Introduction
Intimate partner violence (IPV) is a public health issue as well as a serious social problem. It is estimated that 5.3 million IPV victimizations occur each year.1 More than1 in 4 women experience IPV during their lifetime.2 Abused women are at higher risk for physical and mental health problems, including injury, chronic pain, gynecological and gastrointestinal problems, substance abuse, depression, anxiety, and posttraumatic stress disorder (PTSD).3–7 The CDC estimate that IPV costs society $5.8 billion annually for physical and mental health care, and lost productivity.8
Pregnant women are particularly vulnerable to the harmful effects of IPV, because the violence may affect both maternal and neonatal health. The prevalence of IPV during pregnancy is 0.9 to 26%, depending on variant IPV definitions and study designs.9–11 Violence during pregnancy may be more common than preeclampsia, gestational diabetes, and placenta previa.10, 12 It is well documented that IPV around the time of pregnancy is associated with physical and mental health problems and negative health behaviors. Studies have found an increased risk of maternal injury and death, inadequate prenatal care, smoking and alcohol use in women who experienced IPV.10–13–16
Research on the impact of IPV on neonatal outcomes has yielded mixed results and conflicting findings may be due to variant definitions, different outcome measures, and study designs.10 In a review of 23 studies, 10 studies reported no significant differences or mixed results in birth outcomes between abused and non-abused women. The remaining 13 studies found significant differences in birth outcomes: preterm delivery, low birth weight, fetal death, miscarriage and neonatal intensive care.10 Among studies that found significant differences, the risk was 2 to 4 times greater for delivering a low birth weight infant.17, 18 Abused compared to non-abused women were 1.6 to 2.7 times as likely to have preterm delivery.19, 20 Another systematic review selected 8 studies for meta-analysis that assessed the association between IPV and low birth weight.21 The bivariate association was found in 6 of those studies. However, among the 6 studies, one study did not control for other confounders and 2 studies found no differences after controlling for other factors.
This study aimed to clarify the role of IPV and its association with adverse birth outcomes. We analyzed data from chart reviews of pregnant women, who were screened for IPV at the first prenatal visit and the postpartum visit. Birth outcomes of the abused pregnant women were compared with those of the non-abused pregnant women. We hypothesized that abused pregnant women would have poorer birth outcomes compared to non-abused pregnant women. Outcome measures were preterm delivery, neonatal intensive care, and low birth weight.
Materials and Methods
Study sample
The participants for this study were patients of an urban university affiliated prenatal clinic and its on-site hospital. The prenatal clinic has 12 obstetrics and gynecology faculty and residents who provide medical care to approximately 2,000 pregnant women per year. Inclusion criteria were pregnant women who were seen at the clinic, and who gave birth at the on-site hospital between January 1, 2003 and December 31, 2009. Women without documented IPV screening results, or women who did not give birth at the on-site hospital were excluded.
Procedures
All pregnant women were screened for IPV at the first prenatal visit and the postpartum visit by the providers, and the screening results recorded in the computerized medical charts. We generated a computerized list of women who were seen at the prenatal clinic between January 1, 2003 and December 31, 2009 and have available IPV screening results. This computerized list included information on the names, unique medical record number, IPV screening results, age of the women, and date of visits. If a woman gave birth to more than 1 child during the study time frame, only the most recent pregnancy was included. The unique medical record numbers were used for random selection employing a computer-generated random sequence. Random selection was stratified by victim status. We abstracted from the first prenatal visit to postpartum visits.
Prior to the start of data collection, part-time chart abstractors received online training provided by the university in computerized medical record systems and issues of confidentiality. The research team developed a chart abstraction form. Training materials included explicit criteria for all variables abstracted. One investigator (MV) conducted 10 chart reviews to pilot test the chart abstraction form and training material. The data abstractors received intensive training by an investigator. At the beginning of data collection, interrater reliability for chart abstractors was assessed for the major outcome variables. Each abstractor reviewed a sample of the same 25 charts and a Kappa statistic was calculated. The data abstraction form was modified if the Kappa statistic was less than 0.7; and the data abstractor was retrained to insure the accuracy and reliability of chart abstraction.22, 23 Intrarater reliability was used to accomplish ongoing monitoring of data quality. An investigator (PC) reviewed any discrepancies and corrected the data.
For birth outcomes, data were abstracted from the hospital electronic medical record (EMR) system. After the women gave birth at the on-site hospital, the newborn’s unique medical record number was recorded in the mother’s chart. The newborn also had her or his own chart, containing name, birth date, unique medical record number, mother’s name, and mother’s birth date, and other information. We used mother’s name and mother’s birth date to identify the charts of the newborns of the selected women. We abstracted birth outcomes of the infants from birth to hospital discharge.
Instruments and measures
IPV during pregnancy
Our main independent variable was IPV measured by a 4-item IPV screening tool - HITS (Hurt, Insulted, Threatened with harm, and Screamed at).24 HITS has been developed for use in primary care settings, and tested with diverse populations.24–27 HITS measures IPV in a current relationship and is comprised of the following four items: (1) “How often does your partner physically hurt you?” (2) “How often does your partner insult you or talk down to you?” (3) “How often does your partner threaten you with harm?” and (4) “How often does your partner scream or curse at you?” Answers to each question are based on a 5-point scale from never to frequently (1 to 5). Answers are summed to form an interval scale of the total HITS score from 4 to 20. Using a cutoff score of 10.5, HITS has accurately classified 91% of non-victims and 96% of female victims.24 HITS has demonstrated good reliability and concurrent validity with the Conflict Tactics Scale (CTS), an established gold standard for measuring partner violence. Cronbach’s alpha was 0.80 for HITS and the correlation is 0.85 between HITS and CTS.
Strategies were used in the IPV screening protocols on the study site to minimize underreporting of IPV, including building relationships with the respondents, ensuring privacy and confidentiality, and providing the respondent with multiple opportunities for disclosure. All physicians and medical staff received training on screening techniques and the use of HITS. As most women regularly see the providers for routine exams, a relationship between providers and patients has been established. Women are seen alone in a private room where providers screen for IPV. Providers enter the information into the EMR and the computer automatically calculates the HITS score. The computer then generates a 'pop-up' warning when the patient scores above a particular threshold indicating additional investigation should occur. Physicians then refer victims to an on-site social worker for intervention. The victims were referred to IPV service advocates by the social worker, if needed. This same screening procedure was used throughout the entire time period of chart review.
Maternal and neonatal health
The main outcome variables for maternal and neonatal health were preterm delivery, low birth weight, and neonatal intensive care. Preterm delivery was defined as gestational age less than 38 weeks.28–30 Neonatal intensive care was measured by prevalence of receiving intensive care. Based on published studies, low birth weight was defined as those who are born at <2,500g.16, 21, 28, 29, 31–33 Infants weighing >=2,500g at birth were considered normal weight. As the three outcome measures may be clinically related, we also created a combined birth outcome measure to indicate any of the three poor birth outcomes versus none.
Confounding variables for maternal and neonatal health
Confounding variables for maternal and neonatal health: race/ethnicity, age (<18, 18–34, >35),29 education level, employment status, marital status, insurance type, living arrangements, planned pregnancy, current use of alcohol, weight at the first prenatal visit, and a comorbidity index (0,1,2,3+). The Charlson comorbidity index (CCI) is a weighted index of 19 categories of diseases that have been found to be related to mortality.34, 35 Charlson comorbid conditions (and their corresponding weightings) include myocardial infarction (1), congestive heart failure (1), peripheral vascular disease (1), cerebrovascular disease (1), dementia (1), chronic pulmonary disease (1), connective tissue disease (1), ulcer disease (1), mild liver disease (1), diabetes without complications (1), diabetes with complications (2), hemiplegia (2), renal disease (2), any tumor without metastases (2), leukemia (2), moderate or severe liver disease (3), metastatic solid tumor (3), and acquired immunodeficiency syndrome (6). Increasing scores on the CCI reflect an increasing burden of comorbid conditions.
Statistical analysis
We used descriptive statistics and bivariate analyses to characterize the sample based on victim status. Adverse birth outcomes by victim status were compared using Fisher’s exact test for dichotomized variables and chi-square test for categorical variables. We then assessed the association between each of the other predictor variables with each of the 3 separate outcomes: preterm delivery, low birth weight, and neonatal intensive care. We set statistical significance at the 0.05 level. SPSS, Version 21 was used to conduct the analysis.
To test the hypothesis that abused women are more likely to have adverse birth outcomes, we built a series of models for each of the 3 separate dichotomized outcomes. We analyzed each outcome in separate models. Variables significantly associated with each outcome at the p<0.05 level were retained in the model. Colinearity was checked by correlation analysis to avoid misestimating the contribution of confounders. Adjusted odds ratios and 95% confidence intervals were calculated. As in the bivariate analysis, statistical significance levels were set at 0.05.
Results and Discussion
Participants
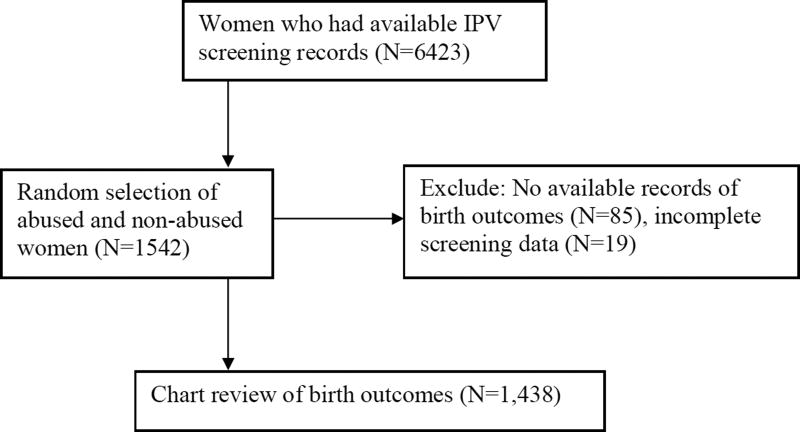
Figure 1 presents the flow chart of the study. During the chart review period from 2008 to 2010, the health informatics department generated a list of 6423 pregnant women seen between 2003 and 2009. Approximately 25% of these charts were randomly selected for review (N=1542). Chart reviews indicated that 85 women did not meet the inclusion criteria and an additional 19 women had incomplete screening records. Therefore, 1,438 pregnant women were included in the final analysis. Of these, 7.5% were victims (N=109) and 92.5% were non-victims (1,433) based on HITS score.
Figure 1.
Flow chart of study design
Table 1 presents the demographic characteristics of participants. The victims and non-victims were similar on all but 2 variables. Victims compared to non-victims were more likely to be current users of alcohol (7.6% vs. 2.7%, p=.012). Victims were more likely to have comorbidities than non-victims (57.8% vs. 43.9%, p=.0035).
Table 1.
Sample characteristics of participants by intimate partner violence screening protocol
| Variable | Victims | Non- victims |
Total | P value |
|---|---|---|---|---|
|
| ||||
| N=109 | N=1329 | N=1,438 | ||
|
| ||||
| Race/ethnicity (%) | ||||
| White | 0.9 | 1.4 | 1.4 | 0.645 |
| African American | 54.1 | 50.7 | 50.9 | |
| Hispanic | 40.4 | 40.3 | 40.3 | |
| Other | 4.6 | 7.6 | 7.4 | |
|
| ||||
| Age (mean, years) | 27.1 | 25.9 | 26.0 | 0.070 |
|
| ||||
| Educational level (%) | 0.703 | |||
| Some high school or less | 47.3 | 44.8 | 45.0 | |
| High school completed | 33.8 | 37.7 | 37.5 | |
| Some college | 9.5 | 10.9 | 10.8 | |
| College completed | 9.5 | 6.5 | 6.7 | |
|
| ||||
| Employed (including part-time) (%) | 25.2 | 28.6 | 28.4 | 0.265 |
|
| ||||
| Marital status (%) | 0.688 | |||
| Single/widowed/divorced | 77.1 | 77.5 | 77.5 | |
| Married | 19.3 | 20.1 | 20.1 | |
| Separated | 3.7 | 2.4 | 2.5 | |
|
| ||||
| Medicare/Medicaid (%) | 0.309 | |||
| Commercial fee for service | 0.0 | 0.5 | 0.5 | |
| Commercial HMO | 1.9 | 4.6 | 4.4 | |
| Medicaid or charity care | 67.6 | 67.6 | 67.6 | |
| Medicaid HMO | 25.7 | 25.1 | 25.1 | |
| Medicare | 4.8 | 2.2 | 2.4 | |
|
| ||||
| Living arrangement | 0.972 | |||
| Participant’s house/apartment | 77.5 | 77.1 | 77.1 | |
| Someone else’s house/apartment | 21.6 | 21.5 | 21.5 | |
| An institution | 1.0 | 1.3 | 1.3 | |
| Other | 0.0 | 0.2 | 0.1 | |
|
| ||||
| Planned pregnancy (%) | 19.6 | 25.1 | 24.7 | 0.227 |
|
| ||||
| Current use of alcohol (%) | 7.6 | 2.7 | 3.1 | 0.012 |
|
| ||||
| Weight at first prenatal visit (%) | 163.5 | 160.8 | 161.0 | 0.534 |
|
| ||||
| Comorbidity (%) | ||||
| 0 | 42.2 | 56.1 | 55.0 | 0.035 |
| 1 | 39.4 | 29.6 | 30.3 | |
| 2 | 11.9 | 10.5 | 10.6 | |
| >=3 | 6.4 | 3.8 | 4.0 | |
Prevalence of poor birth outcomes among victims and non-victims
Table 2 shows significant differences between victims and non-victims in the prevalence of preterm delivery, low birth weight, and neonatal intensive care. Compared to non-abused women, abused women were more likely to have preterm deliveries (18.3% vs. 10.3%; p=.016). Compared to infants of non-victims, infants of victims were more likely to have low birth weight (21.5% vs. 11.0%; p=.003) and to receive neonatal intensive care (23.4% vs. 7.8%; p=.000).
Table 2.
Unadjusted prevalence of preterm deliveries, low birth weight, and neonatal intensive care by IPV victim status
| Variable | Victims | Non- | Total | P value |
|---|---|---|---|---|
| N=109 | N=1329 | N=1,438 | ||
| Preterm deliveries (%) | 18.3 | 10.3 | 10.9 | .016 |
| Low birth weight (%) | 21.5 | 11.0 | 11.8 | .003 |
| Neonatal intensive care (%) | 23.4 | 7.8 | 9.0 | .000 |
| Any of the poor birth outcomes (%) | 34.3 | 17.2 | 18.5 | .000 |
Premature babies were more likely to be low birth weight (p<.001), and low birth weight and premature babies were more likely to need intensive care (p<.001 and p<.001, respectively) (not shown). Victims were twice as likely to have any of the poor birth outcomes (34.3% vs. 17.2%, p<.001).
Multivariate analysis of preterm deliveries, low birth weight, and neonatal intensive care
Multivariate predictors of preterm deliveries, low birth weight, and neonatal intensive care are presented in Table 3. As current use of alcohol and comorbidities were significantly associated with IPV in the bivariate analysis, they were included as confounders in the multivariate analysis. After controlling for current use of alcohol and comorbidity, IPV was associated with preterm deliveries, low birth weight, and neonatal intensive care (p=.049, .007, and .000, respectively). Victims of IPV had 1.72 times the odds of preterm deliveries, 2.03 times the odds of low birth weight, and 3.33 times the odds of neonatal intensive care as non-victims. In addition, victims of IPV had 2.19 times the odds of any of the 3 poor birth outcomes as non-victims (p=.001).
Table 3.
Multivariate analysis of preterm deliveries, low birth weight, and neonatal intensive care
| Preterm deliveries (N=1394) |
Low birth weight (N=1361) |
Neonatal intensive care (N=1341) |
Any poor birth outcomes (N=1318) |
|||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Predictor Variables |
Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
P value | Odds Ratio (95% CI) |
P value |
|
| ||||||||
| IPV | ||||||||
| Non-victims | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Victims | 1.72 | .049 | 2.03 | 0.007 | 3.33 | .000 | 2.19 | .001 |
| (1.00–2.95) | (1.22–3.39) | (1.99–5.58) | (1.40–3.43) | |||||
Discussion
This study showed a significant association between IPV and poor birth outcomes. In this population, approximately one fifth of victims had preterm deliveries and delivered low birth weight babies. Almost one fourth of the infants of victims received neonatal intensive care. As expected, the three outcome measures are all clinically related. Consistent with some previous studies, victims of IPV were 2–3 times more likely to have preterm deliveries, low birth weight, and neonatal intensive care.10, 17–20 Unlike the majority of prior studies, our study did show that the association between IPV and poor birth outcomes exists even after controlling for potential confounders.21 These conflicting findings may be due to variant definitions, different outcome measures, and study designs of previous studies.10
In a recent update, the United States Preventive Services Task Force (USPSTF) recommended that clinicians screen women of childbearing age for IPV and provide or refer women who screen positive for intervention services.36, 37 Previous studies have found that IPV is queried in less than 15% of encounters and interventions are inadequate in up to 90% of cases.38 In this study, although screening and brief intervention were conducted with patients, poor birth outcomes were still found in victims of IPV. Perhaps, more education and promotion strategies are needed to overcome provider and patient barriers. Provider barriers include lack of office protocols, limited time, lack of education, lack of resources, and competing demands.4, 39–41 Access to care and lack of understanding of providers are barriers perceived by patients.42–45 There is some evidence that patient education and treatment intervention leads to increased safety planning, reduced anxiety, and depression among victims of IPV.46–48 Further research is needed to develop office-based intervention for both providers and IPV victims.
Our study has several limitations. First, patients may choose not to disclose their abuse status. Therefore, this study may underestimate the difference between the victims and non-victims in terms of outcomes. Second, the study was conducted in an urban and academic setting so the study results may not be generalized to other clinical settings. Third, this was a chart review study, which is limited by lack of information on certain variables. For instance, income was not documented in the chart. Nonetheless, employment and insurance could be considered proxies for income. Due to the large number of missing data, we did not include some confounders in the analytic sample (e.g., history of child abuse). No imputation of data was done in the analysis. However, there were no significant differences between the analytic sample and the sample with missing data.
Implications
This is one of the few randomized retrospective cohort studies to report the association of IPV to preterm deliveries, low birth weight, and neonatal intensive care, controlling for potential confounders. Our study has considerable strengths. This study would not have been possible were it not for the universal screening that was done using an EMR system. The cohort study design allows us to look at abused women over time throughout the pregnancy, and compare them with their counterparts. This study is based on a large sample of pregnant women. Thus the study has sufficient power to detect statistically significant differences between abused and non-abused women. Unlike the majority of prior studies, this study used a screening tool that demonstrates good validity and reliability. HITS measures verbal, emotional, and physical abuse, thus providing information on various aspects of IPV.
Healthy People 2020 has specified the goals and objectives that address improved maternal and child health, including reduction in low birth weight.49 Besides poor birth outcomes, victims of IPV and their children may suffer long-term negative health effects. It is crucial to establish a standardized comprehensive intervention that promotes birth outcomes for victims of IPV.4, 45
Acknowledgments
Funding statement: National Institute of Health (RO3HD058249).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: The authors report no conflict of interest.
Contributor Information
Ping-Hsin Chen, Email: chenpi@njms.rutgers.edu, Department of Family Medicine, Rutgers New Jersey Medical School, 183 South Orange Avenue, BHSB E1557, Newark, NJ 07103.
Sue Rovi, Department of Family Medicine, Rutgers New Jersey Medical School.
Marielos L. Vega, Department of Family Medicine, Rutgers New Jersey Medical School.
Theodore Barrett, Department of Obstetrics, Gynecology and Women’s Health, Rutgers New Jersey Medical School.
Ko-Yu Pan, Department of Family Medicine, Rutgers New Jersey Medical School.
Mark S. Johnson, Community & Family Medicine, Howard University College of Medicine.
References
- 1.Tjaden P, Thoennes N. Extent, Nature, and Consequences of Intimate Partner Violence: Findings from the National Violence against Women Survey (NCJ187867) Washington, DC: U.S. Department of Justice; 2000. [Google Scholar]
- 2.Black MC, Basile KC, Breiding MJ, et al. The National Intimate Partner and Sexual Violence Survey: 2010 Summary Report. Atlanta: National Center for Injury Prevention and Control, Centers for Disease Control and Prevention; 2011. [Accessed September 26, 2014]. http://www.cdc.gov/violenceprevention/pdf/nisvs_report2010-a.pdf. [Google Scholar]
- 3.Centers for Disease Control and Prevention. [Accessed September 26, 2014];Intimate partner violence: consequences. http://www.cdc.gov/violenceprevention/intimatepartnerviolence/consequences.html.
- 4.Chen P-H, Rovi S, Jacobs A. Intimate partner violence: office screening for victims and perpetrators of IPV. FP Essent. 2013;412:11–17. [PubMed] [Google Scholar]
- 5.Chen PH, Rovi S, Vega M, Jacobs A, Johnson MS. Relation of domestic violence to health status among Hispanic women. J Health Care Poor Underserved. 2009;20(2):569–582. doi: 10.1353/hpu.0.0145. [DOI] [PubMed] [Google Scholar]
- 6.Tandon SD, Parillo KM, Jenkins C, Duggan AK. Formative evaluation of home visitors’ role in addressing poor mental health, domestic violence, and substance abuse among low-income pregnant and parenting women. Maternal & Child Health Journal. 2005 Sep;9(3):273–283. doi: 10.1007/s10995-005-0012-8. [DOI] [PubMed] [Google Scholar]
- 7.Martin SL, Li Y, Casanueva C, Harris-Britt A, Kupper LL, Cloutier S. Intimate partner violence and women’s depression before and during pregnancy. Violence Against Women. 2006 Mar;12(3):221–239. doi: 10.1177/1077801205285106. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Injury Prevention and Control. Costs of Intimate Partner Violence Against Women in the United States. Atlanta (GA): Centers for Disease Control and Prevention; 2003. [Google Scholar]
- 9.Woods SM, Melville JL, Guo Y, et al. Psychosocial stress during pregnancy. Am J Obstet Gynecol. 2010;202:61.e1-7. doi: 10.1016/j.ajog.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boy A, Salihu HM. Intimate partner violence and birth outcomes: a systematic review. International Journal of Fertility & Womens Medicine. 2004 Jul-Aug;49(4):159–164. [PubMed] [Google Scholar]
- 11.Jasinski JL. Pregnancy and domestic violence: a review of the literature. Trauma Violence & Abuse. 2004 Jan;5(1):47–64. doi: 10.1177/1524838003259322. [DOI] [PubMed] [Google Scholar]
- 12.Gazmararian JA, Lazorick S, Spitz AM, Ballard TJ, Saltzman LE, Marks JS. Prevalence of violence against pregnant women. JAMA. 1996;275:1915–1920. [PubMed] [Google Scholar]
- 13.Wilson LM, Reid AJ, Midmer DK, Biringer A, Carroll JC, Stewart DE. Antenatal psychosocial risk factors associated with adverse postpartum family outcomes. CMAJ Canadian Medical Association Journal. 1996 Mar 15;154(6):785–799. [PMC free article] [PubMed] [Google Scholar]
- 14.Bullock L, Bloom T, Davis J, Kilburn E, Curry MA. Abuse disclosure in privately and medicaid-funded pregnant women. Journal of Midwifery & Women’s Health. 2006 Sep-Oct;51(5):361–369. doi: 10.1016/j.jmwh.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Curry MA, Durham L, Bullock L, Bloom T, Davis J. Nurse case management for pregnant women experiencing or at risk for abuse. JOGNN - Journal of Obstetric, Gynecologic, & Neonatal Nursing. 2006 Mar-Apr;35(2):181–192. doi: 10.1111/j.1552-6909.2006.00027.x. [DOI] [PubMed] [Google Scholar]
- 16.McFarlane J, Parker B, Soeken K. Physical abuse, smoking, and substance use during pregnancy: prevalence, interrelationships, and effects on birth weight. JOGNN - Journal of Obstetric, Gynecologic, & Neonatal Nursing. 1996 May;25(4):313–320. doi: 10.1111/j.1552-6909.1996.tb02577.x. [DOI] [PubMed] [Google Scholar]
- 17.Covington DL, Justason BJ, Wright LN. Severity, manifestations, and consequences of violence among pregnant adolescents. Journal of Adolescent Health. 2001 Jan;28(1):55–61. doi: 10.1016/s1054-139x(00)00154-3. [DOI] [PubMed] [Google Scholar]
- 18.Valladares E, Ellsberg M, Pena R, Hogberg U, Persson LA. Physical partner abuse during pregnancy: a risk factor for low birth weight in Nicaragua. Obstetrics & Gynecology. 2002 Oct;100(4):700–705. doi: 10.1016/s0029-7844(02)02093-8. [DOI] [PubMed] [Google Scholar]
- 19.Lipsky S, Holt VL, Easterling TR, Critchlow CW. Impact of police-reported intimate partner violence during pregnancy on birth outcomes. Obstetrics & Gynecology. 2003 Sep;102(3):557–564. doi: 10.1016/s0029-7844(03)00573-8. [DOI] [PubMed] [Google Scholar]
- 20.Covington DL, Hage M, Hall T, Mathis M. Preterm delivery and the severity of violence during pregnancy. Journal of Reproductive Medicine. 2001 Dec;46(12):1031–1039. [PubMed] [Google Scholar]
- 21.Murphy CC, Schei B, Myhr TL, Du Mont J. Abuse: a risk factor for low birth weight? A systematic review and meta-analysis. CMAJ Canadian Medical Association Journal. 2001 May 29;164(11):1567–1572. [PMC free article] [PubMed] [Google Scholar]
- 22.Herman CJ, Speroff T, Cebul RD. Improving compliance with breast cancer screening in older women. Results of a randomized controlled trial. Archives of Internal Medicine. 1995 Apr 10;155(7):717–722. [PubMed] [Google Scholar]
- 23.Fletcher SW, Harris RP, Gonzalez JJ, et al. Increasing mammography utilization: a controlled study. Journal of the National Cancer Institute. 1993 Jan 20;85(2):112–120. doi: 10.1093/jnci/85.2.112. [DOI] [PubMed] [Google Scholar]
- 24.Sherin KM, Sinacore JM, Li XQ, Zitter RE, Shakil A. HITS: a short domestic violence screening tool for use in a family practice setting. Family Medicine. 1998 Jul-Aug;30(7):508–512. [PubMed] [Google Scholar]
- 25.Chen P-H, Rovi S, Vega M, Jacobs A, Johnson MS. Screening for domestic violence in predominantly Hispanic clinical settings. Family Practice, eprint. 2005:1–7. doi: 10.1093/fampra/cmi075. [DOI] [PubMed] [Google Scholar]
- 26.Chen P-H, Rovi S, Washington J, Jacobs A, Vega M, Pan K-Y, Johnson MS. Randomized comparison of 3 methods to screen for domestic violence in family practice. Annals of Family Medicine. 2007;5:430–435. doi: 10.1370/afm.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shakil A, Donald S, Sinacore JM, Krepcho M. Validation of the HITS domestic violence screening tool with males. Fam Med. 2005;37(3):193–198. [PubMed] [Google Scholar]
- 28.Silverman JG, Decker MR, Reed E, Raj A. Intimate partner violence victimization prior to and during pregnancy among women residing in 26 U.S states: associations with maternal and neonatal health. American Journal of Obstetrics & Gynecology. 2006 Jul;195(1):140–148. doi: 10.1016/j.ajog.2005.12.052. [DOI] [PubMed] [Google Scholar]
- 29.Campbell J, Torres S, Ryan J, et al. Physical and nonphysical partner abuse and other risk factors for low birth weight among full term and preterm babies: a multiethnic case-control study. American Journal of Epidemiology. 1999 Oct 1;150(7):714–726. doi: 10.1093/oxfordjournals.aje.a010074. [DOI] [PubMed] [Google Scholar]
- 30.Janssen PA, Holt VL, Sugg NK, Emanuel I, Critchlow CM, Henderson AD. Intimate partner violence and adverse pregnancy outcomes: a population-based study. American Journal of Obstetrics & Gynecology. 2003 May;188(5):1341–1347. doi: 10.1067/mob.2003.274. [DOI] [PubMed] [Google Scholar]
- 31.McFarlane J, Parker B, Soeken K. Abuse during pregnancy: associations with maternal health and infant birth weight. Nursing Research. 1996 Jan-Feb;45(1):37–42. doi: 10.1097/00006199-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002 Jan;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 33.Hack M, Schluchter M, Cartar L, Rahman M, Cuttler L, Borawski E. Growth of very low birth weight infants to age 20 years. Pediatrics. 2003 Jul;112(1 Pt 1):e30–e38. doi: 10.1542/peds.112.1.e30. [DOI] [PubMed] [Google Scholar]
- 34.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 35.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology. 1992 Jun;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 36.US Preventive Services Task Force. US Preventive Task Force Recommendation Statement. Rockville, MD: Agency for Healthcare Research and Quality; 2013. [Accessed September 26, 2014]. Screening for intimate partner violence and abuse of elderly and vulnerable adults. http://www.uspreventiveservicestaskforce.org/uspstf12/ipvelder/ipvelderfinalrs.htm. [Google Scholar]
- 37.Nelson HD, Bougatsos C, Blazina I. Screening Women for Intimate Partner Violence. A Systematic Review to Update the 2004 U.S. Preventive Services Task Force Recommendation. Ann Intern Med. 2012;156(11):796–808. doi: 10.7326/0003-4819-156-11-201206050-00447. [DOI] [PubMed] [Google Scholar]
- 38.Thompson RS, Rivara FP, Thompson DC, et al. Identification and management of domestic violence: a randomized trial. Am J Prev Med. 2000;19(4):253–263. doi: 10.1016/s0749-3797(00)00231-2. [DOI] [PubMed] [Google Scholar]
- 39.Gerber MR, Leiter KS, Hermann RC, Bor DH. How and why community hospital clinicians document a positive screen for intimate partner violence: a cross-sectional study. BMC Fam Pract. 2005;6:48. doi: 10.1186/1471-2296-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flocke SA, Frank SH, Wenger DA. Addressing multiple problems in the family practice office visit. Journal of Family Practice. 2001 Mar;50(3):211–216. [PubMed] [Google Scholar]
- 41.Jaen CR, Stange KC, Nutting PA. Competing demands of primary care: a model for the delivery of clinical preventive services. Journal of Family Practice. 1994 Feb;38(2):166–171. [PubMed] [Google Scholar]
- 42.Mohr DC, Ho J, Duffecy J, et al. Perceived barriers to psychological treatments and their relationship to depression. J Clin Psychol. 2010;66(4):394–409. doi: 10.1002/jclp.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mohr DC, Hart SL, Howard I, et al. Barriers to psychotherapy among depressed and nondepressed primary care patients. Ann Behav Med. 2006;32(3):254–258. doi: 10.1207/s15324796abm3203_12. [DOI] [PubMed] [Google Scholar]
- 44.Dowd MD, Kennedy C, Knapp JF, Stallbaumer-Rouyer J. Mothers’ and health care providers’ perspectives on screening for intimate partner violence in a pediatric emergency department. Arch Pediatr Adolesc Med. 2002;156(8):794–799. doi: 10.1001/archpedi.156.8.794. [DOI] [PubMed] [Google Scholar]
- 45.Iverson KM, Gradus JL, Resick PA, Suvak MK, Smith KF, Monson CM. Cognitive-behavioral therapy for PTSD and depression symptoms reduces risk for future intimate partner violence among interpersonal trauma survivors. J Consult Clin Psychol. 2011:79-193-202. doi: 10.1037/a0022512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson-Whelen S, Hughes RB, Powers LE, Oschwald M, Renker P, Swank PR, Curry MA. Efficacy of a computerized abuse and safety assessment intervention for women with disabilities: a randomized controlled trial. Rehabilitation psychology. 2010;55(2):97–107. doi: 10.1037/a0019422. [DOI] [PubMed] [Google Scholar]
- 47.Coker AL, Smith PH, Whitaker DJ, Le B, Crawford TN, Flerx VC. Effect of an in-clinic IPV advocate intervention to increase help seeking, reduce violence, and improve well-being. Violence Against Women. 2012;18(1):118–131. doi: 10.1177/1077801212437908. [DOI] [PubMed] [Google Scholar]
- 48.Chen P-H, Rovi S, Jacobs A. Intimate partner violence: counseling, community resources, and legal issues for IPV victims and perpetrators. FP Essent. 2013;412:18–23. [PubMed] [Google Scholar]
- 49.Healthy People 2020 Topics & Objectives. [Accessed September 26, 2014]; http://www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=26.