Abstract
25-Hydroxyvitamin D insufficiency and increased cardiovascular risk (CVR) association is still debated. The vitamin D (VitD)-dependent parathyroid hormone (PTH) is considered as the possible actuator of VitD effects on CVR. To investigate the association of CVR, PTH and VitD, we carried out blood pressure measurements and blood samples and collected information on dietary habits, anamnestic, clinical and metabolic data of 451 participants in the Salerno area (Southern Italy) during the World Hypertension Day (17 May). CVR was calculated according to the Framingham CVR charts. The overall population mean age was 51.6 ± 0.7 years, and female sex was slightly prevalent (55%). VitD deficiency (<20 ng/ml) was most frequent (59.7%). In this population, VitD and CVR did not correlate. VitD and PTH inversely correlated (r = −0.265, P < 0.001) as expected. PTH was in direct correlation (r = 0.225, P < 0.001) with CVR. Elevated PTH (75 percentile; ≥49.5 pg/ml) levels identify a population with higher CVR (11.8 ± 0.5 vs. 8.5 ± 0.3, P < 0.001). In a multivariate analysis, both age and PTH correlate to CVR, but not VitD. In conclusion, VitD does not directly affect CVR in the overall population. Rather, increased PTH might be a better predictor of CVR.
Keywords: 25-hydroxyvitamin D cholecalciferol, aging, blood pressure, cardiovascular events, cardiovascular risk
Background
Vitamin D (VitD) and parathyroid hormone (PTH) represent pillars in the homeostasis of calcium and bone metabolism, through their reciprocal regulation. PTH enhances the tubular reabsorption of calcium and stimulates the kidney to transform the 25-hydroxyvitamin D [25(OH)D] in the 1,25-dihydroxyvitamin D, the most metabolically active form of VitD.1,2 VitD favors reabsorption of dietary calcium and phosphorus by increasing the efficiency of intestinal calcium absorption by 30–40% and phosphorus absorption by about 80%,1,3 reducing PTH levels. The 25(OH) VitD is considered the best marker of the VitD status.4 A close inverse relationship between VitD serum levels and PTH exists: indeed, in the case of VitD insufficiency, parathyroid gland is stimulated to release PTH.5–9 Recently, VitD insufficiency has been proposed to be linked to increased cardiovascular risk (CVR) through multiple mechanisms,2,10 including regulation of the renin–angiotensin system,11 alteration of insulin secretion and insulin sensitivity,12,13 impairment of angiogenesis14 and modulation of inflammatory processes inducing atherogenesis.15 Nevertheless, the role of VitD in predicting cardiovascular outcomes is at most controversial, and remains unestablished,16,17 given the fact that controlled clinical trials fail to prove the efficacy of VitD supplementation to reduce CVR and events.18,19 Similarly, a role for serum PTH elevation in CVR derives from data on primary and secondary hyperparathyroidism caused by renal failure: both conditions are associated with hypertension and increased risk of cardiovascular events, including myocardial infarction, stroke and cardiovascular death.20,21 Within these conditions, it is not possible to distinguish the possible role of PTH, given the malignancy associated with primary hyperparathyroidism and chronic kidney failure.
A regulatory role for PTH in the cardiovascular system is based on the findings that PTH receptors are expressed in the heart and the vessels.22 Still, the CVR of elevated PTH and the benefits of PTH-lowering in the general population have not been established.
The aim of the present investigation was to establish the association between serum VitD, PTH and CVR in a general population.
Methods
Study design and population
This is an observational study conducted on the general population in six Southern Italy villages: Castelnuovo Cilento, Salerno, Polla, Sarno, San Gregorio Magno and Satriano di Lucania. A total of 808 participants (45% men and 55% women, 14–85 years) were recruited during the XI, XII and XIII World Hypertension Day that occurs every year on 17 May. The study was approved by the relevant institutional Ethical Committee of Salerno University. Written informed consent was obtained from all participants. The study is registered in the ClincalTrial.gov database (NCT03305276).
Data collection
On the occasion of the World Hypertension Day 2015–2017, boots were organized in the major squares of the mentioned villages, and volunteers of the Medical School of Salerno collected through means of questionnaires the anamnesis and dietary habits. They also measured blood pressure (BP) after 5 min in the sitting position, three times with an interval of 2 min using validated, European Society of Hypertension (ESH) approved electronic oscillometers (A100, Microlife, Padua, Italy), according to the European Society of Cardiology/ESH Guidelines.23 Anamnestic information also included questions on previous cardiovascular conditions or events (coronary heart disease) and cerebrovascular accidents (transient ischemic accidents and stroke). Ongoing therapy and VitD supplementation were also annotated. A venous blood sample was drawn from the antecubital vein, and blood was collected for biochemical analysis at the University Hospital Centralized Service. Data were digitally stored for analysis.
Anamnesis and biochemical data regarding age, sex-specific cholesterol, HDL cholesterol, SBP, cigarette smoking, preexisting conditions and lifestyles were used for the calculation of CVR according to the Framingham Cardiovascular Risk Score.24,25 Familiarity for cardiovascular disease was defined as cardiovascular events in parents and siblings less than 50 years old.
Laboratory assessment of blood samples
A venous blood sample was collected in two tubes of 5.0 ml and centrifuged the same day. The time of the last meal was recorded during the data collection. Blood glucose, insulin, blood urea nitrogen (BUN), creatinine, calcium, phosphorus, total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, VitD and PTH were assessed. Glomerular filtration rate was estimated (eGFR) using the equation of Chronic Kidney Disease Epidemiology Collaboration.26,27
Grouping and statistical analysis
For statistical analysis, out of 808 volunteers, we selected participants aged 20–80 years old, as Framingham risk score can only be calculated up to 80 years old. Availability and quality data check was performed. Furthermore, we used the statistical identification of outliers, and data above or below 3 SD were excluded for VitD, PTH and eGFR (Fig. 1). In the end, the analysis was performed on 451 participants. Participants were divided into three age groups (20–40, 41–60 and 61–80 years old) for selected statistical purposes, as indicated. We used the lowest quartile of VitD and the highest quartile of PTH as the cutoff for the identification of low-VitD (<9 ng/ml) or high-PTH (>49.5 pg/ml) populations.
Fig. 1.
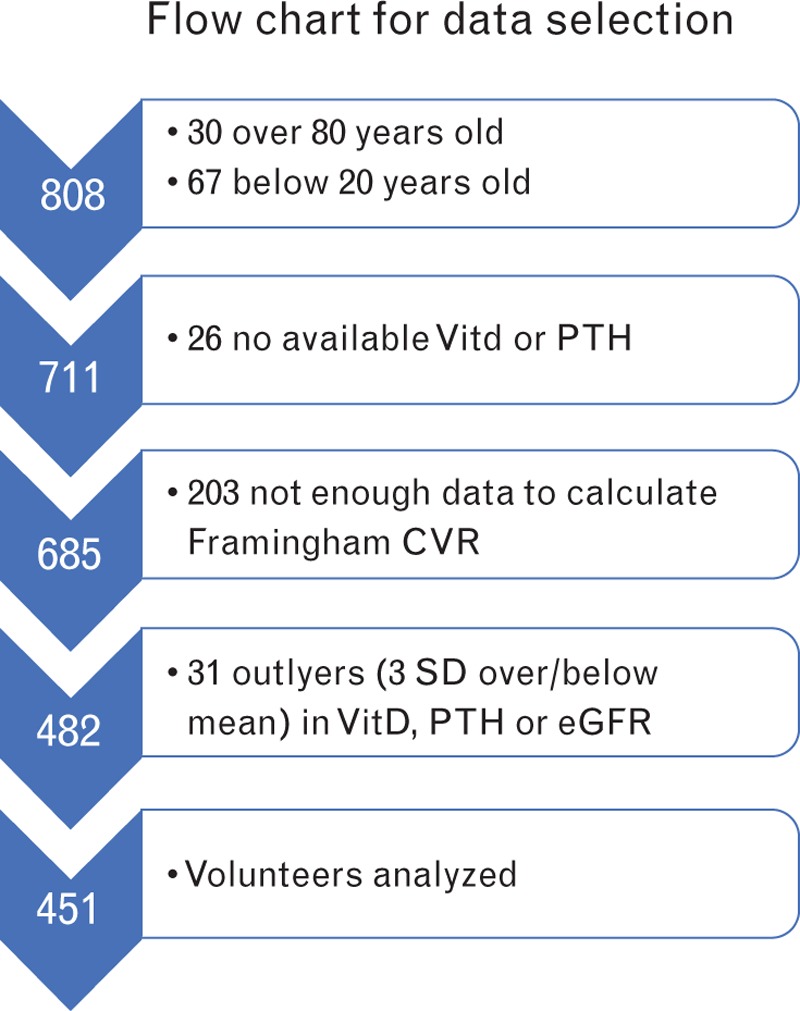
Flowchart describing the selection process leading to the size of population included in the analysis.
Frequencies are reported as a percentage (%), whereas continuous variables are presented as a mean ± standard error. The Student t test was used to compare continuous normally distributed variables, after calculation of the equality of variances with test t of Levene. The Bonferroni method, for the analysis of contrasts in average, was used in the analysis of the variance in presence of more than two populations to compare. The bivariate correlation was made to evaluate the association between the variables of interest. Linear regression was used for the estimation of the expected value of a dependent variable (i.e. PTH and CVR) to fixed factors and covariates (age groups, VitD). The interaction between fixed factors and covariates was also assessed. All data were analyzed by G.I. and R.G. using SPSS IBM Corporation, United States of America version 23.0. The significance was set for values of P less than 0.05.
Results
Baseline characteristics of overall population
Overall population features are shown in Table 1. The prevalence of CVR factors is in line with the European estimates: 47.6% of hypertension, 7% of diabetes and 32.8% of smokers. In the general population, SBP and age have a positive correlation (Pearson correlation r = 0.528, P < 0.001), as expected. A significant share of the population shows low levels of VitD, and the 59.7% of participants have serum levels of VitD below 20.0 ng/ml. In particular, 19.1% has deficiency (below 10.0 ng/ml) and 40.6% has insufficiency (between 10.1 and 20.0 ng/ml). Within the deficient group, women are predominant with a percentage of 66.3%.
Table 1.
Baseline characteristics and laboratory values of the study population
| Age (years) | 51.8 ± 0.73 |
| Male (%) | 44.5% |
| Female (%) | 55.5% |
| Weight (kg) | 73 ± 7.8 |
| BMI (kg/m2) | 27.0 ± 0.24 |
| SBP (mmHg) | 129.2 ± 0.8 |
| DBP (mmHg) | 78.2 ± 0.5 |
| HR (bpm) | 71.8 ± 0.6 |
| Blood glucose (mg/dl) | 81.4 ± 1.1 |
| Triglycerides (mg/dl) | 110.6 ± 3.3 |
| HDL cholesterol (mg/dl) | 59.2 ± 0.73 |
| Total cholesterol (mg/dl) | 198.5 ± 1.9 |
| LDL cholesterol (mg/dl) | 121.1 ± 1.9 |
| BUN (mg/dl) | 32.5 ± 0.4 |
| Creatinine (mg/dl) | 0.84 ± 0.02 |
| eGFR (ml/min/1.73 m2) | 92.4 ± 0.8 |
| 25(OH)D (ng/ml) | 18.3 ± 0.42 |
| PTH (pg/ml) | 38.5 ± 1.13 |
| Smoking (%) | 32% |
| Diabetes (%) | 7% |
| Hypertension (%) | 47.6% |
| Framingham risk score (%) | 5.38 ± 0.32 |
| Prevalence cardiovascular events (%) | 9.8% |
All values are means ± standard errors unless otherwise stated. BMI, body mass index; BUN, blood urea nitrogen; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HR: heart rate; PTH, parathyroid hormone; SBP, systolic blood pressure.
Vitamin D and cardiovascular risk
As expected,28,29 VitD and PTH inversely correlate (Fig. 2). No effect of aging was observed on VitD (data not shown), whereas PTH increases along groups of age (R2 = 0.061, P < 0.001). The relationship between VitD and PTH, though, remains significant at each age group (20–40, 40–60 and 60–80 years, Fig. 2). No effects of age on the relationship between VitD and PTH were observed in a multivariate analysis that included PTH as the dependent variable, and groups of age as a fixed factor and VitD as a covariate (VitD: F = 35.931, P < 0.001; age groups: F = 17.517, P < 0.001; VitD × age groups: F = 1.232, P = 0.293).
Fig. 2.
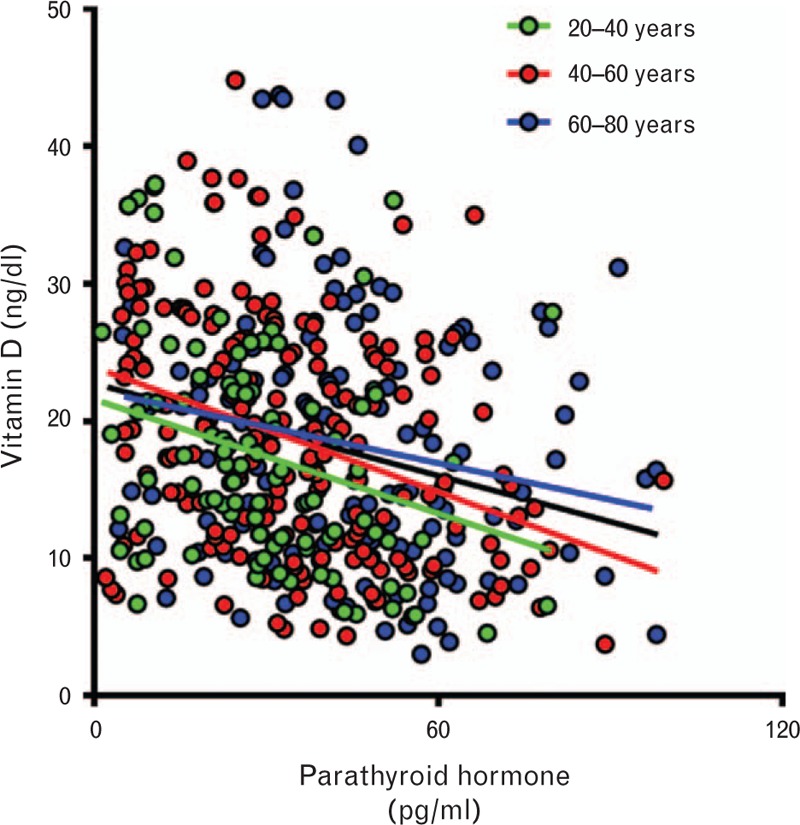
Association between vitamin D and parathyroid hormone in the overall population. There is an inverse correlation, as expected, between the two variables (black line, R2 = 0.07219, F = 28.01; P < 0.001) that is maintained through aging (20–40 years, green color: R2 = 0.08290, F = 9.311, P < 0.003; 41–60 years, red color: R2 = 0.1243, F = 26.84, P < 0.0001; 60–80 years, blue color: R2 = 0.04110, F = 6.343, P < 0.0128).
We then assessed the potential relationship between VitD and CVR. In our population, no linear regression was described between the two variables (B = 0.043; F = 0.818; n.s.). Similarly, low-VitD participants presented the same Framingham Risk Score as the control group (5.5 ± 0.38 vs. 4.9 ± 0.6, respectively, n.s.).
Parathyroid hormone and cardiovascular risk
We then assessed the effect of PTH on CVR. Given the association between age and PTH, it is possible to speculate that PTH serum levels might be a better predictor of CVR. Indeed, in the Framingham CVR calculation, age represents an important determinant. First, we divided the population according to high and low PTH levels in serum, using the above described 75 percentile of serum PTH as a cutoff (49.5 ng/ml). High-PTH participants show higher CVR (7.47 ± 0.7 vs. 4.6 ± 0.35; P < 0.001). Furthermore, PTH and CVR are in linear regression (R = 0.034; beta = 0.184; F = 15.553; P < 0.001). Given the interaction between PTH and age, to identify the independent role of PTH, we tested the effect of PTH on CVR in a model including also age groups (multivariate analysis). Although, as expected, CVR increases with age (F = 124.85, P < 0.001), also PTH effect on CVR is statistically significant (F = 24.231, P < 0.001) and independent from age (PTH × age: F = 0.169, n.s.).
Focus on people with age between 41 and 60 years
We hypothesized that if the driving mechanism of PTH on CVR is older age, this would limit the significance of our results, given the obvious impact of age on CVR. Therefore, to attenuate the effect of aging on the predictive role of PTH on CVR, we decided to compare participants with normal and elevated PTH in an age range that does not affect significantly CVR, that is in the group ranging from 41 to 60 years. At this age, high PTH levels identify participants with double CVR (6.35 ± 1.27 vs. 3.75 ± 0.37, P < 0.001). Apparently, the only determinant of the increased risk is the SBP, all the other parameters being similar between the two groups, including eGFR (Table 2). Confirming what observed in Fig. 2, people with higher PTH also show significantly lower VitD values (Table 2).
Table 2.
Cardiovascular risk factors, renal function and vitamin D status in people with age between 41 and 60 years and parathyroid hormone groups
| Age 41–60 | PTH < 49.5 | PTH ≥ 49.5 | ||
| N = 162 | N = 45 | P value | ||
| Framingham risk score (%) | 5.4 ± 6.7 | 3.7 ± 0.37 | 6.4 ± 1.3 | 0.008 |
| aAge (years) | 51.61 ± 0.39 | 51.3 ± 0.44 | 52.9 ± 0.8 | 0.21 |
| aMale (%) | 36 | 46.2 | 50.0 | 0.752 |
| aFemale (%) | 64 | 65 | 58 | |
| aSmoking (%) | 31 | 31 | 31 | 0.566 |
| aDiabetes (%) | 4 | 4 | 4 | 0.182 |
| Blood glucose (mg/dl) | 81.0 ± 1.51 | 78.5 ± 1.6 | 89.7 ± 4.3 | 0.001 |
| Hypertension (%) | 42 | 42 | 42 | 0.942 |
| aSBP (mmHg) | 128.6 ± 1.61 | 127.5 ± 1.3 | 134.1 ± 2.6 | 0.012 |
| DBP (mmHg) | 80.8 ± 0.70 | 79.8 ± 0.8 | 84.3 ± 1.5 | 0.008 |
| aTotal cholesterol (mg/dl) | 209 ± 2.87 | 206.71 ± 3.1 | 217.8 ± 6.3 | 0.328 |
| aHDL cholesterol (mg/dl) | 59.7 ± 1.05 | 60.0 ± 1.2 | 58.6 ± 2.4 | 0.797 |
| LDL cholesterol (mg/dl) | 129.9 ± 2.5 | 126.4 ± 2.9 | 142.1 ± 5.0 | 0.009 |
| BUN (mg/ml) | 31.5 ± 0.5 | 31.7 ± 0.6 | 30.7 ± 1.1 | 0.654 |
| Creatinine (mg/dl) | 0.79 ± 0.01 | 0.79 ± 0.11 | 0.80 ± 0.02 | 0.729 |
| eGFR (ml/min/1.73 m2) | 93.6 ± 0.8 | 93.9 ± 0.9 | 92.5 ± 1.71 | 0.666 |
| PTH (pg/ml) | 35.4 ± 1.41 | 27.2 ± 1.1 | 64.6 ± 1.7 | 0.001 |
| VitD (ng/ml) | 18.56 ± 0.6 | 19.6 ± 0.7 | 14.8 ± 1.1 | 0.020 |
BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; PTH, parathyroid hormone; VitD, vitamin D.
aIdentifies the variables used for calculation of Framingham Risk Score. All values are means ± standard errors unless otherwise stated. P values are based on Analysis of Variance or Pearson chi-square test for categorical variables.
Significant differences are indicated in bold italics.
Discussion
Our data propose two major advancements of knowledge. First, within the general population, VitD levels fail to associate with CVR. Second, PTH, a VitD dependent hormone, is a more reliable predictor of CVR and affects in particular SBP.
Although increasing evidence accredits PTH as a relevant player in CVR,20,21,28–30 the underlying mechanism is still unclear: a possible explanation relates to the interference with other classical CVR factors. In our population, indeed, we show that PTH increases with age, weight, BMI, SBP, LDL and BUN. In particular, our paper shows for the first time the close relationship between age and PTH. Consequently, also the normality ranges for PTH should consider aging. In our study, we identify 49.5 pg/ml as the upper limit. Using this cutoff, we can draw two major results. First, the group with at least 49.5 pg/ml of PTH presents higher CVR. The inverse correlation between PTH and VitD appears preserved, being the VitD values significantly lower in the group with elevated PTH. In this population, though, the increased CVR can be attributed to aging, which can also be considered as the cause of increased PTH. In particular, age-dependent contraction of the kidney function expressed as the reduction of eGFR and increasing of BUN values can cause altered homeostasis of calcium/phosphate and consequent secondary hyperparathyroidism.31 To exclude an effect of age per se on the increased CVR, we limited our analysis to the population comprised between 41 and 60 years old. In this group, PTH is not influenced by kidney function, which remains similar between high and low PTH groups (Table 2). Moreover, the relationship with VitD is preserved. PTH still predicts the CVR in this subgroup. To finally identify the independent effect of PTH on CVR, we run a multivariable linear modeling, including parameters of aging and PTH levels to correlate significantly and independently with CVR.
The second result is that in a younger and homogenous population, the association between elevated PTH and doubled CVR can be explained by the increased BP, as all other parameters (age, sex, smoking, diabetes, total cholesterol and HDL cholesterol) remain similar between high and low PTH groups. In this group, lower VitD is the only determinant of PTH levels, and therefore its deficiency can lead to increased BP values by increasing PTH.
The use of 49.5 pg/ml as a cutoff for PTH confirms the need to have reference values for each age group for this parameter. We propose that different ranges should be considered for different age groups. Indeed, excluding the elderly and kidney failure, at the age 41–60 years, participants with elevated PTH show significantly higher values of BP, glucose and LDL.
The molecular mechanism underlying the increased BP has not been elucidated. A number of pieces of evidence suggest that PTH has vascular effects. Endothelial dysfunction is one mechanism thought to link PTH to vascular changes: PTH, indeed, may increase serum levels of endothelin-1 and IL-6.32,33 Furthermore, PTH may stimulate the vascular smooth muscle cells to produce factors including collagen and beta-1 integrin which could, in turn, remodel the peripheral vasculature.34 PTH may increase renin release and activate the renin–angiotensin system,35,36 a complex process mediated by serum calcium, renal 1-alpha hydroxylase and resultant changes in 1,25(OH)2D.37,38
Our study does not allow the identification of the mechanisms underlying the increased BP associated with increased PTH; hence this issue deserves further specific investigation.
Perspectives
Although in the general population VitD fails to predict CVR, PTH represents a more reliable and promising biomarker. Although PTH increases with aging, also in younger populations, the cutoff of 49.5 ng/ml can predict increased CVR. The major determinant is the increase of SBP values. Being the variation of PTH values in this age group almost exclusively linked to VitD deficiency, the supplementation of VitD may become an important stronghold to reduce the CVR through the decrease of serum PTH values.
Limitations
Our study, although performed in a significantly large population, has clear geographical limitations, and these results need to be replicated in larger studies including different geographical areas in Italy and in the world. Moreover, a prospective design is not included in our observation, and therefore, we cannot draw outcome implications from our results.
Novelty and significance
What is New: PTH is a better biomarker than VitD to predict CVR.
What is Relevant: Although PTH increases with aging, also in younger populations, the cutoff of 49.5 pg/ml can predict increased CVR. In this younger population, the major determinant of Framingham CVR is the increased SBP. A descending hypothesis is that VitD supplement might reduce PTH levels and BP values, normalizing cardiovascular risk.
Acknowledgements
Conflicts of interest
There are no conflicts of interest.
References
- 1.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004; 80 6 Suppl:1689S–1696S. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266–281. [DOI] [PubMed] [Google Scholar]
- 3.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 2003; 22:142–146. [DOI] [PubMed] [Google Scholar]
- 4.Zerwekh JE. Blood biomarkers of vitamin D status. Am J Clin Nutr 2008; 87:1087S–1091S. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006; 81:353–373. [DOI] [PubMed] [Google Scholar]
- 6.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998; 351:805–806. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med 1998; 338:777–783. [DOI] [PubMed] [Google Scholar]
- 8.Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 1997; 7:439–443. [DOI] [PubMed] [Google Scholar]
- 9.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 2005; 90:3215–3224. [DOI] [PubMed] [Google Scholar]
- 10.Michos ED, Melamed ML. Vitamin D and cardiovascular disease risk. Curr Opin Clin Nutr Metab Care 2008; 11:7–12. [DOI] [PubMed] [Google Scholar]
- 11.Li YC, Qiao G, Uskokovic M, Xiang W, Zheng W, Kong J. Vitamin D: a negative endocrine regulator of the renin–angiotensin system and blood pressure. J Steroid Biochem Mol Biol 2004; 89–90:387–392. [DOI] [PubMed] [Google Scholar]
- 12.Rammos G, Tseke P, Ziakka S. Vitamin D, the renin-angiotensin system, and insulin resistance. Int Urol Nephrol 2008; 40:419–426. [DOI] [PubMed] [Google Scholar]
- 13.Norman AW, Frankel JB, Heldt AM, Grodsky GM. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science 1980; 209:823–825. [DOI] [PubMed] [Google Scholar]
- 14.Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension 1989; 13 (6 Pt 2):954–959. [DOI] [PubMed] [Google Scholar]
- 15.Deluca HF, Cantorna MT. Vitamin D: its role and uses in immunology. FASEB J 2001; 15:2579–2585. [DOI] [PubMed] [Google Scholar]
- 16.Cerit L. Bermuda triangle; heart failure, atrial fibrillation, and vitamin D deficiency. J Cardiovasc Med 2017; 18:121. [DOI] [PubMed] [Google Scholar]
- 17.Cerit L, Kemal H, Gulsen K, Ozcem B, Cerit Z, Duygu H. Relationship between Vitamin D and the development of atrial fibrillation after on-pump coronary artery bypass graft surgery. Cardiovasc J Afr 2017; 28:104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grubler MR, Marz W, Pilz S, et al. Vitamin-D concentrations, cardiovascular risk and events – a review of epidemiological evidence. Rev Endocr Metab Disord 2017; 18:259–272. [DOI] [PubMed] [Google Scholar]
- 19.Al Mheid I, Quyyumi AA. Vitamin D and cardiovascular disease: controversy unresolved. J Am Coll Cardiol 2017; 70:89–100. [DOI] [PubMed] [Google Scholar]
- 20.Rostand SG, Drueke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney Int 1999; 56:383–392. [DOI] [PubMed] [Google Scholar]
- 21.Andersson P, Rydberg E, Willenheimer R. Primary hyperparathyroidism and heart disease – a review. Eur Heart J 2004; 25:1776–1787. [DOI] [PubMed] [Google Scholar]
- 22.Usdin TB, Bonner TI, Harta G, Mezey E. Distribution of parathyroid hormone-2 receptor messenger ribonucleic acid in rat. Endocrinology 1996; 137:4285–4297. [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens 2013; 31:1281–1357. [DOI] [PubMed] [Google Scholar]
- 24.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P. CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA 2001; 286:180–187. [DOI] [PubMed] [Google Scholar]
- 25.Izzo R, de Simone G, Giudice R, Chinali M, Trimarco V, De Luca N, Trimarco B. Effects of nutraceuticals on prevalence of metabolic syndrome and on calculated Framingham Risk Score in individuals with dyslipidemia. J Hypertens 2010; 28:1482–1487. [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu CY. CKD-EPI eGFR categories were better than MDRD categories for predicting mortality in a range of populations. Ann Intern Med 2012; 157:JC5–JC12. [DOI] [PubMed] [Google Scholar]
- 28.Gruson D, Ferracin B, Ahn SA, et al. 1,25-Dihydroxyvitamin D to PTH(1-84) ratios strongly predict cardiovascular death in heart failure. PLoS One 2015; 10:e0135427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson JL, Vanwoerkom RC, Horne BD, et al. Parathyroid hormone, vitamin D, renal dysfunction, and cardiovascular disease: dependent or independent risk factors? Am Heart J 2011; 162:331–339 e2. [DOI] [PubMed] [Google Scholar]
- 30.Garcia de la Torre N, Wass JA, Turner HE. Parathyroid adenomas and cardiovascular risk. Endocr Relat Cancer 2003; 10:309–322. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, Yang S, Chen J, Ma J, Ren Y. Associations of parathyroid hormone levels and mineral parameters with heart rate variability in patients with end-stage renal disease. Int Urol Nephrol 2017; 49:1079–1085. [DOI] [PubMed] [Google Scholar]
- 32.Rashid G, Bernheim J, Green J, Benchetrit S. Parathyroid hormone stimulates endothelial expression of atherosclerotic parameters through protein kinase pathways. Am J Physiol Renal Physiol 2007; 292:F1215–F1218. [DOI] [PubMed] [Google Scholar]
- 33.Isales CM, Sumpio B, Bollag RJ, et al. Functional parathyroid hormone receptors are present in an umbilical vein endothelial cell line. Am J Physiol Endocrinol Metab 2000; 279:E654–E662. [DOI] [PubMed] [Google Scholar]
- 34.Perkovic V, Hewitson TD, Kelynack KJ, Martic M, Tait MG, Becker GJ. Parathyroid hormone has a prosclerotic effect on vascular smooth muscle cells. Kidney Blood Press Res 2003; 26:27–33. [DOI] [PubMed] [Google Scholar]
- 35.Pilz S, Tomaschitz A. Role of vitamin D in arterial hypertension. Expert Rev Cardiovasc Ther 2010; 8:1599–1608. [DOI] [PubMed] [Google Scholar]
- 36.Grant FD, Mandel SJ, Brown EM, Williams GH, Seely EW. Interrelationships between the renin-angiotensin-aldosterone and calcium homeostatic systems. J Clin Endocrinol Metab 1992; 75:988–992. [DOI] [PubMed] [Google Scholar]
- 37.Fitzpatrick LA, Bilezikian JP, Silverberg SJ. Parathyroid hormone and the cardiovascular system. Curr Osteoporos Rep 2008; 6:77–83. [DOI] [PubMed] [Google Scholar]
- 38.Beierwaltes WH. The role of calcium in the regulation of renin secretion. Am J Physiol Renal Physiol 2010; 298:F1–F11. [DOI] [PMC free article] [PubMed] [Google Scholar]


