Abstract
Background
Distal renal tubular acidosis (dRTA) is characterized by an impairment of the urinary acidification process in the distal nephron. Complete or incomplete metabolic acidosis coupled with inappropriately alkaline urine are the hallmarks of this condition. Genetic forms of dRTA are caused by loss of function mutations of either SLC4A1, encoding the AE1 anion exchanger, or ATP6V1B1 and ATP6V0A4, encoding for the B1 and a4 subunits of the vH+ATPase, respectively. These genes are crucial for the function of A-type intercalated cells (A-IC) of the distal nephron.
Summary
Alterations of acid-base homeostasis are variably associated with hypokalemia, hypercalciuria, nephrocalcinosis or nephrolithiasis, and a salt-losing phenotype. Here we report the diagnostic test and the underlying physiopathological mechanisms. The molecular mechanisms identified so far can explain the defect in acid secretion, but do not explain all clinical features. We review the latest experimental findings on the pathogenesis of dRTA, reporting mechanisms that are instrumental for the clinician and potentially inspiring a novel therapeutic strategy.
Key Message
Primary dRTA is usually intended as a single-cell disease because the A-IC are mainly affected. However, novel evidence shows that different cell types of the nephron may contribute to the signs and symptoms, moving the focus from a single-cell towards a renal disease.
Keywords: AE1, Intercalated cells, Metabolic acidosis, Renal tubular acidosis, vH+ATPase
Clinical Aspects
Distal renal tubular acidosis (dRTA) has been the first type of RTA identified and is thus also known as type I RTA. dRTA is characterized by impaired renal acid secretion (with normal glomerular filtration rate) causing metabolic acidosis. On the clinical side, this leads to the excretion of alkaline urine relative to systemic metabolic acidosis [1]. Additional signs associated with dRTA can be hypokalemia, hypercalciuria, nephrocalcinosis or nephrolithiasis, and sometimes salt-losing disease [2]. The term “incomplete dRTA” instead refers to patients with no overt metabolic acidosis at baseline who are screened for dRTA, usually because of nephrolithiasis or nephrocalcinosis, and who can not maximally acidify urine (urinary pH >5.3) following the administration of an acid load [1].
Primary dRTA is a genetic disease and usually manifests during childhood or adolescence [3], while secondary dRTA can occur at any age, either induced by drugs, autoimmune or urological diseases, or dysproteinemia [4, 5]. Mutations in the chloride-bicarbonate exchanger AE1, known as band 3 protein in erythrocytes (RBC), have been found to cause autosomal dominant or recessive dRTA [6]. AE1 is expressed at the basolateral membrane of A-type intercalated cells (A-IC) in the distal nephron, where it is required to transport bicarbonate generated during acid secretion from the cytosol to the renal interstitium [7]. Loss of function mutations of AE1 are not always associated with both renal and hematological phenotype simultaneously, even though band 3 is the most abundant protein of the membrane of RBC [8], revealing a different functional setting in these two cell types. Indeed, AE1 dysfunction leading to hereditary ovalocytosis or spherocytosis is commonly associated with the autosomal recessive form of dRTA, but not with autosomal dominant dRTA [6]. Since autosomal recessive mutations are common in the tropical area, whereas the dominant ones prevail in Western countries, Wrong et al. [9] proposed that this geographic distribution could confer protection against malaria in heterozygous carriers, especially in South Asia.
Mutations in two subunits of the vH+ATPase, namely ATP6V1B1 and ATP6V0A4, also cause autosomal recessive dRTA. Mutations of ATP6V1B1, encoding for the B1 subunit of vH+ATPase, are usually associated with bilateral sensorineural hearing loss [10]. Consistent with this finding, ATP6V1B1 expression is found in the cochlea and endolymphatic sac [11]. Mutations of ATP6V0A4, encoding for the a4 subunit of vH+ATPase, cause autosomal recessive dRTA, associated in some cases with hearing impairment in late childhood [12, 13].
Renal Acidification Mechanisms: From Molecular Mechanisms to Clinical Tests
In order to contribute to the acid-base homeostasis, the kidneys regenerate bicarbonate and excrete nonvolatile acids.
Systemic bicarbonate refilling is dependent on intensive reabsorption of almost all the filtered bicarbonate (FE HCO3− <1%) and on the synthesis of new HCO3− molecules during ammoniagenesis. These mechanisms have been extensively detailed elsewhere [14]. Briefly, bicarbonate reabsorption occurs mainly along the proximal tubule (PT) and the thick ascending limb (TAL) and depends on a similar machinery of proteins [15]. On the apical side, NHE3 and carbonic anhydrase IV mediate intracellular HCO3− intake and, on the basolateral side, NBCe-1 (for the PT) and AE2 (for the TAL) contribute to its final reabsorption in the bloodstream.
Finally, in the PT, new HCO3− and NH4+ are generated during deamination of glutamine and other amino acids, and then NH4+ is secreted in the urine while the bicarbonate is reabsorbed in the bloodstream. Secreted NH4+ is then reabsorbed along the TAL and accumulated in the medullary interstitium as NH3/NH4+ where it contributes to medulla hypertonicity [16]. Final ammonia secretion in the urine is strictly connected to the renal excretion of nonvolatile acids along the distal nephron. Indeed, to maximize H+ excretion, the urine contains different buffer systems. Titratable acids (mainly creatinine, phosphate, and to a lesser extent urate and citrate) and the NH3/NH4+ buffers are the most effective along the outer and inner medulla collecting duct (CD), respectively.
Under normal conditions, the residual buffer capacity of HCO3−/pCO2 is poor in the distal nephron, but it can play a major role when the upstream bicarbonate reabsorption threshold is decreased as during proximal RTA, or saturated as during sodium bicarbonate load. Indeed, by increasing the delivery of bicarbonate to the distal nephron, the HCO3− buffered by the protons secreted locally will increase urinary pCO2 excretion. Since this process is an effect of distal urine proton secretion, the urine to blood pCO2 ratio after a challenge with NaHCO3 load is an efficient method to explore distal urine acidification in humans [17].
The overall urinary evaluation of titratable acidity, NH4+, and HCO3− allows to estimate renal net acid excretion. Clinical physiological tests to diagnose renal acid-base disorders were based on these principles already before the molecular players of acid secretion were known. Indeed, the acid-secreting ability of the kidney was investigated by studying the urine to blood pCO2 ratio after NaHCO3 load [18] or by challenging patients with an acid load through NH4Cl administration [19] and, more recently, by the administration of furosemide/fludrocortisone [20] (Table 1). All these tests are still used in clinics nowadays and their underlying molecular mechanisms are mostly known.
Table 1.
Clinical tests to diagnose renal acid-base disorders
| Challenge | Measure | Positive × dRTA | Ref. |
|---|---|---|---|
| NaHCO3 | (U-B) pCO2 | <20 mm Hg | 15 |
| NH4Cl | urinary pH | >5.3 | 16 |
| Furosemide/fludrocortisone | urinary pH | >5.3 | 17 |
dRTA, distal renal tubular acidosis; U-B, urine to blood.
Acid secretion along the distal nephron mainly occurs through A-IC. These cells are equipped at the apical side with the vH+ATPase and at their basolateral side with the anion exchanger AE1, showing an acid-secreting phenotype. B-type intercalated cells (B-IC) display an opposite polarity in respect to A-IC, by expressing the vH+ATPase at the basolateral membrane and an anion exchanger, namely pendrin, at the apical domain, resembling a base-secreting phenotype. For long time A-IC, B-IC, and non-A non-B cells have been considered a unique pool of extremely plastic cells, able to switch their phenotype and number in response exclusively to different acid-base conditions to adapt renal acid or base secretory capabilities [21]. Recent evidence supports an important role of B-IC in salt reabsorption and regulation of blood pressure as well [7, 22, 23, 24]. The distribution of B-IC and A-IC along the CD is different and responds to several metabolic states. B-IC are mainly located along the cortical and outer stripe of the outer medulla CD, while A-IC are progressively less frequent from the cortical collecting duct (CCD) up to the inner medulla CD (IMCD) [25]. The medullary CD is the site where not only maximal urinary concentration but also acidification occurs, so during the state of metabolic acidosis, the expression of A-IC at this level can significantly increase. Proton secretion is mainly due to the activity of the apical vH+ATPase with a potential additional contribution from the H+/K+-ATPase (hydrogen potassium ATPase). Proton supply results from the catalytic activity of intracellular CAII (carbonic anhydrase type II) that converts CO2 and OH− into H2CO3 and so into H+ and HCO3−. Final bicarbonate reabsorption and intracellular acid-base homeostasis is granted by the basolateral AE1. This relation strictly links AE1 and vH+ATPase function and reflects how a dysfunction of one of them is detrimental for the net acid secretion by A-IC.
The presence of an active transport allows the secretion of protons even in the presence of a steep gradient (about 1 unit of pH). However, the efficacy of the proton secretion requires the presence of a buffer system able to decrease the free proton concentration in the lumen and thus to prevent the gradient-driven back leak of protons. The diffusion of NH3 from the interstitium to the tubular lumen, mainly in the inner stripe of the outer medulla, allows proton buffering and trapping. Hence, NH4+ becomes the molecular readout of efficient distal acid secretion. This mechanism is maximized during acidosis, where not only the abundance of vH+ATPase, but also the number of A-IC is increased in the inner stripe of the outer medulla [8]. This is the rationale for using NH4Cl to evocate a maximal urinary acidification. Indeed, liver conversion of NH4+ to urea dissipates bicarbonate and so induces a metabolic acidosis which persists until the kidney has completely excreted this acid load. Thus, failure in maximal urinary acidification (urinary pH <5.3) during an NH4Cl load is diagnostic for dRTA [19]. Finally, a lumen-negative electric gradient supports the proton secretion by A-IC. This is achieved by electrogenic sodium reabsorption along the principal cells (PC) through the epithelial sodium channel (ENaC) and increases with larger sodium delivery at the CCD [26]. This mechanism suggests, together with a common embryological origin [25], a cooperative function between PC and A-IC potentially explaining the reason of an intercalated distribution pattern along the CD where A-IC are alternated to PC and their potential plasticity when one or the other is injured [27]. The furosemide/fludrocortisone test for the diagnosis of dRTA is based on this mechanism in the original setting. ENaC expression is usually low in normal salt condition in PC, but fludrocortisone, an analog of aldosterone, can upregulate its expression and activity. At the same time, furosemide increases sodium delivery to the distal nephron. In this settings, it is anticipated that the CD reabsorbs the sodium ion avidly through the ENaC, which notably increases the development of lumen-negative transepithelial voltage, and this in turn stimulates proton secretion by electrogenic vH+ATPase. However, recent experimental data propose that furosemide-induced urine acidification may be independent from ENaC activity and rather related to proton secretion by NHE3 in the TAL [28]. Behind the specific physiological mechanisms of action, the furosemide/fludrocortisone and NH4Cl load are clinically considered to be overlapping for the diagnostic accuracy of dRTA [20].
Loss of Function Mutations of AE1 Cause dRTA
Two splice variants of the anion exchanger AE1 are respectively expressed in the RBC (eAE1) and in the kidney (kAE1). Human kAE1 is lacking the first 65 amino acids that are present in human eAE1 [29]. Few mutations give a phenotype both in RBC and in the kidney, suggesting different roles and susceptibilities for eAE1 and kAE1 to cause disease. Only few mutations causing a hematological phenotype have been identified in the extra 65 amino acidic C-terminal tail of the eAE1, thus excluding that this is the only amino acid sequence functional for eAE1. Finally, there is no clear separation, even in terms of hereditability, between the hematologic and the renal phenotype, even though the majority of the mutations affecting the shape and the function of the RBC are inherited as an autosomal recessive trait. So said, it seems that single amino acids are specifically crucial for the global function of either kAE1 or eAE1, suggesting that the ion exchange activity is not the only important role of AE1, but that its membrane anchoring function might be fundamental as well, especially in RBC [30].
How mutations can impair the global function of the kAE1 and lead to dRTA has been investigated extensively. Original in vitro studies demonstrated a trafficking defect to the plasma membrane of both autosomal recessive [31] and autosomal dominant identified mutations [32]. Indeed, Cordat et al. [32] found that dominant dRTA point mutants (kAE1 R589H and S613F) were retained in the endoplasmic reticulum, while recessive mutants could traffic to the Golgi (kAE1 G701D). Based on these results, the authors proposed that intracellular retention of AE1 is the main mechanism causing dRTA. This hypothesis was also strongly supported by observations showing preserved transport activity of the most frequent mutants of AE1 when tested in Xenopus laevis oocytes [33]. Examination of a human biopsy from a patient suffering from dRTA associated with a S613F mutation of AE1 also showed cytosolic retention of mutated AE1 [34]. However, contrasting results were obtained when the mutations were characterized in both polarized and nonpolarized cells. This was the case for the recessive S773P AE1 mutation that showed proper membrane sorting in polarized but not in nonpolarized cells [32].
In vivo studies further explored the role of AE1 in dRTA. Constitutive AE1 knockout (KO) mice recapitulated key renal features of the human dRTA, i.e., spontaneous hyperchloremic metabolic acidosis with deficient stimulation of net acid excretion when challenged with NH4Cl. Surprisingly, the phenotype was striking only in homozygous mice that also presented nephrocalcinosis, hypercalciuria, hyperphosphaturia, and hypocitraturia associated with severe urinary concentration defect, whereas heterozygous KO mice were grossly normal [35]. However, these mice presented with severe failure to thrive and life-threatening hemolytic anemia [36] associated to some extent with an impairment of renal function that could have had some impact on the final renal phenotype. Since AE1 is not expressed at all in this model, it was not possible to assess in this model the intracellular retention of AE1 as shown in the in vitro model.
To recapitulate a mouse model of AE1-dependent dRTA, we recently generated a transgenic mouse expressing one of the most common mutations of the AE1 gene, namely R589H AE1, corresponding to the R607H mutation in mouse AE1 [8]. As humans, these mice do not present RBC abnormalities, but they exhibit incomplete unmasked dRTA when they are challenged with an NH4Cl load. Both homozygous and heterozygous R607H mice fail to properly acidify urine following an acid load, recapitulating the dominant form of dRTA associated with this mutation. R607H AE1 properly localized to the basolateral side of A-IC as well as the wild-type form in control mice. Indeed, to rule out cell line-dependent confounding factors, we tested the localization of human R589H AE1 in a different cell line, namely MDCK M1 cells and IMCD3. Here, R589H AE1 properly localized to the basolateral membrane as in vivo. Since R589H was reported to have a mild reduction in anion exchange activity when tested in Xenopus oocytes [37], we explored the transport activity of AE1 in RBC from control and R607H AE1 mutant mice, and no difference in anion exchange transport was found.
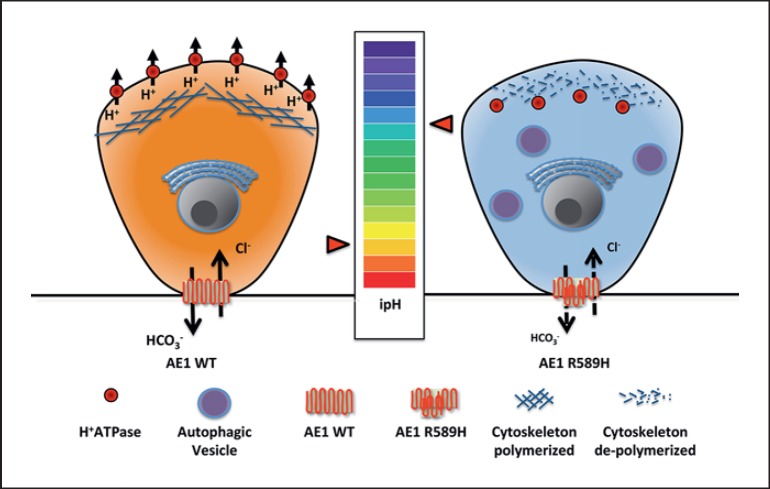
Thus, the major impact of the R607H AE1 mutation was not a transport defect or a mislocalization of the protein, but a severe reduction of A-IC, especially in the renal cortex of both heterozygous and homozygous mice. In addition, in mutant mice, both at baseline and after an acid load, the remnant A-IC showed intracellular retention of vH+ATPase. This is the key protein accounting for acid secretion. In our model, intracellular retention of vH+ATPase was coupled with reduced apical expression in mutant mice. Dysfunction of vH+ATPase was also supported by the presence of an autophagic defect of these cells. As depicted in Figure 1, the model we propose is that lower R607H AE1 expression produces impaired intracellular pH homeostasis, leading to intracellular alkalinization of the A-IC, thereby promoting actin depolymerization, as shown in osteoclasts [38], and ultimately compromising the normal sorting of vH+ATPase to the apical membrane.
Fig. 1.
Schematic representation of the novel identified mechanisms of diseases caused by R589H AE1 mutation. On the left, a normal A-IC is represented, while on the right, an A-IC expressing the R589H AE1 mutation is shown. We hypothesized that reduced expression of R589H AE1 leads to alkalinization of intracellular pH because of intracellular bicarbonate retention. This leads to actin cytoskeleton depolymerization and thus to intracellular retention of vH+ATPase.
Loss of Function Mutations of vH+ATPase Subunits Causes dRTA
vH+ATPase is a complex protein composed of 13 different subunits. It consists of a V0 domain contributing to proton secretion through the plasma membrane and of a V1 domain for ATP hydrolysis. This is a highly conserved protein among species, and it is ubiquitously expressed in epithelial cells either at the plasma membrane or in cytosolic vesicles. Loss of function mutations of the a4 and B1 subunits cause dRTA.
To explore the pathophysiology of B1 subunit-linked dRTA, Finberg et al. [39] generated an Atp6v1b1-deficient mouse strain. This mouse model recapitulates the features of dRTA, failing to properly acidify the urine when challenged with an acid load. However, at baseline, differently from the patients, these mice did not present overt metabolic acidosis in part due to the different dietary regimen, but also because of a compensatory upregulation of the B2 subunit of vH+ATPase. A similar or alternative compensatory mechanism could account for the lack of sensorineural hearing loss observed in these mice in contrast with the patients carrying mutations in Atp6v1b1 [10, 40]. By investigating the sodium reabsorption ability of the Atp6v1b1-deficient mice, a putative mechanism has been demonstrated, explaining the salt-losing phenotype usually affecting dRTA patients. Indeed, Gueutin et al. [41] demonstrated that inactivation of the B1 subunit not only affects A-IC, causing a defect in acid secretion as expected, but also impairs Na and Cl reabsorption along the CCD by primarily impairing the function of B-IC and thus, secondarily, affecting the PC as well. Sodium reabsorption along the CCD is mediated by an electrogenic transport trough the amiloride-sensitive ENaC (in PC) and by an electroneutral thiazide-sensitive pendrin/NDCBE reabsorption systems (in B-IC) [7]. In Atp6v1b1 KO mice, sodium transport in microperfused CCD was abolished both under a normal and a low salt diet, and this was matched by a parallel downregulation of both pendrin and the ENaC in the renal cortex. If pendrin downregulation was a well-known consequence of suppression of the B1 subunit in B-IC, ENaC downregulation in Atp6v1b1 KO mice was unexpected. However, the fact that ENaC downregulation was limited to the renal cortex, where B-IC are present, but not to the renal medulla, where they are not detectable, suggests that local factors may regulate PC activity along the CCD. This autocrine/paracrine regulation of PC function is mediated by an increase in ATP/PGE2 release in Atp6v1b1 KO mice [41]. Indeed, suppression of Atp6v1b1 in B-IC stimulates the release of ATP and then the synthesis and release of PGE2 in the CCD. Finally, these molecules suppress PC activity by decreasing the expression of both the ENaC and AQP2 [41]. Indomethacin, a well-known inhibitor of PGE synthesis, is able to reverse the salt-losing and polyuric phenotype of the Atp6v1b1 KO mice by restoring ENaC and AQP2 expression levels in cortical PC [41]. Defective water and salt reabsorption along the CCD was also responsible for the generation of hypokalemia associated with dRTA in this model. Indeed, as a consequence of increased sodium delivery and urine flow to the medullary CD, PC increase the expression level of the ENaC to maximize medullary sodium reabsorption. ENaC-dependent sodium reabsorption promotes renal outer medullary potassium channel-dependent potassium secretion. In addition, increased medullary urinary flow mediates flow-dependent activation of the BK channel and further stimulates potassium secretion in the tubular lumen. These mechanisms are responsible for the generation of hypokalemia and are reverted by indomethacin administration [41].
Loss of function of Atp6v0a4 coding for the a4 subunit was also recently investigated in a mouse model [42]. These mice are severely affected and die 35 days after birth, showing severe dRTA and deafness. The phenotype of these mice revealed an impairment also of PT function, leading to phosphaturia and proteinuria associated with a pattern of lysosomal storage disease.
Finally, even not identified yet in humans as a cause of primary dRTA, we recently studied the function of the Atp6ap2 subunit of vH+ATPase also known as prorenin receptor [43]. Suppression of Atp6ap2 in the renal epithelial cells induces dRTA as expected by vH+ATPase dysfunction in A-IC, but also a severe urinary concentrating defect. This latter finding was explained by severe alterations of medullary TAL and CD cells because of lysosome swelling and autophagy defect, likely due to absence of functional vH+ATPase in the lysosomes.
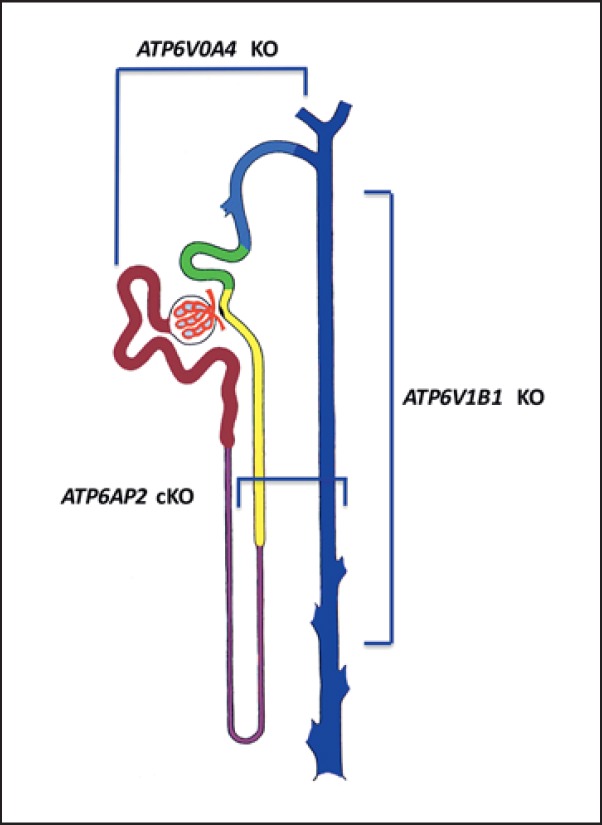
In the general clinical view, dRTA is considered a single-cell disease where only the defect of A-IC is involved. This common meaning does not take into account symptoms that seems to be poorly related to A-IC function, such as salt-losing nephropathy and hypokalemia. The molecular mechanisms described above suggest that the impairment of vH+ATPase is not specific for A-IC, but that it involves other cell types such as B-IC in the Atp6v1b1 KO mice, the PT in the Atp6v0a4 KO model, and finally the TAL and CD in the Atp6ap2 KO model. This supports the concept that vH+ATPase is ubiquitously expressed along the kidneys and so single mutations could preferentially hit different functions also in cell types different from A-IC. Taken together, this evidence supports the concept that dRTA is not a single-cell disease involving only A-IC dysfunction, but that it should be considered a renal disease since other epithelial cells function could be compromised (Fig. 2).
Fig. 2.
Epithelial cell involvement in experimental models of dRTA secondary to vH+ATPase dysfunction. ATP6V0A4 knockout (KO) mice present with signs of dysfunction of proximal tubule cells, while ATP6V1B1 KO mice show an indirect involvement of cortical and medullary principal cells secondary to A-IC dysfunction. Finally, genetic ablation of ATP6AP2 shows a critical role of medullary thick ascending limb and principal cells together with A-IC. This latter vH+ATPase subunit has not been identified yet in humans as a cause of dRTA.
Conclusion
dRTA is usually underdiagnosed even though it can lead to severe consequences such as osteoporosis/osteomalacia and end-stage renal disease secondary to severe nephrolithiasis. These side effects seem to be mainly the result of persistent and sustained metabolic acidosis. Indeed, the therapy of dRTA is mainly direct to treat metabolic acidosis by alkali salt supplementation. Hypokalemia is variably associated with dRTA, and the physiopathological mechanisms behind this condition are not completely disclosed yet.
Finally, the fact that polyuria and salt-losing nephropathy are often associated with a classical dRTA phenotype (both primary and secondary) questions the typical view of a single-cell disease. In view of the recent evidence reported here, A-IC are not the only target of the gene mutation causing dRTA. This suggest that dRTA should be reconsidered as a renal disease involving different renal epithelial cell types.
Conflict of Interest Statement
The authors declare no conflict of interest.
References
- 1.Soriano JR. Renal tubular acidosis: the clinical entity. J Am Soc Nephrol. 2002;13:2160–2170. doi: 10.1097/01.asn.0000023430.92674.e5. [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez-Soriano J, Vallo A. Renal tubular acidosis. Pediatr Nephrol. 1990;4:268–275. doi: 10.1007/BF00857675. [DOI] [PubMed] [Google Scholar]
- 3.Battle D, Haque SK. Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol Dial Transplant. 2012;27:3691–3704. doi: 10.1093/ndt/gfs442. [DOI] [PubMed] [Google Scholar]
- 4.Reddy P. Clinical approach to renal tubular acidosis in adult patients. Int J Clin Pract. 2011;65:350–360. doi: 10.1111/j.1742-1241.2009.02311.x. [DOI] [PubMed] [Google Scholar]
- 5.Gambaro G, Croppi E, Coe F, Lingeman J, Moe O, Worcester E, Buchholz N, Bushinsky D, Curhan GC, Ferraro PM, Fuster D, Goldfarb DS, Heilberg IP, Hess B, Lieske J, Marangella M, Milliner D, Preminger GM, Reis Santos JM, Sakhaee K, Sarica K, Siener R, Strazzullo P, Williams JC, Consensus Conference Group Metabolic diagnosis and medical prevention of calcium nephrolithiasis and its systemic manifestations: a consensus statement. J Nephrol. 2016;29:715–734. doi: 10.1007/s40620-016-0329-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karet FE, Gainza FJ, Gyory AZ, Unwin RJ, Wrong O, Tanner MJ, Nayir A, Alpay H, Santos F, Hulton SA, Bakkaloglu A, Ozen S, Cunningham MJ, Di Pietro A, Walker WG, Lifton RP. Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci USA. 1998;95:6337–6342. doi: 10.1073/pnas.95.11.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambrey R, Trepiccione F. Relative roles of principal and intercalated cells in the regulation of sodium balance and blood pressure. Curr Hypertens Rep. 2015;17:538. doi: 10.1007/s11906-015-0538-0. [DOI] [PubMed] [Google Scholar]
- 8.Mumtaz R, Trepiccione F, Hennings JC, Huebner AK, Serbin B, Picard N, Ullah AKMS, Păunescu TG, Capen DE, Lashhab RM, Mouro-Chanteloup I, Alper SL, Wagner CA, Cordat E, Brown D, Eladari D, Hübner CA. Intercalated cell depletion and vacuolar H+-ATPase mistargeting in an Ae1 R607H knockin model. J Am Soc Nephrol. 2017;28:1507–1520. doi: 10.1681/ASN.2016020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrong O, Bruce LJ, Unwin RJ, Toye AM, Tanner MJ. Band 3 mutations, distal renal tubular acidosis, and Southeast Asian ovalocytosis. Kidney Int. 2002;62:10–19. doi: 10.1046/j.1523-1755.2002.00417.x. [DOI] [PubMed] [Google Scholar]
- 10.Karet FE, Finberg KE, Nelson RD, Nayir A, Mocan H, Sanjad SA, Rodriguez-Soriano J, Santos F, Cremers CW, Di Pietro A, Hoffbrand BI, Winiarski J, Bakkaloglu A, Ozen S, Dusunsel R, Goodyer P, Hulton SA, Wu DK, Skvorak AB, Morton CC, Cunningham MJ, Jha V, Lifton RP. Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet. 1999;21:84–90. doi: 10.1038/5022. [DOI] [PubMed] [Google Scholar]
- 11.Fuster DG, Zhang J, Xie XS, Moe OW. The vacuolar ATPase B1 subunit in distal tubular acidosis: novel mutations and mechanisms for dysfunction. Kidney Int. 2008;73:1151–1158. doi: 10.1038/ki.2008.96. [DOI] [PubMed] [Google Scholar]
- 12.Smith AN, Skaug J, Choate KA, Nayir A, Bakkaloglu A, Ozen S, Hulton SA, Sanjad SA, Al-Sabban EA, Lifton RP, Scherer SW, Karet FE. Mutations in ATP6N1B, in encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet. 2000;26:71–75. doi: 10.1038/79208. [DOI] [PubMed] [Google Scholar]
- 13.Stover EH, Borthwick KJ, Bavalia C, Eady N, Fritz DM, Rungroj N, Giersch AB, Morton CC, Axon PR, Akil I, Al-Sabban EA, Baguley DM, Bianca S, Bakkaloglu A, Bircan Z, Chauveau D, Clermont MJ, Guala A, Hulton SA, Kroes H, Li Volti G, Mir S, Mocan H, Nayir A, Ozen S, Rodriguez Soriano J, Sanjad SA, Tasic V, Taylor CM, Topaloglu R, Smith AN, Karet FE. Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J Med Genet. 2002;39:796–803. doi: 10.1136/jmg.39.11.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capasso G, Unwin R, Rizzo M, Pica A, Giebisch G. Bicarbonate transport along the loop of Henle: molecular mechanisms and regulation. J Nephrol. 2002;15((suppl 5)):S88–S96. [PubMed] [Google Scholar]
- 15.Capasso G, Unwin R, Agulian S, Giebisch G. Bicarbonate transport along the loop of Henle. I. Microperfusion studies of load and inhibitor sensitivity. J Clin Invest. 1991;88:430–437. doi: 10.1172/JCI115322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karim Z, Szutkowska M, Vernimmen C, Bichara M. Recent concepts concerning the renal handling of NH3/NH4+. J Nephrol. 2006;19((suppl 9)):S27–S32. [PubMed] [Google Scholar]
- 17.Kim S, Lee JW, Park J, Na KY, Joo KW, Ahn C, Kim S, Lee JS, Kim GH, Kim J, Han JS. The urine-blood PCO gradient as a diagnostic index of H+-ATPase defect distal renal tubular acidosis. Kidney Int. 2004;66:761–767. doi: 10.1111/j.1523-1755.2004.00801.x. [DOI] [PubMed] [Google Scholar]
- 18.Batlle D, Grupp M, Gaviria M, Kurtzman NA. Distal renal tubular acidosis with intact capacity to lower urinary pH. Am J Med. 1982;72:751–758. doi: 10.1016/0002-9343(82)90540-x. [DOI] [PubMed] [Google Scholar]
- 19.Wrong O, Davies HE. The excretion of acid in renal disease. Q J Med. 1959;28:259–313. [PubMed] [Google Scholar]
- 20.Walsh SB, Shirley DG, Wrong OM, Unwin RJ. Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment: an alternative to ammonium chloride. Kidney Int. 2007;71:1310–1316. doi: 10.1038/sj.ki.5002220. [DOI] [PubMed] [Google Scholar]
- 21.Al-Awqati Q. Terminal differentiation in epithelia: the role of integrins in hensin polymerization. Annu Rev Physiol. 2011;73:401–412. doi: 10.1146/annurev-physiol-012110-142253. [DOI] [PubMed] [Google Scholar]
- 22.Trepiccione F, Soukaseum C, Baudrie V, Kumai Y, Teulon J, Villoutreix B, Cornière N, Wangemann P, Griffith AJ, Byung Choi Y, Hadchouel J, Chambrey R, Eladari D. Acute genetic ablation of pendrin lowers blood pressure in mice. Nephrol Dial Transplant. 2017 doi: 10.1093/ndt/gfw393. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sinning A, Radionov N, Trepiccione F, López-Cayuqueo KI, Jayat M, Baron S, Cornière N, Alexander RT, Hadchouel J, Eladari D, Hübner CA, Chambrey R. Double knockout of the Na+-driven Cl−/HCO3− exchanger and Na+/Cl− cotransporter induces hypokalemia and volume depletion. J Am Soc Nephrol. 2017;28:130–139. doi: 10.1681/ASN.2015070734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trepiccione F, Zacchia M, Capasso G. The role of the kidney in salt-sensitive hypertension. Clin Exp Nephrol. 2012;16:68–72. doi: 10.1007/s10157-011-0489-y. [DOI] [PubMed] [Google Scholar]
- 25.Trepiccione F, Soukaseum C, Iervolino A, Petrillo F, Zacchia M, Schutz G, Eladari D, Capasso G, Hadchouel J. A fate-mapping approach reveals the composite origin of the connecting tubule and alerts on “single-cell”-specific KO model of the distal nephron. Am J Physiol Renal Physiol. 2016;311:F901–F906. doi: 10.1152/ajprenal.00286.2016. [DOI] [PubMed] [Google Scholar]
- 26.Kovacikova J, Winter C, Loffing-Cueni D, Loffing J, Finberg KE, Lifton RP, Hummler E, Rossier B, Wagner CA. The connecting tubule is the main site of the furosemide-induced urinary acidification by the vacuolar H+-ATPase. Kidney Int. 2006;70:1706–1716. doi: 10.1038/sj.ki.5001851. [DOI] [PubMed] [Google Scholar]
- 27.Trepiccione F, Capasso G, Nielsen S, Christensen BM. Evaluation of cellular plasticity in the collecting duct during recovery from lithium-induced nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2013;305:F919–F929. doi: 10.1152/ajprenal.00152.2012. [DOI] [PubMed] [Google Scholar]
- 28.De Bruijn PI, Larsen CK, Frische S, Himmerkus N, Praetorius HA, Bleich M, Leipziger J. Furosemide-induced urinary acidification is caused by pronounced H+ secretion in the thick ascending limb. Am J Physiol Renal Physiol. 2015;309:F146–F153. doi: 10.1152/ajprenal.00154.2015. [DOI] [PubMed] [Google Scholar]
- 29.Wagner S, Vogel R, Lietzke R, Koob R, Drenckhahn D. Immunochemical characterization of a band 3-like anion exchanger in collecting duct of human kidney. Am J Physiol. 1987;253:F213–F221. doi: 10.1152/ajprenal.1987.253.2.F213. [DOI] [PubMed] [Google Scholar]
- 30.Peters LL, Shivdasani RA, Liu SC, Hanspal M, John KM, Gonzalez JM, Brugnara C, Gwynn B, Mohandas N, Alper SL, Orkin SH, Lux SE. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86:917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- 31.Kittanakom S, Cordat E, Akkarapatumwong V, Yenchitsomanus PT, Reithmeier RA. Trafficking defects of a novel autosomal recessive distal renal tubular acidosis mutant (S773P) of the human kidney anion exchanger (kAE1) J Biol Chem. 2004;279:40960–40971. doi: 10.1074/jbc.M405356200. [DOI] [PubMed] [Google Scholar]
- 32.Cordat E, Kittanakom S, Yenchitsomanus PT, Li J, Du K, Lukacs GL, Reithmeier RA. Dominant and recessive distal renal tubular acidosis mutations of kidney anion exchanger 1 induce distinct trafficking defects in MDCK cells. Traffic. 2006;7:117–128. doi: 10.1111/j.1600-0854.2005.00366.x. [DOI] [PubMed] [Google Scholar]
- 33.Walsh S, Borgese F, Gabillat N, Unwin R, Guizouarn H. Cation transport activity of anion exchanger 1 mutations found in inherited distal renal tubular acidosis. Am J Physiol Renal Physiol. 2008;295:F343–F350. doi: 10.1152/ajprenal.00587.2007. [DOI] [PubMed] [Google Scholar]
- 34.Walsh S, Turner MC, Toye A, Wagner CA, Jaeger P, Laing C, Unwin R. Immunohistochemical comparison of a case of inherited distal renal tubular acidosis (with a unique AE1 mutation) with an acquired case secondary to autoimmune disease. Nephrol Dial Transplant. 2007;22:807–812. doi: 10.1093/ndt/gfl662. [DOI] [PubMed] [Google Scholar]
- 35.Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA. Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl−/HCO3− exchanger (slc4a1) J Am Soc Nephrol. 2007;18:1408–1418. doi: 10.1681/ASN.2006101072. [DOI] [PubMed] [Google Scholar]
- 36.Akel A, Wagner CA, Kovacikova J, Kasinathan RS, Kiedaisch V, Koka S, Alper SL, Bernhardt I, Wieder T, Huber SM, Lang F. Enhanced suicidal death of erythrocytes from gene-targeted mice lacking the Cl−/HCO3− exchanger AE1. Am J Physiol Cell Physiol. 2007;292:C1759–C1767. doi: 10.1152/ajpcell.00158.2006. [DOI] [PubMed] [Google Scholar]
- 37.Jarolim P, Shayakul C, Prabakaran D, Jiang L, Stuart-Tilley A, Rubin HL, Simova S, Zavadil J, Herrin JT, Brouillette J, Somers MJ, Seemanova E, Brugnara C, Guay-Woodford LM, Alper SL. Autosomal dominant distal renal tubular acidosis is associated in three families with heterozygosity for the R589H mutation in the AE1 (band 3) Cl−/HCO3− exchanger. J Biol Chem. 1998;273:6380–6388. doi: 10.1074/jbc.273.11.6380. [DOI] [PubMed] [Google Scholar]
- 38.Coury F, Zenger S, Stewart AK, Stephens S, Neff L, Tsang K, Shull GE, Alper SL, Baron R, Aliprantis AO. SLC4A2-mediated Cl−/HCO3−exchange activity is essential for calpain-dependent regulation of the actin cytoskeleton in osteoclasts. Proc Natl Acad Sci USA. 2013;110:2163–2168. doi: 10.1073/pnas.1206392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Finberg KE, Wagner CA, Bailey MA, Paunescu TG, Breton S, Brown D, Giebisch G, Gaibel JP, Lifton RP. The B1-subunit of the H+ATPase is required for maximal urinary acidification. Proc Natl Acad Sci USA. 2005;102:13616–13621. doi: 10.1073/pnas.0506769102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dou H, Finberg KE, Cardell EL, Lifton RP, Choo D. Mice lacking the B1 subunit of H+-ATPase have normal hearing. Hear Res. 2003;180:76–84. doi: 10.1016/s0378-5955(03)00108-4. [DOI] [PubMed] [Google Scholar]
- 41.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, Sohet F, Wagner CA, Eladari D, Chambrey R. Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest. 2013;123:4219–4231. doi: 10.1172/JCI63492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hennings JC, Picard N, Huebner AK, Stauber T, Maier H, Brown D, Jentsch TJ, Vargas-Poussou R, Eladari D, Hubner CA. A mouse model for distal renal tubular acidosis reveals a previously unrecognized role of the V-ATPase a4 subunit in the proximal tubule. EMBO Mol Med. 2012;4:1057–1071. doi: 10.1002/emmm.201201527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trepiccione F, Gerber SD, Grahammer F, Lopez-Cayuqueo KI, Baudrie V, Paunescu TG, Capen DE, Picard N, Alexander RT, Huber TB, Chambrey R, Brown D, Houillier P, Eladari D, Simons M. Renal Atp6ap2/(Pro)renin receptor is required for normal vacuolar H+-ATPase function but not for the renin-angiotensin system. J Am Soc Nephrol. 2016;27:3320–3330. doi: 10.1681/ASN.2015080915. [DOI] [PMC free article] [PubMed] [Google Scholar]