Abstract
This review systematically examines the evidence for shifts in flux through energy generating biochemical pathways in Huntington’s disease (HD) brains from humans and model systems. Compromise of the electron transport chain (ETC) appears not to be the primary or earliest metabolic change in HD pathogenesis. Rather, compromise of glucose uptake facilitates glucose flux through glycolysis and may possibly decrease flux through the pentose phosphate pathway (PPP), limiting subsequent NADPH and GSH production needed for antioxidant protection. As a result, oxidative damage to key glycolytic and tricarboxylic acid (TCA) cycle enzymes further restricts energy production so that while basal needs may be met through oxidative phosphorylation, those of excessive stimulation cannot. Energy production may also be compromised by deficits in mitochondrial biogenesis, dynamics or trafficking. Restrictions on energy production may be compensated for by glutamate oxidation and/or stimulation of fatty acid oxidation. Transcriptional dysregulation generated by mutant huntingtin also contributes to energetic disruption at specific enzymatic steps. Many of the alterations in metabolic substrates and enzymes may derive from normal regulatory feedback mechanisms and appear oscillatory. Fine temporal sequencing of the shifts in metabolic flux and transcriptional and expression changes associated with mutant huntingtin expression remain largely unexplored and may be model dependent. Differences in disease progression among HD model systems at the time of experimentation and their varying states of metabolic compensation may explain conflicting reports in the literature. Progressive shifts in metabolic flux represent homeostatic compensatory mechanisms that maintain the model organism through presymptomatic and symptomatic stages.
Keywords: Huntington’s Disease, homeostasis, intermediate metabolism, flux analysis, mitochondrial dysfunction
ABBREVIATIONS
- Aco
aconitase
- AAT
aspartate amino transferase
- αKG
α-ketoglutarate
- ALAT
alanine amino transferase
- AMPK
AMP kinase
- ANT
adenosine nucleotide translocator
- Aralar
glutamate-aspartate transporter
- Asp
aspartate
- Carn
carnitine
- CAT I
carnitine acyltransferase I
- CAT II
carnitine acyltransferase II
- CI
complex I
- CII
complex II, SDH
- CIII
complex III
- CIV
complex IV, cytochrome oxidase
- CK
creatine kinase
- CMRO2
cerebral metabolic rate of oxygen consumption
- COX
cytochrome oxidase
- Cr
creatine
- CS
citrate synthase
- CV
complex V, F0F1ATPase, ATP synthase
- Cyt C
cytochrome C
- ESC
embryonic stem cells
- ETC
electron transport chain
- FA
Fatty Acid
- FacylCarn
Fatty Acyl-carnitine
- FacylCoA
Fatty Acetyl-Coenzyme A
- FAO
fatty acid oxidation, β-oxidation
- FAS
fatty acid synthase
- FDG
2-[18F] -fluoro-2-deoxy-glucose
- G6PDH
glucose-6-phosphate dehydrogenase
- GABAT
GABA transaminase
- GAD
glutamic acid decarboxylase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GDH
glutarate dehydrogenase
- Glu
glutamate
- Glut1 or 3
glucose transporter 1 or 3
- GS
glutamine synthase
- GSH
glutathione
- HD
Huntington’s Disease
- HIF
hypoxia inducing factor
- HK
hexokinase
- IDH
isocitrate dehydrogenase
- IMM
inner mitochondrial membrane
- IMS
intermembrane space between OMM and IMM
- KGDHC
α-Ketoglutarate dehydrogenase complex
- LDH
lactate dehydrogenase. LDHA favors lactate production. LDHB favors pyruvate production
- Mal
malate
- MRS
magnetic resonance spectroscopy
- MDH
malate dehydrogenase
- Muhtt
mutant huntingtin
- NAA
N-acetyl aspartate
- OMM
outer mitochondrial membrane
- OXPHOS
oxidative phosphorylation
- Pi
phosphate
- PC
pyruvate carboxylase
- PCr
phosphocreatine
- PDH
pyruvate dehydrogenase
- PDHC
pyruvate dehydrogenase complex
- PDK
pyruvate dehydrogenase kinase
- PDP
pyruvate dehydrogenase phosphatase
- PEP
phosphoenolpyruvate
- PFK
phosphofructokinase
- PK
pyruvate kinase
- PM
plasma membrane
- PPP
Pentose Phosphate Pathway
- PTP
permeability transition pore
- PV
parvalbumin
- ROS
reactive oxygen species
- SDH
succinate dehydrogenase
- SIRT
sirtuin
- ST
substrate transporters
- STK
succinyl thiokinase
- TCA
tricarboxylic acid cycle
- UHDRS
unified HD rating system
- UT-B
urea transporter
- VDAC
voltage dependent anion channel
- Xc−
csytine-glutamate exchanger
INTRODUCTION
Energy impairment is an acknowledged part of the Huntington’s disease (HD) phenotype. Yet within the CNS, how and why metabolic changes occur remain controversial. Mechanistically, abnormal energy metabolism in HD results from multiple mechanisms including altered mitochondrial axonal transport, dynamics and biogenesis due to nuclear transcriptional disruption [1–3]. Beyond issues of the size and distribution of mitochondrial mass, controversies remain concerning the existence of abnormalities or imbalances in the flux of metabolites through the various energy generating pathways to produce adequate ATP. Additionally, altered flux may reflect abnormal protein regulation or energy demands. Much attention has been focused upon mitochondrial function, specifically the electron transport chain (ETC), while deficits in glycolysis, the tricarboxylic acid cycle (TCA) and fatty acid oxidation (FAO) have received less attention.
Three different frameworks can be proposed to explain the metabolic dysfunction in HD brains. The first posits that mitochondrial biogenesis, dynamics, trafficking and mitophagy are altered resulting in a dearth of energy generating organelles in appropriate synaptic sites. Each of these mechanisms has been demonstrated in a variety of HD model systems [1–5]. However the presence of these restrictions on mitochondria does not explain how the organism grows initially and continues to survive. The second proposes that the energy generating enzyme complexes in the ETC of CNS mitochondria are compromised due to deficits in expression or functionality. Evidence for alterations in oxidative metabolism comes from post mortem human tissue and mouse models towards the end of their lives [6–8]. However, this finding has not been universal. In postmortem presymptomatic and grade 1 HD striatum and sensorimotor cortex activities of complex I (CI) through complex IV (CIV) were normal, in contrast to their collective reduction reported from grade 3–4 brains [9]. This raises questions about the context and timing of such changes and whether they contribute to initial disease progression or only terminal manifestations of disease. A separate part of this theory includes a consequent increase in mitochondrial calcium loading, which induces the permeability transition pore (PTP). Discussions of the evidence for and against involvement of the PTP goes beyond the scope of this review, but readers are encouraged to consult a recent review by Brustovetsky [10]. A third possibility to consider is that some of the energetic abnormalities arise as a compensatory homeostatic response to transcriptional and proteomic changes induced by mutant huntingtin (muhtt) accumulation. Shifts in flux through interconnected metabolic pathways may compensate for restricted energy production by lowered expression or oxidative damage to select enzymes. The homeostatic response most likely varies over the lifespan with disease progression, dynamically trying to adjust to accumulating consequences of transcriptional and proteostatic disruption. This third framework provides an umbrella which can encompass the events documented by the other two explanatory frames. Furthermore, it can explain continued survival with more subtle but broad shifts across a variety of metabolic pathways.
This review argues from the viewpoint that metabolic abnormalities in HD are compensated by shifts in fluxes through the different branches of the energy generating pathways and that these compensations change over the course of disease. Thus metabolism may appear to function differently in different tissues at distinct disease stages. The context of which mutant htt fragments are active in distinct model systems influences the mechanisms of neuronal demise that ensues [11]. Similar contextual effects should influence how muhtt stresses mitochondrial functions. Electrophysiological changes in HD evolve over time in a regionally specific manner with circuit dysfunction encompassing both progressive and compensatory components [12]. Metabolic changes may follow a similar contextual and temporal progression. Mass action drives shifts in the balance of energy produced by glycolysis or oxidative phosphorylation on a temporal basis. Slowly accumulating oxidative damage may progressively inactivate some metabolic enzymes. Transcriptional dysregulation resulting in abnormal mitochondrial biogenesis and dynamics contribute to the need for compensatory responses. Accumulation of muhtt results in trafficking defects. Enzyme expression changes could result from homeostatic regulation and/or transcriptional dysregulation. Failure of the ETC may occur in CNS mitochondria late in the disease process but does not drive the transition from presymptomatic to symptomatic stages. This dynamic viewpoint can incorporate much of the reported experimental literature, as discussed below. The compilation and synthesis of published works presented here generates hypotheses to be tested in future investigations.
Fragments of muhtt translocate to the nucleus and bind to DNA, altering transcription factor binding and downstream gene expression [13]. This transcriptional dysregulation has been postulated to be both an early-stage event in HD pathogenesis and a later stage disruption of homeostatic compensation for mitochondrial dysregulation, endoplasmic reticulum and oxidative stress responses [14]. However, such homeostatic dysregulation could occur at the level of metabolic enzyme activities that operate on a more rapid and contextual basis than transcription. Indeed tissue specific variations in metabolic enzyme expression and activities support the specific energy demands of tissue specializations [15, 16]. Within the brain, regionally specific energy needs would be similarly expected to be supported by differential gene expression and activity levels, irrespective of muhtt effects.
The regional metabolic phenotypes associated with HD reviewed here occur on a heterogeneous background of energetic profiles that differ among excitatory and inhibitory neurons, the various glial cells and the multiple compartments within these cells [17]. Since the majority of HD mitochondrial studies focus upon regional enzymatic functionality, alterations at this more detailed level remain elusive and results represent the mix of mitochondria from all cells in the harvested samples. For explanations of the various experimental mouse models of HD, the reader is referred to recent reviews [18–20]. Briefly, mouse models vary from the rapidly progressive R6/2 which expresses the human exon 1 fragment with 120 or more polyglutamine repeats, to more slowly progressing knock in models such as the Q111, Q140 and Q175. None of the mouse models discussed appear to have phenotype until the polyglutamine repeats exceed 100 [18–20]. If not explicitly stated, all results reported below were made with respect to appropriate controls detailed in the original reports.
OVERVIEW OF ENERGY-GENERATING PATHWAYS
Energy metabolism must be viewed as a system of interacting biochemical pathways with the electron transport chain and oxidative phosphorylation as the final route to produce ATP (Figs. 1–5). ATP can be generated by glycolysis. Substrates other than glucose can feed into the TCA cycle pathways. Starting in the cytosol, glycolysis converts glucose into pyruvate, producing two ATP molecules per molecule of glucose. Pyruvate must then be transported across both the outer and inner mitochondrial membranes before being metabolized through the TCA cycle to produce the reducing equivalents NADH and FADH plus succinate and malate. Additionally, cytosolic glutamate, malate, Pi, ADP, and NAD have to be transported into the mitochondria. These small molecules then fuel the ETC to build up both mitochondrial membrane potential and the H+ ion gradient that drives oxidative phosphorylation (OXPHOS). Upon exiting the mitochondria, the high energy phosphate in the newly produced ATP is rapidly transferred to creatine (Cr) to form the energy storage molecule phosphocreatine (PCr) via the enzyme creatine kinase (CK). Elsewhere in the cytoplasm when ATP is needed, cytoplasmic CK reverses this latter reaction, moving the phosphate group from PCr back onto ADP.
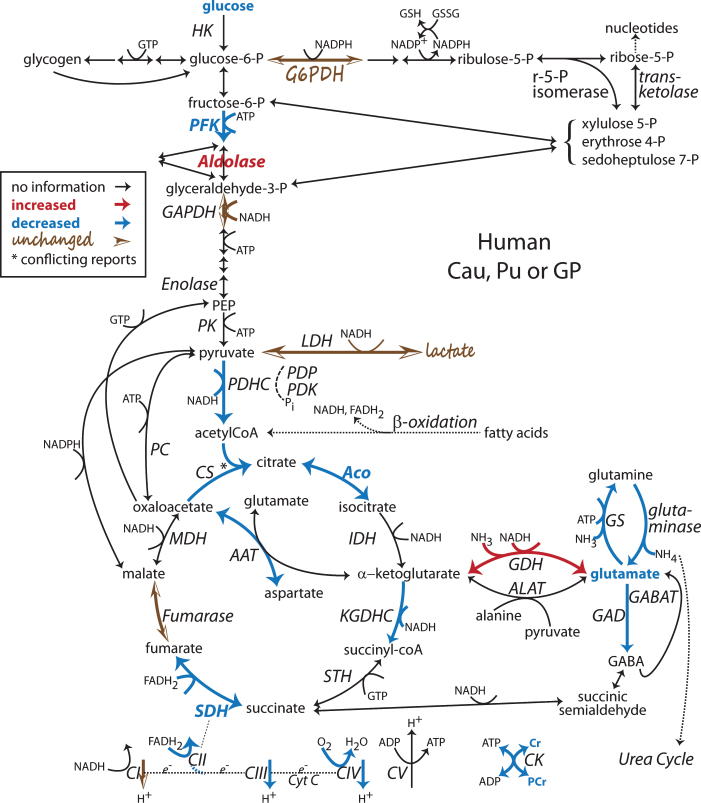
Fig.1.
Alterations found in the human post-mortem HD striatum late in disease in the system of Intermediate Metabolism Pathways. For details and differences between caudate (Cau), putamen (Pu), globus pallidus (GP) and cortex, see the text. Intermediate Metabolism Pathways: Glycolysis, the principle catabolism of glucose to pyruvate is represented centrally. Branching off from glucose-6-P is glycogenolysis to the left and the Pentose Phosphate Pathway (PPP) to the right. In the mitochondria, pyruvate is converted to acetyl CoA which enters the TCA cycle, center bottom. Additional fuel can be generated from β-oxidation of fatty acids, which produces reducing equivalents, NADH and FADH2, and acetyl CoA. Glutamate metabolism, bottom right, can be derived from or provide fuel for TCA intermediates (anaplerosis). The Electron Transport Chain (ETC, very bottom) utilizes the NADH and FADH2 produced in all of these pathways to provide the driving force for converting ADP to ATP. Reactions specific to astrocytes (e.g. GS, PC and GDH) or GABAergic neurons (e.g. GAD, GDH) are included but not specifically separated. Enzymes and metabolites not discussed in this review are not labeled. Diagram adapted from [251]. [6–8, 45, 48–52, 70, 76, 77, 80, 81, 85, 125, 136, 137, 168, 170, 171]. Note: Bold indicates an enzyme expression or activity has been assayed. Bold black or red indicates upregulation. Bold grey or blue indicates downregulation. Bolding of an enzyme or metabolite name indicate protein expression or concentration alterations. Partial bolding or coloring indicates differential expression changes among subunits. Bolding and/or coloring of arrows indicate activity changes. No change is indicated by a brown script font or a half open brown arrowhead. The default sans serif black font and thin black arrows with small heads indicate that no specific information is available. Combinations of symbol changes and asterisks (*) indicate conflicting reports from different laboratories.
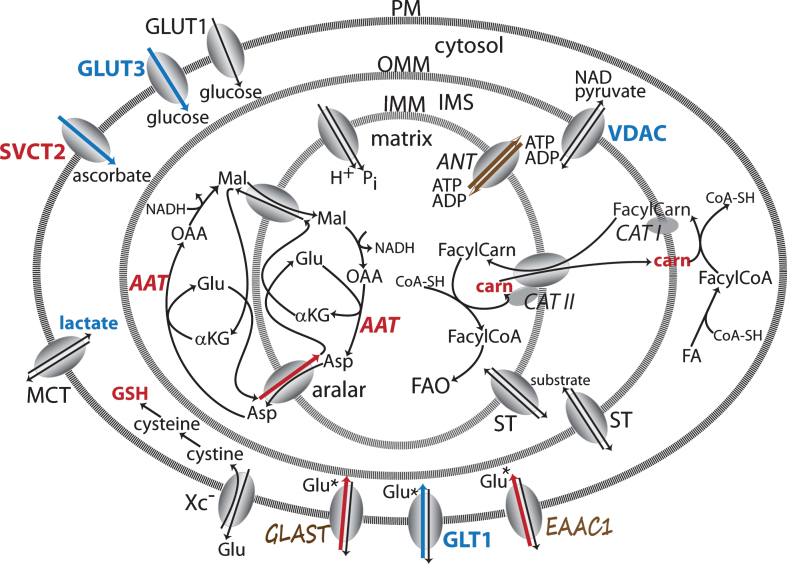
Fig.5.
Transporters and transport pathways for energy substrate movement across plasma and mitochondrial membranes. Key transporters involved in moving metabolites into CNS cells and mitochondria. Changes indicated have been reported in different HD model systems. The malate-aspartate shuttle crosses the IMM on the left. The carnitine shuttle that brings FA from the cytosol into the matrix is on the right. ST represents numerous substrate transporters across the IMM and OMM for pyruvate, glutamate, malate, α-ketoglutarate, aspartate, citrate and succinate, some of which may be neuron or astrocyte specific. GLAST, GLT1 and GLUT1 are localized in astrocytes. EEAC1 and GLUT3 are localized to neurons. Annotations as in Fig. 1 [67, 73, 75, 85, 87, 104–107, 120, 133, 142, 143, 145, 163, 253, 254]. Note: Bold indicates an enzyme expression or activity has been assayed. Bold black or red indicates upregulation. Bold grey or blue indicates downregulation. Bolding of an enzyme or metabolite name indicate protein expression or concentration alterations. Partial bolding or coloring indicates differential expression changes among subunits. Bolding and/or coloring of arrows indicate activity changes. No change is indicated by a brown script font or a half open brown arrowhead. The default sans serif black font and thin black arrows with small heads indicate that no specific information is available. Combinations of symbol changes and asterisks (*) indicate conflicting reports from different laboratories.
Beyond the linked glycolysis, TCA cycle and ETC, additional, important but often overlooked biochemical pathways both divert and contribute energy generating molecules. Diversions include glycogenolysis and the Pentose Phosphate Pathway (PPP). In astrocytes after the first glycolytic reaction, glucose-6-phosphate can be diverted to glycogen production, a storage form of energy. While glycogen-producing enzymes are present in neurons, they are inactivated under physiological conditions [21, 22]. Alternatively, glucose-6-phosphate can enter the PPP, which produces NADPH, an important reducing equivalent for the generation of the antioxidant GSH. End products from the PPP, fructose-6-phosphate and glyceraldehye-3-phosphate then reenter downstream in the glycolytic pathway.
Additional entry points for energy-generating substrates include FAO, also called β-oxidation, glutamate oxidation and the consumption of lactate to produce pyruvate. β-oxidation involves the iterative degradation of fatty acids into acetyl-CoA, the main substrate entering TCA, and the reducing equivalents NADH and FADH2. This occurs through a series of enzymatic reactions initially on the cytosolic side of the outer mitochondrial membrane (OMM) creating fatty acyl-CoAs, which combine with carnitine to cross into the matrix via the acyl-carnitine/carnitine transporter. Once inside the mitochondria, fatty acyl-CoAs are oxidized through a cycle removing 2 carbon atoms at a time to generate NADH, FADH2 and acetyl-CoA. Anaplerotic reactions degrade amino acids into TCA substrates. Glutamate and aspartate can be converted into α-ketoglutarate and oxaloacetate, both TCA intermediates, providing additional entry points for fuel into TCA. In addition acetyl-CoA, the normal substrate entering TCA, also can be diverted to produce cholesterol and fatty acids [23]. As demand for energy generating substrates increases, alternate fuels may be pulled into TCA from β-oxidation or glutamate oxidation.
Regulation of energy generation
Throughout all of these intersecting pathways, energy production is tightly regulated through localized pools, allosteric or substrate inhibition, and feedback pathways that tailor flux to current environmental conditions or demands. In the first step in glycolysis, the interaction of hexokinase with the voltage dependent anion channel (VDAC) in the OMM channels ATP exiting the mitochondria to directly fuel conversion of glucose into glucose-6-phosphate [24]. ATP itself allosterically inhibits phosphofructokinase [25], the second enzyme in glycolysis. The ratio of the concentrations of ATP and ADP ([ATP]/[ADP]) allosterically inhibits pyruvate dehydrogenase (PDH), as do the ratios [NADH]/[NAD+] and [acetyl-CoA]/[CoA]. In the TCA cycle, ATP inhibits isocitrate dehydrogenase (IDH); succinyl-CoA and NADH inhibits α-ketoglutarate dehydrogenase; and all of these plus citrate inhibit citrate synthase (CS). Conversely ADP or AMP activate PDH, CS and IDH in TCA and phosphofructose kinase (PFK) in glycolysis. In PPP, NADP+ stimulates and NADPH can inhibit glucose-6-P dehydrogenase limiting glucose entry into this pathway [25]. Redox balance provides additional control on metabolic fluxes as oxidative changes in the NAD+/NADH ratio determines the pyruvate/lactate ratio through mass action and the direction of flux through PDH [25]. Cytosolic accumulation of NADH could also influence mitochondrial adenine nucleotide supply as NADH reduces VDAC permeability to ADP under physiological conditions [26]. All of these mechanisms would provide local regulatory control of energy production on a minute to minute time scale.
Movement of substrates into and out of the mitochondrial compartment by transporters, many within the large sodium linked carrier family, must be maintained for efficient flux and energy generation. In FAO, the flux through the acyl-carnitine/carnitine transporter responsible for entry of fatty acids into mitochondria becomes the rate limiting factor [25]. Thus bottlenecks for energy generation can occur because of a dearth of transporters as well as at any enzymatic step within these highly interacting pathways. A homeostatic response to a disease process might be expected to activate these regulatory, feedback mechanisms with possible long term consequences for steady state metabolic functioning.
ENERGY DEFICITS IN HD
Rather than talk about all mitochondrial functions lumped together, this review will focus specifically on which pathways are compromised in HD, with a focus on nervous tissue. Abnormalities in other tissues may be noted but will not be systematically explored as energy demand and regulation may be tissue specific [27]. For example, AMP kinase (AMPK), a sensor of energy status on both cellular and whole body scales, may be hypoactive in R6/2 muscle but hyperactive in brain tissue [27]. In addition, the cellular and subcellular compartments specializing in a particular pathway will be indicated. The prevalent idea that gradual mitochondrial dysfunction, through insufficiency or reactive oxygen species (ROS) production, is a driver of the aging process has recently been called into question [28]. Similarly, the idea that less ETC activity may be harmful has also been challenged. In transgenic mice with compromised mitochondrial function lacking the complex I subunit Ndufs4, a lower oxygen environment triggering activation of hypoxia inducing factor (HIF-α) was neuroprotective [29]. In light of these reversals in thinking, a reanalysis of the mechanisms producing energy deficits in the HD pathogenesis may be in order.
Longitudinal changes in energy metabolism in HD
The idea of disease progression inherently contains the notion that the longitudinal path runs uni-directionally towards increasing dysfunction. However, homeostasis, the regulatory processes that maintain biological systems within reasonable operating boundaries, has a great deal of latitude for counterbalancing disruptions to produce proper function and well being. Concomitant expression levels of ion channels can vary greatly and still produce equivalent neuronal electrical activity [30]. Similarly, highly disparate circuit parameters can produce equivalent rhythmic network activity [30]. At each level of complexity, such systemic flexibility underlies homeostatic processes [30]. So when considering the number of enzymes throughout these energy generating pathways, restricted expression or activity of one enzyme would be expected to invoke compensatory shifts in expression levels for many others such that the system continues to provide sufficient energy for survival.
Metabolic changes in premanifest or prodromal HD stages may represent homeostatic response as opposed to signs of irreversible damage. In one cross sectional study of clinical blood biomarkers, the metabolites that were elevated at premanifest stages returned to normal levels during moderate HD [31]. In the DE5 mouse, which only expresses muhtt in medium spiny neurons, respiration utilizing complex II (CII) substrates was reduced presymptomatically but increased symptomatically [32], demonstrating stage-specific shifts in energy production. In the slowly progressing Q111 knock in mouse, increased rates of ATP utilization and reciprocal changes in brain PCr and Cr observed when muhtt is first detectable in the nucleus at 6 wk, were reversed by 13 wk, suggesting a homeostatic restoration occurred [33, 34]. Thus energy pathways appear to be homeostatically regulated during disease progression in both humans and HD mouse models.
A prime example of an energetic physiological response that can either promote disease at late stages or protect at early stages is that of AMPK. By end stage disease in the striatum and cortex of the most severe HD mouse model, R6/2 at 12 weeks, and in human postmortem HD brains, AMPKα1 appears to be translocated to the nucleus, where its increased activity leads to a decrease in the expression of the anti-apoptotic protein Bcl2, contributing to neuronal demise [35]. In contrast, early in disease progression, AMPKα1 may play a different role. The ratio of phosphorylated (activated) AMPK to total AMPK is greater than normal in 4 mo old Q111 mouse brains, one of the slowest progressing models of HD [36] (but see [37]). In mouse striatum expressing virally delivered muhtt, a model where the initial development of toxicity can be followed, simultaneous overexpression of a constitutively active form of AMPK, AMPKα1GOF, reduces lesion size [38]. Similarly, enhancement of AMPK function through overexpression or metformin-induced activation of AMPK-mediated phosphorylation in both nucleus and cytosol are protective [38]. This latter result corroborates studies in cell lines. AMPKα1 mRNA and protein levels in STHdhQ111/1111 cells exceed that of STHdhQ7/7 cells suggesting that AMPK may play a role in maintaining the viability of the STHdhQ111/1111 cells. Low doses of metformin or overexpression of a constitutively active AMPKα1GOF protect STHdhQ111/1111 cells against serum-deprivation induced cell death. AMPKα1GOF also reduces soluble mutant htt levels. This neuroprotection is consistent with the general ability of AMPK to suppress ATP consuming processes and promote ATP production [39] although measures of mitochondrial size or function did not accompany these studies. For whole body regulation of glucose intake and energy production situated in the hypothalamus, AMPK activation decreases synthesis and increases oxidation of fatty acids leading to increased food uptake [40, 41]. When administered in the drinking water of R6/2 mice beginning at 4 wk, metformin reduced clasping time and extended lifespan (but not other measures) of males but not females [42]. Consistent with this, individuals with manifest HD motor symptoms in the ENROLL-HD study who were also taking metformin for diabetes had statistically significant, although quantitatively small, improvements in cognitive performance compared to other HD patients and non-affected age-matched controls [43].
A longitudinal KEGG pathway analysis of altered R6/2 total brain protein expression neatly lays out the oscillating nature of protein expression changes over the course of this mouse’s life [44]. Three or more proteins were altered in glycolysis and OXPHOS at 2, 4, 8, and 12 wks, but not 6 wk of life [44]. Expression of TCA proteins was altered at 2 and 8 wk, while glutamate metabolism changed at 2 wk, PPP expression at 4 wk, and fatty acid metabolism at 8 wk [44](Figs. 2 and 3). Clearly compensatory or regulatory processes of energy metabolism are driving both very early and late disease progression-associated changes. Energy generation was upregulated early in life, in a manner suggesting that fluxes through the entire intermediate metabolism fluctuate in a contextual, stage specific manner [44]. Oscillations in the expression patterns of several enzymes suggest dynamic regulation at this level as well. For example, the expression of SDH subunit Ip is depressed at 2 wk, augmented at 4 wk and depressed again at 8 wk [44]. Fatty acid metabolism was implicated by mRNA changes at 6 and 9 wk [44]. The involvement of PPP, fatty acid and glutamate metabolisms in these changes suggest that a deficit in a single pathway or enzyme cannot explain the energy issues in HD; rather fluxes through the entire system must be considered.
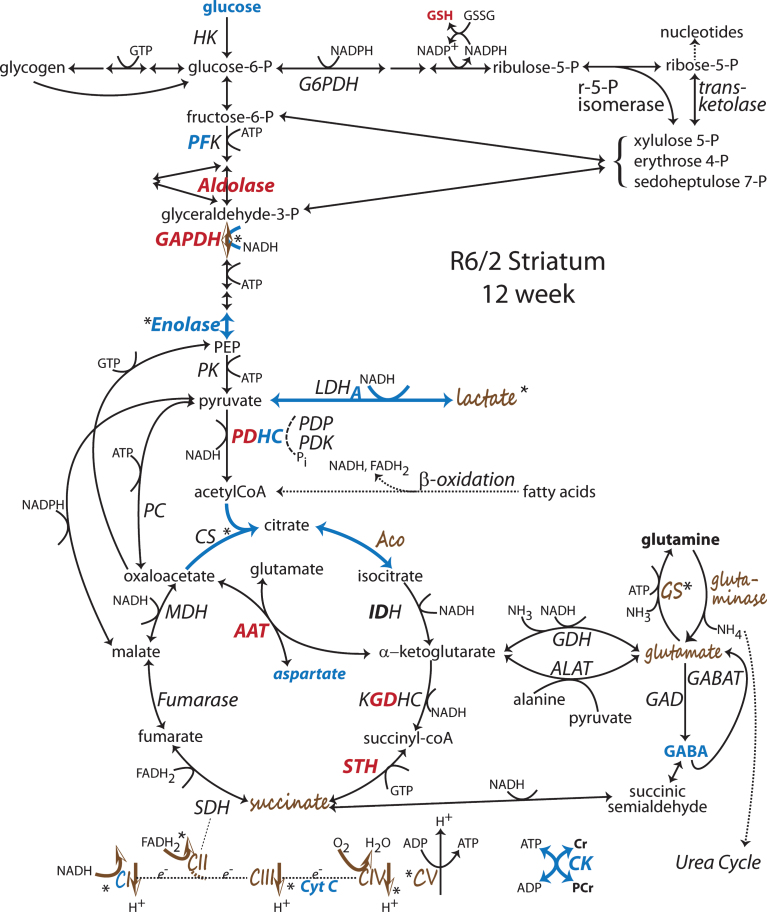
Fig.2.
Changes to metabolic pathways determined from R6/2 striatum late in disease at 12 wk of age. Annotations as in Fig. 1 [53, 54, 56, 67, 68, 70, 73, 92, 104, 105, 110, 111, 113, 134, 136–138, 182, 204, 252, 253]. Note: Bold indicates an enzyme expression or activity has been assayed. Bold black or red indicates upregulation. Bold grey or blue indicates downregulation. Bolding of an enzyme or metabolite name indicate protein expression or concentration alterations. Partial bolding or coloring indicates differential expression changes among subunits. Bolding and/or coloring of arrows indicate activity changes. No change is indicated by a brown script font or a half open brown arrowhead. The default sans serif black font and thin black arrows with small heads indicate that no specific information is available. Combinations of symbol changes and asterisks (*) indicate conflicting reports from different laboratories.
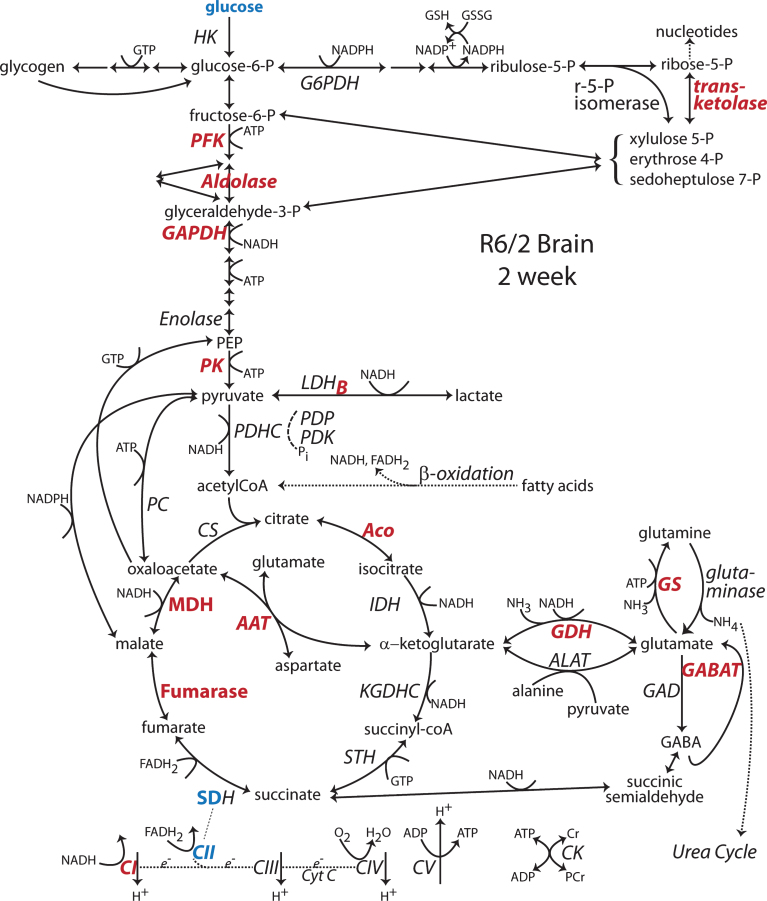
Fig.3.
Changes to metabolic pathways determined from R6/2 brain at 2 wk of age. Annotations as in Fig. 1 [44]. Note: Bold indicates an enzyme expression or activity has been assayed. Bold black or red indicates upregulation. Bold grey or blue indicates downregulation. Bolding of an enzyme or metabolite name indicate protein expression or concentration alterations. Partial bolding or coloring indicates differential expression changes among subunits. Bolding and/or coloring of arrows indicate activity changes. No change is indicated by a brown script font or a half open brown arrowhead. The default sans serif black font and thin black arrows with small heads indicate that no specific information is available. Combinations of symbol changes and asterisks (*) indicate conflicting reports from different laboratories.
Glucose uptake
Consistently, 2-[18F]-fluoro-2-deoxy-glucose (FDG) PET studies have documented progressively decreasing striatal and cortical glucose uptake in presymptomatic [45–47] and symptomatic individuals [45, 48–52]. PET FDG uptake documented decreases in glucose uptake in striatum and frontal and parietal cortices in patients symptomatic for <5 yr spread to additional areas as disease progressed further [50]. Depressed HD patients had lower glucose uptake in thalamus and orbital-frontal inferior prefrontal cortex [51]. In a longitudinal study of symptomatic patients, not only was the rate of decline in FDG uptake correlated with the severity of the UHDRS functional scores, but riluzole treatment was able to reverse both the motor and metabolic declines [52]. However, in presymptomatic HD carriers the story is more complicated. A metabolic network abnormality was identified that included decreased FDG uptake in striatum and anterior cingulate, plus increased FDG uptake in thalamus, vermis and primary motor and visual cortex [47]. Overall glucose uptake increased in this network 18 mo after the initial visit and then declined 26 mo later, suggesting early compensatory shifts in overall brain metabolism followed by decline once compensation was exhausted [47]. While glucose metabolism decreased continually throughout this study, thalamic metabolism first increased and then declined, driving the overall pattern [47].
Similarly, microPET or autoradiographic studies demonstrated reduced glucose uptake in the R6/2 mouse and an HD rat model [53–55] (but see [56, 57]). As in humans, impaired glucose uptake occurs early in the R6/2, with 2DG uptake being reduced in PFC, premotor cortex and striatum of R6/2 mice at 6 wk despite a simultaneously increased relative cerebral blood volume [53]. This may be another instance where metabolic homeostatic mechanisms attempt to compensate for changes induced by muhtt [53]. Notably, in the HD rat, FDG measurements were transiently elevated in sensorimotor cortex only at 2 months, otherwise no differences were observed in glucose uptake compared to controls [57].
Consistent with the PET studies, decreases in expression of glucose transporters (Fig. 5) have been widely reported in both humans and preclinical model systems. In postmortem HD brains at stage 3, but not stage 1, the expression of both the Glut1 (epithelial) and Glut3 (neuronal) glucose transporters is reduced [58]. Most importantly, increased expression of GLUT3 via copy number variations in humans delays the age of HD onset by three years, indicating the impact of boosting energy resources early in this disease [59]. Unfortunately, such copy number variations occur infrequently [59].
Limited glucose uptake, attributable to multiple mechanisms, may be one of the primary, initial restrictions on energy metabolism. Cell surface expression, but not total protein levels, of the GLUT3 glucose transporter is diminished in the striatum and cortex of homozygous Q140 mice compared to controls [60]. In 12 mo old BACHD mice, a decrease in glucose uptake was observed in the striatum, hippocampus and cerebellum pointing to a glycolytic deficit at a time when COX histochemistry, a functional ETC mitochondrial marker, did not reveal any differences attributable to muhtt [61]. Surprisingly, when undergoing whisker stimulation, cortical glucose uptake was greater in presumably presymptomatic BACHD mice at 64 wk than in the controls [61], suggesting a system whose responses are not well modulated. In primary co-cultures of astrocytes and neurons from BACHD mice, 2-deoxyglucose uptake was diminished whenever muhtt expressing astrocytes were present, suggesting that a glycolytic deficit may be attributable to astrocytes rather than neurons [61]. Glucose uptake is attenuated in primary cultures from Q140 neonates as a consequence of elevated rab11 activity [62]. Glucose uptake is decreased in STHdhQ111/111 cells compared to STHdhQ7/7 cells due to decreased mRNA, protein and surface protein levels for the Glut3 transporter [63]. Glut3 mRNA is also reduced in R6/2 striatum as early as 3–4 weeks of age [63]. Glucose and its metabolite glucose-6-phosphate have been identified as stimulators of mTOR-induced autophagy, so a decrease in overall glucose uptake could also downregulate normal autophagy, resulting in more accumulation of mhtt aggregates aggravating disease [64]. Together, these studies suggest that altered Glut3 protein expression and trafficking contribute to the metabolic phenotype in HD.
Glycolysis
In brains of HD patients assayed with PET, the efficiency of conversion of glucose to ATP is maximal in a resting state [65]. Thus when brain activity increases, the ability of the energy generating biochemical pathways to respond rapidly with an increase in ATP production, does not occur or may be limited [66, 67]. Similarly in the occiput of affected individuals, onset of visual stimulation fails to increase Pi/ATP, PCr/ATP or Pi/PCr ratios or lactate accumulation, as observed in control subjects [66]. The glycolytic enzyme phosphofructokinase was diminished in the post mortem HD caudate and putamen, but not in cortex [68] (Fig. 1). The PPP enzyme glucose 6-phosphate dehydrogenase, was unchanged, indicating that the decreased glycolytic flux was not caused by a diversion of glucose into the PPP [68]. Both normal and polyglutamine expanded htt bind to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) but GAPDH activity was not altered in post mortem human caudate, putamen, cortex or cerebellum [69, 70]. However, at 10 wk of age, iodoacetate inhibited GAPDH more in R6/2 striatum and cortex than in control brains, although no mechanism for this increased susceptibility was uncovered [71]. Differential proteomics of samples from stage 4 post mortem brain identified an increase of aldolase, as prominently elevated in both striatum and cortex [72]. Enolase was also carbonylated (oxidized), suggesting its activity would be compromised [72]. Flux through glycolysis may become limited by such protein oxidation as disease progresses. Both neuronal and non-neuronal forms of enolase, one of the later enzymes in the glycolytic pathway, become oxidized in R6/2 striatum at 10 wk, resulting in a lowered overall activity level [73]. Consistent with this, enolase protein levels decreased in both R6/2 cortex and striatum at a time when general protein carbonylation was increased [67]. Additional evidence that striatal neurons survive on glycolytic energy comes from the reduced level of cell death in R6/2 and R6/1 mouse brains induced by malonate, an inhibitor of succinate dehydrogenase, an enzyme that participates in both TCA and ETC [74]. This phenomenon occurs in older, but not younger R6/1 mice, suggesting that a shift (perhaps gradual) from oxidative metabolism to glycolysis occurs with disease progression [74].
While the abnormal polyglutamine expansion may produce a gain of toxic function, the absence of normal htt levels may contribute to a loss of normal function. In human embryonic stem cells (ESC) lacking huntingtin expression, dramatic shifts in energy metabolism, including glycolysis, are characterized by altered metabolite levels [75]. Compared to normal ESC, shifts characteristic of near maximal glycolytic flux include decreases of intracellular glucose, glucose-6-phosphate and fructose-1,6-diphosphate, increases in all glycolytic triose intermediates prior to pyruvate and increases in glucose uptake and pyruvate conversion to lactate [75]. In contrast, ESC expressing Q140/7 muhtt, pyruvate increases at the expense of lactate and many fatty acids and carnitines become overabundant [75]. Consistent with this, nucleotide triphosphate levels drop in htt-/- cells and nucleotide di- and monophosphates increase in the htt-/- cells [75]. Increases in metabolites involved in purine biosynthesis also appear to be part of a homeostatic shift in metabolic pathways to increase ATP synthesis [75]. Surprisingly mitochondrial membrane potential is maintained in both Q140/7 cells and htt-/- cells, the latter presumably at the expense of glycolytically generated ATP [75]. It is noteworthy that the ETC was not altered in either htt-/- or Q140/7 ESC [75]. While it is unclear how the metabolic adaptation to growing in cell culture may be influencing these results, the absence of normal htt function clearly shifts metabolism towards glycolysis and away from TCA and oxidative phosphorylation, not unlike the early phenotype described above in humans [65]. The presence of polyglutamine expanded muhtt also shifts metabolism, but less dramatically and less clearly in this model system [75].
Tricarboxylic acid cycle
The earliest studies of metabolic enzyme deficits in HD realized that activity might change with disease progression. In first assessments of HD postmortem brains, succinate dehydrogenase (SDH) decreased only in the most severe cases [76](Fig. 1). SDH was also depleted beyond what was expected from the agonal state in the caudate but not frontal cortex [77]. Aconitase activity was found to decrease in post mortem caudate, putamen and cortical areas BA 10 and 3, but not cerebellum [70, 72]. Differential proteomics of samples from stage 4 post mortem brain identified a decrease of aconitase and an increase in its oxidation in striatum, but not cortex, leading to a loss of activity in striatal samples [72]. Aconitase activity can be compromised by carbonylation (oxidation) or by aggregation [78, 79]. Tissue transglutaminase, whose activity doubles in grade 3 or 4 HD caudate due to muhtt binding, causes aconitase to aggregate within mitochondria [78, 79]. Similarly CS activity was decreased in putamen and cortex [70]. Activity of the PDH complex was decreased in the caudate, putamen and hippocampus, but not frontal cortex or amygdala of HD brains [80]. The correlation of the loss of caudate PDH activity with disease duration suggested progressive loss with increasing disease burden [77]. α-Ketoglutarate dehydrogenase activity is also depressed in post mortem putamen [81].
In all analyses of post mortem human brain enzyme activities, experimenters have to take into account the effects of age, postmortem tissue interval and quality of tissue preservation. Agonal state, the physiological status of the patient prior to death and the suddenness of death, are rarely reported or considered. Slow deterioration in the last few weeks of life diminished respiratory rates of synaptosomes isolated posthumously from non-demented bronchopneumonia patients compared to those from sudden death cardiac arrest patients [82]. This was attributed to a decline in pH of cortex, CSF and blood presumed to arise from pre-mortem lactic acidosis in the agonal state [83]. In a broad study of mitochondrial DNA changes associated with depression in postmortem human brains, the biggest differences were observed in control brains where tissue pH predicted agonal state [84]. Few studies take into account the agonal state when reporting enzyme activities of SDH, PDH and aspartate amino transferase (AAT) [76, 77, 85]. Agonal state can also influence activity of glutathione reductase and glutathione-S-transferase [86]. While curatorial consistency has improved in contemporary brain banks [87–89], agonal state remains rarely considered.
The most recent metabolomic examination of TCA function in HD was undertaken with the intent of examining all TCA enzymes from the same material, to minimize differences in preparations, laboratories and physiological contexts. Naseri et al. examined the activities and mRNA and some protein levels of all TCA enzymes from postmortem HD parietal cortex and heterozygotic Q175 cortex and striatum at 14-15 mo [90, 91]. In the cortex from mostly grade 2 and 3 cases, an increase in SDH enzyme activity was reported [91]. A similar increase in SDH activity, accompanied by increases in PDH complex and aconitase were observed in Q175 cortex, but not striatum [91]. These results contrast sharply from the late stage post mortem profile, possibly reflecting regionally localized compensatory responses during mid-stage disease. In the Q175, the elevation of PDH activity at the entry to TCA bolsters the subsequent aconitase and SDH steps, representing an attempt to increase energetic flux. Strikingly, no changes were detected in the presumably symptomatic Q175 striatum.
TCA enzyme alterations in the rapidly progressive R6/2 mouse contrast to those in the slower Q175 model (Fig. 2). In R6/2 striatum at 10 wk compared to 4 wk, protein levels of dihydrolipoamide S-succinyltransferase, an E2 subunit of both the PDH and α-ketoglutarate dehydrogenase complexes, increased but PDH protein decreased [73]. This has been interpreted to represent an attempt of one subunit to compensate for loss of activity of the whole complex [73]. Aconitase activity was also reduced in striatum but not cortex of R6/2 at 12 wk [92]. At 10 wk, aconitase protein from R6/2 striatum was more oxidized than at 4 wk, suggesting progressive inactivation, which would impede flux through TCA [67, 73]. By 13 wk, aconitase was oxidized in both regions [93].
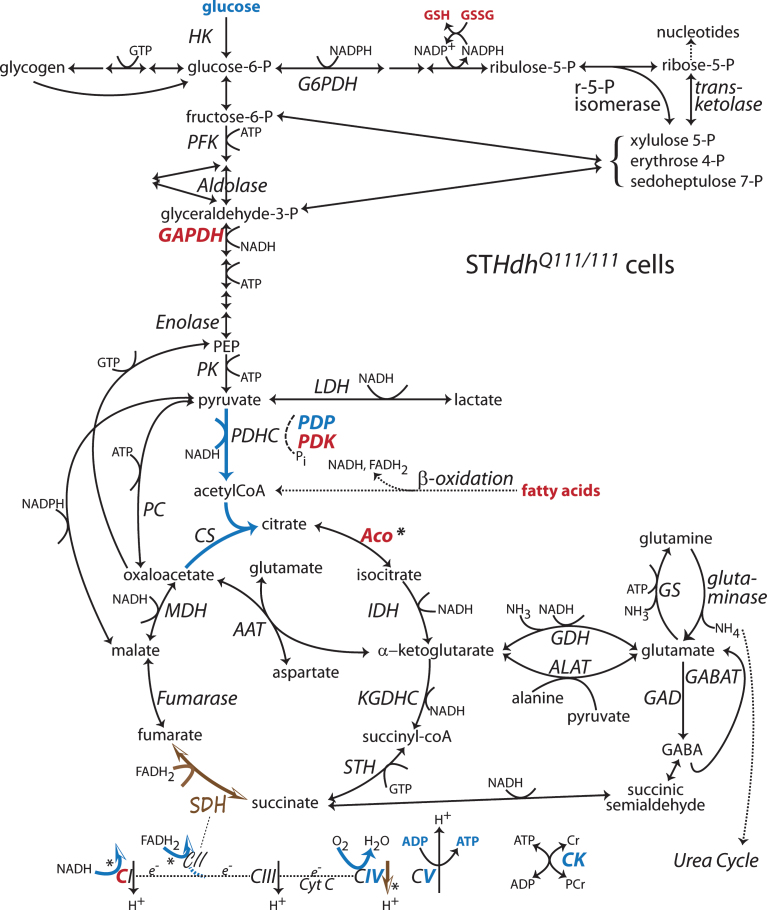
The transcriptional dysregulation generated by muhtt may include increased expression of pyruvate dehydrogenase kinase (PDK), which acts to inhibit pyruvate dehydrogenase (PDH) activity [94]. Mechanistically, PDH activity is reciprocally down or up regulated when phosphorylated or dephosphorylated by isoforms of pyruvate dehydrogenase kinase (PDK) or pyruvate dehydrogenase phosphatase (PDP), respectively [95]. In STHdhQ111/111 cells, observed decreases in PDH activity could be explained by increases in PDK protein levels and decreases in PDP leading to phosphorylation of PDH [94] (Fig. 4). Inhibitors of PDK or the HDAC inhibitor sodium butyrate ameliorated this phenotype and restored PDH activity, mitochondrial respiration and ATP levels [94]. A month long course of sodium butyrate also decreased PDK, phosphorylated PDH and restored motor behavior and relative ATP levels in the cortex of YAC128 mouse at 9 mo [94]. Why PDH activity is depressed in STHdhQ111/111 cells and possibly YAC128 cortex [94] but elevated in Q175 cortex [91] remains unclear. These differences may be attributable to the influence of the metabolic state of background strains or the extent of disease progression at the time the experiments were performed.
Fig.4.
Changes to metabolic pathways determined from STHdhQ111/111 cells compared to STHdhQ7/7 cells. Annotations as in Fig. 1 [63, 87, 94, 119, 120, 134, 204]. Note: Bold indicates an enzyme expression or activity has been assayed. Bold black or red indicates upregulation. Bold grey or blue indicates downregulation. Bolding of an enzyme or metabolite name indicate protein expression or concentration alterations. Partial bolding or coloring indicates differential expression changes among subunits. Bolding and/or coloring of arrows indicate activity changes. No change is indicated by a brown script font or a half open brown arrowhead. The default sans serif black font and thin black arrows with small heads indicate that no specific information is available. Combinations of symbol changes and asterisks (*) indicate conflicting reports from different laboratories.
Anaplerosis and glutamate oxidation
In addition to generating energy, TCA substrates are used in the synthesis of the amino acids glutamate, glutamine, aspartate, and alanine in pathways which branch off of TCA, referred to as glutamate metabolism pathways. These reversible reactions can work in reverse to utilize amino acids as entry points into TCA, for example during glutamate oxidation by GDH. Alterations in these enzymes have also been reported in HD brains and models.
In postmortem HD globus pallidum, GDH was elevated, presumably because of its association with the abundant and increasing presence of astroglia in this brain area [68] (Fig. 1). GDH associates with mitochondria and glutamate transporters to efficiently shuttle exogenous glutamate into the TCA [96]. Within TCA, GDH forms multienzyme complexes with many enzymes to facilitate glutamate utilization as an energy substrate [96]. Their (dys)regulation could contribute to either pathogenic or compensatory shifts in energy production. Astrocyte glutamate synthesis derives from flux through pyruvate carboxylase, an enzyme only found in astrocytes, forming an anaplerotic pathway into TCA [97–99]. While flux analysis tracing carbon movement through this pathway [100] has not been done on HD models, pyruvate carboxylase protein levels were found to be elevated in cultured astrocytes from neonatal BACHD cortex [101], consistent with an increased flux through TCA and increased production of glial glutamate in presymptomatic brain.
Aspartate amino transferase (also known as glutamic oxaloacetic transaminase) reciprocally converts oxaloacetate and glutamate into aspartate and alpha -ketoglutarate. This is the entry point for glutamate conversion into energy via a partial TCA cycle [102, 103]. In R6/2 striatum at 10 wk, aspartate amino transferase expression increased, supporting the idea that flux through the metabolic networks shift in a compensatory manner [73] (Fig. 2). In the face of a reduced flux produced by aconitase oxidation and inactivation, this shortcut would provide an alternative path to supply α-ketoglutarate [73]. An increase in the use of glutamate to fuel this portion of TCA cycle occurs in rat brain synaptosomes under hypoglycemic conditions [102, 103]. While not as severe as the experimentally induced absence of glucose, the reduced glucose uptake in HD could invoke utilization of glutamate as an energy source. HD associated loss of AAT activity in postmortem caudate could indicate that availability of glutamate as an energy substrate was depleted by end stage disease [85] (Fig. 1).
Despite the reported decreases in protein expression for plasma membrane glutamate transporters in HD mice [104–106] (Fig. 5), functional glutamate uptake was normal or increased in YAC128 and R6/2 striatal slices on a synaptic activity time scale [107]. This rapid glutamate clearance could serve as a temporally tuned source of exogenous glutamate for energy generation. Indeed, association of astrocytic glutamate transporters GLT1 and GLAST with GDH and mitochondria, respectively, facilitate use of exogenous glutamate as an energy source [96], suggesting that increased glutamate uptake may be an adaptive anaplerotic response in HD brain.
Electron transport chain
As the set of common final reactions that produce the electrochemical gradient for driving ATP production, the ETC has been investigated as a locus for possible metabolic abnormalities in HD. In a large sample of post mortem grade 3 and 4 HD brains, CII-III activity was depressed in caudate and putamen [6, 9] (Fig. 1). However, in presymptomatic or grade 1 striatum, activity of all the ETC complexes was normal [9], clearly indicating that major compromise of the ETC is a late event in pathogenesis. Largely overlooked in this study were sizable increases in CI and CII-III activity in the cerebellum, a part of the brain comparatively resistant to muhtt [6]. Such shifts support the concept that metabolic disruption is a system–wide phenomena in HD that triggers regionally specific homeostatic compensations.
In mitochondria prepared from postmortem human HD brains, respiration and cytochrome oxidase activity were normal despite unknown post mortem intervals or disease stage at death [8]. A set of recent experiments corroborates this older observation by directly addressing the problem of whether the ETC is functionally impaired in two different HD mice, the R6/2, which expresses an exon 1 muhtt toxic fragment, and the YAC128, which expresses full length muhtt under control of a yeast artificial chromosome [108–110]. In synaptic and nonsynaptic mitochondria from both mouse models at symptomatic stages and in neurons cultured from neonatal brains from these mice, no differences were observed between preparations from muhtt and wild type mice in terms of respiration rates, ATP concentrations or expression of representative proteins of isolated mitochondria [108, 110] (Fig. 1). Independent studies of assembly of ETC complexes and their activity in cortex and striatum uncovered no differences between R6/2 and wild type mice [111]. Similarly, mitochondrial calcium uptake capacity and neuronal oxygen consumption rates were not altered by the presence of muhtt from either model mouse [109, 110]. In the YAC128 model, mitochondrial membrane potential was not altered compared to wild type. Despite these normal mitochondrial functions, the YAC128 and R6/2 mice developed clasping and model-appropriate HD phenotypes [108, 110]. Consistent with these studies, crossing R6/2 with a cyclophilin D knock out mouse, downregulating this positive mediator of the mitochondrial permeability transition pore, resulted in an expected increase in the capacity of mitochondria to sequester calcium, a change that theoretically should be neuroprotective. However, no change in disease progression or phenotype was observed [112]. In R6/2 at 6 wk of age, despite the effects of muhtt impeding protein import into mitochondria, direct effects upon CI or CII respiration were not detected [113], contributing to the idea that other impediments or shifts in energetic or mitochondrial function may contribute to pathogenesis, but not ETC dysfunction. This contrasts with 12 wk old R6/2, near the end of life, in which CIV was decreased in both striatum and cortex [92].
The apparently contradictory results obtained in mice expressing the N171-82Q construct must be viewed by considering that metabolic flux may shift over the course of disease. In young animals only expressing the N171-82Q construct in striatum, CII, but not CI, fueled mitochondrial respiration was reduced presymptomatically, and subsequently increased symptomatically [32]. Similarly, in young or presymptomatic N171-82Q transgenic mice, only CII or lactate/pyruvate fueled respiration decreased [114, 115]. In symptomatic, older N171-82Q mice an increase in CI activity [116] or a decrease in CI, CII or CIV activity have all been reported [114, 117, 118]. These results in the N171-82Q model suggest that metabolic shifts in end stage disease, while distinct from early stages, may be quite variable and dependent upon local husbandry or experimental conditions.
In STHdhQ111/111 cells, activities of all the mitochondrial complexes were equivalent to those from the non-polyglutamine expanded cells, yet mitochondrial respiration on CI and CII substrates (but not CIV substrates) was reduced in situ [119] (Fig. 4). This points to a failure of the entire ETC to produce adequate fluxes in the cellular context. In contrast, another examination of these same cells reported decreases in CI through CIV activities in homozygous cells, but only decreases in CI and CIV in heterozygous cells [120]. Altered expression of two mitochondrial disulfide relay system proteins, responsible for import of Fe-S containing proteins including many components of cytochrome oxidase, was offered as a mechanistic explanation [120]. Importantly, these changes were dependent upon gene dose and passage number [120], demonstrating the contextual nature of energetic deficits.
SDH, or CII, has been proposed as a target for muhtt-induced inhibition since exposure to low doses of chemical inhibitors over long periods mimics some HD phenotypes [121–124]. The fact that inhibition of energy pathways, especially of SDH, mimicked HD phenotypes motivated the search for underlying metabolic abnormalities. Contributing to both TCA and CII activity in ETC, SDH occupies a critical position metabolically. SDH activity, measured as CII, was depressed with respect to citrate synthase in post mortem caudate at presumably end stage disease [7]. In caudate and putamen, but not cortex or cerebellum of stage 1–3 HD brains, expression levels of the Fp and Ip subunits of SDH were depleted [125] (Fig. 1). Similarly, primary neurons transfected with Htt171-82Q had decreased expression of these subunits and consequently lower SDH activity. In addition mitochondrial membrane potential was depleted and TUNEL positive cells were increased, conditions that were rescued by overexpression of the Fp or Ip subunits [125]. Significant differences were not detected in expression of Cox IV, Cyt C or the α subunit of CV [125]. In R6/2, expression of the Ip subunit oscillates over the course of disease [126]. In the R6/1 striatum at 16 wk, Ip subunit expression was reduced while in the N171-82Q mouse, CII expression was reduced beginning at 1 mo of age [114]. In rats with striatal lentiviral N171-82Q injections, SDH activity and Ip expression were depressed [114].
Subtoxic exposure to injurious treatments can produce a protective response termed preconditioning. R6/2 mice consuming low doses of 3-nitroproprionic acid (3NP), a SDH inhibitor, improved their behavioral phenotype, delayed onset of glycosuria and weight loss and increased their lifespan [127]. While no tissue specific metabolic measures were obtained, the metabolic inhibition was hypothesized to produce an upregulation of SDH, perhaps in nonneuronal tissues, until age-related metabolic decline exacerbated the effects of muhtt [127]. An earlier study demonstrated that R6/2 mice receiving subcutaneous injections of 3NP had smaller striatal lesions and fewer overall deaths than controls [128]. Similarly, in both R6/2 mice at 6 and 12 wk and R6/1 mice at 18 wk but not 6 wk, lesions from intrastriatal malonate injection, another SDH inhibitor, were diminished compared to controls [74]. As disease in R6/1 progresses more slowly than in R6/2, this resistance to SDH inhibition appears to progress with disease. Given that SDH inhibition is protective in ischemia-reperfusion injury [129], some of the same shifts in metabolic flux demonstrated by this acute insult may contribute to striatal energetic defects in HD, although this hypothesis remains to be explicitly tested.
Oxidative phosphorylation and ATP production
In humans with early stage HD, 31P magnetic resonance spectroscopy (MRS) of occipital cortex revealed normal basal ATP levels [66]. Only one study reported oxidation of CV in postmortem stage 4 striatum [72]. Variable deficits in basal ATP levels have been reported in preclinical HD models. In symptomatic R6/1 mice at 17 wk, decreases in cortical, but not striatal ATP were noted [130]. On the other hand, in Q150 knock in mice at 14 but not 4 mo, ATP levels were depressed only in synaptosomes, remaining normal in regional brain homogenates [131]. In 9 mo old YAC128 whole brain, ATP/ADP only trended to be decreased [94]. In symptomatic N171-82Q mice, an increase in ATP levels [116] and no change in cortical or striatal ATP/ADP ratios or striatal CV activity [32, 117] have all been reported. ATP was decreased in R6/2 striatum and cortex at 8 and 12 weeks [36]. However, 31P MRS of anesthetized R6/2 failed to find a decrease in the ATP peak [33]. In R6/2 mice at an unspecified age, ATP levels in striatum and cortex did not differ from controls, consistent with no changes in ETC activities, despite a reduction in mtDNA copy numbers [111]. While such differences could be methodological, a more likely explanation might be that the mice were more energetically stressed in one setting compared to another. Whether [36, 130, 131] or not [33, 131, 132] a laboratory finds decreased ATP levels in the HD brain may be dependent upon the physiological status of the animals and tissue at and following sacrifice, often overlooked variables. In human lymphoblast cell lines expressing normal and mutant htt, the ATP/ADP ratio decreased as the polyglutamine expansion lengthened [133]. Total ATP levels in the STHdhQ11/1111 cells have been reported to be decreased or the same as those found in their STHdhQ/77 counterparts [119, 120, 133]. Decreased CV activity was observed in STHdhQ111/111 cells, but not in STHdhQ111/7 cells [120]. CV activity was unchanged in primary neurons transfected with Htt171-82Q [125]. In Neuro2A cells expressing a muhtt exon 1, ATP levels were reduced [134]. In contrast, cultures of embryonic striatum from YAC72 mice exhibited normal ATP levels [132]. How ATP levels are altered in lymphoblasts, muscle, or immortalized cell lines may also depend upon the physiological context of those tissues [119, 120, 133, 135]. In vivo basal energy metabolism, through whatever pathway, may be adequate to supply resting state needs and inadequate during activation [66, 67, 131].
Cytosolic availability of ATP may be limited by muhtt-induced downregulation or oxidation of CK expression [72, 134, 136, 137]. Upon exiting the mitochondria, newly synthesized ATP is converted by CK to PCr, the cytoplasmic high energy storage molecule. Subsequently, cytosolic CK generates ATP from the PCr in response to local energy demands. Disruption of the conversion of PCr back to ATP at cytosolic sites where energy is needed could also restrict or disrupt cellular function and homeostasis. For example, proteostasis, a cellular function requiring local ATP that may be compromised in HD, was restored by CK overexpression [134]. Indeed, CK expression and activity becomes transcriptionally depressed in a longitudinal manner in patients and the R6/2 and Q140 mouse models of HD [134, 137] (Fig. 2). Single time point reductions in CK activity have also been demonstrated in older Q111 and N171-82Q mice [136]. In addition, CK becomes increasingly oxidized over the course of R6/2 disease [73]. As a result of CK depletion, PCr accumulates and local ATP may not be readily available [134, 138]. Under such conditions, one could speculate that cytosolic glycolysis may be called upon to generate ATP to meet transient demands.
Substrate availability
More important than basal ATP levels, cells and the mitochondria within them must have the capacity to respond to an increased energy demand with increased ATP production. Certainly the decline in glucose uptake, discussed above, will place an overall restriction on the availability of the most abundant energy source. In response to visual stimulation, 31P MRS of occipital cortex in HD patients revealed the absence of an increased Pi/PCr ratio, the expected energetic response observed in control subjects [66]. The difference between basal and maximal mitochondrial ATP production is termed spare capacity. Measuring in vivo the rate of mitochondrial ATP production in striatum and cortex using 17O MRS, basal oxygen consumption was comparable between R6/2 and WT mice. When the ETC and ATP production were maximally stimulated, spare capacity was diminished in R6/2 [67]. Surprisingly, CIV activity was normal in these brain regions, leading the investigators to upstream deficits in glycolysis, TCA and possibly substrate availability to explain the constraint on ATP production [67]. Substrate limitations were suggested by a decrease in VDAC1 expression or its oxidation as VDAC1 may be responsible for calcium, NADH, ATP, ADP, Cr, PCr and other substrate transport across the outer mitochondrial membrane [139, 140] (Fig. 5). Oxidation of VDAC1 and a decrease in its expression were separately reported in striata of 10 wk old R6/2, a modification that might alter substrate flux through its channel [67, 73]. Thus the ability to respond to a stimulus with an appropriate increase in energy generation may depend upon the ability to bring substrate into cells and/or their mitochondria.
The idea that mitochondrial substrate availability or uptake capacity may limit energy production has not been directly addressed experimentally. However, the results of many studies could be explained by this mechanism. In cultured embryonic striatal neurons, NMDA-induced elevations in cytosolic calcium led to mitochondrial membrane potential deregulation more among Q111 cells than controls [141]. This observation suggests that the polyQ expansion limited neuronal mitochondrial capacity to generate the ATP needed to effectively restore calcium levels [141]. Since mitochondrial respiratory capacity was unchanged in these cells, mechanistic speculation focused upon an inability of ADP to enter mitochondria [141]. However, limited pyruvate, malate or NADH import could also explain these results.
Indeed, extramitochondrial calcium regulates glutamate fueled respiration by controlling aralar, the glutamate-aspartate transporter involved in the malate-aspartate shuttle, which imports NADH and glutamate and indirectly malate into mitochondria [142, 143] (Fig. 5). Thus, mitochondrial import of ETC substrates appear to be limited by cytosolic calcium. Additional restriction of substrate entry through the malate-aspartate shuttle might arise from an observed decrease in AAT activity in post mortem human caudate [85]. Substrate availability issues have surfaced in intact striatal neurons cultured from embryonic HD rat in which oxygen consumption rates, respiratory control ratio and spare capacity were normal in abundant glucose but decreased when glucose was restricted to 2.5 mM [144]. Alternate energy substrates (lactate and pyruvate) supplemented this respiratory function but did not fully restore it [144]. In STHdhQ111/111 cells, an observed decrease in ATP/ADP ratios was attributed to inadequate ADP uptake into mitochondria despite normal activity of the adenosine nucleotide transporter (ANT) [133], but restricted entry of upstream substrates could also have contributed. Comparing permeabilized STHdhQ111/111 and STHdhQ7/7 cells fueled by CI or CII, but not CIV, substrates, mitochondrial state 3 respiration, respiratory control ratios and the rate of ATP production were decreased with the polyglutamine expansion [119]. Notably under these conditions STHdhQ111/111 cells maintained normal ETC enzyme activity levels and sensitivity to inhibitors of complexes I through IV and normal ATP levels [119] (Fig. 4). The ETC machinery functioned normally but energetic flux appeared to become limited by some unidentified factor [119]. Given that plasma membranes were permeabilized, the limiting factor(s) must reside at the level of substrate transport across either the outer or inner mitochondrial membrane or a paucity of NADH generated by TCA. The pyruvate and phosphate carriers exert reasonably high controls on respiration in isolated brain mitochondria [145] but their function has not been explored in HD models.
Fatty acid β-oxidation
β-oxidation utilizes fatty acids to provide an alternative fuel source for energy production in the brain. Somewhat contradictory evidence suggests that fatty acids may be utilized in the brains or bodies of individuals with HD. Metabolomic studies of symptomatic, but not asymptomatic, HD gene carriers’ plasma under controlled dietary and medicinal conditions revealed lower plasma levels of branched chain amino acids, carnitines, phosphotidylcholines and increased levels of fatty acid breakdown products suggesting that both β-oxidation and amino acids were being shunted into TCA as energy substrates [146]. Mass spectrometry analysis of frontal lobe and striatum from postmortem HD brains identified but-2-enoic acid, an intermediate in fatty acid biosynthesis, as elevated in cortex but depressed in striatum, another indication that β-oxidation may be utilized in a regionally specific fashion [147]. In this same study, metabolites in a number of amino acid synthetic pathways were also reported to be largely downregulated [147]. Analysis of human serum from presymptomatic gene carriers contained some of the same plasma catabolic markers but did not reach statistical significance [148]. In early stage HD patients, one month of dietary consumption of triheptanoin, a triglyceride with three 7-carbon fatty acid chains, improved the occipital Pi/PCR signal detected noninvasively by 31P-MRS in response to visual stimulation [149]. Triheptanoin is metabolized in the liver via FAO to C5 ketone bodies, propionyl-CoA and acetyl-CoA, which can cross the blood brain barrier and serve as fuel sources [149]. This is a dramatic demonstration of the importance of alternative fuel sources for enhancing brain metabolism in HD.
β-oxidation and a pro-catabolic phenotype were implicated in an unbiased principle component analysis of serum metabolites discriminating between presymptomatic (8 wk) and symptomatic (15 wk) N171-82Q mice [148]. However, in N171-82Q mouse serum, mRNA levels of the first enzyme in the β-oxidation pathway, acyl-coenzyme A dehydrogenase, are significantly lower at 13 wk but not at 20 wk compared to controls [115, 117]. L-carnitine, a cofactor required for transport of fatty acids into mitochondria in the rate limiting step in β-oxidation, may also ameliorate symptoms in N171-82Q HD mice after long term IP injections [150] (Fig. 5). N171-82Q mice receiving IP injections of L-carnitine had an extended lifespan, fewer nuclear muhtt aggregates, reduced neuronal loss and improved motor performance [150]. However, whether this protection stemmed from facilitation of β-oxidation or antioxidant properties of L-carnitine was not established.
Cytosolic fatty acid synthesis and mitochondrial beta -oxidation are normally mutually antagonistic cellular processes. Surprisingly, evidence for possible activation of both β-oxidation and FAS stems from a recent unbiased multiomics analysis of the STHdhQ111/111 and STHdhQ7/7 cell lines [151]. This study iteratively matched partially identified differentially expressed mass spec peaks between the two cell lines with an integrated data base of known metabolic pathways, lipids and proteins. This process produced networks of connected, up- or down-regulated metabolites and inferred abnormalities in the biochemical pathways that connected them. Changes in metabolites that were predicted to be abnormally expressed at intermediate positions on the identified pathways were subsequently validated by conventional methods. From the protein fragments in the original mass spec analysis, abnormalities in sphingolipid, fatty acid and steroid metabolism emerged. This includes a demonstration that protein expression of FAS and two predicted long chain fatty acids (EPA & DGHLA) were elevated in STHdhQ111/111 cells compared to STHdhQ7/7 cells [151] (Fig. 4). FAS, responsible for de novo fatty acid synthesis downstream of acetyl-coA, also regulates food intake in the hypothalamus [152]. EPA stabilizes mitochondrial integrity and increases β-oxidation [23]. The prominent alterations in fatty acid metabolism and upregulation of these competing pathways suggests that the STHdhQ111/111 cell line may utilize so much fatty acid as fuel that its general synthesis needs upregulating as well. If these pathways are also increased in human brain, this may be another example of a homeostatic response to muhtt.
Compensatory shifts to generate energy from fatty acids or amino acids may be regulated by sirtuins, which deacetylate enzymes in fatty acid oxidation and amino acid metabolism [153]. SIRT3, whose short form is located in the mitochondria, becomes co-activated by NAD+ to deacetylate enzymes involved in fatty acid oxidation, promoting flux through this energy-generating pathway [153]. NAD+ levels are rate limiting for SIRT3 deacetylation, thus SIRT3 becomes a sensor of metabolic flux. In peripheral tissues, SIRT3 levels increase during caloric restriction or periods of lowered glucose availability and decrease with aging [153]. SIRT3 forms part of a positive feedback pathway to increase energy metabolism through activation of AMPK by increases in AMP levels. AMPK activated CREB and subsequently PCG-1α stimulate SIRT3, which in turn deacetylates LKB1, further stimulating AMPK [153]. SIRT3 also deacetylates both isocitrate dehydrogenase 2, a TCA cycle enzyme producing NADPH, and Mn superoxide dismutase, a mitochondrial antioxidant enzyme, implicating SIRT3 in control of further metabolic flux and antioxidant defenses. During metabolic stress or low glucose availability, SIRT3 deacetylates a long list of mitochondrial proteins involved in TCA, β-oxidation, ETC and oxidative phosphorylation, all in the direction of promoting energy generation [153]. SIRT3 knock out mice are more vulnerable to oxidative stress, while running on a wheel increases SIRT3 expression, producing neuroprotection [154].
However, SIRT3 deacetylation may not be happening in HD due to sirtuin deficiencies, as observed in several HD models [37]. Indeed, muhtt inhibits general cellular deacetylase activity by binding to SIRT1 [155]. Binding of muhtt to SIRT3 has not been shown. Sirt1 overexpression improved functional measures in the N171-82Q mouse, suggesting loss of its regulation may influence pathogenesis [155]. However, Sirt1 levels at different stages of disease were not determined, so at what stage SIRT1 deregulation contributes to pathogenesis remains a mystery. In the STHdhQ111/111 cell model (compared to STHdhQ7/7 control cells), muhtt produces decreases in SIRT3 expression, pAMPK relative to AMPK and NAD+/NADH while increasing acetylated LKB1 and ROS levels [37]. These changes could be reversed by viniferin, a natural analog of resveratrol, an activator of SIRT3 [37]. Thus in these immortalized cells, muhtt expression suppresses the sirtuin pathway involved in compensating for energy deficits. However SIRT3 involvement in regulating fatty acid oxidation and acetylation status of specific enzymes remains to be explored. Furthermore, if muhtt decreases SIRT3 and subsequently decreases the supraregulatory AMPK pathways involving both SIRT3 and PGC1α, this points to an eventual loss of master regulatory control over energy generation, consistent with the severe phenotype encountered at end stage human HD.
Pentose phosphate pathway
Involvement of increased fluxes through the PPP in HD is currently supported only indirectly. In the cortex of heterozygote Q175 mice at 14-15 mo, mRNA levels for transketolase, a rate-limiting step in the PPP, was elevated [156]. Activity levels were not determined [156]. Additional arguments for PPP involvement invoke the need to produce NADPH for compensation for oxidative damage. In samples from postmortem stage 4 HD brains, expression of the antioxidant proteins peroxiredoxins, glutathione peroxidases, SOD and catalase were increased in striatum and cortex [72]. At 2 wk but not later, transketolase expression was upregulated in R6/2 striatum [44] (Fig. 3). These suggest an increased antioxidant need and possible upregulation of the PPP, which produces NADPH for GSH regeneration from GSSG.
In the Drosophila eye model of HD, increased flux through the PPP induced by downregulation of ribose-5-phosphate isomerase was protective, demonstrating that PPP activation could be beneficial in HD [157]. Oxidative stress increases demands for reducing equivalents NADPH and NADH to regenerate the antioxidants thioredoxins and glutathione. Depletion of NADPH pulls glucose through the PPP. Indeed, in a drosophila model of HD, overexpression of the human neuronal glucose transporter (GLUT3) or glucose-6-phosphate dehydrogenase (G6PDH, the enzyme pulling glucose into the PPP) but not phosphofructose kinase (PFK, the second enzyme in glycolysis) extends the muhtt fly lifespan [158]. Moreover, overexpression of all three ameliorate the severe curtailment of lifespan induced by knockout of a subunit of pyruvate dehydrogenase (PDH) or a subunit of CI [158]. This latter observation demonstrates very clearly how endogenous shifts in metabolic flux might compensate for defects in later stages of energy production. A similar mechanism may be at work in human HD as a compensatory homeostatic response, as increased copy number for the SLC2A3 gene encoding GLUT3 increases expression of GLUT3 and correlates with increased age of onset [59]. While these fly studies raise possibilities, upregulation of the PPP in HD has not yet been systematically examined.
In line with this, neonatal (but not adult) iron supplementation in R6/2 mice increases oxidative stress, resulting in accelerated disease progression [159]. The rise in striatal and cortical GSSG at 12 wk associated with iron supplementation since weaning could arise because the PPP failed to compensate. A somewhat transient rise in GSH was found in normally raised R6/2 cortex at 8 wk, as if the system were adjusting homeostatically to attempt to compensate for an increased oxidative load [138]. Consistent with this, a rise in lactate accumulation in cortex of the R6/2 mouse suggests an increase in glucose utilization (beginning at 8 wk, significant by 12 wk) [138], perhaps including augmented flux through the PPP. Comparable changes in lactate concentrations in other models or human studies have not been reported as brain MRS lactate quantification often exceeds the accepted variability limits [160]. Protein oxidation in R6/2 cortex was detected at 10 wk and beyond [67, 161].
The increased oxidative stress in HD may not come solely from an increased production of ROS by mitochondria. Rather the apparent increased oxidative damage may result from a decrease in antioxidant defenses unable to handle the normally generated level of spurious oxidation. In a metabolic environment with a restricted glucose supply, ATP generation may be favored over the PPP production of NADPH. Glutathione synthesis may be limited as transport of cystine, a cysteine precursor, across neuronal plasma membranes may be impaired [162]. In STHdhQ111/111 cells, GSSG, GSH and the glutathione redox cycle activity are increased intracellularly while de novo GSH synthesis and GSH export are depressed, indicating significant disruption of this important antioxidant system [87]. Similarly, shifts in redox status may alter regulatory interactions dependent upon molecules that also have an antioxidant role. For example, ascorbate inhibits glucose uptake and stimulates lactate uptake into neurons via the GLUT3 and SVCT2 transporters, respectively, independent of its redox activity [63, 163] (Fig. 5). This mechanism may be important for routine switching between use of lactate and glucose as fuel sources [63, 163]. However, this switch appears to be inoperative at synapses in the R6/2 striatum at 3–4 wks and beyond [163]. Disruption of SVCT2 transporter cycling into the plasma membrane by muhtt and/or the huntingtin-associated protein-1 may be responsible for the dearth of plasma membrane SVCT2 transporters, contributing to a decrease in intracellular ascorbate, an increase in ROS levels and indirectly promoting glucose uptake over lactate uptake [63, 163].
The full extent of oxidative damage in HD is beyond the scope of this review. The reader is directed to discussions by other authors [161, 164].
Metabolite changes
Metabolomic analysis of steady state levels of small molecules in the brain relevant to energy generation corroborate a view of shifting adjustments at different stages of disease. Changes early in life or presymptomatically may represent homeostatic ones, whereas progressive changes may represent developing dysregulation. Uneven rates of change in metabolite levels were first reported in a longitudinal magnetic resonance spectroscopy (MRS) study of cortex and striatum from R6/2 [138] (Fig. 2). While choline-containing compounds and glutamine increased steadily from 4 to 16 weeks in both regions, elevations in creatine and phosphocreatine jumped initially between 4 and 8 wks and then remained largely stable [138]. N-acetyl aspartate (NAA) decreased beginning at 8 wk but more so in cortex than in striatum [138]. Myo-inositol increased earlier in striatum than cortex, while lactate increased more in cortex, suggesting that metabolic shifts might progress at rates influenced by regional connectivity and composition [138]. Somewhat similar but not identical shifts occur in the slower developing knock in mouse models. At 12 mo in the striatum of Q140 mice, glutamine increased while choline-containing compounds, glutamate, total NAA and taurine decreased [165]. The loss of glutamate was confirmed with chemical exchange saturation transfer imaging and further detected in cortex and corpus callosum as well [165]. In striatum of zQ175 knock-in mice by 12 mo increases in glutamine, taurine, and total creatine, and decreases in NAA were reported [166]. Most interestingly, decreases in glutamate and GABA observed at 4 and 8 mo recovered by 12 mo, another illustration of a homeostatic compensation [166]. An additional study of the zQ175 striatal metabolites reported glutamate decreased at 6 mo, recovered at 9 mo only to decrease again at 12 mo, suggesting a regulatory system that is oscillating as disease progresses [167]. In addition, this study detected decreased NAA from 6 mo, increased glutamine from 9 mo and decreased GABA, and increased myo-inositol and taurine at 12 mo [167]. The differences in the latter two studies suggest that rearing conditions in different laboratories may also contribute variability.
Human MRS metabolite studies are more difficult to compare, as many studies report ratios of metabolite concentrations rather than the absolute values of concentrations. Fewer metabolites are typically detected due to use of lower field strength magnets. Consistent with the observation of early changes in R6/2 cortex, in premanifest to early HD, reduced NAA and glutamate levels in posterior cingulate cortex correlated with a measure of mild cognitive decline [160]. In the putamen, premanifest HD was characterized by a loss of NAA that continued to decline into early HD along with the development of a decrease in total creatine and glutamate and an increase in choline-containing compounds and myo-inositol [168]. The loss of NAA correlated with the total disease burden score and a number of specific motor tasks [168]. The ratio of myo-inositol to NAA correlated with the UHDRS motor score [168]. In a 2 yr follow up longitudinal study, these changes persisted but did not progress making the correlations quite variable [169]. The loss of NAA and creatine have been confirmed by other laboratories [170, 171]. Proton MRS of HD patient CSF detected decreases in both lactate and citrate, but not in glucose, pyruvate, glutamine, alanine, creatine, creatinine, inositol, formate or acetate [172]. The changes in NAA and myo-inositol seen in humans parallel those reported in mice. However, the human MRS measurements remain quite variable and do not extend over a long enough span of disease progression to distinguish compensatory from degenerative changes.
Flux analysis
To truly capture the shifts in energy generating capacities, experiments must move beyond steady state determination of individual metabolite concentrations. Metabolic flux analysis and its derivatives provide a systems level view of reaction rates in enzyme pathways at metabolic steady state [173] and the ability to compare these fluxes across disease progression. Flux analysis applied to E Coli revealed that when and where carbon sources enter a metabolic network determine the overall energetic costs [174]. This suggests that given chronic high energy demands, cells may switch their reliance upon specific energetic pathways attempting to optimize ATP production. Comparing differentiated astrocytes and neural stem cells with 13C flux analysis after differentiation from embryonic stem cells, only astrocytes downregulated their carbon utilization [175]. Given that metabolic rerouting occurs during developmentally appropriate growth, similar metabolic adaptations could be expected to occur upon introduction of abnormal genotypes. With muhtt ubiquitously expressed from conception, tissue-specific metabolic adaptations should be tailored to each developmental stage. In liver glucose metabolism, the range of metabolic regulatory compensation achieved by rapid enzyme phosphorylation or allosteric or hormonal control of enzyme activities was equal to or greater than regulation by longer term alterations in enzyme expression levels [176]. For cerebellar granule neurons maintained in high glucose cultures, flux analysis demonstrated a high proportion of glucose was processed through the pentose phosphate and anaerobic glycolysis pathways [177]. Evolution of flux analysis techniques to in vivo conditions in the context of neuronal-glial interactions and normal electrophysiology, holds promise that the details of how metabolic networks shift in response to disease can indeed be worked out. However, caution must be exercised in identifying initial assumptions, scope and regional specificity when applying these broad systemic analyses [178].
Only one study has tried to look at metabolic shifts in HD from glycolysis through oxidative phosphorylation [116]. Olah et al. [116] went beyond measuring individual enzyme activities and modeled expected changes in fluxes, stating “the flux is a collective property of all the enzymes in the pathway.” In affected parts of the brains of 20 wk old N171-82Q mice, they reported the non-intuitive result that despite a decrease in GAPDH activity, an intermediate glycolytic enzyme, glycolytic flux increased as demonstrated by increases in pyruvate and lactate levels and activities of pyruvate kinase, enolase and hexokinase. The observed direction of changes in these products and enzymes agreed with predictions of their kinetic model [116]. Additionally, this study reported an increase in glutamate dehydrogenase (GDH) activity, an anaplerotic reaction which converts glutamate to α-ketoglutarate, bringing this substrate as fuel into the TCA cycle, bypassing glycolysis [116].
In CNS mitochondria, the rate of ATP production is limited equally by CV and both the phosphate and pyruvate carriers, followed closely by CI [145, 179]. So inhibition of flux at any of these control points could compromise oxidative phosphorylation [145]. Examination of the control coefficients for metabolic flux compared across tissues reveals that brain is more sensitive to compromise of ATP synthase and the phosphate & pyruvate carriers than muscle and heart [145]. Liver is a close second in sensitivity to CV and the carriers, but is more sensitive to CI than brain [145]. Most notably, this study pointed out that the substrate carriers, which are often overlooked, are equally important when considering limitations on energetic flux [145]. A defect in substrate transport could account for the reduced respiratory control ratios in the absence of ETC enzyme deficits in STHdhQ111/111 cells discussed earlier [119]. Defects in substrate availability, due to a decreased VDAC1 expression, were also suggested to underlie the reduction in maximal in vivo cerebral metabolic rate of oxygen consumption (CMRO2) reported for R6/2 striatum and cortex at 10 wk [67].
Analysis of flux control ratios for each of the ETC complexes in R6/2 striatal homogenates at 12 wk uncovered significant but small decreases in CII and CIV control, with decreases in CII exerting more effect than CIV [180]. However, the overall respiratory control ratio was unchanged indicating that basal ETC capacity was normal and CII became the potential bottle neck if it became inhibited [180]. Endogenous inhibition of forebrain SDH occurs due to oxaloacetate binding [181], but this has never been examined in HD. Determination of control coefficients are rarely performed for other aspects of metabolic flux, such as glucose uptake, glycolytic enzymes or other substrate transport. The role of substrate flux, through the pyruvate carrier or the malate-aspartate or glycerol-3-phosphate shuttles that bring in NADH and FADH2, is an overlooked aspect of metabolic function in HD.
Ischemia reperfusion injury provides an informative example of how flux through all these energy generating pathways can shift with changing conditions. In heart, brain, liver and kidney, succinate becomes elevated during in vivo ischemia, returning rapidly to normal levels upon reperfusion [129]. In ischemia, abundant AMP drives its breakdown via the purine nucleotide cycle, producing fumarate. Fumarate however does not accumulate as it can be rapidly converted to succinate via the reversible SDH reaction. Subsequent tracing of labeled carbons, metabolic modeling and manipulation of succinate levels and SDH activity determined that during ischemia, fumarate converts to succinate reversing CII, consuming electrons from CI that were not pulled further through complex III (CIII) and CIV in the absence of oxygen. Upon reperfusion, the accumulated succinate drives normally directed flux through CII, producing excess electron flow that splits between the normal direction to CIII and CIV and reverse flow through CI generating reactive oxygen species [129]. As succinate levels decline, ETC flux returns fully to normal. Mass action shifts in flux through TCA (demonstrated by a decrease in aconitase activity), the alanine amino transferase (ALAT) and AAT reactions and the malate-aspartate shuttle during ischemia also would return to normal directionality as succinate concentrations subside [129]. Such short term temporal variations in metabolites have not been studied in HD model systems. Succinate was observed to decrease in R6/2 cortical and cerebellar, but not striatal, tissue extracts at 12 wk [182]. In presymptomatic HD rats, serum elevation of succinic acid was attributed to SDH inhibition [183]. In acute ischemic injury, expression levels were not altered, only fluxes. In HD, altered expression levels, in addition to mass action effects, will contribute to changes in flux that may be more complex than in ischemic injury. However, the mass action effects, as illustrated in ischemia, have explanatory power that must not be overlooked.
Classical concepts in metabolic regulation include the idea that energy demand drives production. However, recent flux analysis of metabolites and oxygen consumption in retina determined that phototransduction, possibly less energy demanding than maintaining ionic gradients for depolarization in the dark, slowed energy production through glycolysis and less so through TCA cycle [184]. Notably, mouse retinal oxygen consumption after light adaptation operated very near maximum uncoupled rates [184]. Flux analysis of this mostly rod-driven phenomena revealed that retinas relied much more on glycolysis in the dark. In the light, the PPP was stimulated and flux through TCA was slightly slowed [184]. The latter was accomplished through the light-induced decreases in free calcium which down regulated the malate-aspartate shuttle and the activity of α-ketoglutarate dehydrogenase [184]. Thus the metabolic consequences of changing energy demands may not be readily predictable. Similarly unpredictable, visual stimulation of occipital cortex in HD patients did not produce the elevation in Pi/ATP observed in normal subjects [66]. In anesthetized R6/2 in both cortex and striatum, the maximum uncoupled CMRO2 was less than in wild type mice [67]. Both of these experiments suggest that brain metabolism in HD may operate near maximum. While conditions in the HD brain are unlikely to exactly match those in retina, these nuances in retinal metabolism are examples of temporal and contextual energy regulation.
In cancer cells and immortalized cell lines, flux through metabolic pathways shifts to favor aerobic glycolysis over oxidative phosphorylation, a process called the Warburg effect [185, 186]. Induction of anaerobic glycolysis promotes cell longevity, enhancing tumorigenesis [186]. Such shifts have been found in response to synaptic activity in cultured hippocampal neurons on the fairly rapid time scale of hours [187]. Indeed, synaptic activity induced increases in gene expression for glucose and lactate transporters and PDH but a decrease in PDH activity within a few hours [187]. In the AD brain, the Warburg effect coincides with the development of resistance to Aβ accumulation and toxicity on a longer time scale, where resistance was characterized by upregulation of LDHA and PDK favoring lactate production and decreased entry into TCA, respectively [188, 189]. Also associated with the shift to glycolysis and downregulation of oxidative phosphorylation was a decrease in potentially damaging ROS [188, 189]. Hypoxia inducible factor 1 (HIF-1) has been designated as a switch capable of upregulating glycolysis by induction of LDHA and downregulating TCA through reduction of PDK [190]. These AD and cancer studies present two ideas that must be considered in analyzing metabolic shifts in HD. The first is that the immortalized or cultured cells used in many HD studies may already have shifted set points favoring glycolytic activity. To their credit, investigators utilizing such cells compare the polyglutamine expansion to non-expanded cells. The second and more important idea is that the Warburg effect must be considered as a potential baseline condition in HD brain, where surviving neurons generate energy largely by glycolysis. Indeed HIF-1 induced decreases in PDK have been demonstrated in STHdhQ111/111 cells and YAC128 cortex [94].
Urea cycle
Difficulties in buffering brain amines may be an overlooked phenotype of brain metabolism in HD. While the brain contains urea cycle enzymes, normally this pathway is not thought to play a major functional role in nitrogen fixation [191]. Ammonia fixation in the brain largely occurs via amination of glutamate to glutamine by glutamine synthase in astrocytes [99, 192]. Not only was glutamine progressively elevated in the R6/2 striatum and cortex [138, 182] (Fig. 1), but 31P MRS of human brain revealed an increased intracellular pH [193]. Such alkalinization could reflect abnormalities in ammonia fixation in brain or liver or both. Metabolomic analysis of postmortem HD brain samples revealed an elevation of urea uniformly across eleven brain regions, including ones not affected by HD [194]. Consistent with this rise, the urea transporter UT-B present in astrocytes is elevated in postmortem human caudate and cortex, possibly to facilitate removal of the excess urea [194, 195]. A deficit in urea cycle functioning was noted in a prior report demonstrating decreases in post mortem striatal urea indicating additional work is needed to examine enzyme activities and to clarify how the urea cycle may be altered in HD [196].
While often overlooked, pH is a powerful regulator of enzyme activity. For example, phosphofructokinase is stimulated by ammonia while α-ketoglutarate dehydrogenase is inhibited, combining to shift energy flux from oxidative phosphorylation to glycolysis [197]. In the liver, the urea cycle detoxifies ammonia, preventing its accumulation in blood and subsequent widespread alkalinization of body tissues, including brain. Increased levels of ammonia and/or the urea cycle intermediates citrulline and arginine in blood from HD patients, HD sheep, and R6/2 and Q150 mice all point to abnormalities in liver urea cycle function [198, 199]. Indeed, lowering ammonia production with a low protein diet begun before symptom onset ameliorated accumulation of aggregates in the liver and striatum, partially restored liver mRNA levels of a transcription factor for urea cycle enzymes, ameliorated changes in activity of these enzymes, improved motor performance and restored striatal BDNF levels in R6/2 mice [198]. If indeed these peripheral effects of urea cycle abnormalities are early signs of HD onset, as illustrated in the plasma of presymptomatic HD sheep [199], the CNS metabolic defects could reflect a consequence of abnormal pH regulating various metabolic enzymes.
CHANGES AT THE ORGANELLE LEVEL
Mitochondrial biogenesis
The metabolic consequences of decreased PGC1α, a master regulator of mitochondrial biogenesis, have become well established in HD pathogenesis [2, 115, 200]. PGC-1α acts as a co-regulator in conjunction with the nuclear transcription factor PPAR-γ to regulate many genes involved in mitochondrial formation [115]. In humans, two distinct single nucleotide polymorphisms in the PPARGC1A gene modify the age of HD onset in opposite directions [201]. Muhtt interferes with PCG-1α striatal transcription, negatively impacting mitochondrial function [2]. PGC1α mRNA levels were decreased in samples from postmortem human HD striatum as well as in several, but not all, mouse and cell models (Q140, N171-82Q, STHdhQ111/111, but variably or not in R6/2) [2, 115, 202, 203]. Mitochondrial biogenesis decreased in PC12 cells expressing muhtt [204]. In the human samples, compensatory upregulation of mRNA for PPAR-γ and other transcription factors downstream of PGC-1α were also detected (64). PGC-1α overexpression in N171-82Q mice increases expression of mitochondrial enzymes, ameliorated motor phenotype and oxidative stress and prevented aggregate formation and neurodegeneration [117]. Similarly, overexpression of PGC1α restores mitochondrial function and prevents cell death among STHdhQ111/111 cells [2]. Rosigilene, a PPAR-γ agonist, rescues striatal cells from muhtt toxicity and restores motor function, BDNF levels and PCG-1α levels and prevents hypothalamic neuronal loss in the N171-82 mouse model of HD [205]. Stimulation of PPARs with dietary thiazolidinedione or bezafibrate improved mRNA expression of mitochondrial enzymes in brains of R6/2 mice, increased striatal mitochondrial density and ameliorated neuropathology and behavior [206, 207]. Restoration of mTORC1, an upstream positive regulator of PGC1α, also increases expression of PGC1α and associated mitochondrial enzymes and counteracts abnormal behaviors, again in N171-82Q mice [208]. In many of the overexpression rescue experiments, PGC1α and associated mitochondrial enzymes were elevated to greater than normal levels, suggesting that given enough energy, cells can overcome the proteostatic burden of accumulating muhtt.
Despite this strong genetic link to mitochondrial biogenesis, PGC1α knock out mice are viable [202, 209]. In normal development, PGC1α expression colocalizes with and peaks in striatal, cortical and hippocampal GABAergic neurons during the first several weeks postnatally in mouse [210]. In the PGC1α knock out mouse, an early onset but not progressive hyperactive phenotype and clasping are associated with striatal vacuolization and possible white matter loss [202, 209]. These mice differ from R6/2 HD mice in that markers for medium spinal neurons increase in a homeostatic manner, supporting the idea that metabolic pathways do shift to maintain function [202]. Given that in HD, PGC1α and the linked metabolic protein transcription may only be partially depleted [2, 115, 202, 203, 211], compensatory mechanisms may also be present. More importantly, PGC1α appears to more profoundly regulate expression of parvalbumin (PV) and several synaptic proteins critical for transmitter release to a greater extent than mitochondrial proteins [211, 212]. While the transcriptional mechanism for PGC1α suppression of PV has not yet been identified, such regulation suggests PGC1α depletion may cause dysfunction through non-metabolic means [211, 212]. Indeed, mice expressing muhtt exon one in PV expressing neurons exhibit an HD-like hyperactive motor phenotype and age-related changes in inhibitory synaptic events that may be independent of transcriptional dysregulation, suggesting that muhtt and PGC1α driven changes in PV neurons may also be independent of effects upon mitochondrial proteins [213].
Mitochondrial trafficking
The most energy-consuming communication processes of the nervous system occur at synaptic sites, far from the soma. To deliver energy to remote processes, mitochondria must be trafficked to dendrites and axon terminals, a process that can be disrupted by mutant huntingtin [4]. N-terminal mutant huntingtin impairs mitochondrial trafficking in axons of cultured striatal neurons from Q150 knock in and YAC72 mice and primary rat neuronal cultures transfected with muhtt [131, 132, 214]. Both anterograde and retrograde transport were slowed [131, 132]. Full length muhtt slows mitochondrial transport more than an N-terminal fragment [214]. Muhtt impedes axonal transport in its soluble form; aggregates can block axonal transport entirely producing axonal swelling and mitochondrial accumulation upstream of the block [214]. Beyond mitochondria, in vivo retrograde axonal transport of the exogenous marker molecule fluorogold along the striatonigral pathway was also impeded by the presence of mutant htt in YAC72 brain [132]. This interference with mitochondrial trafficking results in a paucity of ATP in synaptosomes harvested from Q150 mouse brains [1, 131]. Thus energy impairments in HD can result from a dearth of mitochondria at presynaptic release sites, limiting information flow and possibly at other sites of high energy demand without compromise of any enzymatic pathway.
Mitochondrial dynamics
Mitochondria in many HD model systems appear to be smaller than in normal tissue. In 6 mo old YAC128 striatum, in muhtt transfected cultured neurons and in fibroblasts from individuals with HD, mitochondria appear more fragmented than in controls [215, 216]. In YAC128 striatum compared to controls, a larger number of mitochondrial cristae occupy a smaller overall volume [216]. In these experiments, the mitochondrial fragmentation was attributed to an increased association between muhtt and DRP1, the protein responsible for fission, as dominant negative DRP1 prevented fragmentation [216]. Increased fragmentation was also observed in the immoratilized STHdhQ111/111 cells who were also more sensitive to staurosporine-induced cell death than controls [120, 216]. Expression of dominant negative DRP1 to prevent fragmentation or overexpression of OPA1 to prevent cristae remodeling, but not overexpression of MFN1 to promote fusion and elongation, rescued Q111 cells, lymphoblasts from HD donors and cultured YAC128 neurons from these phenotypes [216]. Fragmented or enlarged mitochondria were observed in about a quarter of cultured neurons from R6/2 striatum [110].
DRP1-dependent fission can become activated through multiple mechanisms [217, 218], several of which have been demonstrated to contribute to mitochondrial fragmentation in HD. In the STHdhQ111/111 cells, fragmentation appeared to be triggered by increased calcineurin activation of DRP1 [216]. In a number of HD model systems, increased DRP1-induced fragmentation has been attributed to increased dopamine receptor driven calcineurin activity, increased S-nitrosylation of Cdk5 or direct association of muhtt with DRP1 [216]. Aberrant nitric oxide-induced S-nitrosylation of Drp1 also contributes to its activation and mitochondrial fragmentation in muhtt transfected primary neuronal cultures [219]. A selective inhibitor of DRP1 ameliorates mitochondrial fragmentation, restores mitochondrial membrane potential, and improves viability among Q111 cells and both HD patient fibroblasts and induced pluripotent stem cells differentiated into neurons. DRP1 inhibition also ameliorates symptoms in R6/2 mice [220]. Similar results were obtained from downregulation of p53, indicating that p53 might directly initiate DRP1-mediated fission in HD neurons [220].
Cristae disruption appears to be another consequence of DRP1 activation in HD [215, 216]. Cristae from neuronal mitochondria of 6 mo old YAC128 striatum had a higher density of cristae yet lower ratios for cristae to mitochondria surface area and volume [215]. Disorganized cristae were observed in Q111 cells and in primary cultures from neonatal YAC128 striatum [216]. However, fission and these cristae dilations are not incompatible with life as the Q111 cells proliferate and survive in vitro [221]. Further cristae disruptions triggered by apoptotic stimuli were accelerated in Q111 cells suggesting that cytochrome c release may become easier if spaces between cristae become enlarged [216]. In neurons, replenishment of peripheral energy generating capacity through fission of somal and axonal mitochondria is a critical homeostatic function [222]. These fissed mitochondria function normally [222, 223]. Ninety percent of anterogradely transported axonal mitochondria maintain a high membrane potential sensitive to ETC but not glycolytic antagonists [223]. In memory T cells, mitochondrial fusion and associated tight cristae promote OXPHOS while fission and cristae expansion promote glycolysis in effector T cells [224]. The effector T cells are viable, highly functional and metabolically active utilizing aerobic glycolysis and less efficient OXPHOS; such cristae changes are not severe enough to trigger immediate autophagy [224]. Striatal mitochondria express more of the DRP1 receptor FIS1 and are therefore more predisposed to become fissed [225]. If in addition, the observed similar cristae changes in HD neuronal mitochondria induce aerobic glycolysis [215, 216], this would be consistent with the proposed homeostatic shift in energy production towards glycolysis early in disease [65, 67].
Despite the increased incidence of smaller mitochondria in YAC128 striatum [215, 216], isolated YAC128 striatal mitochondria respire normally [108]. Thus these structural changes may increase neuronal sensitivity to apoptosis [216], but they do not do so by compromising ETC function. Other instances of normal mitochondrial bioenergetics function, despite altered shape, have been documented in neurons in which DRP1 activity has been downregulated. Selective depletion of DRP1 in DA neurons in vivo leads to swelling and loss of mitochondrial mass, but not membrane potential, so the degeneration under these conditions may be due to a lack of mitochondria rather than overt dysfunction of intermediate metabolism [226]. Similarly, knock out of DRP1 causes the normally elongated Purkinje cell mitochondria to become large and round with normal cristae morphology, normal membrane potential and ETC activity [227]. Even mitochondria from embryos of DRP1 null mice, which are embryonic lethal, maintain normal ATP levels when supplied with maternal energy sources, again suggesting that enzymatic activities are appropriate [228].
One contradiction remains to be untangled. AMPK, the master energy sensor, is also a regulator of mitochondrial fission. ETC enzyme inhibition of human osteosarcoma cells leads to increases in AMPK activity & consequent mitochondrial fission [229], a phenotype associated with increased uncoupling, a loss of nutrient storage and a decline in ATP production [3]. Since ETC inhibition appears to occur only late in HD progression, a similar mechanism is unlikely to account for mitochondrial fission early in disease progression. AMPK becomes activated by increased AMP/ATP ratios under conditions of cellular stress to increase energy production and decrease energy-consuming processes [230]. For mild energetic stress including exercise or nutritional shortages, AMPK promotes mitochondrial fusion accompanied by increased oxidative phosphorylation [231]. For more severe stress triggered by radiation or toxic chemicals, AMPK promotes mitochondrial fission leading to autophagy and apoptosis [231, 232]. Exactly how AMPK signaling distinguishes between these divergent outcomes remains unclear. HD brains are characterized by normal energy generation at rest but an inability to produce sufficient energy in response to stimulation [66, 67]. Unlike the generic in vitro cell systems used to work out the above roles of AMPK, neuronal mitochondria are not elongated in HD, despite both the activation of AMPK [36] and the arguments that an inability to produce energy upon stimulation [66, 67] would be considered a mild stress, perhaps even milder than starvation. If AMPK activation is producing fission and autophagy, it must be doing so in a highly localized manner since death is not acute or rapid in HD. Also, AMPK may exert disease stage dependent effects [35].
To reconcile the metabolic results from experiments on mitochondrial dynamics with those on isolated mitochondria, one has to ask whether the isolated mitochondria represent fissioned or fused states. Newly isolated mitochondria are typically round and condensed when viewed by EM [108, 233, 234]. This results from the low ionic strength isolation media [233, 234]. When resuspended in osmotically isotonic potassium-containing medium, the condensed state rapidly changes to the orthodox configuration, with corresponding normally structured cristae. Addition of ADP to orthodox isolated mitochondria results in temperature-dependent condensation within 20 sec [234]. It is impossible to assert the original length or size of the mitochondria prior to isolation or if there are changes in size that occur as a result of isolation. The fission and fusion processes can occur within a minute while the centrifugations associated with isolation can take tens of minutes. Nevertheless, the normal ETC activity of isolated mitochondria from striatum and cortex of both the R6/2 and YAC128 mouse models of HD [108, 110] suggests that the original fused or fissed state did not interfere with metabolic capacity of mitochondrial isolates.
Mitophagy
As with increased fission, loss of mitochondrial mass could of itself be sufficient to account for the energetic defects in HD. Indeed, mtDNA copy numbers were decreased in R6/2 striatum (but not cortex) and in STHdhQ111/111 cells [111, 120]. Mitochondrial mass is also decreased in HD induced pluripotent stem cells [5]. Mitochondrial clearance or mitophagy appears to be specialized forms of general autophaghy, which is compromised in HD [235]. Muhtt may also act to alter mitochondrial clearance or mitophagy, although whether the effect is to hasten mitophagy or prevent it is currently disputed [5, 204, 236, 237]. In PC12 cells transfected with Q74 expanded htt and in STHdhQ111/111 cells, oxidized (and therefore inactivated) GAPDH associated with mitochondria targeting them to lysosomes but prevented internalization and mitochondrial degradation [204]. This process increased accumulation of damaged mitochondria and hence mitochondrial mass as evidenced by increased protein levels of VDAC, TOM20 and aconitase relative to enolase [204]. Overexpression of inactive GAPDH restored mitophagy, implicating muhtt as the species inhibiting this form of autophagy [204]. So caution should be applied when interpreting changes in mitochondrial enzyme expression if mitophagy is prevented. Valosin-containing protein (VCP) is a cellular ATPase associated with ER and mitochondrial degradation and autophagy. Muhtt on the mitochondrial outer membrane of STHdhQ111/111 cells and muhtt expressing induced-pluripotent stem cells binds to VCP, accelerating autophagosome formation or mitophagy [5]. This process is associated with loss of mitochondrial membrane potential, shortening of mitochondrial length in neurites, and increased cell death [5]. A synthetic peptide designed to inhibit muhtt-VCP interactions ameliorates these phenotypes plus motor phenotypes in R6/2 and YAC128 mice [5]. However it is not clear from either set of experiments whether the mitochondria are damaged prior to or as a consequence of GAPDH or VCP binding or recruitment. As an inducer of mitophagy, either protein could be causing the depolarization and fission. Thus it is possible that the energy generating pathways remain functional until mitophagy becomes initiated. Further clarification of the controversies surrounding mitophagy are needed to resolve its contribution to energetic defects in HD.
SUMMARY
This discussion has presented an updated, systemic view of shifts in brain energy generating biochemical pathways in models of HD. The idea of a shift in energy status in HD is not new [133]. The proposed contextual homeostatic framework goes a long way towards reconciling the disparate findings across many laboratories. Regulatory feedback mechanisms control flux through the intertwined metabolic pathways, adjusting enzyme activities to meet local energy demands for normal function and the added burdens associated with accumulating muhtt. The ATP supply is sufficient for basal needs, but is limited in times of activation or stress. This loss of spare ATP slowly erodes protein clearance mechanisms, mitochondrial trafficking, and possibly even sodium clearance leading to accumulation of misfolded proteins and aggregates and perhaps hyperosmotic conditions [160]. These disruptions are not immediately lethal; rather they invoke regulatory compensations that shift over the course of disease adjusting to contextual factors.
Mitochondrial shape and size are intimately linked to whether glycolysis or oxidative phosphorylation is favored, adding another dimension to regulation of energy production. Elongated mitochondria favor oxidative phosphorylation and FAO while the fissioned state favors glycolysis and muhtt binds DRP1 promoting fission [153, 216, 224]. Movement of mitochondria within neurons and astrocytes may further limit energy supplies in distal processes. Early in disease, these homeostatic mechanisms may be sufficient to generate and provide the needed energy within the CNS. Transcriptional dysregulation [13, 14] would be expected to further compromise neuronal ability to generate energy, through downregulation of CK expression or PDH activity or other metabolic enzymes. Slowly accumulating oxidative damage to individual metabolic enzymes would also strain capacities for flux through specific pathways, exacerbating energetic dysfunction as disease worsens. Glycolysis, TCA, ETC, and OXPHOS would be compromised by protein oxidation. Both TCA and ETC would be affected by loss of SDH subunit expression. Acetylation, (de)phosphorylation, or subsequent alterations in enzyme expression levels would further compromise fluxes. Oxidative or transcriptional limitations on transporters would limit energy substrates from entering cells or mitochondria, further restricting maximal energy production.
Compromise of the ETC appears not to be the principle or earliest metabolic change in HD pathogenesis. Overt loss of ETC function may not occur until late in HD progression, as seen in the postmortem changes [238]. Early in disease flux through these energy generating pathways may shift towards glycolysis simply due to the rapidity of that response or due to the impediments in mitochondrial trafficking, propensity towards fission and increased mitophagy. Rather, compromise of glucose uptake facilitates glucose utilization to glycolysis and may possibly decrease flux through the PPP, limiting subsequent NADPH and GSH production needed for antioxidant protection. As a result, oxidative damage to key glycolytic and TCA cycle enzymes further restricts energy production so that while basal needs may be met, those of excessive stimulation may not be realized. Later more serious compromises may accumulate, e.g. decreases in SDH expression [238] and/or depression of glycolytic and TCA enzyme activities [65, 70, 72, 77]. Energy production may be further exacerbated by deficits in mitochondrial biogenesis or trafficking [2, 4]. The demonstration that in vivo upstream constrictions in metabolic flux may measurably restrict flux through ETC all the way to oxygen consumption [67] is a possibility not considered by many early energetic studies. Most bench metabolic studies are carried out under resting conditions, as opposed to those imposing a metabolic load, conditions that may better approximate in vivo conditions. The suggestion that substrate availability and transport into mitochondria may also be restricted needs to be explored further. These restrictions on energy production appear to be compensated for by stimulation of fatty acid oxidation and/or glutamate oxidation. The collective shifts in metabolic flux represent homeostatic compensatory mechanisms that maintain model organisms through presymptomatic stages and into symptomatic stages.
Only at end-stage disease would widespread deficits in ETC enzyme activity be expected, as observed in post-mortem regional human brain tissue. Some animal models may mimic this better than others. Surprisingly, in the most severe mouse model, R6/2, measurements of ETC activity, protein expression and ATP production diverge widely among laboratories, despite appropriate technical expertise [32, 33, 36, 66, 110, 111]. Other contextual factors may govern the rate of disease progression in these mice. The longevity of the R6/2 has been reported to vary widely between institutions and in response to environmental effects making it difficult to compare the degree of disease severity at a specific age [138, 239–242]. Stress in handling may be an example of one uncontrolled variable, especially when one considers that mice as a species can be stressed by the presence of scents from male handlers [243]. Diurnal cycle disruptions and the time of day when experiments are performed may be other relevant factors [244, 245]. While difficult to demonstrate so many years after completion of these disparate studies, moving forward, stress-related factors should be carefully considered in the detailed execution of experimental design. The context of which muhtt fragments are active in distinct model systems influences the mechanisms of neuronal demise that ensues [11]. Similarly, contextual effects should influence how muhtt stresses mitochondrial functions.
Future directions
Appropriate questions to ask at this juncture are 1) can such homeostatic shifts be demonstrated all together in a single model system, and 2) what molecular or systemic events upend the homeostasis, sending the system into progressive decline. To answer the first question, a flux analysis approach should be applied longitudinally in several mouse models of HD. The second question addresses the relationship between metabolic dysfunction and clinical disease onset. Is the loss of homeostatic control attributable to mutant huntingtin itself or simply to normal aging? Intriguingly, an important energetic metabolite, NAD+ has been proposed to be a key signal molecule for regulating the aging process [246]. NAD+ is a key cofactor for sirtuins and for PARP reactions [246]. These important life-span regulating proteins compete for NAD+, which acts as an ADP-ribose donor [246]. NAD+ concentrations vary diurnally in mouse liver and decrease in mouse muscle and liver over a lifespan [247, 248]. Hippocampal NAD+ concentration drops after an ischemic insult and can be restored, along with behavioral recovery, by administration of nicotinamide mononucleotide, an activator of the NAD+ salvage synthetic pathway [249]. In normal mouse aging, cortical, hippocampal and striatal NAD+ (along with NADH and NADP+) decrease between 18 and 24 mo as part of the age-associated overall increase in metabolite variability, termed metabolic drift [250]. NAD+ has only been measured in STHdhQ111/111 cells, where the NAD+/NADH ratio increased in the polyglutamine expanded cells [94]. NADP+ levels in dorsal R6/2 brain trended upward at 11 wk [33], but measures of NAD+, NADH, NADP+ and NADPH in other HD mouse brains have not been investigated. Connecting changes in longevity with metabolism may provide an important link for understanding the conflicting views of mitochondrial dysfunction in HD. Upregulating NAD+ is recognized as being another energy boosting strategy without complete neuroprotective powers [246, 249]. However improving endogenous homeostatic responses in metabolic pathways may be a valid treatment strategy.
Conclusion
These results argue strongly that structural compromise of the ETC and the ability to generate ATP are not a direct cause for development of HD pathogenesis. The evidence that the metabolic rates through the various metabolic pathways change over the course of disease also argues that mitochondrial function and regulation are intact and operational over much of the lifespan of effected individuals and mice. The shifts in flux represent homeostatic responses to the changing transcriptional disruptions and increased energy demands for proteostasis. Temporal shifts in flux may occur in situ on a contextual basis without the need to invoke muhtt-induced changes in protein expression. Changes in mitochondrial dynamics and shape may be part of the homeostatic response as mitochondrial form follows function. Disruptions of mitochondrial biogenesis and trafficking further the need for homeostatic responses and add to the energy deficit. These progress until neurons cannot maintain sufficient energy production, eventually undergoing apoptosis. Energy production does however influence the time course of disease in that an increase in GLUT3 copy number delays onset of human disease [59]. Exogenous drugs that boost energy production or upregulate metabolic pathways ameliorate disease phenotypes or extend the lifespan of mouse models. Such interventions prolong the homeostatic compensation but do not address the underlying causes of accumulating muhtt. HD has been described as a disease in which adaptive transcriptional homeostatic mechanisms are disrupted by the polyglutamine expansion [14]. A similar homeostatic perspective should be applied to brain metabolism in HD.
CONFLICT OF INTEREST
The author does not have any conflicts of interest.
ACKNOWLEDGMENTS
I would like to thank Drs. Pierre-Gilles Henry and Gulin Oz for helpful suggestions and critical reading of this manuscript. This work was supported by the Strom Family Fund.
REFERENCES
- [1]. Li XJ, Orr AL, Li S. Impaired mitochondrial trafficking in Huntington’s disease. Biochim Biophys Acta. 2010;1802(1):62–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127(1):59–69. [DOI] [PubMed] [Google Scholar]
- [3]. Schrepfer E, Scorrano L. Mitofusins, from mitochondria to metabolism. Mol Cell. 2016;61(5):683–94. [DOI] [PubMed] [Google Scholar]
- [4]. Guedes-Dias P, Pinho BR, Soares TR, de PJ, Duchen MR, Oliveira JM. Mitochondrial dynamics and quality control in Huntington’s disease. Neurobiol Dis. 2016;90:51–7. [DOI] [PubMed] [Google Scholar]
- [5]. Guo X, Sun X, Hu D, Wang YJ, Fujioka H, Vyas R, et al. VCPrecruitment to mitochondria causes mitophagy impairment andneurodegeneration in models of Huntington’s disease. Nat Commun. 2016;7:12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Browne SE, Bowling AC, MacGarvey U, Baik MJ, Berger SC, Muqit MM, et al. Oxidative damage and metabolic dysfunction in Huntington’sdisease: Selective vulnerability of the basal ganglia. Ann Neurol. 1997;41(5):646–53. [DOI] [PubMed] [Google Scholar]
- [7]. Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann Neurol. 1996;39(3):385–9. [DOI] [PubMed] [Google Scholar]
- [8]. Brennan WAJ, Bird ED, Aprille JR. Regional mitochondrialrespiratory activity in Huntington’s disease brain. J Neurochem. 1985;44(6):1948–50. [DOI] [PubMed] [Google Scholar]
- [9]. Guidetti P, Charles V, Chen EY, Reddy PH, Kordower JH, Whetsell WOJ, et al. Early degenerative changes in transgenic mice expressing mutant huntingtin involve dendritic abnormalities but no impairment of mitochondrial energy production. Exp Neurol. 2001;169(2):340–50. [DOI] [PubMed] [Google Scholar]
- [10]. Brustovetsky N. Mutant huntingtin and elusive defects in oxidative metabolism and mitochondrial calcium handling. Mol Neurobiol. 2016;53(3):2944–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Yu ZX, Li SH, Evans J, Pillarisetti A, Li H, Li XJ. Mutant huntingtin causes context-dependent neurodegeneration in mice with Huntington’s disease. J Neurosci. 2003;23(6):2193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Raymond LA, Andre VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington’s disease: Time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Benn CL, Sun T, Sadri-Vakili G, McFarland KN, DiRocco DP, Yohrling GJ, et al. Huntingtin modulates transcription, occupies gene promoters in vivo, and binds directly to DNA in a polyglutamine-dependent manner. J Neurosci. 2008;28(42):10720–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Kumar A, Vaish M, Ratan RR. Transcriptional dysregulation in Huntington’s disease: A failure of adaptive transcriptional homeostasis. Drug Discov Today. 2014;19(7):956–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Johnson DT, Harris RA, Blair PV, Balaban RS. Functional consequences of mitochondrial proteome heterogeneity. Am J Physiol Cell Physiol. 2007;292(2):C698–C707. [DOI] [PubMed] [Google Scholar]
- [16]. Johnson DT, Harris RA, French S, Blair PV, You J, Bemis KG, et al. Tissue heterogeneity of the mammalian mitochondrial proteome. Am J Physiol Cell Physiol. 2007;292(2):C689–C697. [DOI] [PubMed] [Google Scholar]
- [17]. Dubinsky JM. Heterogeneity of nervous system mitochondria: Location, location, location! Exp Neurol 2009;218(2):293–307. [DOI] [PubMed] [Google Scholar]
- [18]. Chang R, Liu X, Li S, Li XJ. Transgenic animal models for study of the pathogenesis of Huntington’s disease and therapy. Drug Des Devel Ther. 2015;9:2179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Menalled L, Brunner D. Animal models of Huntington’s disease for translation to the clinic: Best practices. Mov Disord. 2014;29(11):1375–90. [DOI] [PubMed] [Google Scholar]
- [20]. Brooks SP, Dunnett SB. Mouse models of Huntington’s disease. Curr Top Behav Neurosci. 2015;22:101–33. [DOI] [PubMed] [Google Scholar]
- [21]. Wiesinger H, Hamprecht B, Dringen R. Metabolic pathways forglucose in astrocytes. GLIA. 1997;21(1):22–34. [DOI] [PubMed] [Google Scholar]
- [22]. Khowaja A, Choi IY, Seaquist ER, Oz G. In vivo magnetic resonancespectroscopy of cerebral glycogen metabolism in animals andhumans. Metab Brain Dis. 2015;30(1):255–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Block RC, Dorsey ER, Beck CA, Brenna JT, Shoulson I. Altered cholesterol and fatty acid metabolism in Huntington disease. J Clin Lipidol. 2010;4(1):17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Gogvadze V, Zhivotovsky B, Orrenius S. The Warburg effect and mitochondrial stability in cancer cells. Mol Aspects Med. 2010;31(1):60–74. [DOI] [PubMed] [Google Scholar]
- [25]. Nelson DL, Cox MM. Lehninger Principles of Biochemistry 6 ed. New York: WH Freeman and Co; 2013. [Google Scholar]
- [26]. Lee AC, Zizi M, Colombini M. Beta-NADH decreases the permeability of the mitochondrial outer membrane to ADP by a factor of 6. J Biol Chem. 1994;269(49):30974–80. [PubMed] [Google Scholar]
- [27]. Ju TC, Lin YS, Chern Y. Energy dysfunction in Huntington’s disease: Insights from PGC-1alpha, AMPK, and CKB. Cell Mol Life Sci. 2012;69(24):4107–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Wang Y, Hekimi S. Mitochondrial dysfunction and longevity in animals: Untangling the knot. Science. 2015;350(6265):1204–7. [DOI] [PubMed] [Google Scholar]
- [29]. Jain IH, Zazzeron L, Goli R, Alexa K, Schatzman-Bone S, Dhillon H, et al. Hypoxia as a therapy for mitochondrial disease. Science. 2016;352(6281):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Marder E. Variability, compensation, and modulation in neurons andcircuits. Proc Natl Acad Sci USA. 2011;108(Suppl 3):15542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Nambron R, Silajdzic E, Kalliolia E, Ottolenghi C, Hindmarsh P, Hill NR, et al. A Metabolic Study of Huntington’s Disease. PLoS One. 2016;11(1):e0146480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Kim SH, Thomas CA, Andre VM, Cummings DM, Cepeda C, Levine MS, et al. Forebrain striatal-specific expression of mutant huntingtin protein in vivo induces cell-autonomous age-dependent alterations in sensitivity to excitotoxicity and mitochondrial function. ASN Neuro. 2011;3(3):e00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Tkac I, Henry PG, Zacharoff L, Wedel M, Gong W, Deelchand D, et al. Homeostatic adaptations in brain energy metabolism in mousemodels of Huntington disease. J Cereb Blood Flow Metab. 2012;32(11):1977–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Wheeler VC, White JK, Gutekunst CA, Vrbanac V, Weaver M, Li XJ, et al. Long glutamine tracts cause nuclear localization of a novel form of huntingtin in medium spiny striatal neurons in HdhQ92 and HdhQ111 knock-in mice. Hum Mol Genet. 2000;9(4):503–13. [DOI] [PubMed] [Google Scholar]
- [35]. Ju TC, Chen HM, Lin JT, Chang CP, Chang WC, Kang JJ, et al. Nuclear translocation of AMPK-alpha1 potentiates striatal neurodegeneration in Huntington’s disease. J Cell Biol. 2011;194(2):209–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Mochel F, Durant B, Meng X, O’Callaghan J, Yu H, Brouillet E, et al. Early alterations of brain cellular energy homeostasis in huntington disease models. J Biol Chem. 2012;287(2):1361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Fu J, Jin J, Cichewicz RH, Hageman SA, Ellis TK, Xiang L, et al. trans-(-)-epsilon-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington disease. J Biol Chem. 2012;287(29):24460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Vazquez-Manrique RP, Farina F, Cambon K, Dolores SM, Parker AJ, Millan JM, et al. AMPK activation protects from neuronal dysfunction and vulnerability across nematode, cellular and mouse models of Huntington’s disease. Hum Mol Genet. 2016;25(6):1043–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39]. Lage R, Dieguez C, Vidal-Puig A, Lopez M. AMPK: A metabolic gauge regulating whole-body energy homeostasis. Trends Mol Med. 2008;14(12):539–49. [DOI] [PubMed] [Google Scholar]
- [40]. Hardie DG. AMPK: A key regulator of energy balance in the single cell and the whole organism. Int J Obes (Lond). 2008;32(Suppl 4):S7–12. [DOI] [PubMed] [Google Scholar]
- [41]. Lopez M, Nogueiras R, Tena-Sempere M, Dieguez C. HypothalamicAMPK: A canonical regulator of whole-body energy balance. Nat RevEndocrinol. 2016;12(7):421–32. [DOI] [PubMed] [Google Scholar]
- [42]. Ma TC, Buescher JL, Oatis B, Funk JA, Nash AJ, Carrier RL, et al. Metformin therapy in a transgenic mouse model of Huntington’sdisease. Neurosci Lett. 2007;411(2):98–103. [DOI] [PubMed] [Google Scholar]
- [43]. Hervas D, Fornes-Ferrer V, Gomez-Escribano AP, Sequedo MD, Peiro C, Millan JM, et al. Metformin intake associates with better cognitive function in patients with Huntington’s disease. PLoS One. 2017;12(6):e0179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Zabel C, Mao L, Woodman B, Rohe M, Wacker MA, Klare Y, et al. A large number of protein expression changes occur early in life and precede phenotype onset in a mouse model for huntington disease. Mol Cell Proteomics. 2009;8(4):720–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45]. Kuhl DE, Markham CH, Metter EJ, Riege WH, Phelps ME, Mazziotta JC. Local cerebral glucose utilization in symptomatic and presymptomatic Huntington’s diesase. Res Publ Assoc Res Nerv Ment Dis. 1985;63:199–209. [PubMed] [Google Scholar]
- [46]. Ciarmiello A, Cannella M, Lastoria S, Simonelli M, Frati L, Rubinsztein DC, et al. Brain white-matter volume loss and glucose hypometabolism precede the clinical symptoms of Huntington’s disease. J Nucl Med. 2006;47(2):215–22. [PubMed] [Google Scholar]
- [47]. Feigin A, Tang C, Ma Y, Mattis P, Zgaljardic D, Guttman M, et al. Thalamic metabolism and symptom onset in preclinical Huntington’s disease. Brain. 2007;130(Pt 11):2858–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Mazziotta JC, Phelps ME, Pahl JJ, Huang SC, Baxter LR, Riege WH, et al. Reduced cerebral glucose metabolism in asymptomatic subjects at risk for Huntington’s disease. N Engl J Med. 1987;316(7):357–62. [DOI] [PubMed] [Google Scholar]
- [49]. Kuhl DE, Phelps ME, Markham CH, Metter EJ, Riege WH, Winter J. Cerebral metabolism and atrophy in Huntington’s disease determined by 18FDG and computed tomographic scan. Ann Neurol. 1982;12(5):425–34. [DOI] [PubMed] [Google Scholar]
- [50]. Martin WR, Clark C, Ammann W, Stoessl AJ, Shtybel W, Hayden MR. Cortical glucose metabolism in Huntington’s disease. Neurology. 1992;42(1):223–9. [DOI] [PubMed] [Google Scholar]
- [51]. Mayberg HS, Starkstein SE, Peyser CE, Brandt J, Dannals RF, Folstein SE. Paralimbic frontal lobe hypometabolism in depression associated with Huntington’s disease. Neurology. 1992;42(9):1791–7. [DOI] [PubMed] [Google Scholar]
- [52]. Squitieri F, Orobello S, Cannella M, Martino T, Romanelli P, Giovacchini G, et al. Riluzole protects Huntington disease patients from brain glucose hypometabolism and grey matter volume loss and increases production of neurotrophins. Eur J Nucl Med Mol Imaging. 2009;36(7):1113–20. [DOI] [PubMed] [Google Scholar]
- [53]. Cepeda-Prado E, Popp S, Khan U, Stefanov D, Rodriguez J, Menalled LB, et al. R6/2 Huntington’s disease mice develop early and progressive abnormal brain metabolism and seizures. J Neurosci. 2012;32(19):6456–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54]. Wang X, Sarkar A, Cicchetti F, Yu M, Zhu A, Jokivarsi K, et al. Cerebral PET imaging and histological evidence of transglutaminase inhibitor cystamine induced neuroprotection in transgenic R6/2 mouse model of Huntington’s disease. J Neurol Sci. 2005;231(1-2):57–66. [DOI] [PubMed] [Google Scholar]
- [55]. von Horsten S, Schmitt I, Nguyen HP, Holzmann C, Schmidt T, Walther T, et al. Transgenic rat model of Huntington’s disease. Hum Mol Genet. 2003;12(6):617–24. [DOI] [PubMed] [Google Scholar]
- [56]. Ooms M, Rietjens R, Rangarajan JR, Vunckx K, Valdeolivas S, Maes F, et al. Early decrease of type 1 cannabinoid receptor binding and phosphodiesterase 10A activity in vivo in R6/2 Huntington mice. Neurobiol Aging. 2014;35(12):2858–69. [DOI] [PubMed] [Google Scholar]
- [57]. Casteels C, Vandeputte C, Rangarajan JR, Dresselaers T, Riess O, Bormans G, et al. Metabolic and type 1 cannabinoid receptor imaging of a transgenic rat model in the early phase of Huntington disease. Exp Neurol. 2011;229(2):440–9. [DOI] [PubMed] [Google Scholar]
- [58]. Gamberino WC, Brennan WA Jr. Glucose transporter isoform expression in Huntington’s disease brain. J Neurochem. 1994;63(4):1392–7. [DOI] [PubMed] [Google Scholar]
- [59]. Vittori A, Breda C, Repici M, Orth M, Roos RA, Outeiro TF, et al. Copy-number variation of the neuronal glucose transporter gene SLC2A3 and age of onset in Huntington’s disease. Hum Mol Genet. 2014;23(12):3129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. McClory H, Williams D, Sapp E, Gatune LW, Wang P, Difiglia M, et al. Glucose transporter 3 is a rab11-dependent trafficking cargo and its transport to the cell surface is reduced in neurons of CAG140 Huntington’s disease mice. Acta Neuropathol Commun. 2014;2:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Boussicault L, Herard AS, Calingasan N, Petit F, Malgorn C, Merienne N, et al. Impaired brain energy metabolism in the BACHD mouse model of Huntington’s disease: Critical role of astrocyte-neuron interactions. J Cereb Blood Flow Metab. 2014;34(9):1500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Li X, Valencia A, McClory H, Sapp E, Kegel KB, Difiglia M. Deficient Rab11 activity underlies glucose hypometabolism in primary neurons of Huntington’s disease mice. Biochem Biophys Res Commun. 2012;421(4):727–30. [DOI] [PubMed] [Google Scholar]
- [63]. Covarrubias-Pinto A, Moll P, Solis-Maldonado M, Acuna AI, Riveros A, Miro MP, et al. Beyond the redox imbalance: Oxidative stress contributes to an impaired GLUT3 modulation in Huntington’s disease. Free Radic Biol Med. 2015;89:1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Ravikumar B, Stewart A, Kita H, Kato K, Duden R, Rubinsztein DC. Raised intracellular glucose concentrations reduce aggregation andcell death caused by mutant huntingtin exon 1 by decreasing mTORphosphorylation and inducing autophagy. Hum Mol Genet. 2003;12(9):985–94. [DOI] [PubMed] [Google Scholar]
- [65]. Powers WJ, Videen TO, Markham J, McGee-Minnich L, Antenor-Dorsey JV, Hershey T, et al. Selective defect of in vivoglycolysis in early Huntington’s disease striatum. Proc Natl AcadSci USA. 2007;104(8):2945–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66]. Mochel F, N’guyen TM, Deelchand D, Rinaldi D, Valabregue R, Wary C, et al. Abnormal response to cortical activation in early stages of Huntington disease. Mov Disord. 2012;27(7):907–10. [DOI] [PubMed] [Google Scholar]
- [67]. Lou S, Lepak VC, Eberly LE, Roth B, Cui W, Zhu XH, et al. Oxygen consumption deficit in Huntington disease mouse brain under metabolic stress. Hum Mol Genet. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68]. Bird ED, Gale JS, Spokes EG. Huntington’s chorea: Post mortem activity of enzymes involved in cerebral glucose metabolism. J Neurochem. 1977;29(3):539–45. [DOI] [PubMed] [Google Scholar]
- [69]. Burke JR, Enghild JJ, Martin ME, Jou YS, Myers RM, Roses AD, et al. Huntingtin and DRPLA proteins selectively interact with the enzyme GAPDH [see comments]. Nat Med. 1996;2(3):347–50. [DOI] [PubMed] [Google Scholar]
- [70]. Tabrizi SJ, Cleeter MW, Xuereb J, Taanman JW, Cooper JM, Schapira AH. Biochemical abnormalities and excitotoxicity in Huntington’s disease brain. Ann Neurol. 1999;45(1):25–32. [DOI] [PubMed] [Google Scholar]
- [71]. Estrada-Sanchez AM, Montiel T, Massieu L. Glycolysis inhibition decreases the levels of glutamate transporters and enhances glutamate neurotoxicity in the R6/2 Huntington’s disease mice. Neurochem Res. 2010;35(8):1156–63. [DOI] [PubMed] [Google Scholar]
- [72]. Sorolla MA, Rodriguez-Colman MJ, Tamarit J, Tamarit J, Ortega Z, Lucas JJ, et al. Protein oxidation in Huntington disease affects energy production and vitamin B6 metabolism. Free Radic Biol Med. 2010;49(4):612–21. [DOI] [PubMed] [Google Scholar]
- [73]. Perluigi M, Poon HF, Maragos W, Pierce WM, Klein JB, Calabrese V, et al. Proteomic analysis of protein expression and oxidative modification in r6/2 transgenic mice: A model of Huntington disease. Mol Cell Proteomics. 2005;4(12):1849–61. [DOI] [PubMed] [Google Scholar]
- [74]. Hansson O, Castilho RF, Korhonen L, Lindholm D, Bates GP, Brundin P. Partial resistance to malonate-induced striatal cell death intransgenic mouse models of Huntington’s disease is dependent onage and CAG repeat length. J Neurochem. 2001;78(4):694–703. [DOI] [PubMed] [Google Scholar]
- [75]. Ismailoglu I, Chen Q, Popowski M, Yang L, Gross SS, Brivanlou AH. Huntingtin protein is essential for mitochondrial metabolism, bioenergetics and structure in murine embryonic stem cells. Dev Biol. 2014;391(2):230–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76]. Butterworth J, Yates CM, Simpson J. Phosphate-activated glutaminase in relation to Huntington’s disease and agonal state. J Neurochem. 1983;41(2):440–7. [DOI] [PubMed] [Google Scholar]
- [77]. Butterworth J, Yates CM, Reynolds GP. Distribution of phosphate-activated glutaminase, succinic dehydrogenase, pyruvate dehydrogenase and gamma-glutamyl transpeptidase in post-mortem brain from Huntington’s disease and agonal cases. J Neurol Sci. 1985;67(2):161–71. [DOI] [PubMed] [Google Scholar]
- [78]. Kim SY, Marekov L, Bubber P, Browne SE, Stavrovskaya I, Lee J, et al. Mitochondrial aconitase is a transglutaminase 2 substrate:Transglutamination is a probable mechanism contributing tohigh-molecular-weight aggregates of aconitase and loss ofaconitase activity in Huntington disease brain. Neurochem Res. 2005;30(10):1245–55. [DOI] [PubMed] [Google Scholar]
- [79]. Zainelli GM, Dudek NL, Ross CA, Kim SY, Muma NA. Mutant huntingtin protein: A substrate for transglutaminase 1, 2, and 3. J Neuropathol Exp Neurol. 2005;64(1):58–65 . [DOI] [PubMed] [Google Scholar]
- [80]. Sorbi S, Bird ED, Blass JP. Decreased pyruvate dehydrogenase complex activity in Huntington and Alzheimer brain. Ann Neurol. 1983;13(1):72–8. [DOI] [PubMed] [Google Scholar]
- [81]. Klivenyi P, Starkov AA, Calingasan NY, Gardian G, Browne SE, Yang L, et al. Mice deficient in dihydrolipoamide dehydrogenase show increased vulnerability to MPTP, malonate and 3-nitropropionic acid neurotoxicity. J Neurochem. 2004;88(6):1352–60. [DOI] [PubMed] [Google Scholar]
- [82]. Wester P, Bateman DE, Dodd PR, Edwardson JA, Hardy JA, Kidd AM, et al. Agonal status affects the metabolic activity of nerve endings isolated from postmortem human brain. Neurochem Pathol. 1985;3(3):169–80. [DOI] [PubMed] [Google Scholar]
- [83]. Hardy JA, Wester P, Winblad B, Gezelius C, Bring G, Eriksson A. The patients dying after long terminal phase have acidotic brains: Implications for biochemical measurements on autopsy tissue. J Neural Transm. 1985;61(3-4):253–64. [DOI] [PubMed] [Google Scholar]
- [84]. Vawter MP, Tomita H, Meng F, Bolstad B, Li J, Evans S, et al. Mitochondrial-related gene expression changes are sensitive to agonal-pH state: Implications for brain disorders. Mol Psychiatry. 2006;11(7):615, 663–15, 679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85]. Butterworth J. Changes in nine enzyme markers for neurons, glia, and endothelial cells in agonal state and Huntington’s disease caudate nucleus. J Neurochem. 1986;47:583–7. [DOI] [PubMed] [Google Scholar]
- [86]. Harish G, Venkateshappa C, Mahadevan A, Pruthi N, Bharath MM, Shankar SK. Mitochondrial function in human brains is affected by pre- and post mortem factors. Neuropathol Appl Neurobiol. 2013;39(3):298–315. [DOI] [PubMed] [Google Scholar]
- [87]. Ribeiro M, Rosenstock TR, Cunha-Oliveira T, Ferreira IL, Oliveira CR, Rego AC. Glutathione redox cycle dysregulation in Huntington’s disease knock-in striatal cells. Free Radic Biol Med. 2012;53(10):1857–67. [DOI] [PubMed] [Google Scholar]
- [88]. Ravid R. Standard Operating Procedures, ethical and legal regulations in BTB (Brain/Tissue/Bio) banking: What is still missing? Cell Tissue Bank. 2008;9(3):151–67. [DOI] [PubMed] [Google Scholar]
- [89]. Tourtellotte WW, Rosario IP, Conrad A, Syndulko K. Human neuro-specimen banking 1961-1992. The National Neurological Research Specimen Bank (a donor program of pre- and post-mortem tissues and cerebrospinal fluid/blood; and a collection of cryopreserved human neurological specimens for neuroscientists). J Neural Transm Suppl. 1993;39:5–15. [PubMed] [Google Scholar]
- [90]. Naseri NN, Bonica J, Xu H, Park LC, Arjomand J, Chen Z, et al. Novel metabolic abnormalities in the tricarboxylic acid cycle in peripheral cells from Huntington’s disease patients. PLoS One. 2016;11(9):e0160384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91]. Naseri NN, Xu H, Bonica J, Vonsattel JP, Cortes EP, Park LC, et al. Abnormalities in the tricarboxylic Acid cycle in Huntington disease and in a Huntington disease mouse model. J Neuropathol Exp Neurol. 2015;74(6):527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92]. Tabrizi SJ, Workman J, Hart PE, Mangiarini L, Mahal A, Bates G, et al. Mitochondrial dysfunction and free radical damage in the Huntington R6/2 transgenic mouse. Ann Neurol. 2000;47(1):80–6. [DOI] [PubMed] [Google Scholar]
- [93]. Zourlidou A, Gidalevitz T, Kristiansen M, Landles C, Woodman B, Wells DJ, et al. Hsp27 overexpression in the R6/2 mouse model of Huntington’s disease: Chronic neurodegeneration does not induce Hsp27 activation. Hum Mol Genet. 2007;16(9):1078–90. [DOI] [PubMed] [Google Scholar]
- [94]. Naia L, Cunha-Oliveira T, Rodrigues J, Rosenstock TR, Oliveira A, Ribeiro M, et al. Histone deacetylase inhibitors protect againstpyruvate dehydrogenase dysfunction in Huntington’s disease. JNeurosci. 2017;37(10):2776–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95]. Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34(Pt 2):217–22. [DOI] [PubMed] [Google Scholar]
- [96]. McKenna MC, Ferreira GC. Enzyme complexes important for the glutamate-glutamine cycle. Adv Neurobiol. 2016;13:59–98. [DOI] [PubMed] [Google Scholar]
- [97]. Brekke E, Morken TS, Walls AB, Waagepetersen H, Schousboe A, Sonnewald U. Anaplerosis for glutamate synthesis in the neonate and in adulthood. Adv Neurobiol. 2016;13:43–58. [DOI] [PubMed] [Google Scholar]
- [98]. Mason GF, Petersen KF, de Graaf RA, Shulman GI, Rothman DL. Measurements of the anaplerotic rate in the human cerebral cortex using 13C magnetic resonance spectroscopy and [1-13C] and [2-13C] glucose. J Neurochem. 2007;100(1):73–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99]. Oz G, Okar DA, Henry PG. Glutamate-glutamine cycle and anaplerosis In: Choi IY, Gruetter R, editors. Neural Metabolism In Vivo. Springer Science+Business Media, LLC, 2012. pp. 921–46. [Google Scholar]
- [100]. Sonnay S, Duarte JM, Just N, Gruetter R. Compartmentalised energy metabolism supporting glutamatergic neurotransmission in response to increased activity in the rat cerebral cortex: A 13C MRS study in vivo at 14.1 T. J Cereb Blood Flow Metab. 2016;36(5):928–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101]. Lee W, Reyes RC, Gottipati MK, Lewis K, Lesort M, Parpura V, et al. Enhanced Ca(2+)-dependent glutamate release from astrocytes of the BACHD Huntington’s disease mouse model. Neurobiol Dis. 2013;58:192–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102]. Sonnewald U, McKenna M. Metabolic compartmentation in cortical synaptosomes: Influence of glucose and preferential incorporation of endogenous glutamate into GABA. Neurochem Res. 2002;27(1-2):43–50. [DOI] [PubMed] [Google Scholar]
- [103]. Yudkoff M, Nelson D, Daikhin Y, Erecinska M. Tricarboxylic acid cycle in rat brain synaptosomes. Fluxes and interactions with aspartate aminotransferase and malate/aspartate shuttle. J Biol Chem. 1994;269(44):27414–20. [PubMed] [Google Scholar]
- [104]. Behrens PF, Franz P, Woodman B, Lindenberg KS, Landwehrmeyer GB. Impaired glutamate transport and glutamate-glutamine cycling: Downstream effects of the Huntington mutation. Brain. 2002;125(Pt 8):1908–22. [DOI] [PubMed] [Google Scholar]
- [105]. Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le GL, et al. Impaired glutamate uptake in the R6 Huntington’s disease transgenic mice. Neurobiol Dis. 2001;8(5):807–21. [DOI] [PubMed] [Google Scholar]
- [106]. Petr GT, Schultheis LA, Hussey KC, Sun Y, Dubinsky JM, Aoki C, et al. Decreased expression of GLT-1 in the R6/2 model ofHuntington’s disease does not worsen disease progression. Eur JNeurosci. 2013;38(3):2477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107]. Parsons MP, Vanni MP, Woodard CL, Kang R, Murphy TH, Raymond LA. Real-time imaging of glutamate clearance reveals normal striatal uptake in Huntington disease mouse models. Nat Commun. 2016;7:11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108]. Hamilton J, Pellman JJ, Brustovetsky T, Harris RA, Brustovetsky N. Oxidative metabolism in YAC128 mouse model of Huntington’s disease. Hum Mol Genet. 2015;24(17):4862–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109]. Pellman JJ, Hamilton J, Brustovetsky T, Brustovetsky N. Ca(2+)handling in isolated brain mitochondria and cultured neuronsderived from the YAC128 mouse model of Huntington’s disease. J Neurochem. 2015;134(4):652–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110]. Hamilton J, Pellman JJ, Brustovetsky T, Harris RA, Brustovetsky N. Oxidative metabolism and Ca2+ handling in isolated brain mitochondria and striatal neurons from R6/2 mice, a model of Huntington’s disease. Hum Mol Genet. 2016;25(13):2762–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111]. Hering T, Birth N, Taanman JW, Orth M. Selective striatal mtDNA depletion in end-stage Huntington’s disease R6/2 mice. Exp Neurol. 2015;266:22–9. [DOI] [PubMed] [Google Scholar]
- [112]. Perry GM, Tallaksen-Greene S, Kumar A, Heng MY, Kneynsberg A, van GT, et al. Mitochondrial calcium uptake capacity as a therapeutic target in the R6/2 mouse model of Huntington’s disease. Hum Mol Genet. 2010;19(17):3354–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113]. Yano H, Baranov SV, Baranova OV, Kim J, Pan Y, Yablonska S, et al. Inhibition of mitochondrial protein import by mutant huntingtin. Nat Neurosci. 2014;17(6):822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114]. Damiano M, Diguet E, Malgorn C, D’Aurelio M, Galvan L, Petit F, et al. A role of mitochondrial complex II defects in genetic modelsof Huntington’s disease expressing N-terminal fragments ofmutant huntingtin. Hum Mol Genet. 2013;22(19):3869–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115]. Weydt P, Pineda VV, Torrence AE, Libby RT, Satterfield TF, Lazarowski ER, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metab. 2006;4(5):349–62. [DOI] [PubMed] [Google Scholar]
- [116]. Olah J, Klivenyi P, Gardian G, Vecsei L, Orosz F, Kovacs GG, et al. Increased glucose metabolism and ATP level in brain tissue of Huntington’s disease transgenic mice. FEBS J. 2008;275(19):4740–55. [DOI] [PubMed] [Google Scholar]
- [117]. Tsunemi T, Ashe TD, Morrison BE, Soriano KR, Au J, Roque RA, et al. PGC-1alpha rescues Huntington’s disease proteotoxicity by preventing oxidative stress and promoting TFEB function. Sci Transl Med. 2012;4(142):142ra97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118]. Bae BI, Xu H, Igarashi S, Fujimuro M, Agrawal N, Taya Y, et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron. 2005;47(1):29–41. [DOI] [PubMed] [Google Scholar]
- [119]. Milakovic T, Johnson GV. Mitochondrial respiration and ATP production are significantly impaired in striatal cells expressing mutant huntingtin. J Biol Chem. 2005;280(35):30773–82. [DOI] [PubMed] [Google Scholar]
- [120]. Napoli E, Wong S, Hung C, Ross-Inta C, Bomdica P, Giulivi C. Defective mitochondrial disulfide relay system, altered mitochondrial morphology and function in Huntington’s disease. Hum Mol Genet. 2013;22(5):989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121]. Beal MF, Brouillet E, Jenkins B, Henshaw R, Rosen B, Hyman BT. Age-dependent striatal excitotoxic lesions produced by the endogenous mitochondrial inhibitor malonate. J Neurochem. 1993;61(3):1147–50. [DOI] [PubMed] [Google Scholar]
- [122]. Beal MF, Brouillet E, Jenkins BG, Ferrante RJ, Kowall NW, et al. Neurochemical and histologic characterization of striatal excitotoxic lesions produced by the mitochondrial toxin 3-nitropropionic acid. J Neurosci. 1993;13(10):4181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123]. Brouillet E, Jenkins BG, Hyman BT, Ferrante RJ, Kowall NW, Srivastava R, et al. Age-dependent vulnerability of the striatum to the mitochondrial toxin 3-nitropropionic acid. J Neurochem. 1993;60(1):356–9. [DOI] [PubMed] [Google Scholar]
- [124]. Brouillet E, Hantraye P, Ferrante RJ, Dolan R, Leroy-Willig A, Kowall, et al. Chronic mitochondrial energy impairment producesselective striatal degeneration and abnormal choreiform movementsin primates. Proc Natl Acad Sci USA. 1995;92(15):7105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125]. Benchoua A, Trioulier Y, Zala D, Gaillard MC, Lefort N, Dufour N, et al. Involvement of mitochondrial complex II defects in neuronaldeath produced by N-terminus fragment of mutated huntingtin. MolBiol Cell. 2006;17(4):1652–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126]. Diedrich M, Mao L, Bernreuther C, Zabel C, Nebrich G, Kleene R, et al. Proteome analysis of ventral midbrain in MPTP-treated normaland L1cam transgenic mice. Proteomics. 2008;8(6):1266–75. [DOI] [PubMed] [Google Scholar]
- [127]. Skillings EA, Morton AJ. Delayed onset and reduced cognitive deficits through pre-conditioning with 3-nitropropionic acid is dependent on sex and CAG repeat length in the R6/2 mouse model of Huntington’s disease. J Huntingtons Dis. 2016;5(1):19–32. [DOI] [PubMed] [Google Scholar]
- [128]. Hickey MA, Morton AJ. Mice transgenic for the Huntington’s disease mutation are resistant to chronic 3-nitropropionic acid-induced striatal toxicity. J Neurochem. 2000;75(5):2163–71. [DOI] [PubMed] [Google Scholar]
- [129]. Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515(7527):431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130]. Lim NK, Hung LW, Pang TY, Mclean CA, Liddell JR, Hilton JB, et al. Localized changes to glycogen synthase kinase-3 and collapsin response mediator protein-2 in the Huntington’s disease affected brain. Hum Mol Genet. 2014;23(15):4051–63. [DOI] [PubMed] [Google Scholar]
- [131]. Orr AL, Li S, Wang CE, Li H, Wang J, Rong J, et al. N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. J Neurosci. 2008;28(11):2783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132]. Trushina E, Dyer RB, Badger JD, Ure D, Eide L, Tran DD, et al. Mutant huntingtin impairs axonal trafficking in mammalian neurons in vivo and in vitro. Mol Cell Biol. 2004;24(18):8195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133]. Seong IS, Ivanova E, Lee JM, Choo YS, Fossale E, Anderson M, et al. HD CAG repeat implicates a dominant property of huntingtin in mitochondrial energy metabolism. Hum Mol Genet. 2005;14(19):2871–80. [DOI] [PubMed] [Google Scholar]
- [134]. Lin YS, Cheng TH, Chang CP, Chen HM, Chern Y. Enhancement of brain-type creatine kinase activity ameliorates neuronal deficits in Huntington’s disease. Biochim Biophys Acta. 18326 742–53. [DOI] [PubMed] [Google Scholar]
- [135]. Lodi R, Schapira AH, Manners D, Styles P, Wood NW, Taylor DJ, et al. Abnormal in vivo skeletal muscle energy metabolism inHuntington’s disease and dentatorubropallidoluysian atrophy. AnnNeurol. 2000;48(1):72–6. [PubMed] [Google Scholar]
- [136]. Zhang SF, Hennessey T, Yang L, Starkova NN, Beal MF, Starkov AA. Impaired brain creatine kinase activity in Huntington’s disease. Neurodegener Dis. 2010;8(4):194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137]. Kim J, Amante DJ, Moody JP, Edgerly CK, Bordiuk OL, Smith K, et al. Reduced creatine kinase as a central and peripheral biomarker in Huntington’s disease. Biochim Biophys Acta. 2010;1802(7-8):673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138]. Zacharoff L, Tkac I, Song Q, Tang C, Bolan P, Mangia S, et al. Cortical metabolites as biomarkers in the R6/2 model of Huntington’s disease. J Cereb Blood Flow Metab. 2012;32(3):502–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139]. Rostovtseva TK, Komarov A, Bezrukov SM, Colombini M. Dynamics of nucleotides in VDAC channels: Structure-specific noise generation. Biophys J. 2002;82(1 Pt 1):193–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140]. Maldonado EN, Lemasters JJ. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion. 2014;19(Pt A):78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141]. Oliveira JM, Jekabsons MB, Chen S, Lin A, Rego AC, Goncalves J, et al. Mitochondrial dysfunction in Huntington’s disease: The bioenergetics of isolated and in situ mitochondria from transgenic mice. J Neurochem. 2007;101(1):241–9. [DOI] [PubMed] [Google Scholar]
- [142]. Gellerich FN, Gizatullina Z, Trumbeckaite S, Nguyen HP, Pallas T, Arandarcikaite O, et al. The regulation of OXPHOS by extramitochondrial calcium. Biochim Biophys Acta. 2010;1797(6-7):1018–27. [DOI] [PubMed] [Google Scholar]
- [143]. Gellerich FN, Gizatullina Z, Nguyen HP, Trumbeckaite S, Vielhaber S, Seppet E, et al. Impaired regulation of brain mitochondria by extramitochondrial Ca2+ in transgenic Huntington disease rats. J Biol Chem. 2008;283(45):30715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144]. Gouarne C, Tardif G, Tracz J, Latyszenok V, Michaud M, Clemens LE, et al. Early deficits in glycolysis are specific to striatal neurons from a rat model of Huntington disease. PLoS One. 2013;8(11):e81528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145]. Rossignol R, Letellier T, Malgat M, Rocher C, Mazat JP. Tissue variation in the control of oxidative phosphorylation: Implication for mitochondrial diseases. Biochem J. 2000;347(Pt 1):45–53. [PMC free article] [PubMed] [Google Scholar]
- [146]. Cheng ML, Chang KH, Wu YR, Chen CM. Metabolic disturbances in plasma as biomarkers for Huntington’s disease. J Nutr Biochem. 2016;31:38–44. [DOI] [PubMed] [Google Scholar]
- [147]. Graham SF, Kumar P, Bahado-Singh RO, Robinson A, Mann D, Green BD. Novel metabolite biomarkers of Huntington’s disease as detected by high-resolution mass spectrometry. J Proteome Res. 2016;15(5):1592–601. [DOI] [PubMed] [Google Scholar]
- [148]. Underwood BR, Broadhurst D, Dunn WB, Ellis DI, Michell AW, Vacher C, et al. Huntington disease patients and transgenic mice have similar pro-catabolic serum metabolite profiles. Brain. 2006;129(Pt 4):877–86. [DOI] [PubMed] [Google Scholar]
- [149]. Adanyeguh IM, Rinaldi D, Henry PG, Caillet S, Valabregue R, Durr A, et al. Triheptanoin improves brain energy metabolism in patients with Huntington disease. Neurology. 2015;84(5):490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150]. Vamos E, Voros K, Vecsei L, Klivenyi P. Neuroprotective effects of L-carnitine in a transgenic animal model of Huntington’s disease. Biomed Pharmacother. 2010;64(4):282–6. [DOI] [PubMed] [Google Scholar]
- [151]. Pirhaji L, Milani P, Leidl M, Curran T, Avila-Pacheco J, Clish CB, et al. Revealing disease-associated pathways by network integration of untargeted metabolomics. Nat Methods. 2016;13(9):770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152]. Lopez M, Vidal-Puig A. Brain lipogenesis and regulation of energy metabolism. Curr Opin Clin Nutr Metab Care. 2008;11(4):483–90. [DOI] [PubMed] [Google Scholar]
- [153]. Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci. 2013;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154]. Cheng A, Yang Y, Zhou Y, Maharana C, Lu D, Peng W, et al. Mitochondrial SIRT3 mediates adaptive responses of neurons to exercise and metabolic and excitatory challenges. Cell Metab. 2016;23(1):128–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155]. Jiang M, Wang J, Fu J, Du L, Jeong H, West T, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med. 2012;18(1):153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156]. Naseri NN, Xu H, Bonica J, Vonsattel JP, Cortes EP, Park LC, et al. Abnormalities in the tricarboxylic Acid cycle in Huntington disease and in a Huntington disease mouse model. J Neuropathol Exp Neurol. 2015;74(6):527–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157]. Wang CT, Chen YC, Wang YY, Huang MH, Yen TL, Li H, et al. Reduced neuronal expression of ribose-5-phosphate isomerase enhances tolerance to oxidative stress, extends lifespan, and attenuates polyglutamine toxicity in Drosophila. Aging Cell. 2012;11(1):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158]. Besson MT, Alegria K, Garrido-Gerter P, Barros LF, Lievens JC. Enhanced neuronal glucose transporter expression reveals metabolic choice in a HD Drosophila model. PLoS One. 2015;10(3):e0118765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159]. Berggren KL, Chen J, Fox J, Miller J, Dodds L, Dugas B, et al. Neonatal iron supplementation potentiates oxidative stress, energetic dysfunction and neurodegeneration in the R6/2 mouse model of Huntington’s disease. Redox Biol. 2015;4:363–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160]. Unschuld PG, Edden RA, Carass A, Liu X, Shanahan M, Wang X, et al. Brain metabolite alterations and cognitive dysfunction in early Huntington’s disease. Mov Disord. 2012;27(7):895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161]. Sorolla MA, Rodriguez-Colman MJ, Vall-llaura N, Tamarit J, Ros J, Cabiscol E. Protein oxidation in Huntington disease. Biofactors. 2012;38(3):173–85. [DOI] [PubMed] [Google Scholar]
- [162]. Li X, Valencia A, Sapp E, Masso N, Alexander J, Reeves P, et al. Aberrant Rab11-dependent trafficking of the neuronal glutamatetransporter EAAC1 causes oxidative stress and cell death inHuntington’s disease. J Neurosci. 2010;30(13):4552–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163]. Acuna AI, Esparza M, Kramm C, Beltran FA, Parra AV, Cepeda C, et al. A failure in energy metabolism and antioxidant uptake precede symptoms of Huntington’s disease in mice. Nat Commun. 2013;4:2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164]. Gao Y, Chu SF, Li JP, Zuo W, Wen ZL, He WB, et al. Do glial cells play an anti-oxidative role in Huntington’s disease? Free Radic Res. 2014;48(10):1135–44. [DOI] [PubMed] [Google Scholar]
- [165]. Pepin J, Francelle L, Carrillo-de Sauvage MA, de LL, Gipchtein P, Cambon K, et al. In vivo imaging of brain glutamate defects in a knock-in mouse model of Huntington’s disease. Neuroimage. 2016;139:53–64. [DOI] [PubMed] [Google Scholar]
- [166]. Heikkinen T, Lehtimaki K, Vartiainen N, Puolivali J, Hendricks SJ, Glaser JR, et al. Characterization of neurophysiological andbehavioral changes, MRI brain volumetry and 1H MRS in zQ175knock-in mouse model of Huntington’s disease. PLoS One. 2012;7(12):e50717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167]. Peng Q, Wu B, Jiang M, Jin J, Hou Z, Zheng J, et al. Characterization of behavioral, neuropathological, brain metabolic and key molecular changes in zQ175 knock-in mouse model of Huntington’s disease. PLoS One. 2016;11(2):e0148839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168]. Sturrock A, Laule C, Decolongon J, Dar SR, Coleman AJ, Creighton S, et al. Magnetic resonance spectroscopy biomarkers in premanifest and early Huntington disease. Neurology. 2010;75(19):1702–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169]. Sturrock A, Laule C, Wyper K, Milner RA, Decolongon J, Dar SR, et al. A longitudinal study of magnetic resonance spectroscopy Huntington’s disease biomarkers. Mov Disord. 2015;30(3):393–401. [DOI] [PubMed] [Google Scholar]
- [170]. Padowski JM, Weaver KE, Richards TL, Laurino MY, Samii A, Aylward EH, et al. Neurochemical correlates of caudate atrophy in Huntington’s disease. Mov Disord. 2014;29(3):327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171]. van den Bogaard SJ, Dumas EM, Teeuwisse WM, Kan HE, Webb A, Roos RA, et al. Exploratory 7-Tesla magnetic resonance spectroscopy in Huntington’s disease provides in vivo evidence for impaired energy metabolism. J Neurol. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172]. Garseth M, Sonnewald U, White LR, Rod M, Zwart JA, Nygaard O, et al. Proton magnetic resonance spectroscopy of cerebrospinal fluid in neurodegenerative disease: Indication of glial energy impairment in Huntington chorea, but not Parkinson disease. J Neurosci Res. 2000;60(6):779–82. [DOI] [PubMed] [Google Scholar]
- [173]. Dalman T, Wiechert W, Noh K. A scientific workflow framework for C metabolic flux analysis. J Biotechnol. 2015. [DOI] [PubMed] [Google Scholar]
- [174]. Waschina S, D’Souza G, Kost C, Kaleta C. Metabolic network architecture and carbon source determine metabolite production costs. FEBS J. 2016;283(11):2149–63. [DOI] [PubMed] [Google Scholar]
- [175]. Sa JV, Kleiderman S, Brito C, Sonnewald U, Leist M, Teixeira AP, et al. Quantification of metabolic rearrangements during neural stem cells differentiation into astrocytes by metabolic flux analysis. Neurochem Res. 2017;42(1):244–53. [DOI] [PubMed] [Google Scholar]
- [176]. Bulik S, Holzhutter HG, Berndt N. The relative importance of kinetic mechanisms and variable enzyme abundances for the regulation of hepatic glucose metabolism–insights from mathematical modeling. BMC Biol. 2016;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177]. Gebril HM, Avula B, Wang YH, Khan IA, Jekabsons MB. (13)Cmetabolic flux analysis in neurons utilizing a model that accountsfor hexose phosphate recycling within the pentose phosphatepathway. Neurochem Int. 2016;93:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178]. Previs SF, Kelley DE. Tracer-based assessments of hepatic anaplerotic and TCA cycle flux: Practicality, stoichiometry, and hidden assumptions. Am J Physiol Endocrinol Metab. 2015;309(8):E727–35. [DOI] [PubMed] [Google Scholar]
- [179]. Davey GP, Peuchen S, Clark JB. Energy thresholds in brain mitochondria. Potential involvement in neurodegeneration. J Biol Chem. 1998;273(21):12753–7. [DOI] [PubMed] [Google Scholar]
- [180]. Aidt FH, Nielsen SM, Kanters J, Pesta D, Nielsen TT, Norremolle A, et al. Dysfunctional mitochondrial respiration in the striatum of the Huntington’s disease transgenic R6/2 mouse model. PLoS Curr. 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181]. Stepanova A, Shurubor Y, Valsecchi F, Manfredi G, Galkin A. Differential susceptibility of mitochondrial complex II to inhibition by oxaloacetate in brain and heart. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 18579 1561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182]. Tsang TM, Woodman B, McLoughlin GA, Griffin JL, Tabrizi SJ, Bates GP, et al. Metabolic characterization of the R6/2 transgenic mousemodel of Huntington’s disease by high-resolution MAS 1H NMRspectroscopy. J Proteome Res. 2006;5(3):483–92. [DOI] [PubMed] [Google Scholar]
- [183]. Verwaest KA, Vu TN, Laukens K, Clemens LE, Nguyen HP, Van GB, et al. (1)H NMR based metabolomics of CSF and blood serum: A metabolic profile for a transgenic rat model of Huntington disease. Biochim Biophys Acta. 2011;1812(11):1371–9. [DOI] [PubMed] [Google Scholar]
- [184]. Du J, Rountree A, Cleghorn WM, Contreras L, Lindsay KJ, Sadilek M, et al. Phototransduction influences metabolic flux and nucleotide metabolism in mouse retina. J Biol Chem. 2016;291(9):4698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185]. Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–14. [DOI] [PubMed] [Google Scholar]
- [186]. Kondoh H. Cellular life span and the Warburg effect. Exp Cell Res. 2008;314(9):1923–8. [DOI] [PubMed] [Google Scholar]
- [187]. Bas-Orth C, Tan YW, Lau D, Bading H. Synaptic activity drives a genomic program that promotes a neuronal Warburg effect. J Biol Chem. 2017;292(13):5183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188]. Newington JT, Rappon T, Albers S, Wong DY, Rylett RJ, Cumming RC. Overexpression of pyruvate dehydrogenase kinase 1 and lactatedehydrogenase A in nerve cells confers resistance to amyloid betaand other toxins by decreasing mitochondrial respiration andreactive oxygen species production. J Biol Chem. 2012;287(44):37245–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189]. Newington JT, Pitts A, Chien A, Arseneault R, Schubert D, Cumming RC. Amyloid beta resistance in nerve cell lines is mediated by the Warburg effect. PLoS One. 2011;6(4):e19191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190]. Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3(3):187–97. [DOI] [PubMed] [Google Scholar]
- [191]. Johnson DT, Harris RA, Blair PV, Balaban RS. Functional consequences of mitochondrial proteome heterogeneity. Am J Physiol Cell Physiol. 2007;292(2):C698–C707. [DOI] [PubMed] [Google Scholar]
- [192]. Cooper AJ, Jeitner TM. Central role of glutamate metabolism in the maintenance of nitrogen homeostasis in normal and hyperammonemic brain. Biomolecules. 2016;6(2):pii: E16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193]. Chaumeil MM, Valette J, Baligand C, Brouillet E, Hantraye P, Bloch G, et al. pH as a biomarker of neurodegeneration in Huntington’s disease: A translational rodent-human MRS study. J Cereb Blood Flow Metab. 2012;32(5):771–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194]. Patassini S, Begley P, Reid SJ, Xu J, Church SJ, Curtis M, et al. Identification of elevated urea as a severe, ubiquitous metabolic defect in the brain of patients with Huntington’s disease. Biochem Biophys Res Commun. 2015;468(1-2):161–6. [DOI] [PubMed] [Google Scholar]
- [195]. Hodges A, Strand AD, Aragaki AK, Kuhn A, Sengstag T, Hughes G, et al. Regional and cellular gene expression changes in human Huntington’s disease brain. Hum Mol Genet. 2006;15(6):965–77. [DOI] [PubMed] [Google Scholar]
- [196]. Graham SF, Kumar PK, Bjorndahl T, Han B, Yilmaz A, Sherman E, et al. Metabolic signatures of Huntington’s disease (HD): (1)H NMR analysis of the polar metabolome in post-mortem human brain. Biochim Biophys Acta. 2016;1862(9):1675–84. [DOI] [PubMed] [Google Scholar]
- [197]. Lichter-Konecki U. Profiling of astrocyte properties in the hyperammonaemic brain: Shedding new light on the pathophysiology of the brain damage in hyperammonaemia. J Inherit Metab Dis. 2008;31(4):492–502 . [DOI] [PubMed] [Google Scholar]
- [198]. Chiang MC, Chen HM, Lee YH, Chang HH, Wu YC, Soong BW, et al. Dysregulation of C/EBPalpha by mutant Huntingtin causes the urea cycle deficiency in Huntington’s disease. Hum Mol Genet. 2007;16(5):483–98. [DOI] [PubMed] [Google Scholar]
- [199]. Skene DJ, Middleton B, Fraser CK, Pennings JL, Kuchel TR, Rudiger SR, et al. Metabolic profiling of presymptomatic Huntington’s disease sheep reveals novel biomarkers. Sci Rep. 2017;7:43030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200]. Tsunemi T, La Spada AR. PGC-1alpha at the intersection of bioenergetics regulation and neuron function: From Huntington’s disease to Parkinson’s disease and beyond. Prog Neurobiol. 2012;97(2):142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201]. Che HV, Metzger S, Portal E, Deyle C, Riess O, Nguyen HP. Localization of sequence variations in PGC-1alpha influence their modifying effect in Huntington disease. Mol Neurodegener. 2011;6(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202]. Lucas EK, Dougherty SE, McMeekin LJ, Trinh AT, Reid CS, Cowell RM. Developmental alterations in motor coordination and medium spiny neuron markers in mice lacking pgc-1alpha. PLoS One. 2012;7(8):e42878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203]. Lee ST, Chu K, Jung KH, Im WS, Park JE, Lim HC, et al. Slowed progression in models of Huntington disease by adipose stem cell transplantation. Ann Neurol. 2009;66(5):671–81. [DOI] [PubMed] [Google Scholar]
- [204]. Hwang S, Disatnik MH, Mochly-Rosen D. Impaired GAPDH-induced mitophagy contributes to the pathology of Huntington’s disease. EMBO Mol Med. 2015;7(10):1307–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205]. Jin J, Albertz J, Guo Z, Peng Q, Rudow G, Troncoso JC, et al. Neuroprotective effects of PPAR-gamma agonist rosiglitazone in N171-82Q mouse model of Huntington’s disease. J Neurochem. 2013;125(3):410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206]. Johri A, Calingasan NY, Hennessey TM, Sharma A, Yang L, Wille E, et al. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Hum Mol Genet. 2012;21(5):1124–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207]. Chiang MC, Chen CM, Lee MR, Chen HW, Chen HM, Wu YS, et al. Modulation of energy deficiency in Huntington’s disease via activation of the peroxisome proliferator-activated receptor gamma. Hum Mol Genet. 2010;19(20):4043–58. [DOI] [PubMed] [Google Scholar]
- [208]. Lee JH, Tecedor L, Chen YH, Monteys AM, Sowada MJ, Thompson LM, et al. Reinstating aberrant mTORC1 activity in Huntington’s disease mice improves disease phenotypes. Neuron. 2015;85(2):303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209]. Lin J, Wu PH, Tarr PT, Lindenberg KS, St Pierre J, Zhang CY, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119(1):121–35. [DOI] [PubMed] [Google Scholar]
- [210]. Cowell RM, Blake KR, Russell JW. Localization of the transcriptional coactivator PGC-1alpha to GABAergic neurons during maturation of the rat brain. J Comp Neurol. 2007;502(1):1–18. [DOI] [PubMed] [Google Scholar]
- [211]. Lucas EK, Dougherty SE, McMeekin LJ, Reid CS, Dobrunz LE, West AB, et al. PGC-1alpha provides a transcriptional framework for synchronous neurotransmitter release from parvalbumin-positive interneurons. J Neurosci. 2014;34(43):14375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212]. Lucas EK, Markwardt SJ, Gupta S, Meador-Woodruff JH, Lin JD, Overstreet-Wadiche L, et al. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1alpha. J Neurosci. 2010;30(21):7227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213]. Dougherty SE, Hollimon JJ, McMeekin LJ, Bohannon AS, West AB, Lesort M, et al. Hyperactivity and cortical disinhibition in mice with restricted expression of mutant huntingtin to parvalbumin-positive cells. Neurobiol Dis. 2014;62:160–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214]. Chang DT, Rintoul GL, Pandipati S, Reynolds IJ. Mutant huntingtin aggregates impair mitochondrial movement and trafficking in cortical neurons. Neurobiol Dis. 2006;22(2):388–400. [DOI] [PubMed] [Google Scholar]
- [215]. Song W, Chen J, Petrilli A, Liot G, Klinglmayr E, Zhou Y, et al. Mutant huntingtin binds the mitochondrial fission GTPase dynamin-related protein-1 and increases its enzymatic activity. Nat Med. 2011;17(3):377–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216]. Costa V, Giacomello M, Hudec R, Lopreiato R, Ermak G, Lim D, et al. Mitochondrial fission and cristae disruption increase the response of cell models of Huntington’s disease to apoptotic stimuli. EMBO Mol Med. 2010;2(12):490–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217]. Cherubini M, Gines S. Mitochondrial fragmentation in neuronal degeneration: Toward an understanding of HD striatal susceptibility. Biochem Biophys Res Commun. 2017;483(4):1063–8. [DOI] [PubMed] [Google Scholar]
- [218]. Pernas L, Scorrano L. Mito-morphosis: Mitochondrial fusion, fission, and cristae remodeling as key mediators of cellular function. Annu Rev Physiol. 2016;78:505–31. [DOI] [PubMed] [Google Scholar]
- [219]. Haun F, Nakamura T, Shiu AD, Cho DH, Tsunemi T, Holland EA, et al. S-nitrosylation of dynamin-related protein 1 mediates mutant huntingtin-induced mitochondrial fragmentation and neuronal injury in Huntington’s disease. Antioxid Redox Signal. 2013;19(11):1173–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220]. Guo X, Disatnik MH, Monbureau M, Shamloo M, Mochly-Rosen D, Qi X. Inhibition of mitochondrial fragmentation diminishes Huntington’s disease-associated neurodegeneration. J Clin Invest. 2013;123(12):5371–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221]. Trettel F, Rigamonti D, Hilditch-Maguire P, Wheeler VC, Sharp AH, Persichetti F, et al. Dominant phenotypes produced by theHD mutation in STHdh(Q111) striatal cells. Hum Mol Genet. 2000;9(19):2799–809. [DOI] [PubMed] [Google Scholar]
- [222]. Amiri M, Hollenbeck PJ. Mitochondrial biogenesis in the axons of vertebrate peripheral neurons. Dev Neurobiol. 2008;68(11):1348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223]. Miller KE, Sheetz MP. Axonal mitochondrial transport and potential are correlated. J Cell Sci. 2004;117(Pt 13):2791–804. [DOI] [PubMed] [Google Scholar]
- [224]. Buck MD, O’Sullivan D, Klein Geltink RI, Curtis JD, Chang CH, Sanin DE, et al. Mitochondrial dynamics controls T cell fate through metabolic programming. Cell. 2016;166(1):63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225]. Guedes-Dias P, de Proen+°a J, Soares TR, Leit+úo-Rocha A, Pinho BgR, Duchen MR, et al. HDAC6 inhibition induces mitochondrial fusion, autophagic flux and reduces diffuse mutant huntingtin in striatal neurons. Biochim Biophys Acta. 185211 2484–93. [DOI] [PubMed] [Google Scholar]
- [226]. Berthet A, Margolis EB, Zhang J, Hsieh I, Zhang J, Hnasko TS, et al. Loss of mitochondrial fission depletes axonal mitochondria in midbrain dopamine neurons. J Neurosci. 2014;34(43):14304–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227]. Fukumitsu K, Hatsukano T, Yoshimura A, Heuser J, Fujishima K, Kengaku M. Mitochondrial fission protein Drp1 regulates mitochondrial transport and dendritic arborization in cerebellar Purkinje cells. Mol Cell Neurosci. 2016;71:56–65. [DOI] [PubMed] [Google Scholar]
- [228]. Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, et al. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186(6):805–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229]. Toyama EQ, Herzig S, Courchet J, Lewis TL Jr, Loson OC, Hellberg K, et al. Metabolism. AMP-activated protein kinase mediatesmitochondrial fission in response to energy stress. Science. 2016;351(6270):275–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230]. Hardie DG. AMPK–sensing energy while talking to other signaling pathways. Cell Metab. 2014;20(6):939–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231]. Wang C, Youle R. Cell biology: Form follows function for mitochondria. Nature. 2016;530(7590):288–9. [DOI] [PubMed] [Google Scholar]
- [232]. Zhang CS, Lin SC. AMPK promotes autophagy by facilitating mitochondrial fission. Cell Metab. 2016;23(3):399–401. [DOI] [PubMed] [Google Scholar]
- [233]. Sorgo R, Zhang CJ, Tedeschi H. The internal concentration of K+ in isolated rat liver mitochondria. Biochem Biophy Acta. 1985;806(2):272–6. [DOI] [PubMed] [Google Scholar]
- [234]. Lang RD, Bronk JR. A study of rapid mitochondrial structural changes in vitro by spray-freeze-etching. J Cell Biol. 1978;77(1):134–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235]. Martin DD, Ladha S, Ehrnhoefer DE, Hayden MR. Autophagy in Huntington disease and huntingtin in autophagy. Trends Neurosci. 2015;38(1):26–35. [DOI] [PubMed] [Google Scholar]
- [236]. Khalil B, El FN, Aouane A, Cabirol-Pol MJ, Rival T, Lievens JC. PINK1-induced mitophagy promotes neuroprotection in Huntington’s disease. Cell Death Dis. 2015;6:e1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237]. Wong YC, Holzbaur EL. The regulation of autophagosome dynamics by huntingtin and HAP1 is disrupted by expression of mutant huntingtin, leading to defective cargo degradation. J Neurosci. 2014;34(4):1293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238]. Liot G, Valette J, Pepin J, Flament J, Brouillet E. Energy defects in Huntington’s disease: Why “in vivo” evidence matters. Biochem Biophys Res Commun. 2017;483(4):1084–95. [DOI] [PubMed] [Google Scholar]
- [239]. Skillings EA, Wood NI, Morton AJ. Beneficial effects of environmental enrichment and food entrainment in the R6/2 mouse model of Huntington’s disease. Brain Behav. 2014;4(5):675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240]. Wood NI, Glynn D, Morton AJ. “Brain training” improves cognitive performance and survival in a transgenic mouse model of Huntington’s disease. Neurobiol Dis. 2011;42(3):427–37. [DOI] [PubMed] [Google Scholar]
- [241]. Wood NI, Carta V, Milde S, Skillings EA, McAllister CJ, Ang YL, et al. Responses to environmental enrichment differ with sex and genotype in a transgenic mouse model of Huntington’s disease. PLoS One. 2010;5(2):e9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242]. Menalled LB, Patry M, Ragland N, Lowden PA, Goodman J, Minnich J, et al. Comprehensive behavioral testing in the R6/2 mouse model of Huntington’s disease shows no benefit from CoQ10 or minocycline. PLoS One. 2010;5(3):e9793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243]. Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, et al. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11(6):629–32. [DOI] [PubMed] [Google Scholar]
- [244]. Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levine MS, et al. Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington’s disease mice. Neurosci. 2008;157(1):280–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245]. Morton AJ, Wood NI, Hastings MH, Hurelbrink C, Barker RA, Maywood ES. Disintegration of the sleep-wake cycle and circadian timing in Huntington’s disease. J Neurosci. 2005;25(1):157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246]. Verdin E. NAD(+) in aging, metabolism, and neurodegeneration. Science. 2015;350(6265):1208–13. [DOI] [PubMed] [Google Scholar]
- [247]. Peek CB, Affinati AH, Ramsey KM, Kuo HY, Yu W, Sena LA, et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science. 2013;342(6158):1243417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248]. Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Canto C, et al. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154(2):430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249]. Park JH, Long A, Owens K, Kristian T. Nicotinamide mononucleotide inhibits post-ischemic NAD(+) degradation and dramatically ameliorates brain damage following global cerebral ischemia. Neurobiol Dis. 2016;95:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250]. Ivanisevic J, Stauch KL, Petrascheck M, Benton HP, Epstein AA, Fang M, et al. Metabolic drift in the aging brain. Aging (Albany NY). 2016;8(5):1000–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251]. Hawkins RA, Mans AM. Intermediary metabolism of carbohydrates and other fuels. In: Lajtha A, editor. Metabolism in the Nervous System. 2nd ed NY: Plenum Press; 1982. pp. 259–94. [Google Scholar]
- [252]. Fox JH, Kama JA, Lieberman G, Chopra R, Dorsey K, Chopra V, et al. Mechanisms of copper ion mediated Huntington’s disease progression. PLoS One. 2007;2(3):e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253]. Choo YS, Mao Z, Johnson GV, Lesort M. Increased glutathione levels in cortical and striatal mitochondria of the R6/2 Huntington’s disease mouse model. Neurosci Lett. 2005;386(1):63–8. [DOI] [PubMed] [Google Scholar]
- [254]. Berkich DA, Ola MS, Cole J, Sweatt AJ, Hutson SM, LaNoue KF. Mitochondrial transport proteins of the brain. J Neurosci Res. 2007;85(15):3367–77. [DOI] [PubMed] [Google Scholar]