Abstract
Background:
Huntington’s disease (HD) is a progressive neurodegenerative disorder that results in a gradual decline in mobility and balance. Increasing evidence has documented an important role of executive function in the safe ambulation of the elderly and people with a variety of neurological disorders. Little is known about the contribution of cognitive deficits to decline in mobility over time in HD.
Objective:
This study examined the relationships of mobility, motor and cognitive function measures at baseline, and of mobility and cognitive measures over four years.
Methods:
A retrospective chart review was performed on 70 patients with genetically confirmed HD (age 20–75 years old) across 121 HD clinic visits. Correlations between Unified Huntington’s Disease Rating Scale – Total Motor, Tinetti Mobility Test (TMT), and cognitive measures (Letter Verbal Fluency, Symbol Digit Modalities Test (SDMT), and Stroop Test) were analyzed. Longitudinal relationships between TMT and cognitive measures were examined using mixed effect regression models.
Results:
Gait and balance measures representing domains of mobility (TMT scores) were significantly correlated with each of the cognitive measures with the exception of the Verbal Fluency score. Mixed effects regression modeling showed that the Stroop Interference sub-test and SDMT were significant predictors (p-values <0.01) of TMT total scores.
Conclusions:
Impairments in executive function measures correlate highly with measures of gait, balance and mobility in individuals with HD. Interventions designed to improve mobility and decrease fall risk should also address issues of cognitive impairments with particular consideration given to interventions that may focus on motor-cognitive dual task training.
Keywords: Tinetti mobility test, cognition, stroop, symbol digit modalities test, motor function, Huntington’s disease
INTRODUCTION
Huntington’s disease (HD) is a genetic neurodegenerative disorder that results in involuntary movements (chorea), balance and gait impairments, changes in behavior and declines in cognition. Cognitive decline occurs early in the disease course, occasionally preceding chorea and motor impairment, and continues to progress throughout the disease process [1]. Cognitive deficits include difficulty with executive function including trouble with planning and organizing, problems with working, visual and verbal memory, and impaired concentration [2–4]. While gait dysfunction is typically thought to arise primarily from damage to motor circuitry of the basal ganglia, studies in elderly populations and other neurologic populations indicate that gait dysfunction may also be related to changes in cognitive function [5, 6]. As the clinical diagnosis of HD is not commonly made until the onset of motor symptoms, a better understanding of the relationship of cognitive function to motor function and mobility over time is critical. Understanding the interactions between cognitive and motor changes in HD may lead to sounder interventions to decrease mobility impairments, reduce fall risk, and improve referrals for targeted rehabilitation.
Safe ambulation requires both adequate motor control and attention to the variations in the surrounding environment. Prior work indicates a strong relationship between cognitive function and ambulation; in particular, poorer executive function and attention have been correlated with slower gait speed and an increased risk of falls in older adults and in persons with dementia and Parkinson’s disease [7–10]. In the context of a neurological disease, previously automatic movements, such as walking or balancing, may become attention demanding [10, 11] further implicating cognitive impairment as a contributor to compromised mobility. Not surprisingly, individuals with HD demonstrate impairments performing tasks that require simultaneous motor and cognitive processing (i.e., motor-cognitive dual-tasks) [12]. Divided attention is necessary to successfully complete dual-tasks and dual-task skills are required for safe ambulation, especially in more complex environments. Given the relatively early involvement of prefrontal and frontal lobe dysfunction in HD [3, 13, 14] and the significant decline in ambulation, it is likely that cognitive function impacts gait function in individuals with HD. Indeed, frontal lobe atrophy has been linked with both gait and balance dysfunction and executive function in elderly adults, [15] and executive function in particular is a significant predictor of gait velocity over the course of aging [16].
Recent work has linked clinically observable motor function (i.e., grip strength) with cognitive function over time in older adults, [17] yet this relationship has not been explored in individuals with HD with respect to mobility. The purpose of this study was to investigate the longitudinal relationship between mobility measures and cognitive measures in individuals with HD. We hypothesized that 1) executive function measures would have a moderate to strong correlation with mobility measures; and 2) mobility and cognitive measures would have a moderate to strong correlation with disease severity as measured by the Unified Huntington’s Disease Rating Scale Total Motor Score (UHDRS-TMS) [18]; and 3) there would be a slow decline in mobility over time as measured by the Tinetti Mobility Test (TMT) in individuals with HD. A better understanding of the relationship of cognition and mobility and the predictive value of these measures will help investigators to devise interventions that improve mobility and safety in individuals with HD.
2. MATERIALS AND METHODS
Participants
A retrospective review of the medical records of ambulatory patients (21–75 years old) with confirmed HD who attended the Movement Disorders Clinic from 2009 to 2013 was performed. Individuals who had a documented diagnosis of any other neurologic disease or orthopedic condition that negatively impacted their cognition or gait were excluded. We extracted demographic data (age, sex, diagnosis), the UHDRS-TMS, time since diagnosis, years of education, cognitive scores on the standard UHDRS cognitive battery, and performance on the TMT from the database for each appointment for each individual. Not all individuals had both cognitive testing and the TMT performed at all visits and these visits were excluded from the analysis. All procedures were reviewed and approved by the Institutional Review Board at The Ohio State University.
Cognitive measures
The cognitive battery administered as part of the UHDRS consists of three tests: Letter Verbal Fluency Task, Symbol Digit Modalities Test (SDMT), and the three part Stroop Tests (color, word, and interference). These measures have been used routinely in persons with HD [18]. The SDMT and the Stroop tests are measures of executive function. The Stroop Test is also considered to be a measure of processing speed and selective attention [19]. Cognitive testing for all patients was administered by the same individual (AMD).
Mobility measures
The Tinetti Mobility Test (TMT) consists of balance and gait subscales with higher scores out of 28 possible indicating better performance. It is reliable and predicts falls among those with Parkinson’s disease and HD [20–22]. Participants were observed and rated by one of three raters (AK, DK, NF) on their performances of the TMT which is comprises a series of functional maneuvers (e.g., getting up from and sitting down in a chair, standing with and without eyes closed, responding to a nudge, walking and turning). These raters have established inter-rater reliability.
Disease specific motor measures
The UHDRS-TMS for each patient at each visit was administered by the same motor rater certified neurologist (SK).
Statistical analysis
Data were analyzed for patient visits in which both TMT and cognitive measures were obtained. Of primary interest was the change in TMT over time and the effect of cognitive measures on TMT. A mixed effects modeling framework was used for all longitudinal analyses including a random intercept and slope by participant and unstructured covariance. The first observation on each patient was defined as t0. A linear trend in time from t0 was included as a fixed effect in all models and the following additional covariates were considered for inclusion: age, years of education, gender and years from diagnosis. In order to evaluate the impact of the cognitive measures on TMT in the presence of the temporal trend, the cognitive measures were disaggregated into “between” and “within” components [23, 24]. Thus, an average score for the cognitive measure was calculated (“between”) and included in the model as a fixed effect along with the raw value (“within”). Pearson correlation coefficients were also used to examine the relationship between TMT total scores and cognitive measures and the UHDRS-TMS at baseline. The criteria used to evaluate Pearson correlation coefficients were fair (values of 0.25–0.50), moderate to good (values of 0.50–0.75) and excellent (values of 0.75 and above) [25]. All analyses were conducted in SAS v9.4 (SAS Institute, Cary, NC) and R (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Seventy patients (n = 40 females, 57.1%) met the inclusion criteria of this retrospective chart review with cognitive and mobility data across 121 visits. Table 1 provides the patient characteristics. Eleven patients were missing diagnosis date information. A total of 36 patients had only one visit (51.4%), 20 had two (28.6%), 10 had three (14.3%) and 4 had four visits (5.7%). For the 34 patients with multiple visits, the average time between consecutive visits was 1.3 years and the average total follow up time was 1.8 years.
Table 1.
Patient characteristics
| Variable | N | Mean | Std Dev | Minimum | Maximum |
| Age at first exam | 70 | 50.9 | 14.6 | 19.8 | 82.7 |
| Age at diagnosis | 59 | 44.6 | 14.9 | 15.0 | 77.2 |
| Years of education | 70 | 13.0 | 2.7 | 5.0 | 19.0 |
| UHDRS Total Motor Score | 70 | 45.83 | 15.90 | 11.0 | 81.0 |
| Verbal Fluency | 70 | 21.50 | 11.91 | 3.00 | 47.00 |
| SDMT | 70 | 22.91 | 9.66 | 5.00 | 48.00 |
| Stroop Color | 70 | 42.61 | 15.36 | 8.00 | 76.00 |
| Stroop Word | 70 | 54.00 | 21.30 | 14.00 | 110.00 |
| Stroop Interference | 70 | 23.24 | 9.81 | 2.00 | 43.00 |
| Tinetti Mobility Total Score | 70 | 19.64 | 5.78 | 3.00 | 28.00 |
| Number of visits per patient | 70 | 1.7 | 0.9 | 1 | 4 |
| Overall study time for patients with multiple visits (y) | 34 | 1.8 | 0.9 | 0.4 | 3.2 |
SDMT, Symbol Digit Modalities Test; UHDRS, Unified Huntington’s disease rating scale.
3.1. Baseline relationships
Correlation of TMT gait and balance measures with cognitive measures
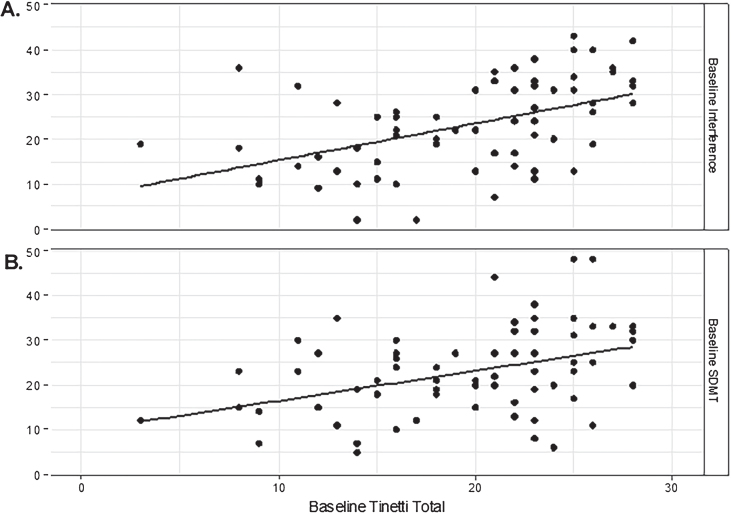
Baseline TMT total score was significantly correlated with each of the cognitive measures (rp = 0.40 SDMT; rp = 0.45 Stroop Color; rp = 0.45 Stroop Word; rp = 0.48 Stroop Interference, all p < 0.001), with the exception of Verbal Fluency (rp = 0.23, p = 0.060). Among the cognitive measures, the measures reflecting executive function including the Stroop and the SDMT (Fig. 1) had the strongest relationship with the TMT. The Stroop Interference sub-test had the strongest relationship with TMT total scores with better performance (i.e., higher scores) correlating with better performance (i.e., higher scores) on the TMT.
Fig.1.
Correlations between the Tinetti total score and A) Stroop Interference. (rp = 0.48; p < 0.001) and B) Symbol Digit Modalities (rp = 0.40, p < 0.001) test scores at baseline (t0).
Correlation of mobility and cognitive measures with the UHDRS-TMS
Both mobility and cognitive performance were correlated with disease motor severity, measured with the UHDRS-TMS. Baseline TMT score was strongly correlated with UHDRS-TMS (rp = –0.62; p < 0.001), indicating that as disease severity worsens, TMT performance declines. Similarly, SDMT (rp = –0.55; p < 0.001) and Stroop (Color: rp = –0.59; p < 0.001; Word: rp = –0.59; p < 0.001; Interference: rp = –0.49; p < 0.001) were strongly correlated with disease severity, while Verbal Fluency was moderately correlated (rp = –0.40; p < 0.001), indicating that greater disease severity is more closely linked with worsening cognitive performance across executive function, processing speed and selective attention domains.
3.2. Longitudinal relationships
Changes in TMT scores over time
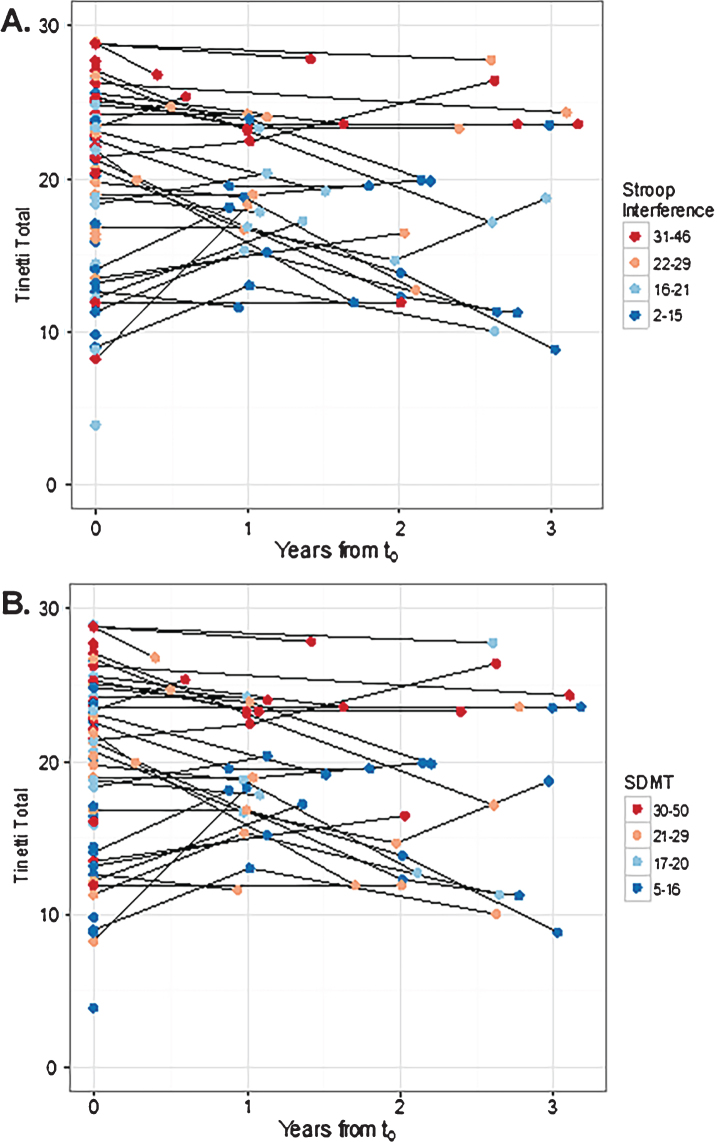
Although there was wide variability in performance on the TMT at baseline (t0) across patients, in general there was relatively low within patient variability in TMT scores over time (Fig. 2). Mixed-effects regression modeling showed an unadjusted decrease of 0.64 points per year (p = 0.13). Including gender, years of education, and years from diagnosis to t0 as covariates in the model had a negligible impact on the temporal trend and were not statistically significant (p-values >0.1). However, age decreased the effect of the temporal trend by roughly 9% (to 0.58 points per year) and was statistically significant (p = 0.044). Thus, in subsequent models, only age was retained in the longitudinal models exploring the effect of the cognitive measures on TMT.
Fig.2.
Three-dimensional scatterplot indicating the relationship of TMT to A) Stroop Interference and B) SDMT over time. Each point on the figure represents a participant visit and the shade of each point represents the A) Stroop Interference score or B) SDMT score, with warmer colors (i.e., reds) indicating better performance and cooler colors (i.e., blues) indicating poorer performance. Visually, the decline in TMT scores over time is evident, and a stable relationship of TMT performance to cognitive performance is maintained over time. Cognitive measures were binned into quartiles indicating performance at 0–25%, 26–50%, 51–75% and 76% -maximum value. SDMT, Symbol Digit Modalities Test; TMT, Tinetti Mobility Test.
Relationship of cognitive and mobility measures over time
Multivariable mixed-effects regression modeling showed that the cognitive measures explained a significant amount of the variability in TMT total scores that remained after adjusting for the temporal trend and age (Table 2). Considering the between patient component, the strongest predictors for TMT total scores were the Stroop Interference sub-test (p = 0.0006) and the SDMT (p = 0.0017). The within patient components of the cognitive measures were not significant in any of the models. However, only 34 patients’ assessments included multiple longitudinal measurements, likely hampering our ability to detect changes within a patient after adjusting for the average. Additionally, the total follow up for patients with multiple measurements was relatively short (mean of 1.8 years, maximum of 3.2 (Table 1)).
Table 2.
Multivariable model results for the Tinetti total score outcome, adjusting for age, time and the between and within components of the Stroop measures (n = 70)
| Cognitive Measure | Predictor | Effect (95% CI) | P-value |
| Stroop Color | Years from t0 | –0.631 (–1.524, 0.262) | 0.1593 |
| Age at t0 | –0.093 (–0.168, –0.017) | 0.0165 | |
| Between | 0.191 (0.041, 0.341) | 0.0135 | |
| Within | –0.013 (–0.144, 0.118) | 0.8421 | |
| Stroop Word | Years from t0 | –0.527 (–1.375, 0.321) | 0.2125 |
| Age at t0 | –0.102 (–0.176, –0.028) | 0.0077 | |
| Between | 0.124 (0.029, 0.219) | 0.0115 | |
| Within | 0.016 (–0.063, 0.095) | 0.6854 | |
| Stroop Interference | Years from t0 | –0.726 (–1.571, 0.119) | 0.0894 |
| Age at t0 | –0.049 (–0.127, 0.028) | 0.2073 | |
| Between | 0.343 (0.151, 0.535) | 0.0006 | |
| Within | –0.057 (–0.208, 0.095) | 0.4532 | |
| Word Fluency | Years from t0 | –0.673 (–1.526, 0.180) | 0.1168 |
| Age at t0 | –0.116 (–0.200, –0.032) | 0.0077 | |
| Between | 0.195 (0.007, 0.383) | 0.0427 | |
| Within | –0.040 (–0.199, 0.119) | 0.6147 | |
| SDMT | Years from t0 | –0.724 (–1.565, 0.117) | 0.0931 |
| Age at t0 | –0.079 (–0.157, –0.000) | 0.0489 | |
| Between | 0.297 (0.115, 0.480) | 0.0017 | |
| Within | –0.047 (–0.185, 0.091) | 0.4505 |
CI, Confidence Interval; SDMT, Symbol Digit Modalities test; significant values are bolded.
DISCUSSION
Motor and cognitive performance are commonly impaired in persons with HD, yet relatively little is known about how they are related to each other. In this study, we examined the utility of the TMT to assess mobility over time as well as its relationships with cognition and disease motor severity at baseline and with cognition over time. The significance of this work is underscored by findings from the TRACK-HD and PREDICT-HD studies demonstrating that both cognitive and quantitative motor impairment are detectable in pre-manifest HD [1, 26, 27]. The TMT is an established measure of mobility in individuals with HD; indeed, low TMT scores have been linked with higher risk of falls [21]. Given that a change of 4 points or greater on the TMT is clinically meaningful, [28, 29] our findings that TMT scores decreased on average 0.58 points per year suggests that individuals with HD would demonstrate significant declines in mobility over approximately 7 years. The low within patient variability indicates that TMT scores are generally stable and decline slowly over time. Although further study is required, these findings suggest that the TMT may be a valid measure of change over time in mobility function in clients with HD
Executive function (Stroop Interference and SDMT) measures were moderately correlated with balance and gait function (TMT) in individuals with HD (rp 0.48 and 0.40 respectively), indicating that better cognitive performance was linked with better mobility. These cognitive function measures also predicted longitudinal TMT total scores after adjusting for time and age, with average 1 unit higher scores on the Stroop Interference and SDMT tests predicting 0.34 and 0.29 unit higher TMT scores respectively. These results are consistent with previous studies showing moderate to strong correlations between measures of executive function and measures of ambulation in aging and in dementia [7–9]. The cognitive processes underlying executive function are believed to be critical to dual tasking during gait, [8] which may explain why individuals with HD often have difficulty with dual task performance [12]. It is likely that cognitive decline found in HD negatively impacts the individual’s ability to perform mobility related tasks required for the TMT and everyday life such as thinking about navigating the environment and processing instructions from a clinician while walking.
Interestingly, TMT score changes across time within patients within this study were not related to cognitive scores. This may be due to the short time frames across which most assessments were conducted and the limited number of participants who were tested repeatedly. Given the results of this study showing a 0.58 point change a year it would take a longer time span across assessments to demonstrate within patient relationships.
Motor and cognitive function at baseline were related with disease severity, measured by UHDRS-TMS. Poorer performance on the TMT was linked with higher disease severity (rp = –0.62; p < 0.001), demonstrating that ambulation skill is related to disease severity. This finding is in agreement with our previous work [21]. Similarly, poorer cognitive performance was linked with higher disease severity (rp ranging from –0.39 to –0.59; p < 0.001). These results are highly consistent with the findings of the Huntington’s Study Group (1996) [18], and build upon work demonstrating strong negative correlations between the frontal assessment battery and the UHDRS-TMS, [30] and strong relationships among UHDRS-TMS and motor-cognitive dual-task performance in individuals with HD [12]. Recent work suggests that composite cognitive scores as compared to a single measure may improve tracking of disease progression in HD, [31] and that poorer motor and cognitive performance are linked to smaller caudate and putamen volumes [1].
An interesting finding was that among the comparisons between the TMT and Stroop Word, Color and Interference tests the TMT showed the highest correlation with the Interference sub-test. Recent attempts to identify measures that best correlate as markers for HD progression have identified the highest correlations with the Word reading sub-test [32]. While the Word reading sub-test correlation is also highly significant in our analyses, the correlation with the Interference sub-test may be more revealing related to the types of skills needed to maintain mobility. The Interference test requires an additional component of attention and deductive skills as it requires a mental conversion between words and colors. These features are more akin to more complex dual tasking. In support of this hypothesis, Fritz and colleagues reported that timed performance on the Walking While Talking Test (WWTT) simple (walking 40 feet while saying the alphabet) and complex (walking while saying every other letter of the alphabet) conditions, a measure of dual-task ability, had the highest correlations with the Stroop Interference test scores among the three Stroop sub-tests [12]. Slower time on the WWTT was moderately correlated for the simple (r = –0.38, p < 0.05) and complex (r = –0.51, p < 0.01) conditions with worse performance on the Stroop Interference test [12].
The primary limitations of this study are the relatively small number of participants with more than one assessment time point and the short follow-up time. This limits the ability to generalize the findings of this study to the HD population as a whole. Further studies on larger cohorts across multiple sites are indicated to verify the results of this study in the HD population as a whole and to better assess within participant change across time in mobility and cognition. The findings may also have been impacted by the subjectivity of TMT ratings; however, all raters established interrater reliability prior to the start of the study, which may mitigate this limitation. In addition the raters were blinded to previous scores when assessing the TMT.
In conclusion, there is a moderately strong correlation between cognitive and motor dysfunction in HD that is reflected in impairments in gait and balance on the TMT. Impairments in gait, balance and mobility lead to increasing fall risks and decreased independence. The TMT in combination with cognitive assessment tools may help predict the need for specific interventions. A recent systematic review of motor-cognitive dual-task training in individuals with a variety of neurological disorders other than HD, suggested that these types of interventions may affect gait velocity, stride length and balance [33]. After further study of larger cohorts over longer periods focused therapeutic interventions may be designed specific to the deficits and needs of those with HD.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
The authors would like to acknowledge Kasia Schuen for her contributions to data collection and entry. This project was supported by the Robert A. Vaughan Fund.
REFERENCES
- [1]. Misiura MB, Lourens S, Calhoun VD, Long J, Bockholt J, Johnson H, et al. Cognitive control, learning, and clinical motor ratings are most highly associated with basal ganglia brain volumes in the premanifest Huntington’s disease phenotype. J Int Neuropsychol Soc. 2017;23(2):159–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Harrington DL, Smith MM, Zhang Y, Carlozzi NE, Paulsen JS, PREDICT-HD Investigators of the Huntington Study Group. Cognitive domains that predict time to diagnosis in prodromal Huntington disease. J Neurol Neurosurg Psychiatry. 2012;83(6):612–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Unschuld PG, Liu X, Shanahan M, Margolis RL, Bassett SS, Brandt J, et al. Prefrontal executive function associated coupling relates to Huntington’s disease stage. Cortex. 2013;49(10):2661–73. [DOI] [PubMed] [Google Scholar]
- [4]. Morkl S, Muller NJ, Blesl C, Wilkinson L, Tmava A, Wurm W, et al. Problem solving, impulse control and planning in patients with early- and late-stage Huntington’s disease. Eur Arch Psychiatry Clin Neurosci. 2016;266(7):663–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. D’Orio VL, Foley FW, Armentano F, Picone MA, Kim S, Holtzer R. Cognitive and motor functioning in patients with multiple sclerosis: Neuropsychological predictors of walking speed and falls. J Neurol Sci. 2012;316(1-2):42–6. [DOI] [PubMed] [Google Scholar]
- [6]. Al-Yahya E, Dawes H, Smith L, Dennis A, Howells K, Cockburn J. Cognitive motor interference while walking: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2011;35(3):715–28. [DOI] [PubMed] [Google Scholar]
- [7]. Mielke MM, Roberts RO, Savica R, Cha R, Drubach DI, Christianson T, et al. Assessing the temporal relationship between cognition and gait: Slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68(8):929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Amboni M, Barone P, Hausdorff JM. Cognitive contributions to gait and falls: Evidence and implications. Mov Disord. 2013;28(11):1520–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Kearney FC, Harwood RH, Gladman JR, Lincoln N, Masud T. The relationship between executive function and falls and gait abnormalities in older adults: A systematic review. Dement Geriatr Cogn Disord. 2013;36(1-2):20–35. [DOI] [PubMed] [Google Scholar]
- [10]. Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: Which aspects of gait are attention demanding? Eur J Neurosci. 2005;22(5):1248–56. [DOI] [PubMed] [Google Scholar]
- [11]. Yogev-Seligmann G, Hausdorff JM, Giladi N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–42; quiz 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Fritz NE, Hamana K, Kelson M, Rosser A, Busse M, Quinn L. Motor-cognitive dual-task deficits in individuals with early-mid stage Huntington disease. Gait Posture. 2016;49:283–89. [DOI] [PubMed] [Google Scholar]
- [13]. Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology. 2005;65(5):745–7. [DOI] [PubMed] [Google Scholar]
- [14]. Delmaire C, Dumas EM, Sharman MA, van den Bogaard SJ, Valabregue R, Jauffret C, et al. The structural correlates of functional deficits in early huntington’s disease. Hum Brain Mapp. 2013;34(9):2141–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Kerber KA, Enrietto JA, Jacobson KM, Baloh RW. Disequilibrium in older people: A prospective study. Neurology. 1998;51(2):574–80. [DOI] [PubMed] [Google Scholar]
- [16]. Holtzer R, Verghese J, Xue X, Lipton RB. Cognitive processes related to gait velocity: Results from the Einstein Aging Study. Neuropsychology. 2006;20(2):215–23. [DOI] [PubMed] [Google Scholar]
- [17]. Fritz NE, McCarthy CJ, Adamo DE. Handgrip strength as a means of monitoring progression of cognitive decline - A scoping review. Ageing Res Rev. 2017;35:112–23. [DOI] [PubMed] [Google Scholar]
- [18]. Huntington’s Study Group. Unified Huntington’s Disease Rating Scale: Reliability and consistency. Mov Disord. 1996;11(2):136–42. [DOI] [PubMed] [Google Scholar]
- [19]. Hanes KR, Andrewes DG, Smith DJ, Pantelis C. A brief assessment of executive control dysfunction: Discriminant validity and homogeneity of planning, set shift, and fluency measures. Arch Clin Neuropsychol. 1996;11(3):185–91. [PubMed] [Google Scholar]
- [20]. Kegelmeyer DA, Kloos AD, Thomas KM, Kostyk SK. Reliability and validity of the Tinetti Mobility Test for individuals with Parkinson disease. Phys Ther. 2007;87(10):1369–78. [DOI] [PubMed] [Google Scholar]
- [21]. Kloos A, Kegelmeyer DA, Young G, Kostyk S. Fall Risk Assessment using the Tinetti Mobility Test in individuals with Huntington’s disease. Mov Disord. 2010;25(16):2838–44. [DOI] [PubMed] [Google Scholar]
- [22]. Busse M, Quinn L, Khalil H, McEwan K. Optimising mobility outcome measures in Huntington’s disease. J Huntingtons Dis. 2014;3(2):175–88. [DOI] [PubMed] [Google Scholar]
- [23]. Begg MD, Parides MK. Separation of individual-level and cluster-level covariate effects in regression analysis of correlated data. Stat Med. 2003;22(16):2591–602. [DOI] [PubMed] [Google Scholar]
- [24]. Curran PJ, Bauer DJ. The disaggregation of within-person and between-person effects in longitudinal models of change. Annu Rev Psychol. 2011;62:583–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Portney L, Watkins M. Foundations of clinical research: Applications to practice 3rd ed Upper Saddle River: Pearson Prentice Hall; 2009. [Google Scholar]
- [26]. Tabrizi SJ, Scahill RI, Durr A, Roos RA, Leavitt BR, Jones R, et al. Biological and clinical changes in premanifest and early stage Huntington’s disease in the TRACK-HD study: The 12-month longitudinal analysis. Lancet Neurol. 2011;10(1):31–42. [DOI] [PubMed] [Google Scholar]
- [27]. Aylward EH, Harrington DL, Mills JA, Nopoulos PC, Ross CA, Long JD, et al. Regional atrophy associated with cognitive and motor function in prodromal Huntington disease. J Huntingtons Dis. 2013;2(4):477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Kloos AD, Fritz NE, Kostyk SK, Young GS, Kegelmeyer DA. Clinimetric properties of the Tinetti Mobility Test, Four Square Step Test, Activities-specific Balance Confidence Scale, and spatiotemporal gait measures in individuals with Huntington’s disease. Gait Posture. 2014;40(4):647–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Quinn L, Khalil H, Dawes H, Fritz NE, Kegelmeyer D, Kloos AD, et al. Reliability and minimal detectable change of physical performance measures in individuals with pre-manifest and manifest Huntington disease. Phys Ther. 2013;93(7):942–56. [DOI] [PubMed] [Google Scholar]
- [30]. Rodrigues GR, Souza CP, Cetlin RS, de Oliveira DS, Pena-Pereira M, Ujikawa LT, et al. Use of the frontal assessment battery in evaluating executive dysfunction in patients with Huntington’s disease. J Neurol. 2009;256(11):1809–15. [DOI] [PubMed] [Google Scholar]
- [31]. Jones R, Stout JC, Labuschagne I, Say M, Justo D, Coleman A, et al. The potential of composite cognitive scores for tracking progression in Huntington’s disease. J Huntingtons Dis. 2014;3(2):197–207. [DOI] [PubMed] [Google Scholar]
- [32]. Stout JC, Jones R, Labuschagne I, O’Regan AM, Say MJ, Dumas EM, et al. Evaluation of longitudinal 12 and 24 month cognitive outcomes in premanifest and early Huntington’s disease. J Neurol Neurosurg Psychiatry. 2012;83(7):687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Fritz NE, Cheek FM, Nichols-Larsen DS. Motor-cognitive dual-task training in persons with neurologic disorders: A systematic review. J Neurol Phys Ther. 2015;39(3):142–53. [DOI] [PMC free article] [PubMed] [Google Scholar]