Supplemental Digital Content is available in the text.
Keywords: adventitia, cell differentiation, stem cells, vascular diseases, vascular remodeling
Abstract
Objective—
Vascular adventitial Sca1+ (stem cell antigen-1) progenitor cells preferentially differentiate into smooth muscle cells, which contribute to vascular remodeling and neointima formation in vessel grafts. Therefore, directing the differentiation of Sca1+ cells toward the endothelial lineage could represent a new therapeutic strategy against vascular disease.
Approach and Results—
We thus developed a fast, reproducible protocol based on the single-gene transfer of ETV2 (ETS variant 2) to differentiate Sca1+ cells toward the endothelial fate and studied the effect of cell conversion on vascular hyperplasia in a model of endothelial injury. After ETV2 transduction, Sca1+ adventitial cells presented a significant increase in the expression of early endothelial cell genes, including VE-cadherin, Flk-1, and Tie2 at the mRNA and protein levels. ETV2 overexpression also induced the downregulation of a panel of smooth muscle cell and mesenchymal genes through epigenetic regulations, by decreasing the expression of DNA-modifying enzymes ten-eleven translocation dioxygenases. Adventitial Sca1+ cells grafted on the adventitial side of wire-injured femoral arteries increased vascular wall hyperplasia compared with control arteries with no grafted cells. Arteries seeded with ETV2-transduced cells, on the contrary, showed reduced hyperplasia compared with control.
Conclusions—
These data give evidence that the genetic manipulation of vascular progenitors is a promising approach to improve vascular function after endothelial injury.
The vascular adventitia is a niche for multipotent and lineage-restricted resident progenitor cells, positive for Sca1 (stem cell antigen-1), cd34, c-kit, or Flk-11,2 and able to differentiate into the various cell types forming the vascular wall, including smooth muscle cells (SMCs), endothelial cells (EC), and mesenchymal cells. Recent evidence suggests that the vascular progenitor cells, firstly identified in the adventitia of the aortic root of ApoE (apolipoprotein E)-deficient mice—a model of native atherosclerosis, contribute to vascular diseases and remodeling.1 In vein graft and wire injury models, adventitial Sca1+ progenitor cells (AdvSca1) grafted on the perivascular side of the vessel, migrated to the neointima, newly expressed SMC markers, including SM22α (smooth muscle protein 22-alpha) and enhanced atherosclerotic progression.1,3 A recent study showed that 50% of neointimal SMCs of injured vessels were derived from adventitial mesenchymal stem cells.4 Reciprocally, Majesky et al5 demonstrated that a proportion of AdvSca1 cells originated in situ from differentiated medial SMCs and that after vascular injury, SMC-derived AdvSca1 cells expanded and were involved in adventitial remodeling. These findings implicate that the vascular adventitial stem/progenitor cell population could play an important role in vascular diseases.
See cover image
AdvSca1 cells are preferentially specified toward an SMC fate in vitro,2 and collagen IV or PDGF-BB (platelet-derived growth factor-BB) strongly induced the expression of SMC genes, including αSMA (α-smooth muscle actin) and SM22α.1,6 AdvSca1 cells showed to a lesser extent the potential to give rise to adipocytes, osteoblasts, and chondrocytes.6 When incubated with VEGF (vascular endothelial growth factor), only a subset of AdvSca1 cells, also expressing αSMA, expressed EC marker cd31.2 Resveratrol promoted in vitro the differentiation of AdvSca1 cells toward the endothelial lineage through inhibition of the β-catenin pathway. In vivo, a resveratrol-enhanced diet also reduced neointima formation and promoted reendothelization in a murine model of vein graft,7 and similar results were observed when VEGF was applied to the adventitial side of decellularized vessel grafts.8 These results encouraged us to develop a robust and reproducible protocol to direct AdvSca1 cell differentiation toward EC as a therapeutic approach, and thus, preventing the differentiation of vascular progenitor cells into SMCs during the development of vascular diseases.
ETV2 (ETS variant 2)—a member of the ETS (E26 transformation-specific) transcription factor family—is a strong inducer of EC differentiation during early vascular development9 and has been used in several studies, alone or in combination with other factors, to transdifferentiate in vitro mouse and human fibroblasts into EC.10,11 In the current study, we investigated whether we could direct AdvSca1 cells toward the endothelial lineage by overexpressing ETV2 and how the cell conversion would affect vascular hyperplasia. Our results showed that ETV2 transduction increased EC gene expression in AdvSca1 cells. ETV2 also downregulated SMC/mesenchymal genes expression by decreasing the expression of epigenetic modifiers TET (ten-eleven translocation) enzymes. AdvSca1 cells transduced with ETV2 in an endothelial injury model lost the ability to enhance neointima formation or vascular remodeling and promoted reendothelization.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
AdvSca1 Cells Are Specified Toward an SMC/Mesenchymal Fate In Vitro
To assess the potential of AdvSca1 cells to differentiate into EC in vitro without gene manipulation, we cultured the cells for 3 days in different conditions: in Dulbecco modified Eagle medium with 10% of fetal bovine serum (FBS) and supplemented with LIF (leukemia inhibitory factor; to keep the undifferentiated state of AdvSca1 cells) or without LIF, with and without VEGF; or in Dulbecco modified Eagle medium with 10% of serum replacement (SR) with and without VEGF. Cells cultured in SR displayed a change of morphology (Figure 1A; Figure IA in the online-only Data Supplement) with reduced proliferation as shown by measurement of Edu incorporation and cell cycle distribution by flow cytometry analysis (Figure IIA and IIB in the online-only Data Supplement). AdvSca1 cells cultured in SR (Adv-SR) showed an increase in the expression of cd34 expression compared with undifferentiated AdvSca1 cells by quantitative real-time polymerase chain reaction (RT-PCR). However, endothelial gene VE-cadherin (cdh5), cd31, and Flk-1 expression were not increased in any culture conditions, and VEGF did not further promote EC differentiation in SR medium (Figure IB in the online-only Data Supplement). Cd34 is expressed by ECs and vascular wall progenitor cells with endothelial potential,12 but its expression was also reported on progenitors with smooth muscle and mesenchymal potential.13 Consequently, we also analyzed the expression of markers specific for other lineages and noticed a strong induction of SMC/mesenchymal genes, such as αSMA (acta2), Pdgfrβ, cdh11, and col1a, in Adv-SR cells (Figure 1C). Immunofluorescence staining confirmed the induction of αSMA in Adv-SR cells at the protein level (Figure 1B). We concluded that on LIF and serum removal, AdvSca1 cells autonomously and preferentially commit to an SMC/mesenchymal fate, and VEGF alone is insufficient to induce EC differentiation in vitro.
Figure 1.
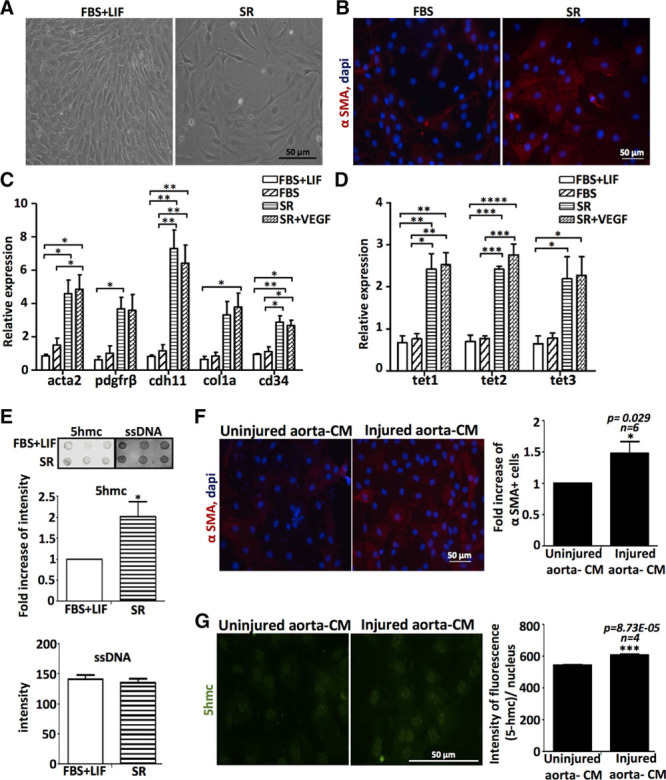
Adventitial Sca1+ (AdvSca1) progenitor cells differentiate into smooth muscle cells/mesenchymal cells and undergo epigenetic changes in vitro and ex vivo in response to vascular injury. A, Morphology of AdvSca1 cells cultured for 3 d in medium with fetal bovine serum (FBS)+LIF (leukemia inhibitory factor) or with serum replacement (SR). B, Immunofluorescence staining of AdvSca1 cells cultured for 5 d in medium with FBS or SR for αSMA (α-smooth muscle actin; red). DAPI (4’,6-diamidino-2-phenylindol) stains nuclei (blue). Quantitative real-time polymerase chain reaction of (C) acta2, Pdgfrβ, cdh11, col1a, cd34, and (D) TET (ten-eleven translocation) expression in AdvSca1 cells cultured in different conditions (experiment done in triplicates; *P<0.05, **P<0.01, ***P<0.001, ****P<0.001; data representative of 2 independent experiments). E, Dot blot for global enrichment for 5hmc using genomic DNA (n=3 independent experiments) isolated from AdvSca1 cells cultured in FBS+LIF or SR. Single-strand DNA (ssDNA) antibody shows equal loading. Densitometry quantification was performed using ImageJ software. Mouse AdvSca1 cells were cultured for 5 d in presence of conditioned media (CM) from uninjured or injured sections of the same rat artery. F, Immunofluorescence staining of AdvSca1 cells for αSMA (red) and increase of αSMA+ cells (n=6 arteries; *P<0.05 vs uninjured aorta-CM; 5×20 magnification fields were counted for each experiment). G, Immunofluorescence staining of AdvSca1 cells for 5hmc (green). Intensity of fluorescence per nucleus and per ×40 magnification field, n=4 magnification fields, ***P<0.001 vs uninjured aorta-CM. The histogram is representative of results from 4 different arteries.
TET and 5hmC Are Upregulated During AdvSca1 Cell Differentiation Into SMC/Mesenchymal Cells In Vitro and in Response to Vascular Injury
We next investigated the potential mechanisms associated with the SMC/mesenchymal differentiation of AdvSca1 cells. Several studies support the involvement of epigenetic alterations in vascular diseases. Recently, DNA-modifying enzymes TET were linked to SMC plasticity and the development of atherosclerotic lesions in patients and mice models.14 We measured TET (tet1, tet2, and tet3) mRNA levels in AdvSca1 cells after culture in different conditions (Figure 1D). Because TET enzymatic activity is to convert 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC), we also measured 5hmC levels in genomic DNA from undifferentiated AdvSca1 cells and differentiated Adv-SR cells (Figure 1E; Figure IC in the online-only Data Supplement). Our results demonstrated that elevated TET mRNA and 5hmC levels were associated with increased differentiation toward an SMC/mesenchymal phenotype. We next investigated the effect of vascular injury on AdvSca1 cells differentiation using an aorta-conditioned medium (CM)-based differentiation assay described in a recent study.15 AdvSca1 cells were incubated in CM prepared from an ex vivo-injured or uninjured ascending aorta for 5 days. Immunostaining using a specific antibody for αSMA (Figure 1F; Figure ID in the online-only Data Supplement) and quantitative RT-PCR for col1a (Figure IE in the online-only Data Supplement) revealed that CM from injured aorta stimulated the expression of these SMC/mesenchymal genes in AdvSca1 cells at the protein and RNA levels, respectively. Quantitative RT-PCR to measure TET expression showed a significant increase in Tet3 expression in AdvSca1 cells incubated with CM from injured aorta compared with CM from uninjured aorta (Figure IF in the online-only Data Supplement). Immunofluorescence staining using a specific antibody for 5hmc also confirmed increased DNA hydroxymethylation in AdvSca1 in presence of injured aorta-CM compared with uninjured aorta-CM (Figure 1G). Altogether, our results showed that AdvSca1 cells differentiate preferentially into SMC/mesenchymal cells in vitro and in response to vascular injury conditions. We also showed that epigenetic events are associated with SMC/mesenchymal differentiation.
ETV2 Directs AdvSca1 Cells Toward an Endothelial Fate
To force the differentiation of the cells toward the endothelial lineage instead of the SMC/mesenchymal lineage, we transduced AdvSca1 cells with adenoviral particles overexpressing ETV2—an EC transcription factor, strong inducer of EC differentiation, used in previous studies to transdifferentiate fibroblasts into EC.10,11 The cells were placed in SR medium with VEGF to facilitate VE-cadherin and cd34 expression. After 7 days, the cells were harvested, and ETV2 overexpression was confirmed by immunostaining (Figure 2A). AdvSca1 cells overexpressing ETV2 (AdvSca1+ cells differentiated in SR and transduced with ETV2 virus [Adv-ETV2]) displayed a more endothelial-like morphology compared with undifferentiated AdvSca1 (AdvSca1) and to the cells differentiated with the adenovirus control (AdvSca1+ cells differentiated in SR and transduced with null virus [Adv-null]; Figure 2B) and could take up red fluorescent-labeled acetylated LDL (low-density lipoprotein; Figure 2C). Global gene expression analysis revealed that Adv-ETV2 cells obtained from 2 independent experiments clustered with 2 different EC lines as illustrated by hierarchical clustering (Figure 2E) and were enriched for a full panel of endothelial-specific genes (Figure 2D). We confirmed the strong induction of EC-specific markers, including VE-cadherin, flk-1 (VEGFR2), and tie2, and of genes involved in angiogenesis and vascular development, such as Rasip1 at the mRNA level (Figure 2F and 2G) compared with undifferentiated AdvSca1 cells, AdvSca1+ cells differentiated in SR+VEGF, and Adv-null. Flk-1, VE-cadherin, and Rasip1 induction were confirmed at the protein level (Figures 2H and 4D; Figure VA in the online-only Data Supplement). Therefore, our results proved that ETV2 alone is sufficient to direct the differentiation of AdvSca1 cells toward the endothelial lineage.
Figure 2.
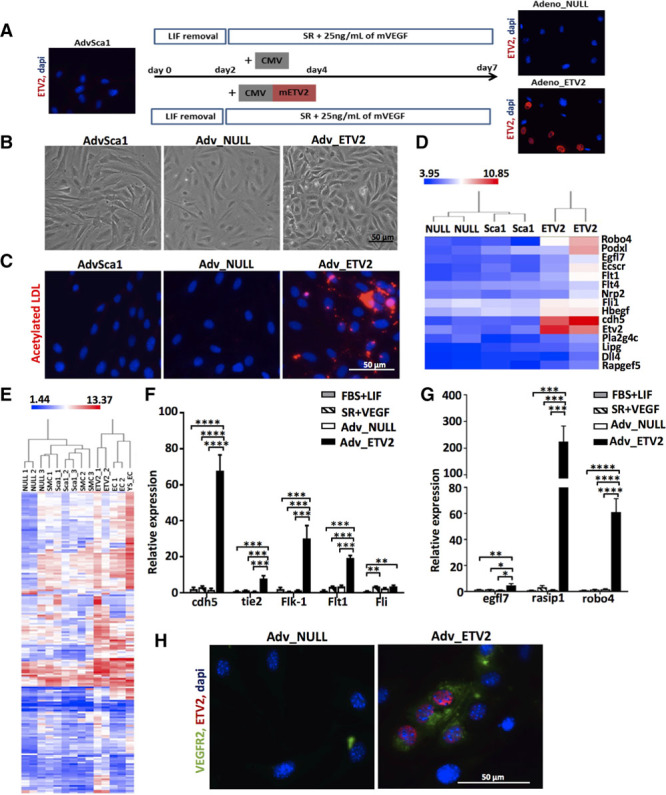
Adenovirus-mediated ETV2 (ETS variant 2) overexpression promotes endothelial differentiation of adventitial Sca1+ (AdvSca1) cells. A, Schematic of the differentiation protocol and immunofluorescence staining for ETV2 (red). B, Morphology of AdvSca1, adventitial Sca1+ cells differentiated in serum replacement (SR) and transduced with null virus (Adv-null), and adventitial Sca1+ cells differentiated in SR and transduced with ETV2 virus (Adv-ETV2) cells at day 7. C, Ability to take up acetylated LDL (low-density lipoprotein; red fluorescence). D, Heat map for selected genes shows enriched expression for endothelial cell (EC) genes in Adv-ETV2 cells based on the microarray results. Color bar indicates gene expression in scale. E, Hierarchical clustering of global gene expression after microarray. Postnatal mouse smooth muscle cell, mouse EC lines CRL2581 (YS-EC) and MS1 (EC) were used as controls. Quantitative real-time polymerase chain reaction of (F) EC receptors cdh5, tie2, Flk-1, and Flt1, transcription factor Fli, and of (G) EC-specific genes Egfl7, Rasip1, and Robo4 in Adv-ETV2, Adv-null cells, and AdvSca1 cells cultured in fetal bovine serum (FBS)+LIF (leukemia inhibitory factor) or SR+VEGF (*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; n=4 independent experiments). H, Immunofluorescence staining of Adv-null and Adv-ETV2 cells for VEGFR2 (green) and ETV2 (red).
Figure 4.
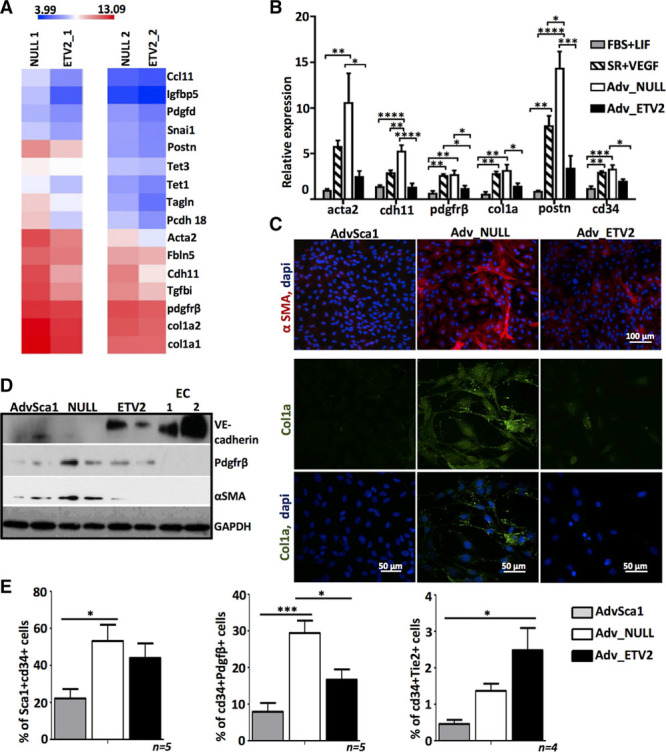
ETV2 (ETS variant 2) overexpression inhibits the differentiation of adventitial Sca1+ (AdvSca1) cells into smooth muscle cells/mesenchymal cells. A, Heat map for selected genes shows enriched expression for mesenchymal and profibrotic genes in adventitial Sca1+ cells differentiated in serum replacement (SR) and transduced with null virus (Adv-null) cells compared with adventitial Sca1+ cells differentiated in SR and transduced with ETV2 virus (Adv-ETV2), from 2 separate experiments based on microarray. Color bar indicates gene expression in scale. B, Validation by quantitative real-time polymerase chain reaction of the mRNA changes for the indicated genes in Adv-ETV2, Adv-null cells, and AdvSca1 cultured in fetal bovine serum (FBS)+LIF (leukemia inhibitory factor) or SR+VEGF (n=4 independent experiments; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001). C, Immunofluorescence staining of AdvSca1, Adv-null, and Adv-ETV2 cells for αSMA (α-smooth muscle actin) and collagen1. D, Western blot analysis of VE-cadherin, Pdgfrβ, and αSMA expression in AdvSca1, Adv-null, and Adv-ETV2 cells. GAPDH antibody was used for loading control. Protein extracts from mouse endothelial cells MS1 (1) and CRL2581 (2) were used as positive controls for endothelial cell (EC) protein profile expression. E, Quantification by flow cytometry of cd34+ Sca1+, cd34+ Pdgfrβ+ and cd34+ Tie2+ populations in undifferentiated AdvSca1, Adv-null, and Adv-ETV2 cells (*P<0.05, ***P<0.001; n=4–5 independent experiments).
Adv-ETV2 Cells Form Capillaries In Vivo and Ex Vivo
To compare the potential of AdvSca1, Adv-null and Adv-ETV2 cells to form capillaries in vivo, the cells were stably labeled by infection with histone H2b-RFP (red fluorescent protein) lentiviral particles to allow their tracing and used in a subcutaneous angiogenesis Matrigel assay. As shown previously,5 undifferentiated AdvSca1 cells have a limited ability to spontaneously form capillaries in an in vivo Matrigel assay. After ETV2 reprogramming, Adv-ETV2 cells displayed increased vasculogenic properties and could be seen incorporated in a much denser network of vessels (Figure 3A). Similar to our in vitro results, Adv-ETV2 RFP cells still expressed EC markers VE-cadherin and VEGFR2 in vivo as shown by immunofluorescence. The cells also newly expressed EC marker cd31, showing that the cells had further differentiated (Figure 3A; Figure VI in the online-only Data Supplement). The presence of blood cells in the lumen indicating a functional capillary network could only be seen in vessels from Matrigel plugs seeded with Adv-ETV2 cells (Figure VI in the online-only Data Supplement), confirming that Adv-ETV2 cells were behaving like functional EC in vivo. Almost no vessel formation occurred within the Matrigel plug with Adv-null cells, indicating that the cells do not have the ability to differentiate into EC or to promote vasculogenesis (Figure 3A). We next investigated whether ETV2 could promote EC differentiation of AdvSca1 cells using the ex vivo mouse aortic ring assay to study angiogenesis. Aortic ring explants were isolated from Sca1-GFP (green fluorescent protein) transgenic mice to allow easy detection by fluorescence of the AdvSca1 cells in the vascular wall. The mice used in this study were also from ApoE−/− background because it was described that a high number of Sca1+ cells was observed in the adventitia of aorta of ApoE null mice.1 The aortic rings cultured in the presence of ETV2 overexpressing adenovirus for 7 days displayed increased sprouting compared with explants cultured with null adenovirus (Figure 3B; Figure VIIA in the online-only Data Supplement). ETV2 overexpression was confirmed in the tissue and in Sca1+ GFP cells by immunofluorescence staining (Figure VIIB in the online-only Data Supplement), and a quantification revealed an increase of GFP cells expressing cd31 after ETV2 transduction (Figure 3C and 3D). We concluded that the overexpression of ETV2 in adventitial progenitor cells leads to an increase of cd31+ cells with proangiogenic properties in the vascular wall.
Figure 3.
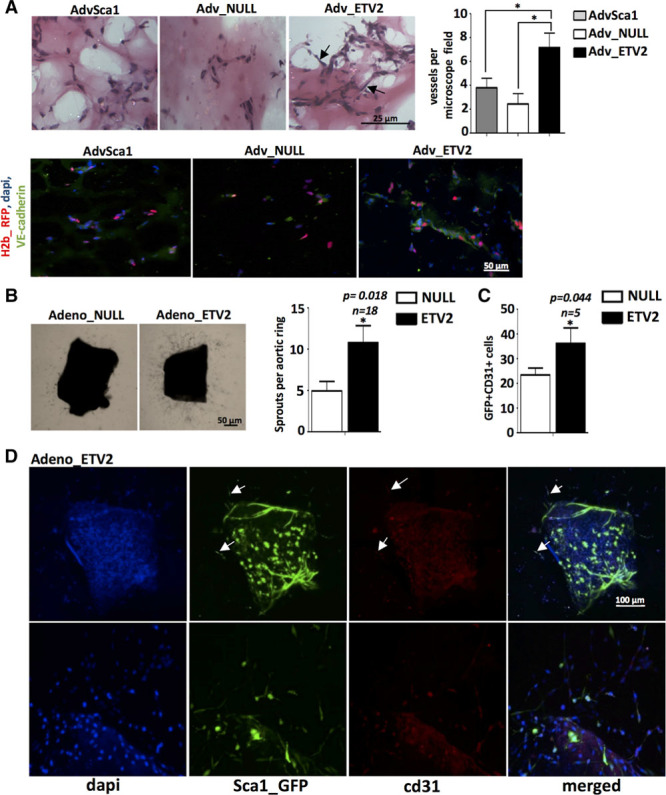
ETV2 (ETS variant 2) overexpression in adventitial Sca1+ (AdvSca1) cells promotes angiogenesis. A, LV H2b-RFP (red fluorescent protein) stably transduced AdvSca1, adventitial Sca1+ cells differentiated in serum replacement (SR) and transduced with null virus (Adv-null), or adventitial Sca1+ cells differentiated in SR and transduced with ETV2 virus (Adv-ETV2) cells were used for in vivo matrigel plug assays. Adv-ETV2 cells formed a denser network of capillary-like structures (black arrows) compared with the 2 other groups (*P<0.05; 5 microscope fields were counted for each plug; n=5 plugs for each population). RFP Adv-ETV2 express VE-cadherin (green) in newly formed capillaries after immunofluorescence staining. B, Representative bright field pictures and quantification of vascular sprouting observed with aortic ring explants from ApoE Sca1-GFP (green fluorescent protein) mice cultured with ETV2 overexpressing adenovirus compared with the control null adenovirus (n=18 rings for each group from 3 independent arteries/experiments; *P<0.05). C, Quantification of GFP+cd31+ cells (n=5 rings from 2 independent arteries/experiments; *P<0.05). D, Representative pictures of aortic ring explants from Sca1-GFP transgenic mice, stained for cd31 (red) after culture with ETV2 overexpressing adenovirus. Arrows indicate cd31-positive GFP cells.
ETV2 Prevents SMC/Mesenchymal Differentiation of AdvSca1 Cells
In accordance with the upregulation of SMC/mesenchymal genes in Adv-SR cells (Figure 1C), quantitative RT-PCR revealed that several genes of SMC/mesenchymal lineage, including acta2, cdh11, pdgfrβ, postn, and profibrotic gene col1a, were upregulated in Adv-null and AdvSca1 cells placed in SR+VEGF compared with undifferentiated AdvSca1 cells kept in FBS+LIF (Figure 4B). The increase in αSMA, pdgfrβ, and col1a were also confirmed at the protein level (Figure 4C and 4D). Gene ontology analysis of the differentially regulated genes between Adv-null and undifferentiated AdvSca1 cells according to the microarray data confirmed an upregulation of biological processes linked to extracellular matrix organization and SMC function (Figure III in the online-only Data Supplement). Global gene expression analysis and quantitative RT-PCR showed that ETV2 overexpression resulted in the inhibition of SMC/mesenchymal gene expression in Adv-ETV2 cells, including acta2, cdh11, and col1a (Figure 4A and 4B). Immunostainings and Western blot analysis demonstrated that αSMA and col1a expressions were also lower at the protein level in Adv-ETV2 versus Adv-null cells (Figure 4C and 4D). Gene ontology analysis confirmed the downregulation of genes linked to extracellular matrix organization and control of the vascular diameter and blood pressure in Adv-ETV2 compared with Adv-null cells (Figure IV in the online-only Data Supplement). Surprisingly, quantitative RT-PCR revealed a decrease of cd34 mRNA expression in Adv-ETV2 cells (Figure 4B). Cd34 is a marker of EC and endothelial progenitors, and its inhibition could be a barrier for EC differentiation. We performed flow cytometry using an antibody directed against cd34 together with an antibody against specific lineage markers Pdgfrβ or Tie2. This allowed us to compare how AdvSca1, Adv-null, and Adv-ETV2 cells distributed into the mesenchymal and EC lineages and the expression of cd34 in the 2 lineages. Our results showed that when removed from their maintenance medium, Adv-null cells differentiated into a cd34+ Pdgfrβ+ mesenchymal cell type (from 7.9±5.4% SD to 29±7.5% SD) and that only few of them became cd34+ Tie2+ (1.4±0.4% SD) or Tie2+ (1.6±0.5% SD; Figure 4E; Figure VB in the online-only Data Supplement). These results are in accordance with gene expression analysis (Figures 1C and 4B). ETV2 promoted the differentiation of AdvSca1 cells toward the EC fate by downregulating the number of cd34+ Pdgfrβ+ cells (16.7±6.2% SD) and increasing by 3 folds the ratio of cd34+ Tie2+ versus cd34+ Pdgfrβ+ cells (Figure 4E; Figures VB and XI in the online-only Data Supplement).
ETV2 Prevents SMC/Mesenchymal Gene Expression Through the Downregulation of an IGFBP-5-TET Axis
To investigate the involvement of epigenetic events in ETV2-mediated reprogramming, we compared TET expression profiles in AdvSca1 cells cultured in FBS+LIF or SR+VEGF, Adv-null, and Adv-ETV2 cells using quantitative RT-PCR. As expected, all TETs were upregulated in AdvSca1+ cells differentiated in SR+VEGF and Adv-null compared with AdvSca1 progenitor cells. We also discovered that tet1 and tet3 mRNA levels were significantly lower in Adv-ETV2 cells compared with Adv-null, whereas Tet2 levels seemed unchanged after ETV2 overexpression (Figure 5A). Tet1 and Tet3 protein levels appeared also lower in the nuclear and perinuclear regions of Adv-ETV2 cells compared with Adv-null cells as shown by immunofluorescence staining (Figure 5B; Figure VIII in the online-only Data Supplement). We next performed small interfering RNA (siRNA)-mediated knockdown of each Tet isoform in Adv-SR cells. SiRNA knockdown of Tet1 and Tet2 led to concomitant downregulation of the other isoforms most likely because of redundancy in their sequences. Nevertheless, an efficient specific downregulation of Tet3 was achieved, with no reduction of Tet1 and Tet2 expression. This allowed us to identify the role of Tet3 in the regulation of SMC/mesenchymal genes expression because Tet3 siRNA transfection in Adv-SR led to a decrease of αSMA, Pdgfrβ, and col1a mRNA levels compared with Adv-SR cells transfected with siRNA control (Figure 5C and 5D). Altogether, our results imply that the downregulation of Tet3 expression after ETV2 overexpression is involved in the downregulation of SMC/mesenchymal genes observed in Adv-ETV2 cells. Nevertheless, further investigation will be needed to determine whether the other TETs, and in particular, Tet1, which is also downregulated in Adv-ETV2 cells, play a role in SMC/mesenchymal differentiation of AdvSca1 cells. The screening of our microarray data and quantitative RT-PCR also revealed a strong downregulation of Igfbp-5 (insulin-like growth factor-binding protein 5) expression in Adv-ETV2 cells (Figures 4A and 5E). IGFBP-5 protein is synthetized by vascular smooth muscles cells and can be secreted or translocated in the nucleus to act as a transcriptional activator.16 IGFBP-5 is strongly induced in Adv-SR cells, and its inhibition using specific siRNA in Adv-SR cells decreased SMC/mesenchymal genes expression (Figure 5F and 5G; Figure IXA in the online-only Data Supplement). IGFBP-5 KD Adv-SR cells also expressed lower levels of TET mRNA, especially tet1 and tet3, indicating that IGFBP-5 regulates TET expression (Figure 5H). Our results demonstrated that IGFBP-5 acts upstream of TET to regulate SMC/mesenchymal gene expression and that ETV2 prevents mesenchymal/SMC differentiation of AdvSca1 cells by downregulating the IGFBP-5-TET pathway and excessive DNA hydroxymethylation. Interestingly, AdvSca1 cells incubated for 5 days in CM prepared from an ex vivo-injured aorta expressed higher levels of nuclear IGFBP-5 compared with AdvSca1 cells in CM from uninjured aortas (Figure IXB in the online-only Data Supplement).
Figure 5.
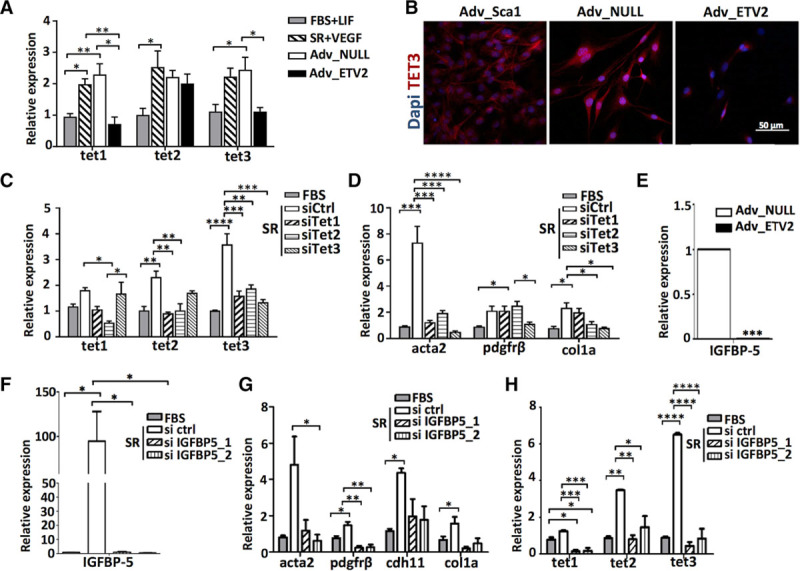
Identification of an IGFBP (insulin-like growth factor-binding protein)-5–TET (ten-eleven translocation) pathway involved in the differentiation of the adventitial Sca1+ (AdvSca1) cells into smooth muscle cells (SMCs)/mesenchymal cells. A, Quantitative real-time polymerase chain reaction (RT-PCR) of tet1, tet2, and tet3 mRNA expression in AdvSca1 cells cultured in fetal bovine serum (FBS)+LIF (leukemia inhibitory factor), serum replacement (SR)+VEGF, adventitial Sca1+ cells differentiated in SR and transduced with null virus (Adv-null), and adventitial Sca1+ cells differentiated in SR and transduced with ETV2 (ETS variant 2) virus (Adv-ETV2) cells (n=3 independent experiments; *P<0.05, **P<0.01). B, Immunofluorescence staining of AdvSca1, Adv-null, and Adv-ETV2 cells for TET3 (red). DAPI (4’,6-diamidino-2-phenylindol) stains nuclei (blue). Quantitative RT-PCR of (C) tet and (D) SMC genes acta2, pdgfrβ, and col1a expression in AdvSca1 cells after 3 d in FBS or SR with transfection of ctrl siRNA or siRNA against tet1, tet2, and tet3 (triplicate; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; data representative of 2 independent experiments). E, IGFBP-5 mRNA expression in Adv-null and Adv-ETV2 cells. Quantitative RT-PCR of (F) IGFBP-5, (G) SMC genes acta2, pdgfrβ, cdh11, and col1a, and (H) TET gene expression in AdvSca1 cells after 3 d in FBS or SR with transfection of ctrl siRNA or siRNA IGFBP-5 (triplicate; *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001; data representative of 2 independent experiments).
Adv-ETV2 Cells Improve Vascular Remodeling in a Model of Femoral Artery Wire Injury
To study the effect of AdvSca1, Adv-null, and Adv-ETV2 cells in a context of vascular injury, the cells were stably labeled with nuclear H2b-RFP to allow tracing and seeded on the adventitial side of wire-injured femoral arteries. One week after the injury, RFP cells could be seen in the adventitia for all 3 conditions. Arteries transplanted with undifferentiated AdvSca1 cells also displayed RFP cells in the neointima, which had already been observed in a previous study using the same experimental model with stably GFP-labeled AdvSca1 cells (Figure 6A).3 The 3 layers of the vascular wall of the arteries were measured 2 weeks post-injury using histological sections and compared with injured arteries where no cells were grafted. Similarly to what had been described in the work by Yu et al,3 the grafting of a high number of exogenous AdvSca1 cells led to an increase of the neointima lesion. Adv-null cells stayed localized in the adventitia, did not migrate to the neointima, and did not increase neointimal hyperplasia but induced a thickening of the vascular wall (Figure 6B). We hypothesized that the difference of localization of the cells in the vascular wall was because of differences in migratory properties. We performed in vitro transwell assays, which demonstrated that AdvSca1 cells differentiated into SMC/mesenchymal cells (Adv-SR) had reduced basal migration ability compared with undifferentiated AdvSca1 cells (Figure IIC and IID in the online-only Data Supplement). Our microarray results and gene ontology analysis also confirmed the upregulation of cell adhesion and downregulation of cell migration processes in Adv-null cells compared with AdvSca1 cells, which are not differentiated yet, possibly through changes in their extracellular matrix composition, such as higher collagen1a levels, and in the expression profile of their integrins and chemokines receptors (data not shown).
Figure 6.
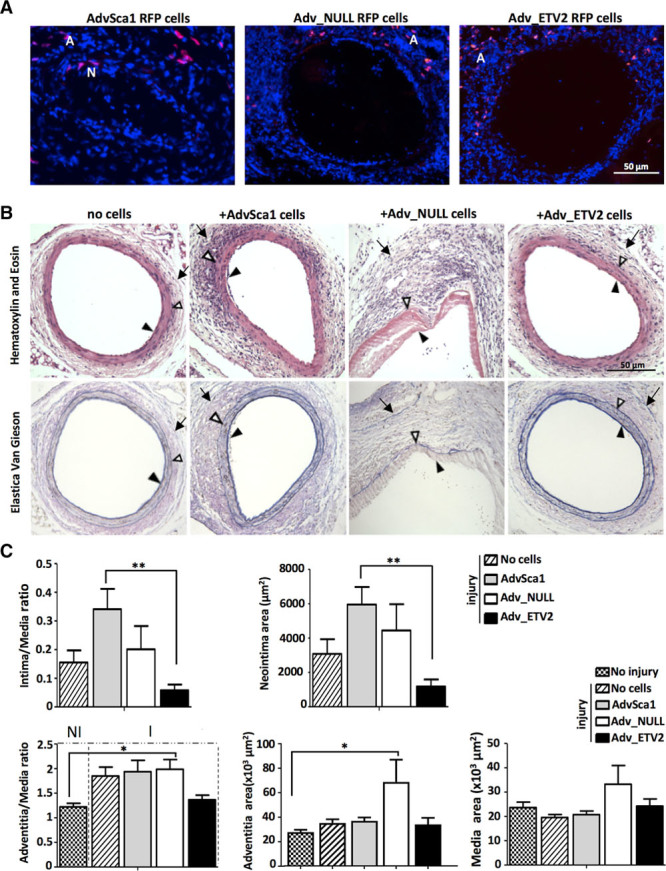
Adventitial Sca1+ cells differentiated in serum replacement (SR) and transduced with ETV2 (ETS variant 2) virus (Adv-ETV2) cells improve vascular remodeling in a model of arterial injury. A, LV H2b-RFP (red fluorescent protein) stably transduced adventitial Sca1+ (AdvSca1), adventitial Sca1+ cells differentiated in SR and transduced with null virus (Adv-null), and Adv-ETV2 cells were seeded on the adventitial side of a wire-injured femoral artery. One week after the injury, RFP cells could be seen in the adventitia of all groups. RFP cells could also be seen in the neointima of arteries where AdvSca1 cells were transplanted. (A, adventitia; N, Neointima). B, Representative image of Hematoxylin and Eosin (top) and elastin (bottom) staining performed on histological sections, 2 wk post-injury for each group: arteries with no cells, seeded with AdvSca1 cells, Adv-null, or Adv-ETV2 cells. Arrows indicate the external limit of the intima, media, and adventitia. C, Measurements of the adventitia, media, and neointima and calculation of the intima/media and adventitia/media ratios were made on 3 sections per injured artery (n=6 arteries for each group; *P<0.05, **P<0.01). Measurements of adventitia, media, and adventitia/media ratio for uninjured arteries are also indicated (3 sections were analyzed per artery; n=4 arteries).
Arteries transplanted with Adv-ETV2 showed decreased vascular wall remodeling and neointimal hyperplasia compared with all the other groups, including the group of arteries with no grafted cells as indicated by reduced adventitia/media and intima/media ratios (Figure 6B and 6C). One component of vascular hyperplasia is cell proliferation. After injury, cells in the media and at the external border of the media, negatives for Sca1 expression showed increased proliferation (Figure XA and XB in the online-only Data Supplement), and this effect was amplified with the grafting of Adv-null cells but not Adv-ETV2 as seen by PCNA (proliferating cell nuclear antigen) immunostaining followed by quantification (Figure 7A). After 2 weeks, few RFP cells could still be detected (data not shown), so we hypothesized that the effect of the progenitors on hyperplasia could be explained by paracrine effects on SMC proliferation. We performed coculture experiments where SMCs were plated in a transwell on top of Sca1+ adventitial cells. After Edu incorporation, SMC were harvested, fixed, and analyzed for cell cycle distribution. Our results showed that adding undifferentiated AdvSca1 cells to the culture could decrease SMC proliferation but that after SMC/mesenchymal differentiation, the cells (Adv-SR) had lost their antiproliferative effect (Figure XC in the online-only Data Supplement). Similarly, SMC cultured with Adv-null cells showed more proliferation than when cultured with undifferentiated AdvSca1 cells. Overexpressing ETV2 in the adventitial progenitors partially rescued the antiproliferative effect on SMC (Figure 7B). Immunostaining using cd31 antibody showed that 1 week post-injury, reendothelization was improved in arteries grafted with Adv-ETV2 (Figure 7C). We only detected occasionally RFP Adv-ETV2 cells in the endothelial layer of the arteries 1 week post-injury indicating that Adv-ETV2 indirectly contributed to the reendothelization process. Therefore, the positive effect of Adv-ETV2 cells on vascular remodeling could be explained by a combination of antiproliferative and proangiogenic properties.
Figure 7.
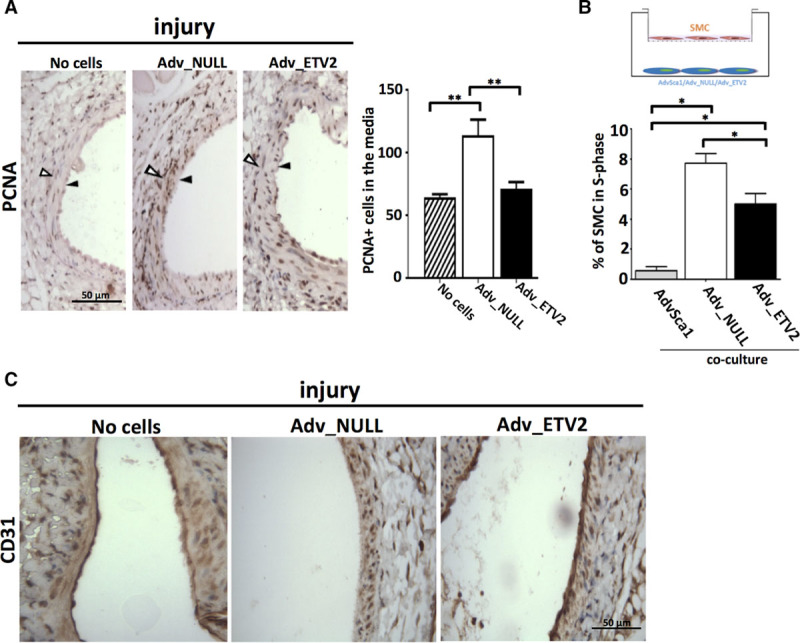
Adventitial Sca1+ cells differentiated in serum replacement (SR) and transduced with ETV2 (ETS variant 2) virus (Adv-ETV2) cells induce less smooth muscle cell (SMC) proliferation in vitro and in vivo and facilitate reendothelization of injured arteries. A, Representative picture of immunohistochemistry using PCNA (proliferating cell nuclear antigen) antibody performed on sections of 2 wk postinjury arteries where no cells, adventitial Sca1+ cells differentiated in SR and transduced with null virus (Adv-null), or Adv-ETV2 cells were seeded (n=4 arteries). Arrows indicate the external limit of the intima and media. PCNA+ cells of the media were quantified for each experimental group (**P<0.01; n=4). B, SMCs were cultured on transwells on top of undifferentiated adventitial Sca1+, Adv-null, or Adv-ETV2 cells for 3 d. SMCs were incubated with Edu for 2 h, harvested, fixed, and analyzed for cell cycle distribution using Fx cycle (*P<0.05, **P<0.01 ***P<0.001; n=3; data representative of 2 independent experiments). C, Immunostaining using cd31 antibody of paraffin section of 1-wk postinjury arteries where no cells, Adv-null, or Adv-ETV2 cells were seeded.
Discussion
In accordance to previous findings, we observed that AdvSca1 cells grafted on the adventitial side of a wire-injured femoral artery migrated within the vascular wall toward the luminal side of the vessel and increased neointimal hyperplasia.3 We also showed that the overexpression of ETV2 in AdvSca1 cells was sufficient to direct the cells toward the endothelial lineage while preventing them from acquiring an αSMA+ phenotype with pathological properties. This work presents the first evidence that the transdifferentiation of AdvSca1 cells into ECs, that is, controlling the cell fate of vascular wall resident progenitor cells by gene transfer, is a promising therapeutic approach against vascular diseases.
Twenty percent of the AdvSca1 cells used in this study expressed cd34 marker, 20% expressed Pdgfrβ, and <1% expressed Tie2. Majesky et al5 have recently described the heterogeneity within AdvSca1 cells. Using in vivo fate mapping, they showed that the AdvSca1 pool is composed of 2 separate populations. One population originated from differentiated SMC (SMC-derived AdvSca1 cells) and contributed to adventitial remodeling after injury. Several subpopulations were identified within SMC-derived AdvSca1 cells depending on the expression of markers, such as Pdgfrβ. The AdvSca1 cells used in our study share several similarities with SMC-derived AdvSca1 cells, including common markers and a predisposition to differentiate into SMC and mural cells rather than ECs. When AdvSca1 cells were placed in culture without LIF and with SR for 5 days to promote their differentiation, a strong increase in the number of Sca1+ cd34+ cells could be measured. Cd34 has been found to be expressed on the surface of mesenchymal,13,17 SMC,1 and EC progenitors12 and could be identifying cells that have left the multipotent progenitor state Sca1+ to a more lineage committed one Sca1+ cd34+. These observations are based on in vitro experiments. Therefore, after successfully generating a Sca1 knock-in inducible Cre mice, our next step is to confirm in vivo that Sca1+ cd34+ cells originate from Sca1+ cells. A recent study showed that adventitial Gli1+ cd34+ Pdgfrβ+ cells could migrate to the neointima of injured arteries and differentiate into SMC-like cells expressing αSMA and calponin.4 cd34+ Tie2+ VEGFR2+ VE-cadherin+ cd31− cells located in the vasculogenic zone between the adventitia and the media of human thoracic arteries, on the opposite, were identified as endothelial precursors able to form sprouts ex vivo.12 Our results implicate that ETV2 overexpression promotes in vitro the commitment of AdvSca1 cells toward an endothelial progenitor fate by inhibiting the differentiation into cd34+ Pdgfrβ+ cells while promoting the emergence of cd34+ Tie2+ cells (Figure XI in the online-only Data Supplement).
Both AdvSca1 (data not shown) and Adv-null cells increased cell proliferation within the vascular wall in vivo but in different layers because of different localization. Based on our in vitro coculture proliferation assays and ex vivo CM differentiation assays, we postulate that in the context of vascular injury, AdvSca1 cells differentiate toward a mesenchymal fate promoting SMC proliferation through paracrine effect, leading to enhanced vascular hyperplasia. PDGF-DD could mediate this effect because our microarray indicated it is expressed in Adv-null cells. It was shown that PDGF-DD is overexpressed in ApoE-deficient mice and can induce KLF4 (Kruppel-like factor 4) expression and phenotypic modulation in SMC.18 ETV2 overexpression decreased Pdgfd gene expression and reduced the proliferative effect on SMC.
ETV2 is a critical transcriptional activator of EC gene expression during vascular development.9 ChIP-seq analysis indicated that ETV2 directly binds to promoters and enhancers of endothelial genes, including Flk-1, Fli1, Tie2, VE-cadherin, Dll4, and Nrp2.19 Several studies also demonstrated that ETV2 is important for the specification of the mesoderm: in the absence of ETV2, endothelial and endocardial progenitors differentiate into cardiomyocytes.20 Mechanistically, one report showed that miR-130a—a direct target of ETV2—promoted the endothelial program at the expense of the cardiac program by blocking Pdgfrα expression and signaling.21 In a recent study, ETV2 overexpression alone was sufficient to convert human adult fibroblasts into EC. In accordance with our findings, a downregulation of fibrotic genes, such as col1a2, could be observed together with the increase of EC genes expression in the reprogrammed cells.11 The authors also suggested that ETV2 functions through the recruitment of cofactors, such as FoxC2 (Forkhead box C2), and of epigenetic modifiers but did not specifically investigate the mechanisms by which ETV2 downregulated the fibroblastic signature. In this report, we showed that ETV2 can repress SMC/mesenchymal gene expression by decreasing DNA hydroxymethylation in the AdvSca1 cells through the downregulation of an IGFBP-5-TET axis. DNA hydroxymethylation has been linked to SMC plasticity and vascular remodeling. A recent study has reported the loss of Tet2 expression and a reduction of 5hmc marks in SMC in response to injury and in coronary atherosclerotic plaques.14 Reduced Tet2 expression leads to reduced 5hmc, increased 5mc, reduction of SMC differentiation marker genes, and increased vascular remodeling.22 Our results showed that in AdvSca1 cells, the increase of tet3 expression and of 5hmc enrichment promotes SMC/mesenchymal gene expression and that ETV2 counteracts this effect by inhibiting IGFBP-5 expression. IGFBP-5 is known to be expressed and secreted by SMC and to modulate extracellular IGF1 (insulin-like growth factor 1) availability and effect on SMC proliferation and migration.23 It was also shown that IGFBP-5 can translocate to the nucleus of porcine SMC, contains a transactivation domain, and functions as a transcriptional activator independently of IGF signaling.16 We identified here a new role for IGFPP-5 in the transcriptional regulation of AdvSca1 gene expression. Interestingly, IGFBP-5 mRNA expression is increased in atherosclerotic pig aortas compared with normal arteries24 and is also overexpressed in lung fibrosis where it was shown to induce the transcription of collagen and fibronectin.25 Our results showed that AdvSca1 cells incubated in CM prepared from an ex vivo-injured aorta expressed higher levels of nuclear IGFBP-5 compared with AdvSca1 cells in CM from uninjured aortas. Further investigation will be needed to establish whether IGFBP-5 could serve as marker of atherosclerosis.
Our work could have potential future clinical implications. After vascular injury, excessive vascular wall remodeling, including activation of the adventitia and intimal hyperplasia, can lead to restenosis. In this report, we confirmed that AdvSca1 cells can enhance vascular remodeling, and we showed that their transdifferentiation into EC using single-gene delivery of ETV2 can prevent this pathological effect. The transplantation of Adv-ETV2 cells on wire-injured femoral arteries even improved the remodeling compared with control arteries where no cells were grafted, by preventing SMC proliferation and promoting reendothelization.
Previous studies have shown that an increase in adventitial angiogenesis could have negative consequences on plaque growth and stability.26 For instance, the adenoviral delivery of proangiogenic factors, such as VEGF-A and VEGF-D, led to a significant increase in the intima/media ratio and a significant positive correlation was observed between the size of the adventitial vasa vasorum network and intimal hyperplasia,27 whereas antiangiogenic molecules, such as endostatin, have been shown to reduce intimal neovascularization and plaque growth in ApoE null mice.28 Consequently, the short- and long-term effects of a gene therapy involving cell fate modification of vascular progenitor cell to promote their EC commitment and prevent their SMC/mesenchymal differentiation still need to be investigated to evaluate the positive and negative implications this strategy could have on the progression of vascular diseases.
We established a protocol based on the adenovirus-mediated single-gene delivery of ETV2. As revealed by the variations in the gene changes between duplicate samples submitted for microarray analysis, the efficiency of ETV2 transduction varies, and differences in the level of remaining cells nontranduced in the Adv-ETV2 groups might create individual EC and SMC/mesenchymal gene variations (Figures 2D, 2E, and 4A). A positive selection of the transduced cells would allow the generation of a more homogenous EC-like population. The focus of our study was, however, not to generate a pure endothelial population but to prove that the single-gene delivery of ETV2 was sufficient to promote the differentiation of AdvSca1 cells toward the endothelial fate, which could have translational applications for in vivo reprogramming and gene therapy.
We think our results are encouraging for the development of future cell and gene therapies approaches targeting AdvSca1 cells to improve vascular remodeling after angioplasty or stenting. The localization of AdvSca1 cells on the outer layer of the vessel also make them interesting candidates for gene manipulation strategies or in vivo reprogramming. Our findings may open new therapeutic perspectives for other vascular diseases, including hypertension, or for pathologies linked to fibrosis because it was shown that Sca1+ matrix-producing cells contribute to aortic fibrosis.29
Acknowledgments
We would like to thank Dr Matteo Beretta for his help with the animal work. This work has been funded by the British Heart Foundation and the Oak Foundation.
Sources of Funding
This work has been funded by the British Heart Foundation (RG/14/6/31144) and the Oak Foundation.
Disclosures
None.
Supplementary Material
Nonstandard Abbreviations and Acronyms
- 5hmC
- 5-hydroxymethylcytosine
- 5mC
- 5-methylcytosine
- Adv-ETV2
- adventitial Sca1+ cells differentiated in SR and transduced with ETV2 virus
- Adv-null
- adventitial Sca1+ cells differentiated in SR and transduced with null virus
- AdvSca1
- adventitial Sca1+ progenitor cell
- Adv-SR
- adventitial Sca1+ cells differentiated in SR
- ApoE
- apolipoprotein E
- CM
- conditioned medium
- EC
- endothelial cell
- ETV2
- ETS variant 2
- IGF1
- insulin-like growth factor 1
- IGFBP
- insulin-like growth factor-binding protein
- LIF
- leukemia inhibitory factor
- RT-PCR
- real-time polymerase chain reaction
- Sca1
- stem cell antigen-1
- αSMA, acta2
- α-smooth muscle actin
- SMC
- smooth muscle cell
- SR
- serum replacement
- TET
- ten-eleven translocation
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.117.309853/-/DC1.
Highlights.
Adventitial Sca1+ (stem cell antigen-1) progenitor cells are specified toward smooth muscle cell/mesenchymal fate.
The transduction of endothelial-specific transcription factor ETV2 (ETS variant 2) can direct the differentiation of adventitial Sca1+ cells toward the endothelial lineage.
ETV2 decreases the expression of smooth muscle cell/mesenchymal gene expression by downregulating IGFBP (insulin-like growth factor-binding protein)-5 and TET (ten-eleven translocation) expression.
Sca1+ cells transduced with ETV2 improve vascular remodeling when seeded on wire-injured femoral arteries.
References
- 1.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA. 2008;105:93, 49–9354. doi: 10.1073/pnas.0711382105. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu B, Wong MM, Potter CM, Simpson RM, Karamariti E, Zhang Z, Zeng L, Warren D, Hu Y, Wang W, Xu Q. Vascular stem/progenitor cell migration induced by smooth muscle cell-derived chemokine (C-C Motif) ligand 2 and chemokine (C-X-C motif) ligand 1 contributes to neointima formation. Stem Cells. 2016;34:2368–2380. doi: 10.1002/stem.2410. doi: 10.1002/stem.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, Gratwohl S, Madhurima K, Hutcheson JD, Jain S, Aikawa E, Humphreys BD. Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell. 2016;19:628–642. doi: 10.1016/j.stem.2016.08.001. doi: 10.1016/j.stem.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Majesky MW, Horita H, Ostriker A, Lu S, Regan JN, Bagchi A, Dong XR, Poczobutt J, Nemenoff RA, Weiser-Evans MC. Differentiated smooth muscle cells generate a subpopulation of resident vascular progenitor cells in the adventitia regulated by Klf4. Circ Res. 2017;120:296–311. doi: 10.1161/CIRCRESAHA.116.309322. doi: 10.1161/CIRCRESAHA.116.309322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Wong MM, Campagnolo P, Simpson R, Winkler B, Margariti A, Hu Y, Xu Q. Adventitial stem cells in vein grafts display multilineage potential that contributes to neointimal formation. Arterioscler Thromb Vasc Biol. 2013;33:1844–1851. doi: 10.1161/ATVBAHA.113.300902. doi: 10.1161/ATVBAHA.113.300902. [DOI] [PubMed] [Google Scholar]
- 7.Campagnolo P, Hong X, di Bernardini E, Smyrnias I, Hu Y, Xu Q. Resveratrol-induced vascular progenitor differentiation towards endothelial lineage via MiR-21/Akt/β-catenin is protective in vessel graft models. PLoS One. 2015;10:e0125122. doi: 10.1371/journal.pone.0125122. doi: 10.1371/journal.pone.0125122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tsai TN, Kirton JP, Campagnolo P, Zhang L, Xiao Q, Zhang Z, Wang W, Hu Y, Xu Q. Contribution of stem cells to neointimal formation of decellularized vessel grafts in a novel mouse model. Am J Pathol. 2012;181:362–373. doi: 10.1016/j.ajpath.2012.03.021. doi: 10.1016/j.ajpath.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Oh SY, Kim JY, Park C. The ETS factor, ETV2: a master regulator for vascular endothelial cell development. Mol Cells. 2015;38:1029–1036. doi: 10.14348/molcells.2015.0331. doi: 10.14348/molcells.2015.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han JK, Chang SH, Cho HJ, Choi SB, Ahn HS, Lee J, Jeong H, Youn SW, Lee HJ, Kwon YW, Cho HJ, Oh BH, Oettgen P, Park YB, Kim HS. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation. 2014;130:1168–1178. doi: 10.1161/CIRCULATIONAHA.113.007727. doi: 10.1161/CIRCULATIONAHA.113.007727. [DOI] [PubMed] [Google Scholar]
- 11.Morita R, Suzuki M, Kasahara H, Shimizu N, Shichita T, Sekiya T, Kimura A, Sasaki K, Yasukawa H, Yoshimura A. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc Natl Acad Sci USA. 2015;112:160–165. doi: 10.1073/pnas.1413234112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Kilic N, Ergün S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 13.Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Kränkel N, Katare R, Angelini G, Emanueli C, Madeddu P. Human adult vena saphena contains perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu R, Jin Y, Tang WH, Qin L, Zhang X, Tellides G, Hwa J, Yu J, Martin KA. Ten-eleven translocation-2 (TET2) is a master regulator of smooth muscle cell plasticity. Circulation. 2013;128:2047–2057. doi: 10.1161/CIRCULATIONAHA.113.002887. doi: 10.1161/CIRCULATIONAHA.113.002887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li C, Zhen G, Chai Y, Xie L, Crane JL, Farber E, Farber CR, Luo X, Gao P, Cao X, Wan M. RhoA determines lineage fate of mesenchymal stem cells by modulating CTGF-VEGF complex in extracellular matrix. Nat Commun. 2016;7:11455. doi: 10.1038/ncomms11455. doi: 10.1038/ncomms11455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Q, Li S, Zhao Y, Maures TJ, Yin P, Duan C. Evidence that IGF binding protein-5 functions as a ligand-independent transcriptional regulator in vascular smooth muscle cells. Circ Res. 2004;94:E46–E54. doi: 10.1161/01.RES.0000124761.62846.DF. doi: 10.1161/01.RES.0000124761.62846.DF. [DOI] [PubMed] [Google Scholar]
- 17.Corselli M, Chen CW, Sun B, Yap S, Rubin JP, Péault B. The tunica adventitia of human arteries and veins as a source of mesenchymal stem cells. Stem Cells Dev. 2012;21:1299–1308. doi: 10.1089/scd.2011.0200. doi: 10.1089/scd.2011.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas JA, Deaton RA, Hastings NE, Shang Y, Moehle CW, Eriksson U, Topouzis S, Wamhoff BR, Blackman BR, Owens GK. PDGF-DD, a novel mediator of smooth muscle cell phenotypic modulation, is upregulated in endothelial cells exposed to atherosclerosis-prone flow patterns. Am J Physiol Heart Circ Physiol. 2009;296:H442–H452. doi: 10.1152/ajpheart.00165.2008. doi: 10.1152/ajpheart.00165.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Li D, Yu YY, Kang I, Cha MJ, Kim JY, Park C, Watson DK, Wang T, Choi K. Induction of hematopoietic and endothelial cell program orchestrated by ETS transcription factor ER71/ETV2. EMBO Rep. 2015;16:654–669. doi: 10.15252/embr.201439939. doi: 10.15252/embr.201439939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palencia-Desai S, Kohli V, Kang J, Chi NC, Black BL, Sumanas S. Vascular endothelial and endocardial progenitors differentiate as cardiomyocytes in the absence of Etsrp/Etv2 function. Development. 2011;138:4721–4732. doi: 10.1242/dev.064998. doi: 10.1242/dev.064998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh BN, Kawakami Y, Akiyama R, Rasmussen TL, Garry MG, Gong W, Das S, Shi X, Koyano-Nakagawa N, Garry DJ. The Etv2-miR-130a network regulates mesodermal specification. Cell Rep. 2015;13:915–923. doi: 10.1016/j.celrep.2015.09.060. doi: 10.1016/j.celrep.2015.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhuang J, Luan P, Li H, Wang K, Zhang P, Xu Y, Peng W. The yin-yang dynamics of DNA methylation is the key regulator for smooth muscle cell phenotype switch and vascular remodeling. Arterioscler Thromb Vasc Biol. 2017;37:84–97. doi: 10.1161/ATVBAHA.116.307923. doi: 10.1161/ATVBAHA.116.307923. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh T, Gordon RE, Clemmons DR, Busby WH, Jr, Duan C. Regulation of vascular smooth muscle cell responses to insulin-like growth factor (IGF)-I by local IGF-binding proteins. J Biol Chem. 2003;278:42886–42892. doi: 10.1074/jbc.M303835200. doi: 10.1074/jbc.M303835200. [DOI] [PubMed] [Google Scholar]
- 24.Zheng B, Duan C, Clemmons DR. The effect of extracellular matrix proteins on porcine smooth muscle cell insulin-like growth factor (IGF) binding protein-5 synthesis and responsiveness to IGF-I. J Biol Chem. 1998;273:8994–9000. doi: 10.1074/jbc.273.15.8994. [DOI] [PubMed] [Google Scholar]
- 25.Yasuoka H, Hsu E, Ruiz XD, Steinman RA, Choi AM, Feghali-Bostwick CA. The fibrotic phenotype induced by IGFBP-5 is regulated by MAPK activation and egr-1-dependent and -independent mechanisms. Am J Pathol. 2009;175:605–615. doi: 10.2353/ajpath.2009.080991. doi: 10.2353/ajpath.2009.080991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camaré C, Pucelle M, Nègre-Salvayre A, Salvayre R. Angiogenesis in the atherosclerotic plaque. Redox Biol. 2017;12:18–34. doi: 10.1016/j.redox.2017.01.007. doi: 10.1016/j.redox.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhardwaj S, Roy H, Heikura T, Ylä-Herttuala S. VEGF-A, VEGF-D and VEGF-D(DeltaNDeltaC) induced intimal hyperplasia in carotid arteries. Eur J Clin Invest. 2005;35:669–676. doi: 10.1111/j.1365-2362.2005.01555.x. doi: 10.1111/j.1365-2362.2005.01555.x. [DOI] [PubMed] [Google Scholar]
- 28.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Montaniel KR, Saleh MA, Xiao L, Chen W, Owens GK, Humphrey JD, Majesky MW, Paik DT, Hatzopoulos AK, Madhur MS, Harrison DG. Origin of matrix-producing cells that contribute to aortic fibrosis in hypertension. Hypertension. 2016;67:461–468. doi: 10.1161/HYPERTENSIONAHA.115.06123. doi: 10.1161/HYPERTENSIONAHA.115.06123. [DOI] [PMC free article] [PubMed] [Google Scholar]


