Abstract
Background
Normal anion gap metabolic acidosis is a common but often misdiagnosed clinical condition associated with diarrhea and renal tubular acidosis (RTA). Early identification of RTA remains challenging for inexperienced physicians, and diagnosis and treatment are often delayed.
Summary
The presence of RTA should be considered in any patient with a high chloride level when the CL−/Na+ ratio is above 0.79, if the patient does not have diarrhea. In patients with significant hyperkalemia one should evaluate for RTA type 4, especially in diabetic patients, with a relatively conserved renal function. A still growing list of medications can produce RTA.
Key Messages
This review highlights practical aspects concerning normal anion gap metabolic acidosis.
Keywords: Acidosis, Anion gap, Hyperchloremia, Urine pH, Osmolal gap, Renal-tubular acidosis
Introduction
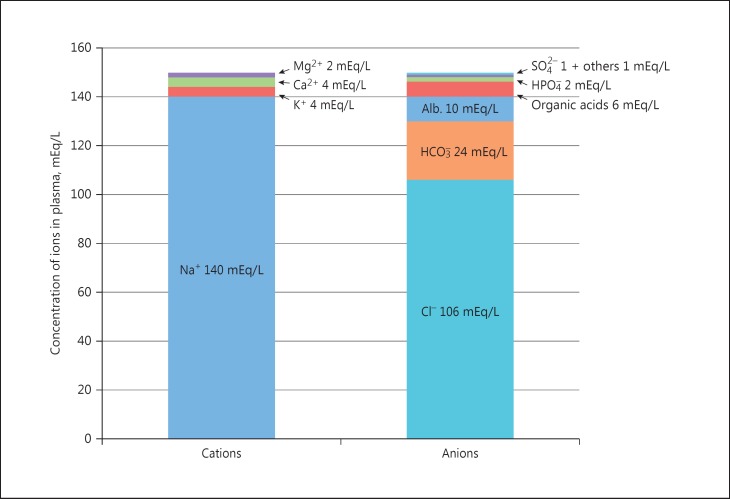
For more than 40 years, clinicians have used the anion gap (AG) as a major tool to evaluate acid-base disorders [1, 2, 3, 4, 5]. An increased value of the AG suggests a possible organic acidosis due to endogenous acids or exposure to exogenous acids [1]. Although the concept of the AG was described in 1936 by James Gamble [6], it did not gain widespread recognition by physicians until the 1970s after the introduction of autoanalyzers and the rapid availability of multiple analytes [3]. According to Gamble [6], electrical neutrality in solution demands that the sum of the cations is equal to the sum of the anions, also represented in a gamblegram (Fig. 1). Sodium, chloride, bicarbonate, and albumin are quantitatively the major ions in the extracellular fluid compartment and are therefore used to calculate the AG. A true “ion gap,” however, does not exist in vivo which makes the AG a fundamental tool to evaluate acid-base disorders [1]. From Figure 1 it is obvious that the elements used in the AG equation are quantitatively the most prominent electrolytes in plasma:
Fig. 1.
Gamblegram: balance between anions and cations in plasma.
Reference Ranges of the AG
Many reference ranges of the AG have been reported from about 5 to 14 mEq/L, with autoanalyzers using an ion-selective electrode. However, the AG value is dependent on the type of instrument used to measure its components. In the study reported by Roberts et al. [3], the AG value was 5–10 mEq/L with the Synchron CX3 analyzer (Beckman Coulter), 9–14 mEq/L for the Hitachi 717 analyzer (Boehringer Mannheim, Mannheim, Germany), and 8–13 mEq/L for the Vitros 950 analyzer (Johnson & Johnson, New Brunswick, NJ, USA). In another study in 4,525 healthy adults, AG varied from 8.5 to 15.2 mEq/L, with a mean value of 11.6 mEq/L [5]. Therefore, one should know the reference range of the analyzer used and, if known, the patient's baseline AG, too. One should also realize that reference ranges are usually based upon venous bicarbonate values that may be about 1–2 mEq/L higher than arterial values [7]. Changes in sodium concentration are dependent on the water content of plasma and go hand in hand with the chloride concentration, and, therefore, AG evaluation remains a useful tool in dysnatremia.
Serum AG is affected by the concentrations of all anions and cations which are not included in its calculations: i.e., albumin, globulin, potassium, calcium, magnesium, and organic and inorganic acids. Because of the narrow extracellular concentration, most ions are omitted from the calculation. Nevertheless, one should correct the AG for hypoalbuminemia. To appreciate the relevance of this correction, one can consider a healthy individual with the following serum values: [Na+] = 140 mEq/L, [Cl−] = 106 mEq/L, [HCO3−] = 24 mEq/L. The AG = 10 mEq/L, representing primarily albumin. The correction factor for albumin is 2.3–2.5 × [albumin], in g/dL [1]. Therefore, each g/dL albumin decline will decrease the AG with about 2.5 mEq/L. To appreciate these facts, the AG formule should be: [Na+] − [Cl−] − [HCO3−] − 2.5 [albumin, in g/dL]. This equation is about zero in health, to stress the balance of ions, and also shows the relevance of albumin as a negative ion.
Normal AG Metabolic Acidosis
Nomenclature
In the acid-base literature, a nomenclature has evolved that confused many experienced clinicians as well as trainees and students [8]. Concerning normal AG acidosis, one can find the terms “normal AG,” “non-AG,” and “hyperchloremic” metabolic acidosis, all three implicating the same disorder. However, the verb “non-AG” implicates an AG of zero, which is incorrect because one, in fact, refers to an AG below 10–12 mEq/L. Hyperchloremia will be present in a patient with shock and dehydration with hypernatriemia, however, with a high AG. Sodium and chloride will increase because of dehydration and little water intake. Hyperchloremic metabolic acidosis is, therefore, not synonymous with a normal AG. Therefore, the preferred terminology is normal AG metabolic acidosis.
Another confusing subject is the so-called “dilution acidosis” suggesting that a fall in serum bicarbonate concentration is solely due to expansion of the extracellular fluid volume with large volumes of intravenous fluids like normal saline. However, dilution acidosis is a misnomer because the reason for the decrease in bicarbonate in chloride-rich fluids is the necessity of electroneutrality and not merely “dilution” of bicarbonate. Therefore, hyperchloremic metabolic acidosis can also be the result of chlorine inhalation [9], because an increased Cl−/Na+ ratio above 0.79 will lead to a normal AG metabolic acidosis [10, 11].
The classification of renal tubular acidosis (RTA) may also be confusing as the defect in the proximal and distal tubules is named paradoxically type 2 and type 1, respectively (Table 1).
Table 1.
Characteristics of normal anion gap (AG) metabolic acidosis diseases
| Serum bicarbonate, mEq/L | Plasma K+ | Ca2+ excretion | Urine AG mEq/L | Urine osmolol gap, mosm/kg in metabolic acidosis | Urinary NH+4, mEq/day | Minimal urine pH | Ability to acidify urine in response to acidemia | Urine-blood pCO2, mm Hg | Comment | |
|---|---|---|---|---|---|---|---|---|---|---|
| Health | Normal | Normal | Normal | +20 to +90 | 10 – 100 | 30–40 | 4.5 – 6 | Yes | >30 | |
| Severe diarrhea | <24 | Low | Normal | –20 to −50 | >200 | High | >5.5 | Yes | ||
| Toluene/hippurate | <24 | Low | Normal | Positive | >200 | High | Yes | |||
| Defective CA II activity/proximal RTA (type 2) | 12 – 20 | Low/normal | Normal | Negative −20 to −50 | >150 | Normal | <5.5 | Yes | Urine pH >6.5 during early phase with bicarbonaturia | |
| Fanconi syndrome/proximal RTA (type 2) | 12 – 18 | Low | ↑ | Negative −20 to −50 | >150 | Normal | <5.5 | Yes | Hypophosphatemia/phosphaturia, hypouricemia/hyperuricosuria renal glucosuria (glucosuria with a normal serum glucose concentration), aminoaciduria | |
| Hypokalemic distal RTA (type 1) | 10 – 20 | Low | ↑ | Positive | <150 (usually <50 – 100) | Low | >5.5 (often >6.5) | No | <30 | |
| Back diffusion | 8 – 15 | Low | ? | Positive | ? | Low | No | |||
| Hyperkalemic distal RTA (voltage-dependent RTA) | 8 – 15 | High | Normal or ↑ | Positive | <150(usually <50 – 100) | Low | >5.5(often >6.5) | No | ||
| RTA type 3 | Low | Low | ↑ | Low | >5.5 | No | ||||
| RTA type 4 | 16 – 22 | High | Normal | Positive | <150(usually <50 – 100) | Low | <5.5 | Yes | Often increased creatinine | |
CA, carbonic anhydrase; RTA, renal tubular acidosis; urine-blood pCO2, urine-blood pCO2 after bicarbonate loading.
Case Definition and Pathophysiology
In case of a normal AG metabolic acidosis, bicarbonate loss is replaced by chloride and the AG equation ([Na+] − [Cl−] − [HCO3−]) will, therefore, remain the same, or “normal.” In other words, if bicarbonate drops 10 mEq/L and chloride raises 10 mEq/L, the sum of the anions remains the same. The real question in normal AG metabolic acidosis is consequently: in what circumstances will there be an exchange of bicarbonate and chloride, or – in other words – when will the decrease or loss of bicarbonate be replaced by chloride – to maintain electroneutrality. This exchange of bicarbonate and chloride occurs in diseases of the gastrointestinal tract and the kidneys.
Gastrointestinal Cause of Normal AG Metabolic Acidosis
Severe diarrhea is the most common cause of gastrointestinal loss of bicarbonate. Because the concentration of bicarbonate in diarrheal fluid is generally greater than that in plasma, large amounts can be lost in severe diarrhea or ileostomy [12]. Ureteral diversion can also lead to a normal AG metabolic acidosis. Ureteral implantation into the sigmoid colon or the replacement of the urinary bladder using a short loop of ileum will lead to the exposure of urine to the gastrointestinal mucosa, which will cause gastrointestinal bicarbonate loss and retention of chloride [13]. Cholestyramine is a nonabsorbable anion exchange resin used to bind bile acids in the gut. It has been used in the treatment of hypercholesterolemia, pruritus associated with elevated levels of bile acids, and diarrhea due to bile acid malabsorption in the setting of ileal disease or resection [14]. It swaps chloride anions for bile acids in the lumen of the small intestine, resulting in bile acid complexes that are fecal excreted instead of being reabsorbed in the ileum. This exchange causes gastrointestinal secretion of bicarbonate and absorption of chloride. If the kidneys cannot compensate by increasing chloride excretion and bicarbonate retention because of impaired urinary acidification such as renal insufficiency and aldosterone antagonism a normal AG metabolic acidosis develops [14].
Renal Tubular Acidosis
There are three major forms of RTA: proximal RTA (type 2), distal RTA (type 1), and hyperkalemic RTA (type 4). Hyperkalemic RTAs include hypoaldosteronism (type 4) and a voltage-dependent RTA, which is caused by defects in distal sodium reabsorption and is perhaps a subtype of distal RTA. RTA type 3 is a mixed RTA form of type 1 and type 2 [15].
Tubular defects are inherited or acquired. This review will focus on the acquired forms of RTA.
Proximal Tubule Dysfunction
To recognize RTA, one should know how tubular dysfunction will lead to metabolic acidosis. The proximal tubule absorbs approximately 85–90% of the filtered bicarbonate and 60% of the filtered sodium along with water, phosphate, amino acids, and glucose. The loop of Henle reabsorbs around 10% of the filtered bicarbonate and the remaining 5–10% is reabsorbed in the collecting tubules. The process of bicarbonate reabsorption in the distal tubule involves an HCO3−/Cl− exchanger [16].
Isolated Carbonic Anhydrase Defect
An isolated acquired proximal tubular acid-base defect (RTA type 2) can be caused by failing of the enzyme carbonic anhydrase (CA) IV by enzyme blockers like acetazolamide and topiramate. CAs are a family of zinc metalloenzymes found in all organisms, catalyzing the reversible reaction of CO2 hydration to bicarbonate and a proton [17]. An anhydrase is defined as an enzyme that catalyzes the removal of a water molecule from a compound, and so it is this “reverse” reaction that gives CA its name, because it removes a water molecule from carbonic acid. H2CO3 is formed in the body by the reaction of CO2 with H2O:
H2O + CO2 ↔ H2CO3 ↔ H+ + HCO3−.
This reaction is slow, and small amounts of H2CO3 are formed unless the enzyme CA is present. This enzyme is especially abundant in the walls of the lung alveoli, where CO2 is released, and also in the epithelial cells of the renal tubules, where CO2 reacts with H2O to form H2CO3. In many organisms, CA enzymes are involved in crucial physiological processes connected with respiration and transport of CO2/bicarbonate, pH, and CO2 homeostasis, electrolyte secretion in a variety of tissues of organs, biosynthetic reactions (e.g., gluconeogenesis, lipogenesis, and ureagenesis), bone resorption, calcification, tumorigenicity, and many other physiologic or pathologic processes [16, 17].
Four different CAs are expressed in the human nephron and two isoforms of CA deficiency have been described (CA II and CA IV) [18]. CA II is cytoplasmic and found in the proximal and distal tubule. CA IV is located in the apical membrane of the proximal tubule. In the proximal tubular lumen, filtered bicarbonate reacts with hydrogen ions to form carbonic acid (H2CO3) that splits into CO2 and H2O by the action of luminal enzyme CA IV. CO2 diffuses back into the cells where it reacts with H2O in the presence of cytoplasmic CA II and generates HCO3− and H+. HCO3− is transported into the blood, in exchange of chloride, while H+ is secreted into the lumen. Bicarbonate absorption by this mechanism is saturable, so whenever the normal level of 24 mmol/L is reached, loss of bicarbonate in the urine develops. Under normal conditions, however, there is virtually no bicarbonate in the urine [17, 19]. Hypokalemia is very common in proximal RTA because of excess urinary loss of K+. This is due to the increased delivery of Na+ and HCO3− to the distal nephron, where Na+/K+ exchange occurs. Also, volume depletion induces aldosterone secretion that will contribute to K+ wastage. One should realize that proximal RTA is a self-limiting disorder and bicarbonate wasting can be transient depending on the intake of bicarbonate-forming substances. Because the bicarbonate reabsorptive capacity is reduced from the normal level of approximately 24 mEq/L, a new steady state will be present as all the filtered bicarbonate can now be reabsorbed, and the patient is able to excrete the daily acid load. However, the bicarbonate concentration will remain low as the urinary bicarbonate wasting continues until the plasma HCO3− level reaches a new low level of about 12–18 mEq/L [20, 21]. The plasma bicarbonate concentration usually does not fall below 12 mEq/L in patients with proximal RTA as distal renal tubules have substantial bicarbonate reabsorptive capacity [21].
Fanconi Syndrome
A generalized proximal tubular dysfunction may cause the so-called Fanconi syndrome characterized by a complex transport defect of the proximal tubule that results in decreased reabsorption of glucose, amino acids, bicarbonate, uric acid, and phosphate. Therefore, bicarbonaturia, tubular proteinuria, aminoaciduria, phosphaturia, glucosuria, and uric acid and sodium wasting may be present [22]. Fanconi syndrome can occur as an inborn error or an acquired disorder, including multiple myeloma and specific medications, including tenofovir and ifosfamide (Table 2).
Table 2.
Causes of renal tubular acidosis (RTA)
| Causes of proximal RTA |
Causes of distal RTA |
Causes of RTA type 4 | ||
|---|---|---|---|---|
| isolated defect | generalized defect | with hypokalemia | with hyperkalemia | |
|
Autosomal dominant Proximal RTA from unknown gene mutation |
Primary (genetic) inborn errors of metabolism (Cystinosis, Wilson disease, galactosemia, hereditary fructose intolerance, methylmalonic acidemia, glycogen storage diseases) |
Calcium-induced tubular damage Idiopathic hypercalciuria Primary hyperparathyroidism Hypervitaminosis D Medullary sponge kidney |
||
|
Autosomal recessive Sodium bicarbonate symporter (NBC1) protein mutation in the SLC4A4 gene |
Dysproteinemic states (Myeloma, monoclonal gammopathy) |
Autoimmune diseases Sjögren syndrome Rheumatoid arthritis SLE Polyarteritis nodosa Thyroiditis Primary biliary cirrhosis Chronic active hepatitis Cryoglobulinemia |
Decreased effective intravascular volume of any cause Sickle cell disease Urinary tract obstruction SLE Renal transplant rejection Amyloidosis |
Aldosterone deficiency Addison disease 21-Hydroxylase deficiency Hyporeninemia Diabetic nephropathy Tubulointerstitial disease HIV IgM monoclonal gammopathy |
| Inherited CA II deficiency caused by mutations in the CA2 gene – associated with mental retardation, cerebral calcifications and osteopetrosis (Sly syndrome) | Honeybee stings |
Idiopathic causes Marfan syndrome Wilson disease Ehlers-Danlos syndrome |
Aldosterone resistance Obstructive uropathy Sickle cell nephropathy Amyloidosis Diabetic nephropathy Lupus nephritis Pseudohypoaldosteronism |
|
| Secondary hyperparathyroidism with chronic hypocalcemia Vitamin D deficiency |
||||
| Tubulointerstitial diseases (Sjögren syndrome, medullary cystic disease, renal transplantation) | ||||
| Nephrotic syndrome | ||||
| Amyloidosis | ||||
| Paroxysmal nocturnal hemoglobinuria | ||||
| Toxins (lead, mercury, copper, cadmium, glue sniffing) | ||||
| Drugs as causes of proximal RTA |
Drugs as causes of distal RTA |
Drugs as causes of RTA type 4 | ||
|---|---|---|---|---|
| isolated defect | generalized defect | with hypokalemia | ||
| CA inhibitors | Ifosfamide, aminoglycosides, expired tetracycline, streptozocin, azacitidine (antimetabolites), mercaptopurine, valproic acid, ranitidine, lead, cadmium, mercury, antiretroviral drugs, propylene glycol-containing drugs | Amphotericin B, lithium carbonate, methicillin (meticillin), foscarnet, ifosfamide, toluene |
Reduced NH4+ production (hypoaldosteronism) K+-sparing diuretics (spironolactone, eplerenone, amiloride, triamterene), cotrimoxazole, ACEI, angiotensin II receptor type 1 antagonists, renin inhibitors, NSAIDs, ciclosporin, tacrolimus, heparin |
|
ACEI, angiotensin-converting enzyme inhibitors; CA, carbonic anhydrase; SLE, systemic lupus erythematosus.
Distal Tubular Dysfunction
In the distal tubules, acid excretion (H+) is counterbalanced by K+ retention, by H+/K+ ATPase, leading to hypokalemia and metabolic acidosis when there is a defect in this exchange. Another defect may be an increased permeability of the luminal membranes to secreted protons. This results in back diffusion of H+ despite the presence of an effective pump. This gradient defect is classically seen in patients treated with amphotericin B [23, 24]. Before the back diffusion occurs, the secreted H+ ions in the tubular lumen bind HCO3− to form H2CO3. As there is no luminal CA, H2CO3 is slowly dehydrated to CO2 and water causing transient high urine pCO2 levels. This is the only type of classic RTA with high urine pCO2 levels (> 65 mm Hg) and corresponding high urine-blood pCO2 difference (>25 mm Hg) [23]. A small number of patients with distal RTA have a voltage defect in the distal tubules leading to hyperkalemia rather than hypokalemia [24]. The necessary transepithelial voltage gradient for the exchange of H+/K+ cannot be maintained, forcing retention of both K+ and H+ in exchange for Na+, as can be seen in aldosterone-related RTA type 4 (discussed below). The entities are distinct because, in contrast with distal RTA due to a voltage defect in the distal tubules, patients with RTA type 4 maintain their ability to acidify urine in response to acidemia [24].
RTA Type 4
Aldosterone plays an essential role in the maintenance of fluid and electrolyte homeostasis in the collecting duct, where Na+ is exchanged for H+ and K+. The kidney accounts for about 90% of excreted potassium, primarily governed by plasma aldosterone and delivery of sodium and water to the distal secretory site. Hypoaldosteronism (RTA type 4) is caused by reductions in aldosterone secretion or responsiveness resulting in an increase in plasma H+ and K+ concentrations. Well-known causes are chronic interstitial nephritis and the most common RTA type: hyporeninemic hypoaldosteronism due to diabetic nephropathy. Many drugs will decrease the activity of aldosterone, including angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and direct renin inhibitors (Table 2) [23].
Normal AG Acidosis due to Saline Infusion
Numerous severely ill patients admitted to the hospital will develop an iatrogenic normal AG metabolic acidosis caused by fluid resuscitation with normal saline (NaCL 0.9%). As an example, all patients with severe diabetic ketoacidosis treated with NaCl 0.9% will have a combined high AG and normal AG metabolic acidosis soon after admission [29]. Hyperchloremia develops rapidly, increasing to 50% by 4 h in a previous study [25]. Patients treated with therapeutic plasma exchange with a replacement solution of 4% human albumin with a high chloride concentration can also develop a normal AG metabolic acidosis [26]. Another rare cause in this respect may be the use of NaCl 0.9% for total gut irrigation through the nasogastric route method as a bowel preparation in children undergoing colorectal surgeries [27]. Normal saline has a pH of 5.5 and a chloride content of 154 mmol/L and sodium of 154 mmol/L. The low pH has little influence on the development of acidosis after resuscitation. Because plasma has a sodium content of about 140 mmol/L and much lower chloride content of about 106 mmol/L, the chloride increase will be relatively higher than the sodium increase with the infusion of NaCl 0.9%. Because of this increase in chloride, a decrease in bicarbonate follows to maintain electroneutrality. Serum chloride is responsible for about one third of the extracellular fluid tonicity and two thirds of all anionic charges in plasma. Because of its high concentration, chloride is the most important anion to balance extracellular cations [28]. All bodily fluids conform to the principle of electrical neutrality, containing an equivalent number of positively and negatively charged ions. Therefore, to maintain electrical neutrality in the face of rising serum chloride anions from normal saline, the serum loses an equal amount of bicarbonate anions resulting in normal AG metabolic acidosis [29].
Diagnostic Evaluation of Normal AG Acidosis
Functional Tests
The first step is to exclude severe diarrhea or the use of medications that can cause RTA.
Urinary Ammonium/Urine AG
If the kidney function is intact, hyperchloremic acidosis should lead to increased renal excretion of ammonium, and measurement of urinary ammonium can, therefore, be used to differentiate between renal and extrarenal causes of normal AG acidosis. However, since few laboratories measure urinary ammonium routinely, the urinary AG ([Na+] + [K+] – [Cl−]) and urinary osmolal gap ([Na+] + [K+] + glucose + BUN – [Cl−] in mmol/l) are often used as surrogate measures of excretion of urinary ammonium [30, 31]. When individuals ingest a typical western diet, the quantity of sodium and potassium absorbed by the gastrointestinal tract will be higher than the absorbed chloride, and the urine AG will have a positive value (about +20 to +90 mmol/L) in healthy individuals. Ammonium is excreted as NH4Cl, and, therefore, the chloride excretion should be high in metabolic acidosis and the urinary AG ([Na+] + [K+] – [Cl−]) must become negative in severe diarrhea (about −30 to −50 mEq/L). In RTA type 1 and type 4, the urine AG will become positive because of the low chloride excretion as a result of the impaired urinary ammonium (NH4+) excretion as ammonium chloride [NH4Cl]). This defect can be seen in renal failure, distal RTA, or hypoaldosteronism [30, 31]. In proximal RTA, bicarbonate resorption is defective, but the ammonium excretion remains intact, and, therefore, a negative urinary AG will be found in RTA type 2 [32].
The Urinary Osmolal Gap
The urinary AG becomes unreliable when polyuria is present, when the urine pH exceeds 6.5 [31], or when urinary ammonium is excreted with an anion other than chloride (e.g., keto acids, acetylsalicylic acid, d-lactic acid, and large quantities of penicillin [30]. Furthermore, the acidification of the urine requires adequate distal delivery of sodium; thus, the usefulness of the urinary AG is questionable when the urinary sodium level is less than 20 mmol/L [33]. In such cases, the urinary osmolal gap is generally more reliable. The urinary osmolal gap determines the difference between measured and calculated urinary osmolality. The urinary osmolality is calculated as follows: (2 [Na+] + 2 [K+]) + (urine urea nitrogen [in mg/dL]/2.8) + (urine glucose [in mg/dL]/18) or (in mmol/L): (2 [Na+] + 2 [K+]) + (urine urea nitrogen) + (urine glucose). In patients without diabetes, the glucose concentration is often omitted from this calculation. A normal urine osmolal gap is approximately 10–100 mosm/kg, with urinary ammonium excretion being approximately one half of this value (5–50 mmol/L) due to accompanying anions. A high urine osmolal gap >200 mEq/L suggests a high urine ammonium concentration, and a nonrenal cause for the acidosis like diarrhea (often >300–400 mEq/L) is more likely than RTA [34, 35]. A urinary osmolal gap below 40 mmol per liter in normal anion-gap acidosis indicates impairment in urinary ammonium excretion. The urinary osmolal gap usually reflects the level of ammonium, except in the presence of large quantities of a nondissociated acid, such as β-hydroxybutyric acid in ketoacidosis and hippurate in toluene intoxication. The urinary osmolal gap, as compared with the urinary AG, has a better correlation with the urinary ammonium value [30, 31].
Urine pH
The urine pH reflects the hydrogen ion concentration and, therefore, the degree of acidification of the urine. Because urine can achieve a hydrogen ion concentration 1,000× greater than blood, its pH ranges from 4.5 to 8 (average pH 5–6) [36]. Though the gold standard of measurement is with a pH electrode, dipsticks offer the convenience of cost and ease of use [37]. However, many limitations concerning the urine pH level exist. The pH determined by dipsticks covers a pH range from 5.0 to 8.5 or to 9.0. Using dipsticks, however, significant deviations from the true pH are observed for values below 5.5 and above 7.5 [38]. The pH of urine is dependent on the time of day, the prandial state, diet, health status, and medications. A high protein diet is associated with acidic urine, and a vegetarian diet typically produces more alkaline urine because of bicarbonate formation from fruits, especially citrus, and vegetables. Highly diluted urine and a very low concentration of urinary sodium may interfere with the achievement of a minimum pH despite a normal renal acidification function. Often, the urine specimen becomes contaminated preanalytically with bacteria during collection [36]. While midstream urine collection after cleansing of the genitalia may be the preferred method, most urine collections are made without this practice. Both the male and female urethras are colonized with microorganisms. While urine collected by conventional voiding is often bacterially contaminated, even midstream random urine collections, whether with or without prior cleansing of external genitalia, have bacterial contamination rates of up to 30% [36, 38]. Left standing, the pH of a bacterial-contaminated, unpreserved urine specimen will continue to increase. Bacterial contamination of urine with microorganisms that split urea may yield urinary pH values >8.0 because of bacterial decomposition of urea to ammonia. A urea-splitting microorganism can produce alkaline urine through the following mechanism [36]:
Urea (H2NCONH2) + H2O → 2NH3 + CO2 → 2NH4+ + 2OH−.
Therefore, the measurement of pH in fresh urine samples and a pH meter should be used if an accurate measurement is necessary.
In metabolic acidosis and acidemia, the urine pH should decrease below 5.3 when the kidney function is intact. A higher value may indicate the presence of RTA. A normal adult under a common western diet eliminates about 40 mEq/day of NH4+. In case of chronic metabolic acidosis, the urinary NH4+ can increase up to 5–8 times the normal value, causing a maximum decrease in the urine pH to 4.5 [40]. Sometimes, it may be challenging to differentiate between diarrhea and distal RTA as a cause of normal AG acidosis and an elevated urine pH. Hypokalemia due to diarrhea can result in an inappropriately elevated urine pH that is due to a decrease in collecting duct H+ secretion because hypokalemia is a potent stimulus of renal NH3 production. The excess NH3 in the urine binds to secreted H+, thereby elevating the urine pH despite adequate tubular H+ secretion. When the hypokalemia is treated, H+ production by the kidney decreases, and the urine pH decreases appropriately. In contrast, in distal RTA (type 1), correction of hypokalemia has no effect on urine pH [41].
Ammonium Chloride Load
Administration of NH4Cl to induce metabolic acidosis with assessment of the renal response by serial measurement of urine pH has been often utilized in the past. It has classically been considered a crucial test in the diagnosis of distal RTA. Nowadays, its clinical application is quite restricted because the NH4Cl test is poorly tolerated since it induces nausea and vomiting. Also, the ability to acidify the urine may be assessed with less aggressive explorations [42].
Furosemide and Fludrocortisone
An alternative way to test the capacity for distal acidification is to administer furosemide and the mineralocorticoid fludrocortisone simultaneously [39]. The combination of both increased distal Na+ delivery, and the mineralocorticoid effect will stimulate distal H+ secretion by both an increase in the luminal electronegativity and a direct stimulation of H+ secretion. Normal subjects will lower urine pH to values below 5.5 with either maneuver [39, 42].
The Urine-Blood pCO2 during NaHCO3 Loading
Urine pCO2 after alkalization is a measure of the capacity of the proton pump to maximally secrete H+ because alkaline urine provides a favorable gradient for H+ secretion. The urine-blood pCO2 during NaHCO3 loading is, therefore, an excellent diagnostic index of H+-ATPase defect distal RTA [43]. In the NaHCO3 loading test, 2.75% NaHCO3 solution should be infused intravenously at a rate of 4 mL/kg/h. Urine and blood samples are taken at 2-h intervals until the plasma bicarbonate concentration reaches 24 mmol/L. Urine and blood pCO2 are measured then using a blood gas analyzer. The urine-blood pCO2 is ≤30 mm Hg in patients with distal RTA and a H+-ATPase defect but >30 mm Hg in health.
Bicarbonate Load
When patients are in a steady state and there is no hypokalemia, sodium bicarbonate 0.5–1.0 mEq/kg/h can be infused until the plasma HCO3− increases to the threshold and bicarbonaturia ensues resulting in a high urine pH and high fractional HCO3− excretion [21]:
A urine pH >7.5 or fractional excretion of HCO3− >15% is diagnostic of proximal RTA after bicarbonate loading. Urine pH will be unchanged in normal patients or those with distal RTA. A fractional excretion of HCO3− <5% excludes proximal RTA, and a value of 5–15% is indeterminate.
Decreased or Negative AG with or without Acidosis
Increased Concentration of Cations
The result of the equation [Na+] – [Cl−] – [HCO3−] will become low in several clinical cases where the measured chloride increases. To maintain electrical neutrality, hyperchloremia develops when high levels of cations exist, as seen in lithium toxicity, monoclonal IgG gammopathy, or disorders characterized by high levels of calcium or magnesium.
Pseudohyperchloremia
Pseudohyperchloremia is a phenomenon where there is a normal actual chloride concentration, but a high laboratory result is obtained for a chloride assay due to interference by a “chloride look-alike” in the form of a halogen ion such as bromine [Br−] or iodine [I−]. In these cases, chloride measurements, up to 175 mEq/L and an AG as low as −55 have been reported [44, 45]. Salicylates can potentially represent another cause for falsely elevated chloride levels and thus an extremely negative AG [46].
Practical Considerations
To bring the above information into practice, 3 case examples are discussed.
Patient 1
A 60-year-old man was admitted to the surgery ward for an inguinal hernia operation. He was known with long-standing diabetes mellitus type 2, with retinopathy and incompliance. His medication consisted of insulin, amlodipine, and simvastatin. On examination, the blood pressure was 133/94 mm Hg and edema was present. The remainder of the examination was unrevealing. The hemoglobin was 11 g%, sodium 139 mEq/L, potassium 5.9 mEq/L, creatinine 19 mg%, and glucose 176 mg%; urine dipstick was albumin positive.
Discussion. We are often confronted with insufficient information, so we must make a tentative diagnosis to guide further evaluation. In this case, the combination of moderate diabetic retinopathy, diabetic nephropathy with proteinuria, and hyperkalemia without prescription of drugs directly affecting the renin-angiotensin-aldosterone system, the diagnosis of RTA type 4 is almost certain. The most important causal factor of chronic hyperkalemia in patients with diabetes is the syndrome of hyporeninemic hypoaldosteronism [47]. In one study, RTA type 4 was very common in patients having significant hyperkalemia, with an incidence of 42% in patients admitted to a university hospital with a potassium level ≥6 mEq/L [48]. In our patient, other laboratory results were: Na 138 mEq/L, K 6.4 mEq/L, chloride 113 mEq/L, arterial blood gas: pH 7.36, pCO2 35 mm Hg, pO2 109 mm Hg, bicarbonate 19 mEq/L, albumin 2.0 g/dL, urine pH 6.3, glucose negative, and spot urine Na 61 mEq/L, K 35 mEq/L, and chloride 57 mEq/L on the next day. The serum AG is 6 mEq/L; to correct it for albumin, one should add 2.5 mEq/L per g/dL albumin to the calculated AG, so in this case add 5 mEq/L. An AG of 11 mEq/L is probably normal. Therefore, we have a normal AG metabolic acidosis, with urine AG (Na+ – K+ – Cl−) = 61 + 37 – 57 = 40 mEq/L. A positive urine AG adds to the diagnosis of RTA type 4.
Patient 2
You are consulted for a patient with diabetic ketoacidosis because of decreasing bicarbonate level during therapy. Laboratory results of days 1 and 2 are listed in Table 3. On day 2, the AG was increased 3 mEq/L and bicarbonate was decreased 9 mEq/L. In ketoacidosis, we expect that the increase in AG is about the same as the decrease in bicarbonate. Because the bicarbonate was substantially lower than expected on day 2, there must be a high and normal AG metabolic acidosis. The latter is caused by saline infusions. We must know this so-called delta-delta concept has many limitations [49].
Table 3.
Laboratory results of patient 2
| Plasma concentration | Day 1 | Day 2 |
|---|---|---|
| Sodium | 133 | 137 |
| Chloride | 96 | 107 |
| Bicarbonate (used normal value 24 mEq/L) | 17 | 15 |
| Anion gap (used upper value 12 mEq/L) | 20 | 15 |
Patient 3
A female patient was admitted to hospital because of intermittent vomiting, weight loss, and dizziness. She was treated for 18 years with HIV, and, recently, her CD4 count was high and the viral load very low. She was dehydrated, and laboratory tests revealed a plasma sodium of 138 mEq/L, chloride of 110 mEq/L, and glucose 110 mg%. An important clue was provided by the urine dipstick: glucose in the urine was high, while the serum glucose was normal. The serum chloride level was out of proportion for the sodium level with a CL−/Na+ ratio above 0.79 (0.797) suggesting a normal AG metabolic acidosis. The renal glucosuria was pointing to a proximal RTA, and, indeed, the bicarbonate level was 17 mEq/L, and low levels of uric acid and phosphate were noticed. After oral administration of sodium bicarbonate, the urine pH increased from 6.5 to 7.5, and bicarbonaturia was also confirmed quantitatively. In this case, tenofovir caused the proximal RTA, and after termination of the drug the patient recovered completely.
Conclusion
As can be seen by the 3 cases, making a diagnosis in normal AG metabolic acidosis is quite straightforward with clinical signs and additional simple laboratory tests (Tables 1, 2). The presence of RTA should be considered in any patient with a high chloride level, in particular when the CL−/Na+ ratio is above 0.79 and the patient does not have diarrhea. In patients with significant hyperkalemia, especially in diabetic patients with a relatively conserved renal function, one should evaluate for RTA type 4. A still growing list of medications can produce RTA.
Conflict of Interest Statement
The author has no conflict of interest to disclose.
Acknowledgment
I am indebted to Jan-Willem Boldingh for making the gamblegram (Fig. 1).
References
- 1.Berend K, de Vries AP, Gans RO. Physiological approach to assessment of acid-base disturbances. N Engl J Med. 2015;372:195. doi: 10.1056/NEJMc1413880. [DOI] [PubMed] [Google Scholar]
- 2.Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2:162–174. doi: 10.2215/CJN.03020906. [DOI] [PubMed] [Google Scholar]
- 3.Roberts WL, Johnson RD. Serum anion gap. Has the reference value really fallen? Arch Pathol Lab Med. 1997;121:568–572. [PubMed] [Google Scholar]
- 4.Sadjadi SA, Manalo R, Jaipaul N, McMillan J. Ion-selective electrode and anion gap range: What should the anion gap be? Int J Nephrol Renovasc Dis. 2013;6:101–105. doi: 10.2147/IJNRD.S44689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farwell WR, Taylor EN. Serum anion gap, bicarbonate and biomarkers of inflammation in healthy individuals in a national survey. CMAJ. 2010;182:137–141. doi: 10.1503/cmaj.090329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamble JL. Extracellular fluid and its maintenance. N Engl J Med. 1936;250:1150–1152. [Google Scholar]
- 7.Kelly A, McAlpine R, Elizabeth E. Agreement between bicarbonate measured on arterial and venous blood gases. Emerg Med Australas. 2004;16:407–409. doi: 10.1111/j.1742-6723.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- 8.Berend K. Acid-base pathophysiology after 130 years: confusing, irrational and controversial. J Nephrol. 2013;26:254–265. doi: 10.5301/jn.5000191. [DOI] [PubMed] [Google Scholar]
- 9.Szerlip HM, Singer I. Hyperchloremic metabolic acidosis after chlorine inhalation. Am J Med. 1984;77:581–582. doi: 10.1016/0002-9343(84)90127-x. [DOI] [PubMed] [Google Scholar]
- 10.Durward A, Skellett S, Mayer A, Taylor D, Tibby SM, Murdoch IA. The value of the chloride: sodium ratio in differentiating the aetiology of metabolic acidosis. Intensive Care Med. 2001;27:828–835. doi: 10.1007/s001340100915. [DOI] [PubMed] [Google Scholar]
- 11.Berend K, van Hulsteijn LH, Gans RO. Chloride: the queen of electrolytes? Eur J Intern Med. 2012;23:203–211. doi: 10.1016/j.ejim.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Ratnam S, Kaeny W, Shapiro JI. Pathogenesis and management of metabolic acidosis and alkalosis. In: Schrier RW, editor. Renal and Electrolyte Disorders. ed 7. Philadelphia: Lippincott, Williams & Wilkins; 2010. pp. 86–121. [Google Scholar]
- 13.Hall MC, Koch MO, McDougal WS. Metabolic consequences of urinary diversion through intestinal segments. Urol Clin North Am. 1991;18:725–735. [PubMed] [Google Scholar]
- 14.Kamar FB, McQuillan RF. Hyperchloremic metabolic acidosis due to cholestyramine: a case report and literature review. Case Rep Nephrol. 2015 doi: 10.1155/2015/309791. 309791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santos F, Ordóñez FA, Claramunt-Taberner D, Gil-Peña H. Clinical and laboratory approaches in the diagnosis of renal tubular acidosis. Pediatr Nephrol. 2015;30:2099–2107. doi: 10.1007/s00467-015-3083-9. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal M, McKenna R. Update on carbonic anhydrase inhibitors: a patent review (2008–2011) Expert Opin Ther Pat. 2012;22:903–915. doi: 10.1517/13543776.2012.707646. [DOI] [PubMed] [Google Scholar]
- 17.Supuran CT. How many carbonic anhydrase inhibition mechanisms exist? J Enzyme Inhib Med Chem. 2016;31:345–360. doi: 10.3109/14756366.2015.1122001. [DOI] [PubMed] [Google Scholar]
- 18.Oosterwijk E. Carbonic anhydrase expression in kidney and renal cancer: implications for diagnosis and treatment. Subcell Biochem. 2014;75:181–198. doi: 10.1007/978-94-007-7359-2_10. [DOI] [PubMed] [Google Scholar]
- 19.Curthoys NP, Moe OW. Proximal tubule function and response to acidosis. Clin J Am Soc Nephrol. 2014;9:1627–1638. doi: 10.2215/CJN.10391012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rennke H, Denker BM. Renal Pathophysiology. ed 4. Philadelphia: Lippincott Williams & Wilkins; 2014. pp. 153–173. chapt 6: Metabolic Acidosis. [Google Scholar]
- 21.Reddy P. Clinical approach to renal tubular acidosis in adult patients. Int J Clin Pract. 2011;65:350–360. doi: 10.1111/j.1742-1241.2009.02311.x. [DOI] [PubMed] [Google Scholar]
- 22.Garashi T, Sekine T, Inatomi J, Seki G. Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J Am Soc Nephrol. 2002;13:2171–2177. doi: 10.1097/01.asn.0000025281.70901.30. [DOI] [PubMed] [Google Scholar]
- 23.Liamis G, Milionis HJ, Elisaf M. Pharmacologically-induced metabolic acidosis. Drug Saf. 2010;33:371–391. doi: 10.2165/11533790-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Yaxley J, Pirrone C. Review of the diagnostic evaluation of renal tubular acidosis. Ochsner J. 2016;16:525–530. [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor D, Durward A, Tibby SM, Thorburn K, Holton F, Johnstone IC, Murdoch IA. The influence of hyperchloraemia on acid base interpretation in diabetic ketoacidosis. Intensive Care Med. 2006;32:295–301. doi: 10.1007/s00134-005-0009-1. [DOI] [PubMed] [Google Scholar]
- 26.Ritzenthaler T, Grousson S, Dailler F. Hyperchloremic metabolic acidosis following plasma exchange during myasthenia gravis crisis. J Clin Apher. 2016;31:479–480. doi: 10.1002/jca.21432. [DOI] [PubMed] [Google Scholar]
- 27.Bala I, Dwivedi D, Jain D, Mahajan JK. Hyperchloremic metabolic acidosis following total gut irrigation with normal saline in pediatric patients: a rare occurrence. Indian J Crit Care Med. 2017;21:55–56. doi: 10.4103/0972-5229.198329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thongprayoon C, Cheungpasitporn W, Cheng Z, Qian Q. Chloride alterations in hospitalized patients: prevalence and outcome significance. PLoS One. 2017;12:e0174430. doi: 10.1371/journal.pone.0174430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahler SA, Conrad SA, Wang H, Arnold TC. Resuscitation with balanced electrolyte solution prevents hyperchloremic metabolic acidosis in patients with diabetic ketoacidosis. Am J Emerg Med. 2011;29:670–674. doi: 10.1016/j.ajem.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 30.Reddy P, Mooradian AD. Clinical utility of anion gap in deciphering acid-base disorders. Int J Clin Pract. 2009;63:1516–1525. doi: 10.1111/j.1742-1241.2009.02000.x. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez Soriano J. Renal tubular acidosis: the clinical entity. J Am Soc Nephrol. 2002;13:2160–2170. doi: 10.1097/01.asn.0000023430.92674.e5. [DOI] [PubMed] [Google Scholar]
- 32.Katzir Z, Dinour D, Reznik-Wolf H, Nissenkorn A, Holtzman E. Familial pure proximal renal tubular acidosis – a clinical and genetic study. Nephrol Dial Transplant. 2008;23:1211–1215. doi: 10.1093/ndt/gfm583. [DOI] [PubMed] [Google Scholar]
- 33.Finkel KW, Dubose TF. Metabolic acidosis. In: Dubose T Jr, Hamm L, editors. Acid Base and Electrolyte Disorders: A Companion to Brenner & Rector's, The Kidney. Philadelphia: Saunders; 2002. pp. 55–66. [Google Scholar]
- 34.Rastegar M, Nagami GT. Non-anion gap metabolic acidosis: a clinical approach to evaluation. Am J Kidney Dis. 2017;69:296–301. doi: 10.1053/j.ajkd.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 35.Soleimani M, Rastegar A. Pathophysiology of renal tubular acidosis: core curriculum. Am J Kidney Dis. 2016;68:488–498. doi: 10.1053/j.ajkd.2016.03.422. [DOI] [PubMed] [Google Scholar]
- 36.Cook JD, Strauss KA, Caplan YH, Lodico CP, Bush DM. Urine pH: the effects of time and temperature after collection. J Anal Toxicol. 2007;31:486–496. doi: 10.1093/jat/31.8.486. [DOI] [PubMed] [Google Scholar]
- 37.Kwong T, Robinson C, Spencer D, Wiseman OJ, Karet Frankl FE. Accuracy of urine pH testing in a regional metabolic renal clinic: is the dipstick accurate enough? Urolithiasis. 2013;41:129–132. doi: 10.1007/s00240-013-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delanghe J, Speeckaert M. Preanalytical requirements of urinalysis. Biochem Med (Zagreb) 2014;24:89–104. doi: 10.11613/BM.2014.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh S, Shirley D, Wrong O, Unwin R. Urinary acidification assessed by furosemide and fludrocortisone treatment: an alternative to ammonium chloride. Kidney Int. 2007;71:1310–1316. doi: 10.1038/sj.ki.5002220. [DOI] [PubMed] [Google Scholar]
- 40.Wrong O. Distal renal tubular acidosis: the value of urinary pH, PCO2 and NH4+ measurements. Pediatr Nephrol. 1991;5:249–255. doi: 10.1007/BF01095966. [DOI] [PubMed] [Google Scholar]
- 41.Galla JH, Kurtz I, Kraut JA, Lipschik GY, Macrae JP. Acid-base disorders. In: Lerma E, Berns J, Nissenson A, editors. Current Essentials of Diagnosis & Treatment in Nephrology & Hypertension. ed 1. New York: McGraw-Hill Education/Medical; 2012. pp. 42–59. chapt 5. [Google Scholar]
- 42.Santos F, Ordóñez FA, Claramunt-Taberner D, Gil-Peña H. Clinical and laboratory approaches in the diagnosis of renal tubular acidosis. Pediatr Nephrol. 2015;30:2099–2107. doi: 10.1007/s00467-015-3083-9. [DOI] [PubMed] [Google Scholar]
- 43.Kim S, Lee JW, Park J, Na KY, Joo KW, Ahn C, Kim S, Lee JS, Kim GH, Kim J, Han JS. The urine-blood PCO gradient as a diagnostic index of H+-ATPase defect distal renal tubular acidosis. Kidney Int. 2004;66:761–767. doi: 10.1111/j.1523-1755.2004.00801.x. [DOI] [PubMed] [Google Scholar]
- 44.Emmett M. Approach to the patient with a negative anion gap. Am J Kidney Dis. 2016;67:143–150. doi: 10.1053/j.ajkd.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 45.Danel VC, Saviuc PF, Hardy GA, Lafond JL, Mallaret MP. Bromide intoxication and pseudohyperchloremia. Ann Pharmacother. 2001;35:386–387. doi: 10.1345/aph.10156. [DOI] [PubMed] [Google Scholar]
- 46.Zimmer BW, Marcus RJ, Sawyer K, Harchelroad F. Salicylate intoxication as a cause of pseudohyperchloremia. Am J Kidney Dis. 2008;51:346–347. doi: 10.1053/j.ajkd.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 47.Sousa AG, Cabral JV, El-Feghaly WB, de Sousa LS, Nunes AB. Hyporeninemic hypoaldosteronism and diabetes mellitus: pathophysiology assumptions, clinical aspects and implications for management. World J Diabetes. 2016;7:101–111. doi: 10.4239/wjd.v7.i5.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haas CS, Pohlenz I, Lindner U, Muck PM, Arand J, Suefke S, Lehnert H. Renal tubular acidosis type IV in hyperkalaemic patients – a fairy tale or reality? Clin Endocrinol (Oxf) 2013;78:706–711. doi: 10.1111/j.1365-2265.2012.04446.x. [DOI] [PubMed] [Google Scholar]
- 49.Rastegar A. Use of the ΔAG/ΔHCO3− ratio in the diagnosis of mixed acid-base disorders. J Am Soc Nephrol. 2007;18:2429–2431. doi: 10.1681/ASN.2006121408. [DOI] [PubMed] [Google Scholar]