with AC3/cAMP/PKA signaling [49].
HNF1B and PCBD1
HNF1B (hepatocyte nuclear factor 1 homeobox B) and PCBD1 (pterin-4α-carbinolamine dehydratase/ dimerization cofactor of hepatocyte nuclear factor 1 homeobox A) are the two known transcriptional regulatory factors for FXYD2 expression, especially in the distal tubules [57, 58]. HNF1B is involved in organogenesis and the formation of tubular structures in the liver, pancreas, lung, and kidney, and its expression seems restricted to epithelial cells [59, 60]. It has also been shown to transcriptionally activate PKHD1, the gene which, when mutated, causes autosomal recessive cystic kidney disease [61]. Indeed, mutations in HNF1B are emerging as the most frequent monogenic cause of developmental renal abnormalities [62]. PCBD1 is a dimerization cofactor for HNF1B in the nucleus [63, 64], as well as a cytosolic enzyme involved in the regeneration of tetrahydrobiopterin (BH4) [65, 66]. Gene expression studies combined with immunohistochemical analyses of the kidney have shown that Pcbd1 is highly expressed in the DCT, where Pcbd1 transcript levels are upregulated by a low-Mg2+ diet [58, 67]. PCBD1 increases HNF1B-induced FXYD2 transcription by 50% [58]. Mutant PCBD1 proteins were not capable of increasing FXYD2 transcription [58].
KCNJ10
Kir4.1 (potassium voltage-gated channel subfamily J member 10), encoded by KCNJ10, is expressed in the basolateral membrane of the DCT [68, 69]. Kir4.1 mutation interferes with basolateral K+ recycling, compromising Na-K-ATPase function and generation of the apical membrane potential. Convincing evidence has shown that CaSR, coexpressed in the same region, inhibits cell surface expression of Kir4.1 [70] via a mechanism dependent on Gq and caveolin-1 [70, 71]. Reduced Na-K-ATPase activity due to Kir4.1 mutations causes a reduced apical NCC activity and apical membrane voltage, the major driving force for Mg2+ influx [72]. Importantly, CaSR is able to modulate Kir4.1-mediated potassium extrusion in response to the physiological range of the extracellular Ca2+ concentration (EC50 Ca2+ of 1.0 mm) [70]. Thus, through CaSR, Kir4.1 regulates the distal nephron NaCl transport and apical membrane potential physiologically. Interestingly, in contradiction to classic teaching, no measurable CaSR-mediated ROMK inhibition could be demonstrated [70].
KCNA1
Kv1.1 (potassium voltage-gated channel subfamily A member 1), encoded by KCNA1[73], is an apical membrane K+ channel contributing to the sustained transapical membrane voltage by extruding K+ into the lumen. A mutation in Kv1.1 was identified as a causative factor for isolated autosomal dominant hypomagnesemia due to renal Mg2+ wasting [73]. A recent study by San-Cristobal et al. [74] showed that the function of Kv1.1 can be regulated by ankyrin-3 (ANK3), a member of adaptor proteins that link the cytoskeletal network to the cytoplasmic domain of plasma membrane proteins [75]. Mice on a high-Mg2+ diet have been shown to double their fractional urine Mg2+ excretion, associated with a 1.8-fold increase in renal ANK3 expression. Using the carboxy terminal domain of Kv1.1 to screen murine kidney lysates revealed that Kv1.1 function can be inhibited by ANK3. Biophysical studies have shown that ANK3 is able to functionally block the channel opening of Kv1.1 without affecting its plasma membrane expression. Thus, Kv1.1 is involved in establishing a positive and favorable electrical apical membrane gradient to drive Mg2+ entry through TRPM6 in the epithelial cells of the DCT.
Notably, the ANK3-binding motif is not present in the Kv1.1 channel. Whether ANK3 binds the Kv1.1 channel directly or through other proteins in a macromolecular complex is currently not known. Nonetheless, the data clearly demonstrate a role for ANK3 in regulating the biophysical properties of Kv1.1, contributing to a physiologically relevant Mg2+ regulatory pathway.
CNNM2 and SLC41A3
The exact mechanism of basolateral Mg2+ efflux from the renal tubular cells is currently unknown. Studies have suggested that SLC41A3 [76, 77] and cyclin M2 (CNNM2) [78] may act as Mg2+ efflux channels. CNNM2 is also expressed in the basolateral membrane of the TAL [78]. CNNM2 contains two highly conserved CBS (cystathionine-β-synthase) domains, critical for dimerization with other transporters and also for the Mg2+-dependent gating of the magnesium transporter E channel [79], a homolog of the SLC41 family of transporters [80]. Protein topology and homology modeling of a conserved CBS domain, however, suggest that CNNM2 might function as a cytoplasmic Mg2+ sensor [81, 82, 83]. The mechanism regulating the functions of CNNM2 and SLC41A has yet to be fully elucidated.
Other Transporters Affecting Mg2+ Homeostasis
Mutations in the genes encoding NCC, ROMK, ClC-Kb, and barttin (a subunit of ClC-Kb) have been characterized in more detail under the category of salt-losing nephropathies [84, 85, 86, 87, 88, 89, 90] (Table 1).
Dysmagnesemia due to Transporter/Protein Mutations in the DCT
Hypomagnesemia with Secondary Hypocalcemia
TRPM6 mutations are responsible for HSH, also known as primary intestinal hypomagnesemia. HSH patients typically present during the first few months of life with neurological symptoms of tetany and seizures associated with profound hypomagnesemia and secondary hypocalcemia due to parathyroid failure. HSH is associated with the most severe hypomagnesemia among all genetic forms of channelopathy, in the range of 0.05–0.20 mm in a recent study [48], due to the dual defects in gastrointestinal and renal Mg2+ absorption. To date, fewer than 50 unique mutations in TRPM6 have been recorded [48]. Most of the mutations are predicted to give rise to a premature termination of TRPM6 in approximately 85% of the mutations tested. The remaining mutations include missense mutations and mutations affecting the TRPM gating property or interfering with its plasma membrane trafficking. Thus far, most TRPM6 mutant products tested in vitro in cellular systems have shown a dramatic decrease in channel currents, with the exception of the Q1663R variant, which functions almost the same way as the wild-type TRPM6[48]. Overall, there has been no clear pattern of genotype-phenotype association. It should be noted that in a number of cases, the diagnosis and consequent Mg2+ administration were delayed because the Mg2+ level was not measured on initial presentation, and the patients suffered from repeated convulsions leading to permanent brain damage and mental retardation [30, 41, 42].
Isolated Recessive Hypomagnesemia
Isolated recessive hypomagnesemia is an autosomal recessive disorder [91] caused by a mutation in the EGF gene, c.3209C>T (p.Pro1070Leu), causing a defect in the basolateral trafficking of pro-EGF, in turn leading to a defect in soluble EGF elaboration and in EGF-mediated apical TRPM6 expression [50]. Affected individuals present with seizures during infancy and mental retardation due to severe hypomagnesemia. Ca2+ derangement is typically spared [91]. Similarly, cetuximab, an EGFR antibody used as an anticancer agent, has been associated with severe hypomagnesemia due to lack of EGF receptor-mediated TRPM6 trafficking to the apical membrane and lack of Mg2+ absorption through TRPM6 [92, 93].
EAST Syndrome and SeSAME Syndrome
Mutations in the KCNJ10 gene, encoding Kir4.1, were shown to cause a syndrome named EAST (epilepsy, ataxia, sensorineural deafness, and tubulopathy) and SeSAME (seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance) by two independent groups [69, 94]. The syndrome is characterized by seizures, sensorineural deafness, and ataxia, as well as by electrolyte alterations akin to those in Gitelman syndrome including salt wasting, hypokalemic metabolic alkalosis, and hypomagnesemia. The serum Ca2+ concentration, however, tends to be reduced [69, 94]. Because mental retardation has not been a clinical feature, the term “EAST syndrome” seems appropriate and is used clinically. Kir4.1 mutations, by interfering with Na-K-ATPase activity, compromise apical Na+ entry via the NCC channel, thus partially depolarizing the apical membrane and diminishing the favorable driving force for Mg2+ influx via the TRPM6 channels.
Autosomal Dominant Hypomagnesemia
Kv1.1 is a Shaker-related voltage-gated K+ channel encoded by KCNA1. Its mutation, c.763A>G (p.Asn255Asp), has been shown to cause autosomal dominant hypomagnesemia [73]. It is posited that the substitution of the highly conserved asparagine for aspartic acid renders the Kv1.1 channel nonfunctional. The mutation was identified in a large Brazilian family. Of the 46 family members, 21 carried the mutation and were affected by severe hypomagnesemia. Affected individuals experience muscle cramps, tetanic episodes, muscle weakness, and myokymia [73]. Remarkably, Kv1.1 mutations have been identified previously and have been associated with episodic ataxia without Mg2+ alteration [95]. The previously described mutations are adjacent to the mutations that are responsible for hypomagnesemia. It is hypothesized that replacement of a neutral amino acid (asparagine) with an acidic (aspartic) acid – which alters the structure of Kv1.1, resulting in a defect in apical K+ efflux via Kv1.1 – compromises the generation of the transapical membrane potential, leading to a defect in Mg2+ absorption.
Isolated Dominant Hypomagnesemia
To date, a total of 3 families with hypomagnesemia related to an FXYD2 mutation have been identified. These 3 families have the identical c.115G>A (p.Gly41Arg) mutation. The first family reported with this mutation and hypomagnesemia was a Dutch family [56, 96, 97]. Recently, 2 additional families were identified, from Belgium and the Netherlands [98]. The affected individuals show hypomagnesemia and hypocalcemia. Some of them have polyuria and hypokalemia. All of the affected individuals complained of muscle cramps and generalized weakness. Kidney failure was reported in one of the probands. Interestingly, haplotype analyses suggest that the 3 families have a common founder, but genealogy failed to identify a common ancestor up to 1700 [98].
Hypomagnesemia and Maturity-Onset Diabetes of the Young
Patients with dominant mutations in the HNF1B gene or recessive mutations in the PCBD1 gene can develop hypomagnesemia and maturity-onset diabetes of the young [58, 99]. These clinical findings could be explained at the molecular level by the role of PCBD1 as a transcriptional coactivator of HNF1B. HNF1B mutations have also been associated with polycystic kidney disease and urogenital malformations, consistent with the role of HNF1B in transcriptional activation of PKHD1[61] and organogenesis [59, 60, 62]. Not all patients harboring these mutations, however, develop hypomagnesemia [58, 100]. Further studies are necessary to better understand the underlying genotype-phonotype relation.
Dominant Hypomagnesemia due to CNNM2 Mutations
Two unrelated families with dominant hypomagnesemia were found to carry two mutations in the CNNM2 gene [78, 101]. One is the heterozygous deletion c.117delG (p.Ile40SerfsX15), and the other is the heterozygous missense mutation c.1703C>T (p.Thr568Ile). The deletion causes truncated proteins and the missense mutation causes a substitution of an amino acid for one of the two highly conserved CBS domains. Significant intrafamilial phenotypic variation was observed. In the family with the truncating mutation, the proband developed symptomatic hypomagnesemia at the age of 2 years, while his mother developed symptoms in her teenage years.
Remaining Questions and Future Research
Although our understanding of Mg2+ regulation, especially in the kidneys, has improved tremendously in the space of the last 20 years, a large number of questions remain. For instance, most of the Mg2+ transporter-mediated signaling pathways have not been fully elucidated; the underlying reasons for the large and complex variations in genotype and phenotype are unknown, as are the epigenetic factors and genetic modifiers that may influence the clinical phenotypes. Moreover, whether the other TAL-expressed claudins (i.e., claudin-10) perform a role in Mg2+ homeostasis is unclear. Mg2+ dysregulation in mitochondrial diseases has not been well characterized. Lastly, many proposed disease mechanisms are speculative (i.e., the pathophysiology of renal failure in FHHNC). That said, with emerging modern technologies – i.e., high-resolution microscopy and crystallography, which has resolved the crystal structures of claudin [102] and Mg2+ transporter proteins (Mrs2 and SLC41A) [79, 103, 104, 105], newer electrophysiology techniques, genome-wide association studies, next-generation sequencing technology, and analytical tools – it is foreseeable that, before long, more information on Mg2+ regulation and dysregulation will be revealed. Studies on genetic alterations would help us to manage the common clinical patients with dysmagnesemia.
Conclusion
Mg2+ is a critical cation, inextricably intertwined with numerous cellular functions to maintain cell viability and tissue integrity. Dysmagnesemia and polymorphisms of genes related to Mg2+ regulation have been associated with a number of diseases including cancer, diabetes, nephrolithiasis, osteoporosis, and an array of neurological abnormalities. The genetics of Mg2+ dysregulation are heterogeneous and complex. Although significant progress has been made, the quest for more details and for the remaining unknowns continues. These efforts are well justified, since understanding the underlying regulations and abnormalities will facilitate a better diagnosis, prevention, and management of nongenetic forms of dysmagnesemia which are common and can be associated with devastating consequences [2].
Conflict of Interest Statement
The authors have no competing interests.
References
- 1.Chan KH, Chacko SA, Song Y, et al. Genetic variations in magnesium-related ion channels may affect diabetes risk among African American and Hispanic American women. J Nutr. 2015;145:418–424. doi: 10.3945/jn.114.203489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheungpasitporn W, Thongprayoon C, Qian Q. Dysmagnesemia in hospitalized patients: prevalence and prognostic importance. Mayo Clin Proc. 2015;90:1001–1010. doi: 10.1016/j.mayocp.2015.04.023. [DOI] [PubMed] [Google Scholar]
- 3.Piskacek M, Zotova L, Zsurka G, et al. Conditional knockdown of hMRS2 results in loss of mitochondrial Mg2+ uptake and cell death. J Cell Mol Med. 2009;13:693–700. doi: 10.1111/j.1582-4934.2008.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 5.Thorleifsson G, Holm H, Edvardsson V, et al. Sequence variants in the CLDN14 gene associate with kidney stones and bone mineral density. Nat Genet. 2009;41:926–930. doi: 10.1038/ng.404. [DOI] [PubMed] [Google Scholar]
- 6.Shen B, Sun L, Zheng H, et al. The association between single-nucleotide polymorphisms of TRPM7 gene and breast cancer in Han Population of Northeast China. Med Oncol. 2014;31:51. doi: 10.1007/s12032-014-0051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin JY, Chung SY, Lin MC, et al. Effects of magnesium sulfate on energy metabolites and glutamate in the cortex during focal cerebral ischemia and reperfusion in the gerbil monitored by a dual-probe microdialysis technique. Life Sci. 2002;71:803–811. doi: 10.1016/s0024-3205(02)01738-1. [DOI] [PubMed] [Google Scholar]
- 8.Smith DA, Connick JH, Stone TW. Effect of changing extracellular levels of magnesium on spontaneous activity and glutamate release in the mouse neocortical slice. Br J Pharmacol. 1989;97:475–482. doi: 10.1111/j.1476-5381.1989.tb11975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasina L, Zanotta D, Puricelli S, et al. Acute neurological symptoms secondary to hypomagnesemia induced by proton pump inhibitors: a case series. Eur J Clin Pharmacol. 2016;72:641–643. doi: 10.1007/s00228-016-2024-2. [DOI] [PubMed] [Google Scholar]
- 10.Chardain A, Mesnage V, Alamowitch S, et al. Posterior reversible encephalopathy syndrome (PRES) and hypomagnesemia: a frequent association? Rev Neurol (Paris) 2016;172:384–388. doi: 10.1016/j.neurol.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 11.Ebel H, Günther T. Magnesium metabolism: a review. J Clin Chem Clin Biochem. 1980;18:257–270. doi: 10.1515/cclm.1980.18.5.257. [DOI] [PubMed] [Google Scholar]
- 12.Le Grimellec C. Micropuncture study along the proximal convoluted tubule. Electrolyte reabsorption in first convolutions. Pflugers Arch. 1975;354:133–150. doi: 10.1007/BF00579944. [DOI] [PubMed] [Google Scholar]
- 13.Morita K, Furuse M, Fujimoto K, et al. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci USA. 1999;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon DB, Lu Y, Choate KA, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- 15.Konrad M, Schaller A, Seelow D, et al. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou J, Renigunta A, Konrad M, et al. Claudin-16 and claudin-19 interact and form a cation-selective tight junction complex. J Clin Invest. 2008;118:619–628. doi: 10.1172/JCI33970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong Y, Renigunta V, Himmerkus N, et al. Claudin-14 regulates renal Ca++ transport in response to CaSR signalling via a novel microRNA pathway. EMBO J. 2012;31:1999–2012. doi: 10.1038/emboj.2012.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou J, Paul DL, Goodenough DA. Paracellin-1 and the modulation of ion selectivity of tight junctions. J Cell Sci. 2005;118((pt 21)):5109–5118. doi: 10.1242/jcs.02631. [DOI] [PubMed] [Google Scholar]
- 19.Breiderhoff T, Himmerkus N, Stuiver M, et al. Deletion of claudin-10 (Cldn10) in the thick ascending limb impairs paracellular sodium permeability and leads to hypermagnesemia and nephrocalcinosis. Proc Natl Acad Sci USA. 2012;109:14241–14246. doi: 10.1073/pnas.1203834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Díaz-Soto G, Rocher A, García-Rodríguez C, et al. The calcium-sensing receptor in health and disease. Int Rev Cell Mol Biol. 2016;327:321–369. doi: 10.1016/bs.ircmb.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox ER, Burton QL, Naz S, et al. Mutations in the gene encoding tight junction claudin-14 cause autosomal recessive deafness DFNB29. Cell. 2001;104:165–172. doi: 10.1016/s0092-8674(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 22.Kitajiri SI, Furuse M, Morita K, et al. Expression patterns of claudins, tight junction adhesion molecules, in the inner ear. Hear Res. 2004;187:25–34. doi: 10.1016/s0378-5955(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 23.Ho C, Conner DA, Pollak MR, et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat Genet. 1995;11:389–394. doi: 10.1038/ng1295-389. [DOI] [PubMed] [Google Scholar]
- 24.Simon DB, Karet FE, Hamdan JM, et al. Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 25.Castrop H, Schiessl IM. Physiology and pathophysiology of the renal Na-K-2Cl cotransporter (NKCC2) Am J Physiol Renal Physiol. 2014;307:F991–F1002. doi: 10.1152/ajprenal.00432.2014. [DOI] [PubMed] [Google Scholar]
- 26.Finer G, Shalev H, Birk OS, et al. Transient neonatal hyperkalemia in the antenatal (ROMK defective) Bartter syndrome. J Pediatr. 2003;142:318–323. doi: 10.1067/mpd.2003.100. [DOI] [PubMed] [Google Scholar]
- 27.Simon DB, Bindra RS, Mansfield TA, et al. Mutations in the chloride channel gene, CLCNKB, cause Bartter's syndrome type III. Nat Genet. 1997;17:171–178. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 28.Birkenhäger R, Otto E, Schürmann MJ, et al. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat Genet. 2001;29:310–314. doi: 10.1038/ng752. [DOI] [PubMed] [Google Scholar]
- 29.Simon DB, Karet FE, Rodriguez-Soriano J, et al. Genetic heterogeneity of Bartter's syndrome revealed by mutations in the K+ channel, ROMK. Nat Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 30.Walder RY, Shalev H, Brennan TM, et al. Familial hypomagnesemia maps to chromosome 9q, not to the X chromosome: genetic linkage mapping and analysis of a balanced translocation breakpoint. Hum Mol Genet. 1997;6:1491–1497. doi: 10.1093/hmg/6.9.1491. [DOI] [PubMed] [Google Scholar]
- 31.Weber S, Schneider L, Peters M, et al. Novel paracellin-1 mutations in 25 families with familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2001;12:1872–1881. doi: 10.1681/ASN.V1291872. [DOI] [PubMed] [Google Scholar]
- 32.Bardet C, Courson F, Wu Y, et al. Claudin-16 deficiency impairs tight junction function in ameloblasts, leading to abnormal enamel formation. J Bone Miner Res. 2016;31:498–513. doi: 10.1002/jbmr.2726. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguti PM, Neves FA, Hotton D, et al. Amelogenesis imperfecta in familial hypomagnesaemia and hypercalciuria with nephrocalcinosis caused by CLDN19 gene mutations. J Med Genet. 2017;54:26–37. doi: 10.1136/jmedgenet-2016-103956. [DOI] [PubMed] [Google Scholar]
- 34.Konrad M, Hou J, Weber S, et al. CLDN16 genotype predicts renal decline in familial hypomagnesemia with hypercalciuria and nephrocalcinosis. J Am Soc Nephrol. 2008;19:171–181. doi: 10.1681/ASN.2007060709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Müller D, Kausalya PJ, Claverie-Martin F, et al. A novel claudin 16 mutation associated with childhood hypercalciuria abolishes binding to ZO-1 and results in lysosomal mistargeting. Am J Hum Genet. 2003;73:1293–1301. doi: 10.1086/380418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Godron A, Harambat J, Boccio V, et al. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis: phenotype-genotype correlation and outcome in 32 patients with CLDN16 or CLDN19 mutations. Clin J Am Soc Nephrol. 2012;7:801–809. doi: 10.2215/CJN.12841211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blair JW, Carachi R. Neonatal primary hyperparathyroidism – a case report and review of the literature. Eur J Pediatr Surg. 1991;1:110–114. doi: 10.1055/s-2008-1042470. [DOI] [PubMed] [Google Scholar]
- 38.Glaudo M, Letz S, Quinkler M, et al. Heterozygous inactivating CaSR mutations causing neonatal hyperparathyroidism: function, inheritance and phenotype. Eur J Endocrinol. 2016;175:421–431. doi: 10.1530/EJE-16-0223. [DOI] [PubMed] [Google Scholar]
- 39.Watanabe S, Fukumoto S, Chang H, et al. Association between activating mutations of calcium-sensing receptor and Bartter's syndrome. Lancet. 2002;360:692–694. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 40.Hannan FM, Babinsky VN, Thakker RV. Disorders of the calcium-sensing receptor and partner proteins: insights into the molecular basis of calcium homeostasis. J Mol Endocrinol. 2016;57:R127–R142. doi: 10.1530/JME-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walder RY, Landau D, Meyer P, et al. Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nat Genet. 2002;31:171–174. doi: 10.1038/ng901. [DOI] [PubMed] [Google Scholar]
- 42.Schlingmann KP, Weber S, Peters M, et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat Genet. 2002;31:166–170. doi: 10.1038/ng889. [DOI] [PubMed] [Google Scholar]
- 43.Voets T, Nilius B, Hoefs S, et al. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 44.Chubanov V, Waldegger S, Mederos y Schnitzler M, et al. Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci USA. 2004;101:2894–2899. doi: 10.1073/pnas.0305252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz C, Dorovkov MV, Zhao X, et al. The channel kinases TRPM6 and TRPM7 are functionally nonredundant. J Biol Chem. 2005;280:37763–37771. doi: 10.1074/jbc.M509175200. [DOI] [PubMed] [Google Scholar]
- 46.Li M, Jiang J, Yue L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J Gen Physiol. 2006;127:525–537. doi: 10.1085/jgp.200609502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xie J, Sun B, Du J, et al. Phosphatidylinositol 4,5-bisphosphate (PIP2) controls magnesium gatekeeper TRPM6 activity. Sci Rep. 2011;1:146. doi: 10.1038/srep00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lainez S, Schlingmann KP, van der Wijst J, et al. New TRPM6 missense mutations linked to hypomagnesemia with secondary hypocalcemia. Eur J Hum Genet. 2014;22:497–504. doi: 10.1038/ejhg.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blanchard MG, Kittikulsuth W, Nair AV, et al. Regulation of Mg2+ reabsorption and transient receptor potential melastatin type 6 activity by cAMP signaling. J Am Soc Nephrol. 2016;27:804–813. doi: 10.1681/ASN.2014121228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Groenestege WM, Thébault S, van der Wijst J, et al. Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest. 2007;117:2260–2267. doi: 10.1172/JCI31680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poulsen SS, Nexø E, Olsen PS, et al. Immunohistochemical localization of epidermal growth factor in rat and man. Histochemistry. 1986;85:389–394. doi: 10.1007/BF00982668. [DOI] [PubMed] [Google Scholar]
- 52.Breyer MD, Redha R, Breyer JA. Segmental distribution of epidermal growth factor binding sites in rabbit nephron. Am J Physiol. 1990;259((pt 2)):F553–F558. doi: 10.1152/ajprenal.1990.259.4.F553. [DOI] [PubMed] [Google Scholar]
- 53.Thebault S, Alexander RT, Tiel Groenestege WM, et al. EGF increases TRPM6 activity and surface expression. J Am Soc Nephrol. 2009;20:78–85. doi: 10.1681/ASN.2008030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mishra NK, Peleg Y, Cirri E, et al. FXYD proteins stabilize Na,K-ATPase: amplification of specific phosphatidylserine-protein interactions. J Biol Chem. 2011;286:9699–9712. doi: 10.1074/jbc.M110.184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arystarkhova E, Sweadner KJ. Splice variants of the gamma subunit (FXYD2) and their significance in regulation of the Na,K-ATPase in kidney. J Bioenerg Biomembr. 2005;37:381–386. doi: 10.1007/s10863-005-9475-y. [DOI] [PubMed] [Google Scholar]
- 56.Meij IC, Koenderink JB, van Bokhoven H, et al. Dominant isolated renal magnesium loss is caused by misrouting of the Na+,K+-ATPase γ-subunit. Nat Genet. 2000;26:265–266. doi: 10.1038/81543. [DOI] [PubMed] [Google Scholar]
- 57.Ferrè S, Veenstra GJ, Bouwmeester R, et al. HNF-1B specifically regulates the transcription of the γa-subunit of the Na+/K+-ATPase. Biochem Biophys Res Commun. 2011;404:284–290. doi: 10.1016/j.bbrc.2010.11.108. [DOI] [PubMed] [Google Scholar]
- 58.Ferrè S, de Baaij JH, Ferreira P, et al. Mutations in PCBD1 cause hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol. 2014;25:574–586. doi: 10.1681/ASN.2013040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Coffinier C, Barra J, Babinet C, et al. Expression of the vHNF1/HNF1β homeoprotein gene during mouse organogenesis. Mech Dev. 1999;89:211–213. doi: 10.1016/s0925-4773(99)00221-x. [DOI] [PubMed] [Google Scholar]
- 60.Kolatsi-Joannou M, Bingham C, Ellard S, et al. Hepatocyte nuclear factor-1β: a new kindred with renal cysts and diabetes and gene expression in normal human development. J Am Soc Nephrol. 2001;12:2175–2180. doi: 10.1681/ASN.V12102175. [DOI] [PubMed] [Google Scholar]
- 61.Hiesberger T, Bai Y, Shao X, et al. Mutation of hepatocyte nuclear factor-1β inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest. 2004;113:814–825. doi: 10.1172/JCI20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clissold RL, Hamilton AJ, Hattersley AT, et al. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol. 2015;11:102–112. doi: 10.1038/nrneph.2014.232. [DOI] [PubMed] [Google Scholar]
- 63.Johnen G, Kaufman S. Studies on the enzymatic and transcriptional activity of the dimerization cofactor for hepatocyte nuclear factor 1. Proc Natl Acad Sci USA. 1997;94:13469–13474. doi: 10.1073/pnas.94.25.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sourdive DJ, Transy C, Garbay S, et al. The bifunctional DCOH protein binds to HNF1 independently of its 4-α-carbinolamine dehydratase activity. Nucleic Acids Res. 1997;25:1476–1484. doi: 10.1093/nar/25.8.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Citron BA, Davis MD, Milstien S, et al. Identity of 4a-carbinolamine dehydratase, a component of the phenylalanine hydroxylation system, and DCoH, a transregulator of homeodomain proteins. Proc Natl Acad Sci USA. 1992;89:11891–11894. doi: 10.1073/pnas.89.24.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Werner ER, Blau N, Thöny B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J. 2011;438:397–414. doi: 10.1042/BJ20110293. [DOI] [PubMed] [Google Scholar]
- 67.de Baaij JH, Groot Koerkamp MJ, Lavrijsen M, et al. Elucidation of the distal convoluted tubule transcriptome identifies new candidate genes involved in renal Mg2+ handling. Am J Physiol Renal Physiol. 2013;305:F1563–F1573. doi: 10.1152/ajprenal.00322.2013. [DOI] [PubMed] [Google Scholar]
- 68.Reichold M, Zdebik AA, Lieberer E, et al. KCNJ10 gene mutations causing EAST syndrome (epilepsy, ataxia, sensorineural deafness, and tubulopathy) disrupt channel function. Proc Natl Acad Sci USA. 2010;107:14490–14495. doi: 10.1073/pnas.1003072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bockenhauer D, Feather S, Stanescu HC, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cha SK, Huang C, Ding Y, et al. Calcium-sensing receptor decreases cell surface expression of the inwardly rectifying K+ channel Kir4.1. J Biol Chem. 2011;286:1828–1835. doi: 10.1074/jbc.M110.160390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang C, Sindic A, Hill CE, et al. Interaction of the Ca2+-sensing receptor with the inwardly rectifying potassium channels Kir4.1 and Kir4.2 results in inhibition of channel function. Am J Physiol Renal Physiol. 2007;292:F1073–F1081. doi: 10.1152/ajprenal.00269.2006. [DOI] [PubMed] [Google Scholar]
- 72.Sala-Rabanal M, Kucheryavykh LY, Skatchkov SN, et al. Molecular mechanisms of EAST/SeSAME syndrome mutations in Kir4.1 (KCNJ10) J Biol Chem. 2010;285:36040–36048. doi: 10.1074/jbc.M110.163170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Glaudemans B, van der Wijst J, Scola RH, et al. A missense mutation in the Kv1.1 voltage-gated potassium channel-encoding gene KCNA1 is linked to human autosomal dominant hypomagnesemia. J Clin Invest. 2009;119:936–942. doi: 10.1172/JCI36948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.San-Cristobal P, Lainez S, Dimke H, et al. Ankyrin-3 is a novel binding partner of the voltage-gated potassium channel Kv1.1 implicated in renal magnesium handling. Kidney Int. 2014;85:94–102. doi: 10.1038/ki.2013.280. [DOI] [PubMed] [Google Scholar]
- 75.Bennett V. Ankyrins. Adaptors between diverse plasma membrane proteins and the cytoplasm. J Biol Chem. 1992;267:8703–8706. [PubMed] [Google Scholar]
- 76.Quamme GA. Molecular identification of ancient and modern mammalian magnesium transporters. Am J Physiol Cell Physiol. 2010;298:C407–C429. doi: 10.1152/ajpcell.00124.2009. [DOI] [PubMed] [Google Scholar]
- 77.Kolisek M, Nestler A, Vormann J, et al. Human gene SLC41A1 encodes for the Na+/Mg2+ exchanger. Am J Physiol Cell Physiol. 2012;302:C318–C326. doi: 10.1152/ajpcell.00289.2011. [DOI] [PubMed] [Google Scholar]
- 78.Stuiver M, Lainez S, Will C, et al. CNNM2, encoding a basolateral protein required for renal Mg2+ handling, is mutated in dominant hypomagnesemia. Am J Hum Genet. 2011;88:333–343. doi: 10.1016/j.ajhg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hattori M, Iwase N, Furuya N, et al. Mg2+-dependent gating of bacterial MgtE channel underlies Mg2+ homeostasis. EMBO J. 2009;28:3602–3612. doi: 10.1038/emboj.2009.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sahni J, Scharenberg AM. The SLC41 family of MgtE-like magnesium transporters. Mol Aspects Med. 2013;34:620–628. doi: 10.1016/j.mam.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.de Baaij JH, Stuiver M, Meij IC, et al. Membrane topology and intracellular processing of cyclin M2 (CNNM2) J Biol Chem. 2012;287:13644–13655. doi: 10.1074/jbc.M112.342204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Corral-Rodríguez MA, Stuiver M, Abascal-Palacios G, et al. Nucleotide binding triggers a conformational change of the CBS module of the magnesium transporter CNNM2 from a twisted towards a flat structure. Biochem J. 2014;464:23–34. doi: 10.1042/BJ20140409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salminen A, Anashkin VA, Lahti M, et al. Cystathionine β-synthase (CBS) domains confer multiple forms of Mg2+-dependent cooperativity to family II pyrophosphatases. J Biol Chem. 2014;289:22865–22876. doi: 10.1074/jbc.M114.589473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Seyberth HW. An improved terminology and classification of Bartter-like syndromes. Nat Clin Pract Nephrol. 2008;4:560–567. doi: 10.1038/ncpneph0912. [DOI] [PubMed] [Google Scholar]
- 85.Alfandary H, Landau D. Future considerations based on the information from Barrter's [sic!] and Gitelman's syndromes. Curr Opin Nephrol Hypertens. 2017;26:9–13. doi: 10.1097/MNH.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 86.Simon DB, Nelson-Williams C, Bia MJ, et al. Gitelman's variant of Bartter's syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 87.Balavoine AS, Bataille P, Vanhille P, et al. Phenotype-genotype correlation and follow-up in adult patients with hypokalaemia of renal origin suggesting Gitelman syndrome. Eur J Endocrinol. 2011;165:665–673. doi: 10.1530/EJE-11-0224. [DOI] [PubMed] [Google Scholar]
- 88.Berry MR, Robinson C, Karet Frankl FE. Unexpected clinical sequelae of Gitelman syndrome: hypertension in adulthood is common and females have higher potassium requirements. Nephrol Dial Transplant. 2013;28:1533–1542. doi: 10.1093/ndt/gfs600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Glaudemans B, Yntema HG, San-Cristobal P, et al. Novel NCC mutants and functional analysis in a new cohort of patients with Gitelman syndrome. Eur J Hum Genet. 2012;20:263–270. doi: 10.1038/ejhg.2011.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Moes AD, van der Lubbe N, Zietse R, et al. The sodium chloride cotransporter SLC12A3: new roles in sodium, potassium, and blood pressure regulation. Pflugers Arch. 2014;466:107–118. doi: 10.1007/s00424-013-1407-9. [DOI] [PubMed] [Google Scholar]
- 91.Geven WB, Monnens LA, Willems JL, et al. Isolated autosomal recessive renal magnesium loss in two sisters. Clin Genet. 1987;32:398–402. doi: 10.1111/j.1399-0004.1987.tb03157.x. [DOI] [PubMed] [Google Scholar]
- 92.Schrag D, Chung KY, Flombaum C, et al. Cetuximab therapy and symptomatic hypomagnesemia. J Natl Cancer Inst. 2005;97:1221–1224. doi: 10.1093/jnci/dji242. [DOI] [PubMed] [Google Scholar]
- 93.Tejpar S, Piessevaux H, Claes K, et al. Magnesium wasting associated with epidermal-growth-factor receptor-targeting antibodies in colorectal cancer: a prospective study. Lancet Oncol. 2007;8:387–394. doi: 10.1016/S1470-2045(07)70108-0. [DOI] [PubMed] [Google Scholar]
- 94.Scholl UI, Choi M, Liu T, et al. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci USA. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Imbrici P, D'Adamo MC, Grottesi A, et al. Episodic ataxia type 1 mutations affect fast inactivation of K+ channels by a reduction in either subunit surface expression or affinity for inactivation domain. Am J Physiol Cell Physiol. 2011;300:C1314–C1322. doi: 10.1152/ajpcell.00456.2010. [DOI] [PubMed] [Google Scholar]
- 96.Meij IC, Saar K, van den Heuvel LP, et al. Hereditary isolated renal magnesium loss maps to chromosome 11q23. Am J Hum Genet. 1999;64:180–188. doi: 10.1086/302199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Meij IC, Koenderink JB, De Jong JC, et al. Dominant isolated renal magnesium loss is caused by misrouting of the Na+,K+-ATPase γ-subunit. Ann NY Acad Sci. 2003;986:437–443. doi: 10.1111/j.1749-6632.2003.tb07226.x. [DOI] [PubMed] [Google Scholar]
- 98.de Baaij JH, Dorresteijn EM, Hennekam EA, et al. Recurrent FXYD2 p.Gly41Arg mutation in patients with isolated dominant hypomagnesaemia. Nephrol Dial Transplant. 2015;30:952–957. doi: 10.1093/ndt/gfv014. [DOI] [PubMed] [Google Scholar]
- 99.Adalat S, Woolf AS, Johnstone KA, et al. HNF1B mutations associate with hypomagnesemia and renal magnesium wasting. J Am Soc Nephrol. 2009;20:1123–1131. doi: 10.1681/ASN.2008060633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Faguer S, Decramer S, Chassaing N, et al. Diagnosis, management, and prognosis of HNF1B nephropathy in adulthood. Kidney Int. 2011;80:768–776. doi: 10.1038/ki.2011.225. [DOI] [PubMed] [Google Scholar]
- 101.Arjona FJ, de Baaij JH, Schlingmann KP, et al. CNNM2 mutations cause impaired brain development and seizures in patients with hypomagnesemia. PLoS Genet. 2014;10 doi: 10.1371/journal.pgen.1004267. e1004267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Suzuki H, Nishizawa T, Tani K, et al. Crystal structure of a claudin provides insight into the architecture of tight junctions. Science. 2014;344:304–307. doi: 10.1126/science.1248571. [DOI] [PubMed] [Google Scholar]
- 103.Eshaghi S, Niegowski D, Kohl A, et al. Crystal structure of a divalent metal ion transporter CorA at 2.9 angstrom resolution. Science. 2006;313:354–357. doi: 10.1126/science.1127121. [DOI] [PubMed] [Google Scholar]
- 104.Lunin VV, Dobrovetsky E, Khutoreskaya G, et al. Crystal structure of the CorA Mg2+ transporter. Nature. 2006;440:833–837. doi: 10.1038/nature04642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Payandeh J, Pai EF. A structural basis for Mg2+ homeostasis and the CorA translocation cycle. EMBO J. 2006;25:3762–3773. doi: 10.1038/sj.emboj.7601269. [DOI] [PMC free article] [PubMed] [Google Scholar]