Abstract
Background
Viridans group streptococcal (VGS) bacteremia is common among neutropenic patients. Although VGS bacteremia occurs in non-neutropenic patients, risk factors are not well established. We conducted a case-case-control study to identify risk factors for VGS among neutropenic and non-neutropenic patients.
Methods
Patients with VGS bacteremia between January 2009 and December 2014 in our 200-bed clinical research hospital were identified using microbiology records. Neutropenic and non-neutropenic patients at the time of positive culture were matched 1:1 to controls on the basis of neutrophil count (ANC), ward, and length of stay. We extracted demographic, laboratory, medication, and other clinical data from chart reviews. Data were analyzed using McNemar’s test, Wilcoxon signed-rank test, and conditional logistic regression modeling.
Results
Among 101 patients, 63 were neutropenic and 38 non-neutropenic at the time of VGS bacteremia. In multivariable analysis of neutropenic patients, only lower ANC predicted VGS bacteremia (odds ratio [OR], 0.16; 95% confidence interval [CI], 0.05–0.59; P = 0.006). Recent use of vancomycin was protective (OR, 0.23; 95% CI, 0.07–0.73; P = 0.013). No clinical factors were associated with VGS in the non-neutropenic cases.
Conclusions
Only lower ANC nadir increased the risk for VGS bacteremia in the neutropenic group, and vancomycin was protective. Other previously described factors (chemotherapy, radiation, oral conditions) related to neutropenia were not independently associated with VGS bacteremia. No tested clinical factors predicted infection in the non-neutropenic group. Our results suggest that VGS bacteremia should be anticipated when making antimicrobial choices in profoundly neutropenic patients, and merit further exploration in non-neutropenic patients.
Keywords: bacteremia, neutropenia, Streptococcus mitis, viridans
Viridans group streptococci (VGS) are part of the normal flora of the mouth and GI tract. In the past, bacteremia caused by these organisms was limited largely to patients who had valvular abnormalities or were undergoing dental procedures. VGS bacteremia has, however, become a common source of morbidity among neutropenic patients [1–3] as the organisms are thought to translocate into the bloodstream when mucosal barriers are compromised in the setting of neutropenia, chemotherapy-induced mucositis, and alteration in the stomach pH [1].The clinical course of VGS bacteremia can be complicated by acute respiratory distress syndrome and a toxic shock–like picture known as “VGS shock syndrome,” resulting in high mortality [1–5].
Gram-negative bacteremias carry a high rate of mortality in the setting of neutropenia, and thus the empirical antibiotic therapy and prophylaxis in this population have traditionally focused on Gram-negative pathogens. In recent decades, Gram-positive organisms have become the dominant etiology, responsible for up to 70% of bacteremias in this population [6, 7]. VGS are isolated in approximately one-quarter of these cases, and their rise has been associated with the increased use of ciprofloxacin and TMP-SMX prophylaxis in patients who have prolonged neutropenia [2, 8].
Several studies have described risk factors for VGS bacteremia among neutropenic patients, including mucositis, use of proton pump inhibitors, cytosine arabinoside, and fluoroquinolone prophylaxis [1, 4, 9–11]. Gram-positive antimicrobial coverage is protective [9, 12]. We observed, however, that almost 40% of patients with VGS bacteremia at our center were not neutropenic at the time of positive culture. Risk factors for such patients are not well established, and identifying modifiable risk factors would potentially allow for preventive interventions. Our goal was to identify and contrast the risk factors for VGS bacteremia among neutropenic and non-neutropenic patients in our 200-bed clinical research hospital.
METHODS
Study Design and Identification of Cases and Controls
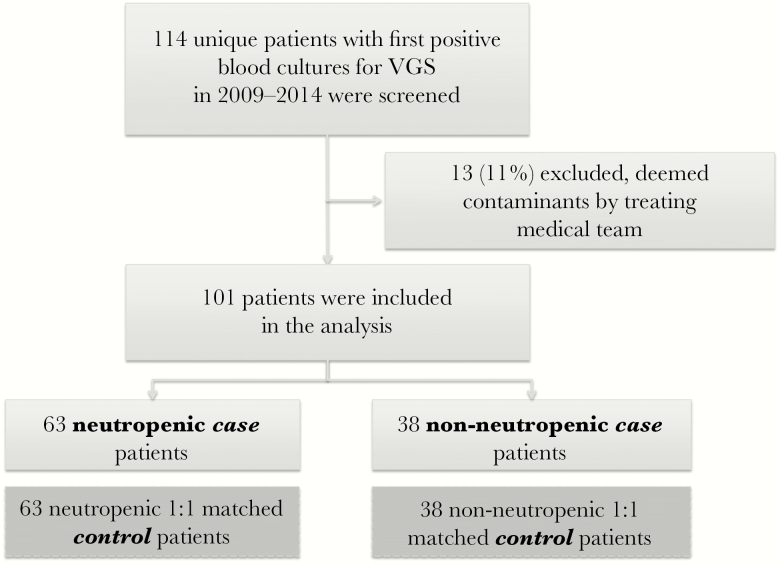
To compare and contrast the risk factors between 2 defined patient groups (neutropenic and non-neutropenic), we designed a retrospective case-case-control study [13], in which 2 case-control studies were conducted in parallel. Patients who had VGS bacteremia between January 2009 and December 2014 were identified using microbiology records on the basis of at least 1 positive blood culture. This retrospective electronic chart review was exempt from ethics review. A preliminary chart review was used to exclude patients whose cultures were deemed by clinicians at the time to be contaminants. Only the first bacteremic episode per patient was analyzed. We divided patients into neutropenic and non-neutropenic case groups based on absolute neutrophil count (ANC) ≤ or >1000 cells/mm3 at the time of culture positivity.
Controls were selected on the basis of ANC, hospital ward at the time of case patient VGS bacteremia, and length of stay (LOS), and they were matched 1:1 to case patients, yielding 2 case and 2 control groups (Figure 1). For controls, the day of positive culture of the matched case patient was used as the “reference date” for the purpose of ascertaining data and clinical parameters.
Figure 1.
Screening and inclusion of patients in the case-case-control design. Abbreviation: VGS = viridans group streptococci.
Data Collection
We extracted clinical data from electronic medical record review, including demographic characteristics, microbiologic data, antibiotic exposure during the 3-week period prior to VGS bacteremia (or reference date for controls), and other clinical parameters. Speciation was confirmed by MALDI-TOF mass spectrometry (Bruker Daltonics Inc., Billerica, MA). Antibiotic susceptibility testing was performed with the BD Phoenix (Becton-Dickinson) and Etest (bioMérieux) systems.
ANC was recorded on the day of first positive culture (or reference date for controls) and categorized by degree of neutropenia (<200 cells/mL, 201–500/mL, 501–1000/mL) and non-neutropenia (>1000/mL). Other potential risk factors were abstracted for the 3 weeks prior to VGS bacteremia (or reference date for controls), including number of days of neutropenia, receipt and days of radiation therapy, chemotherapeutic agents, mucositis and other oral conditions, dental symptoms, dental procedures, sinus computed tomography (CT) with abnormality consistent with sinusitis, neutropenic enterocolitis diagnosed by abdominal CT, C. difficile infection, gastrointestinal graft-vs-host disease (confirmed by histology), stem cell transplantation, and use of H2 blocker and/or proton pump inhibitor (PPI).
Antibiotic exposure data for the 3 weeks prior to VGS bacteremia (or reference date for controls) included receipt of and days of therapy with antibacterial and antifungal agents. For descriptive purposes, clinical outcome data were collected, including need for supplementary oxygen, intensive care unit (ICU) level of care or mechanical ventilation within the 5 days following culture positivity, and 30-day mortality.
In addition, underlying conditions were noted, and the Charlson comorbidity index was calculated for each patient [14]. The Pitt bacteremia score, a prognostic score (0–14 points, worse prognosis at higher scores) that uses clinical parameters such as fever, hypotension, need for mechanical ventilation, mental status, and cardiac arrest, was also calculated for both case groups [15].
Statistical Analysis
Data are described as frequencies and percentages, medians (interquartile [25th–75th percentile] ranges), and odds ratios (ORs) and 95% confidence intervals (CIs). Analyses were conducted separately for each case-control study/patient group, and then compared between them. Paired data within each study were analyzed using the paired t test or Wilcoxon signed rank test, or McNemar’s test, as appropriate. Data were compared between studies by the 2-sample t test or Wilcoxon rank-sum test, and the Fisher exact test. For each case-control study, conditional univariable and multivariable logistic regression analyses were carried out for assessing the relation between risk factors, antibiotic exposures, and clinical outcomes, and VGS bacteremia. A P value <0.05 and 95% CIs excluding 1.0 were considered statistically significant. Data were analyzed using SAS v9.4 (SAS Institute, Inc, Cary, NC).
RESULTS
Study Population
Over the 6-year study period, 114 distinct patients who had VGS bacteremia were identified. Thirteen of these cultures were deemed to be contaminants, leaving 101 cases for analysis, including 63 neutropenic case patients and 38 non-neutropenic case patients (Figure 1), each with their respective 1:1 matched controls. No patient was found to have endocarditis. Central lines were present in 97% of neutropenic case patients and 71% of non-neutropenic case patients. Baseline characteristics can be seen in Table 1.
Table 1.
Characteristics of Neutropenic and Non-neutropenic Patients Who Had VGS Bacteremia and Their Respective Matched Controls
| Neutropenic Cases (n = 63) |
Neutropenic Controls (n = 63) |
P Value (Cases vs Controls) |
Non-neutropenic Cases (n = 38) |
Non-neutropenic Controls (n = 38) |
P Value (Cases Vs Controls) |
P Value (Cases vs Cases) |
P Value (Controls vs Controls) |
|
|---|---|---|---|---|---|---|---|---|
| Female, n (%) | 34 (54%) | 25 (40%) | 0.12 | 10 (26%) | 20 (53%) | 0.018 | 0.008 | 0.22 |
| Age, median (IQR), y | 41.3 (24–56) | 42.6 (25–56) | 0.65 | 50 (30–64) | 47.2 (36–57) | 0.63 | 0.028 | 0.14 |
| Age < 18 y | 10 (16%) | 9 (14%) | 0.32 | 1 (3%) | 2 (5%) | 0.32 | 0.049 | 0.20 |
| LOS, median (IQR), d | 30 (23–63) | 34 (24–61) | 0.24 | 17 (7–30) | 18.5 (8–24) | 0.99 | <0.001 | <0.001 |
| Pitt score, median (IQR) | 1 (0–3) | N/A | N/A | 1 (0–2) | N/A | N/A | 0.29 | N/A |
| Charlson index, median (IQR) | 2 (2–3) | 2 (1–4) | 0.69 | 4 (2–6) | 3.5 (2–7) | 0.94 | 0.033 | 0.019 |
| Underlying condition | 0.76 | 0.89 | <0.001 | <0.001 | ||||
| Solid tumor | 6 (10%) | 4 (6%) | 16 (42%) | 15 (39%) | ||||
| Hematologic malignancy | 39 (62%) | 40 (63%) | 10 (26%) | 11 (29%) | ||||
| HIV | 1 (2%) | 0 | 2 (5%) | 2 (5%) | ||||
| Severe aplastic anemia | 15 (24%) | 12 (19%) | 1 (3%) | 2 (5%) | ||||
| Other | 2 (3%) | 7 (11%) | 9 (24%) | 8 (21%) |
The sum of percentages may not equal 100% due to rounding.
Abbreviations: IQR = interquartile range (25th–75th percentile); LOS = length of stay; VGS = viridans group streptococci.
Neutropenic vs Non-Neutropenic Patients
Non-neutropenic patients had a significantly higher proportion of solid organ malignancy and a lower proportion of hematologic malignancy (P < 0.001), higher Charlson comorbidity index (P = 0.033 for cases and P = 0.019 for controls), and shorter length of stay (P < 0.001 for both cases and controls) than neutropenic patients (Table 1). Non-neutropenic cases were older than neutropenic cases (median age, 50 years; range, 30–64 years; vs median age, 41.3 years; range, 24–56 years, P = 0.028), but there was no significant age difference between the 2 groups’ controls (Table 1).
Pitt bacteremia scores and median lengths of stay prior to positive blood culture did not differ significantly between neutropenic and non-neutropenic cases.
Cases vs Controls
There was no significant difference between the median age of cases and respective controls for either the neutropenic or non-neutropenic groups (Table 1). There were fewer female non-neutropenic cases than respective controls (26.3% vs 52.6%; P = 0.018). The proportions of pediatric patients (age < 18 years) were 15.9% and 14.3% (P = 0.32) among the neutropenic cases and controls, respectively, and 2.6% and 5.3% (P = 0.32) among non-neutropenic cases and controls, respectively. The median length of stay was also similar between cases and controls in each group, as described in Table 1. The Charlson comorbidity index did not differ significantly between cases and controls for either neutropenic or non-neutropenic groups.
Microbiological Features
Among neutropenic and non-neutropenic case patients, 54.0% and 32.0%, respectively, grew VGS from additional blood cultures within 7 days of the first positive VGS blood culture; 76.2% and 71.1% had multiple positive blood cultures that grew VGS with or without additional organisms. In the neutropenic group, S. mitis represented 75.8% of VGS isolates, S. salivarius 16.7%, S. oralis 6.1%, and S. gordonii 1.5%. Isolates in the non-neutropenic group were 58.5% S. mitis, 19.5% S. salivarius, 7.3% S. anginosus, 4.9% S. bovis, and 2.4% for each of the following: S. constellatus, S. gallolyticus, S. intermedius, and S. lutetiensis.
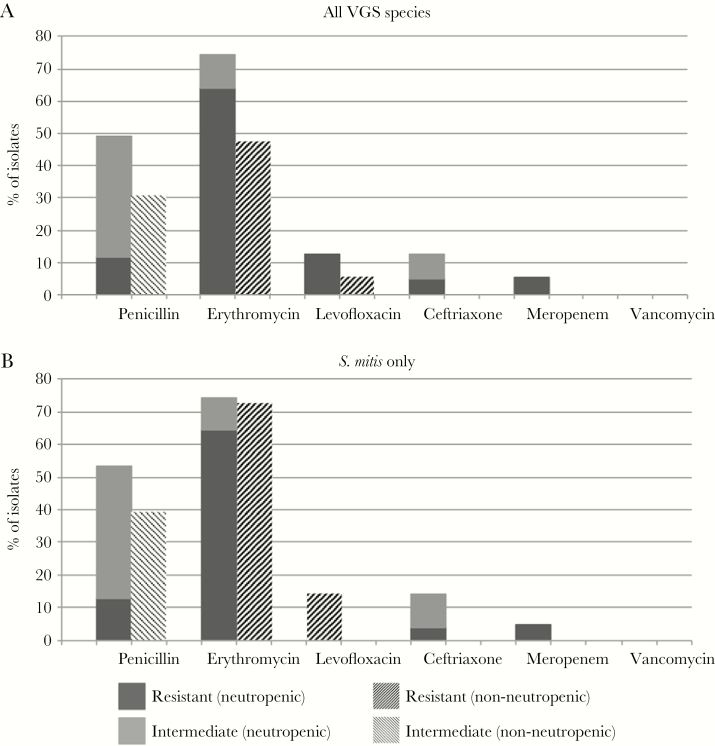
The antimicrobial resistance patterns of the case patients’ VGS isolates are described in Figure 2A. While we observed a higher frequency of antimicrobial resistance among VGS isolates from neutropenic patients, only cephalosporin resistance was significantly more common in this group than among matched controls (P = 0.022). The susceptibility pattern of the most common species, S. mitis, is shown in Figure 2B. S. salivarius was the second most frequently isolated species, with 11 isolates in the neutropenic group and 8 in the non-neutropenic group. Among 19 S. salivarius isolates, nonsusceptibility was observed for penicillin (n = 3), ceftriaxone (n = 1), erythromycin (n = 3), levofloxacin (n = 1), and meropenem (n = 1) in the neutropenic group, and for penicillin (n = 3), erythromycin (n = 1), and levofloxacin (n = 1) in the non-neutropenic group.
Figure 2.
Antibiotic resistance pattern of viridans group streptococcal isolates per neutropenic and non-neutropenic groups. Graphic (A) shows data for all VGS and (B) shows data for only S. mitis. Abbreviation: VGS = viridans group streptococci.
Predictive Clinical Associations
In conditional univariable models, receipt of cyclophosphamide (P = 0.011) or fludarabine (P = 0.023), receipt of radiation (P = 0.027) and duration of radiation therapy (P = 0.036), mucositis or other oral condition (P < 0.001), and ANC at the time of positive culture (P < 0.001) were all significantly predictive of VGS bacteremia among neutropenic patients (Table 2). The duration of neutropenia prior to bacteremia was not significantly associated with VGS bacteremia. Receipt of cephalosporins (P < 0.001), carbapenems (P = 0.027), and vancomycin (P < 0.001) in the 3 weeks prior to positive culture was protective in this group. Ceftazidime accounted for most of the cephalosporin use in neutropenic patients (8 of 9 cases and 25 of 29 controls). However, while still statistically significant, the apparent protective role of cephalosporins was weaker when adjusted for vancomycin use (OR, 0.32; 95% CI, 0.12–0.81; P = 0.017). The receipt of fluoroquinolones was not found to be significantly associated with VGS bacteremia in this group.
Table 2.
Description of Conditional Logistic Regression Models of Potential Risk Factors for VGS Bacteremia in Neutropenic and Non-neutropenic Patients
| Univariable Models | Multivariable Modela | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Neutropenic Patients | Non-neutropenic Patients | Neutropenic Patients | |||||||
| Covariateb | OR | 95% CI | P Value | OR | 95% CI | P Value | OR | 95% CI | P Value |
| Radiation therapy | 3.20 | 1.12–11.17 | 0.027 | 2.00 | 0.104–117.99 | NS | 7.96 | 0.16–406.81 | 0.30 |
| Radiation days | 1.41 | 1.02–2.05 | 0.036 | 1.12 | 0.62–2.59 | NS | 0.70 | 0.23–2.19 | 0.54 |
| Mucositis/oral condition | 4.50 | 1.82–13.33 | <0.001 | 2.00 | 0.29–22.11 | NS | 4.08 | 0.73–22.64 | 0.11 |
| ANCc | 0.11 | 0.02–0.34 | <0.001 | — | — | — | 0.16 | 0.05–0.59 | 0.006 |
| Cyclophosphamide | 3.60 | 1.29–12.40 | 0.011 | 0.40 | 0.04–2.44 | NS | 6.82 | 0.85–54.56 | 0.07 |
| Fludarabine | 3.00 | 1.14–9.23 | 0.023 | 0.50 | 0.05–3.49 | NS | 0.68 | 0.11–4.27 | 0.68 |
| Cephalosporins | 0.23 | 0.08–0.57 | <0.001 | 0.57 | 0.12–2.25 | NS | 0.24 | 0.04–1.43 | 0.12 |
| Carbapenems | 0.31 | 0.09–0.89 | 0.027 | 0.67 | 0.06–5.82 | NS | 1.28 | 0.15–10.88 | 0.82 |
| Vancomycind | 0.21 | 0.07–0.53 | <0.001 | 0.60 | 0.09–3.08 | NS | 0.16 | 0.02–1.66 | 0.12 |
Abbreviations: ANC = absolute neutrophil count; CI = confidence interval; OR = odds ratio; VGS = viridans group streptococci.
aThe condition for inclusion into the multivariable model was set to P < 0.1 by univariable analysis.
bIn the 3 weeks prior to culture positivity for cases; for controls, the day of positive culture of the matched case was used as the “reference date” for the purpose of ascertaining data and clinical parameters. Only covariates that were statistically significant for at least 1 of the groups are listed.
cANC was recorded on the day of first positive culture (or reference date for controls) and categorized by level (<200 cell/mm3, 201–500 cell/mm3, 501–1000 cell/mm3) for analysis. This analysis is only applicable to neutropenic patients as ANC level was >1000 cell/mm3 for all cases and controls in the non-neutropenic group. Using actual ANC counts (all >1000 cell/mm3) for the non-neutropenic group yielded nonsignificant results (OR, 0.98; 95% CI, 0.89–1.07; P = 0.61) in univariable analysis.
dOR=0.23, 95% CI: 0.07-0.73; P=0.013 when ANC was the only other covariate after iteratively removing least explanatory covariates from the multivariable model.
No factor was independently found to be significantly associated with development of VGS bacteremia among non-neutropenic cases (Table 2).
Other variables that were not associated with VGS bacteremia in either group included other chemotherapeutic agents, other antimicrobial drugs, dental symptoms or procedures, abnormal sinus CT, neutropenic enterocolitis, C. difficile infection, intestinal graft-vs-host disease, stem cell transplantation, PPI or H2 blocker use, need for supplementary oxygen or mechanical ventilation, ICU admission, and 30-day mortality.
Variables with P < 0.1 in univariable analyses were included in the conditional multivariable logistic regression model (Table 2). No variable met this criterion in the non-neutropenic group.
In the multivariable model, lower ANC nadir was the sole predictor of VGS bacteremia in the neutropenic group (OR, 0.16; 95% CI, 0.05–0.59; P = 0.006). Detailed analysis suggested that the risk of VGS bacteremia decreased by 0.8% per each 1-cell/mm3 increase in ANC nadir. In a multivariable model including only the 2 most explanatory variables, vancomycin use and ANC, exposure to vancomycin in the previous 3 weeks was also found to be protective (OR, 0.23; 95% CI, 0.07–0.73; P = 0.013).
Outcomes
Although neutropenic case patients were more likely to be admitted to the ICU in the 30 days following bacteremia than neutropenic controls in the 30 days following the reference date (17.5% vs 4.8%; P = 0.033), 30-day mortality (1 vs 3 patients, P = 0.32) and need for supplemental oxygen (22.2% vs 16.1%, P = 0.32) did not differ significantly between neutropenic cases and controls. In the non-neutropenic group, there was no significant difference between case and control patients in ICU admissions (26.3% vs 13.2%; P = 0.10), death (0 vs 1 patient; P = 1.0), or oxygen requirement (29.7% vs 23.7%; P = 0.37).
DISCUSSION
Among neutropenic patients, lower ANC increased risk of VGS bacteremia, and recent vancomycin use was protective. The degree of neutropenia was also found to be an independent risk factor in past studies [1]. The duration of neutropenia leading up to the bacteremic episode was not found to be a significant risk factor, which is surprising given that patients expected to have prolonged neutropenia are targeted for antibiotic prophylaxis. While many other factors have been described in previous studies, it seems that the degree of neutropenia plays a critical role in host susceptibility to these organisms and is closely linked with other described associations such as mucositis, chemotherapy, and radiation therapy.
Exposure to vancomycin in the 3 weeks prior was found to be protective in our study, consistent with earlier studies showing reduced VGS bacteremia with early administration of vancomycin [12, 16]. Recent exposure to antibiotics with Gram-positive coverage may reduce oral and gastrointestinal VGS colonization, making it less likely for these commensals to translocate into the bloodstream. In our study, the only antibiotic shown to be an independent protective factor was vancomycin, while other types of Gram-positive coverage (such as penicillins and cephalosporins) were not associated with a lower frequency of bacteremia, despite the overall high susceptibility to beta-lactam antibiotics among the isolates.
The use of fluoroquinolones has been implicated as a risk factor for VGS bacteremia in prior studies [1], attributed to the relatively high rate of VGS resistance to fluoroquinolones. We did not observe this finding in our study. One possible explanation could be the more frequent use of levofloxacin as it has a broader spectrum for Gram-positive organisms than earlier-generation fluoroquinolones. Levofloxacin has been recommended as the fluoroquinolone of choice in neutropenic patients with particularly high risk for mucositis and VGS infection [17].
No tested clinical factors predicted infection in the non-neutropenic group. VGS bacteremia in non-neutropenic hosts is traditionally considered an infection occurring primarily after oral procedures or in the setting of endocarditis, but we did not find oral conditions to be an independent risk factor, and no enrolled patient had endocarditis. It is a consideration, however, that the non-neutropenic population was heterogeneous in underlying condition, which may make identification of common risk factors more challenging in a small sample size. Another possibility is that some VGS bacteremias in non-neutropenic patients represented contaminants, despite their subsequent treatment as bona fide infections.
Vancomycin retained excellent in vitro activity for S. mitis among our patients, consistent with previous literature [18]. Isolates from neutropenic patients were overall less susceptible to ceftriaxone, a firstline treatment for VGS bacteremia [19], than were those from non-neutropenic patients (85% vs 100%). This finding supports the continued practice of using vancomycin as empirical therapy for VGS bacteremia in neutropenic patients. Fewer than 75% of isolates in both groups were fully susceptible to penicillin, in accord with the literature that penicillin susceptibility can no longer be assumed in VGS, with reduced susceptibility reported to be as high as 60% in some reports [20].
There was no significant difference in ICU admission, oxygen requirement, or 30-day mortality between cases and controls in both groups. The lack of difference in outcomes might be related to having a mixed control group, which included both bacteremic and nonbacteremic patients, as well as a relatively small sample size and few adverse events (3 neutropenic patients developed viridans streptococcal shock syndrome, and 4 neutropenic patients died within 30 days of VGS bacteremia).
Limitations of our study include a low ratio of cases to controls. We matched only 1 control per case because a higher ratio was not possible with our matching criteria in a small hospital. We cannot entirely exclude a possible misclassification of some VGS cultures as contaminants or true bacteremias. In some past studies, a case definition required 2 or more positive blood cultures of the same VGS species or clear signs and symptoms of bacteremia [1, 4, 10]; in contrast, our determination of a true bacteremia was largely guided by the clinical decisions of the medical teams. It is also worth noting that previous studies examining risk factors for VGS bacteremia have been heterogeneous regarding their control selection. For instance, Elting et al. used patients with Gram-positive bacteremia as controls, while Bochud et al. used nonbacteremic controls [2, 3]. The different control choice can account for some of the variability in risk factors reported among studies.
We did not exclude patients who had bacteremia due to other organisms in our control groups because the goal was to identify risk factors for VGS bacteremia as compared with the rest of the neutropenic or non-neutropenic patient populations. Excluding infected patients in the control groups could have made controls less representative of the “source” population from which cases were obtained. However, we recognize that choosing infected or noninfected control groups may help identify distinct risk factors [10]. This may have been possible in the setting of a larger institution.
The results of this single case-case-control study suggest that VGS bacteremia in neutropenic patients should continue to be treated as a distinct entity that must be anticipated in a subset of profoundly neutropenic patients. Vancomycin retains very good activity in vitro against these pathogens. VGS bacteremia in non-neutropenic hospitalized patients is likely a heterogeneous condition, rather than a single entity, and deserves further exploration in larger studies to identify opportunities for prevention.
Acknowledgments
The authors are grateful to Dr. Juan C. Gea-Banacloche for his editorial comments.
Financial support. This study was supported by the National Institutes of Health Clinical Center and the intramural research program of the National Institute of Allergy and Infectious Diseases.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentations. The preliminary results of this study were presented at IDWeek 2015 (San Diego, CA, October 2015).
References
- 1. Elting LS, Bodey GP, Keefe BH. Septicemia and shock syndrome due to Viridans streptococci: a case-control study of predisposing factors. Clin Infect Dis 1992; 14:1201–7. [DOI] [PubMed] [Google Scholar]
- 2. Bochud PY, Calandra T, Francioli P. Bacteraemia due to Viridans streptococci in neutropenic patients: a review. Am J Med 1994; 97:256–64. [DOI] [PubMed] [Google Scholar]
- 3. Tunkel AR, Sepkowitz KA. Infections caused by Viridans streptococci in patients with neutropenia. Clin Infect Dis 2002; 34:1524–9. [DOI] [PubMed] [Google Scholar]
- 4. Gassas A, Grant R, Richardson S et al. Predictors of viridans streptococcal shock syndrome in bacteremic children with cancer and stem-cell transplant recipients. J Clin Oncol 2004; 22:1222–7. [DOI] [PubMed] [Google Scholar]
- 5. Marron A, Carratalà J, González-Barca E et al. Serious complications of bacteremia caused by Viridans streptococci in neutropenic patients with cancer. Clin Infect Dis 2000; 31:1126–30. [DOI] [PubMed] [Google Scholar]
- 6. Aust C, Tolfvenstam T, Broliden K et al. Bacteremia in Swedish hematological patients with febrile neutropenia: bacterial spectrum and antimicrobial resistance patterns. Scand J Infect Dis 2013; 45:285–91. [DOI] [PubMed] [Google Scholar]
- 7. Miedema KG, Winter RH, Ammann RA et al. Bacteria causing bacteremia in pediatric cancer patients presenting with febrile neutropenia–species distribution and susceptibility patterns. Support Care Cancer 2013; 21:2417–26. [DOI] [PubMed] [Google Scholar]
- 8. Zinner SH. Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on gram-positive and resistant bacteria. Clin Infect Dis 1999; 29:490–4. [DOI] [PubMed] [Google Scholar]
- 9. Bochud P-Y, Eggiman P, Calandra T et al. Bacteraemia due to Viridans streptococcus in neutropenic patients with cancer: clinical spectrum and risk factors. Clin Infect Dis 1994; 18:25–31. [DOI] [PubMed] [Google Scholar]
- 10. Paganini H, Staffolani V, Zubizarreta P et al. Viridans streptococci bacteraemia in children with fever and neutropenia: a case-control study of predisposing factors. Eur J Cancer 2003; 39:1284–9. [DOI] [PubMed] [Google Scholar]
- 11. Ruescher TJ, Sodeifi A, Scrivani SJ et al. The impact of mucositis on alpha-hemolytic streptococcal infection in patients undergoing autologous bone marrow transplantation for hematologic malignancies. Cancer 1998; 82:2275–81. [PubMed] [Google Scholar]
- 12. Seo SK, Xiao K, Huang YT et al. Impact of peri-transplant vancomycin and fluoroquinolone administration on rates of bacteremia in allogeneic hematopoietic stem cell transplant (HSCT) recipients: a 12-year single institution study. J Infect 2014; 69:341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaye KS, Harris AD, Samore M, Carmeli Y. The case-case-control study design: addressing the limitations of risk factor studies for antimicrobial resistance. Infect Control Hosp Epidemiol 2005; 26:346–51. [DOI] [PubMed] [Google Scholar]
- 14. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994; 47:1245–51. [DOI] [PubMed] [Google Scholar]
- 15. Paterson DL, Ko WC, Von Gottberg A et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial infections. Ann Intern Med 2004; 140:26–32. [DOI] [PubMed] [Google Scholar]
- 16. Jaffe D, Jakubowski A, Sepkowitz K et al. Prevention of peritransplantation Viridans streptococcal bacteremia with early vancomycin administration: a single-center observational cohort study. Clin Infect Dis 2004; 39:1625–32. [DOI] [PubMed] [Google Scholar]
- 17. Freifeld AG, Bow EJ, Sepkowitz KA et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52:e56–93. [DOI] [PubMed] [Google Scholar]
- 18. Marron A, Carratalà J, Alcaide F et al. High rates of resistance to cephalosporins among viridans-group streptococci causing bacteraemia in neutropenic cancer patients. J Antimicrob Chemother 2001; 47:87–91. [DOI] [PubMed] [Google Scholar]
- 19. Baddour LM. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young. Circulation 2005; 394–434. [DOI] [PubMed] [Google Scholar]
- 20. Han SB, Bae EY, Lee JW et al. Clinical characteristics and antimicrobial susceptibilities of viridans streptococcal bacteraemia during febrile neutropenia in patients with hematologic malignancies: a comparison between adults and children. BMC Infect Dis 2013; 13:273. [DOI] [PMC free article] [PubMed] [Google Scholar]