ABSTRACT
Serum lactate levels are traditionally interpreted as a marker of tissue hypoxia and often used clinically as an indicator of severity and outcome of sepsis/septic shock. Interestingly, recent studies involving the effects of tumor-derived lactate suggest that lactate itself may have an immunosuppressive effect in its local environment. This finding adds to the recent advances in immunometabolism that shed light on the importance of metabolism and metabolic intermediates in the regulation of innate immune and inflammatory responses in sepsis. In this article, we summarize recent studies, showing that the activation of immune cells requires aerobic glycolytic metabolism and that lactate produced by aerobic glycolysis may play an immunosuppressive role in sepsis.
Keywords: Aerobic glycolysis, immunosuppression, lactate, sepsis/septic shock
INTRODUCTION
Sepsis is a clinical syndrome characterized by systemic inflammatory response to infection (1–3). In the early stage of sepsis, activated innate immune cells initiate a significant increase in both innate immune and inflammatory responses to clear invading pathogens from the host. If the initial response is not properly controlled, it will result in exaggerated innate immune and inflammatory responses that could damage organs (2, 4) and increase septic mortality (1). Early clinical efforts are focused on controlling the inflammatory responsive phase of sepsis. Unfortunately, over the last 20 years unanimously poor results have been obtained from clinical trials using anti-inflammatory targets (5). Recent data show that the mortality of sepsis has been significantly reduced due to improvements in the treatment protocols (6). Furthermore, those who survive from the acute hyperinflammatory responsive phase remain at an increased risk for secondary and/or nosocomial infection, and consequential late-stage mortality (7, 8). However, the mechanisms are incompletely understood. Hotchkiss, Payen, and Pickkers as well as others recently hypothesized that an immunosuppressive phase may exist in sepsis/septic patients (3, 9–15) and may alternatively be a better candidate for therapy (16, 17). Although this hypothesis seems controversial (18, 19), there are number of factors that contribute to immunosuppression, including the apoptosis of innate immune cells and most T-cell populations (9), a decrease in the number of lymphoid progenitors (20), a reduction in bone marrow cell production (21), and a resultant state of immune tolerance/paralysis in which many immune cells are reprogrammed via epigenetic alterations to an unresponsive phenotype (22). In addition, metabolic reprogramming of immune cells may also contribute to the development of immune dysfunction during sepsis (23). Importantly, increasing evidence shows that extracellular lactate may have an important regulatory effect on a variety of immune cells (24). In addition, excellent review articles have been published showing that aerobic glycolytic metabolism is necessary for the activation of immune cells (23, 25–28). In this article, we briefly discuss lactate's potential inhibitory role in the systemic immune response to sepsis.
SERUM LACTATE LEVELS IN SEPSIS
The measurement of serum lactate levels is often incorporated in the clinical management of critical illness, particularly in cases of severe sepsis and septic shock (29). In this context, serum lactate is typically used to evaluate disease severity, treatment response, and prognosis (30). The recent sepsis-3 guidelines recommend that persistence of a serum lactate more than 2 mmol/L, despite adequate fluid resuscitation, should be included as a new criterion when clinically defining septic shock (31). This recommendation is based on the recognition that lactate levels correlate strongly and positively with disease severity, morbidity, and mortality in the context of sepsis (32, 33). Published literature has shown that high concentrations of serum lactate could be a predictor of mortality, whereas reduced lactate levels have been reported to be associated with improved clinical outcomes (15, 29, 34–38). Vincent et al. (39) recently published an excellent review article regarding the value of blood lactate kinetics in critically ill patients. The authors systematically searched the published literatures, collected data from 96 studies, and concluded that a better outcome was associated with decreasing blood lactate concentrations. This was not limited to septic patients, suggesting that the value of lactate kinetics seems to be valid regardless of the initial value (39). The authors preferred using the term lactate kinetics that reflect the greater lactate production than clearance (39). Indeed, recent studies highlight the important role of immune cells in the production of lactate through aerobic glycolytic metabolism (25, 26, 40, 41).
INCREASED LACTATE PRODUCTION BY ACTIVATED IMMUNE CELLS THROUGH AEROBIC GLYCOLYSIS
Over half a century ago, Dr. Otto Warburg observed that various cancer cells metabolize glucose directly to lactate despite the presence of abundant oxygen in the environment (42). Initially termed the Warburg effect, this phenomenon is now more commonly referred to as aerobic glycolysis. Recently, the transient utilization of aerobic glycolysis has been observed in many activated immune cells (43, 44). Although it is significantly less favorable energetically, there are several advantages to this type of metabolism with regard to immune function. First, an adequate immune response requires rapid energy production, and aerobic glycolysis provides the essential ATP immediately (45). Second, aerobic glycolysis and parallel increases in the pentose phosphate pathway provide important precursors for the synthesis of lipids, amino acids, and nucleotides that are required for rapid cellular growth and proliferation (25). In addition to supporting the basic energy requirements of the dividing cell, altered metabolism is now known to play an important and direct role in regulating changes in immune cell phenotype. For example, recent studies have shown that metabolic enzymes and their products can stimulate the release of alarmins from cells (46), act as bacterial component receptors (47), promote epigenetic modification of histones for trained immunity (48), regulate microRNA expression (49), and participate in various other immunoregulatory processes. Collectively, these findings suggest that the transient adoption of aerobic glycolysis in the immune response may also play an active role in regulating cell phenotype (50).
Indeed, the switch to aerobic glycolysis seems to play an important role in the inflammatory response by the innate immune system. Toll-like receptors (TLRs) are critical in the induction of innate immune and inflammatory responses. TLRs could bind with their ligands, including bacterial components and endogenous ligands, and initiate a cellular signaling cascade that ultimately results in transcriptional regulatory changes within the cell (51, 52). For example, lipopolysaccharides (LPS) binds with TLR4 on dendritic cells (DCs) and macrophages, leading to a metabolic transition from oxidative phosphorylation to aerobic glycolysis and resulting in a proinflammatory phenotype (53, 54, 40). Suzuki et al. recently outlined the differential reliance of proinflammatory (M1) macrophages on aerobic glycolysis. This is in contrast to alternative (M2) phenotypes that rely more heavily on oxidative phosphorylation (55). In addition to these observations, in vitro inhibition of glycolysis seems to reprogram innate immune cells to a more anti-inflammatory state, further highlighting the importance of metabolism in cell phenotype (56, 57).
In addition, adaptive immunity also plays an important role in mediating the pathogen-specific and delayed response in sepsis. The metabolic regulation of adaptive immune cells has been reported in subsets of both B and T lymphocytes that adopt a Warburg-like metabolism upon activation (58–60). In lymphocytes, metabolic regulation seems to be different between T-effector (Teff) cells and T-regulatory (Treg) cells. Michalek et al. recently reported that Teff cells exhibit greater expression of the glucose transporter 1 (GLUT1) and elevated levels of glycolysis, while Treg cells primarily rely on fatty acid oxidation (61). This finding may highlight a characteristic metabolic difference between predominantly proinflammatory cells. For example, Teff and M1 macrophage subtypes rely primarily on glycolysis, whereas Treg and M2 macrophage subtypes use fatty acid oxidation. Ultimately, these variations may represent an important feature by which immune cells can respond differently to metabolites present in their surroundings.
DECREASED PRODUCTION OF LACTATE IMPROVES SURVIVAL OUTCOME OF SEPTIC MICE
Critical illness usually causes a metabolic shift from mitochondrial oxidative phosphorylation to aerobic glycolysis. This transition is associated with lactate production, multiple organ dysfunction, and poor outcomes. As previously mentioned, utilization of aerobic glycolytic metabolism by activated immune cells could contribute to increase lactate production. Nalos et al. used transcriptomic analysis to examine the cellular metabolism of circulating blood cells from nonhypoxic critically ill patients and observed a significant reprogramming of metabolic pathways during critical illness. These authors concluded that aerobic glycolysis does exist in nonhypoxic cells during critical illness (62). The increased lactate production may also indicate a metabolic shift to an inflammatory glycolysis. Palsson-McDermott et al. have shown that stimulation of macrophages with LPS significantly increased the expression of pyruvate kinase M2 (PKM2), a critical modulator of IL-1β production, macrophage polarization, glycolytic reprogramming, and Warburg metabolism (63). Furthermore, activation of PKM2 attenuated LPS-induced proinflammatory M1 macrophage phenotype and promoted traits typical of an M2 macrophage (63). Xie et al. (64) from the same group reported that PKM2-mediated glycolysis promotes inflammasome activation by modulating EIF1AK2 phosphorylation in macrophages. In accordance with these findings, pharmacological inhibition of the PKM2-EIF2AK2 pathway has been shown to protect mice from lethal endotoxemia and polymicrobial sepsis (64). Inhibition of aerobic glycolysis by either 2-deoxy-d-glycose (2-DG) or PKM2 inhibitor also markedly improves survival outcome in polymicrobial sepsis, and reduces serum lactate levels and HMGB1 release (46). Wang et al. reported a similar observation that inhibition of aerobic glycolysis by 2-DG significantly improved survival outcome in bacterial sepsis (65) and reduced LPS-induced inflammation in vivo(66). Recently, Zheng et al. (67) reported that sepsis-increased glycolysis also contributes to cardiomyopathy and mortality. Inhibition of glycolysis by 2-DG markedly improves cardiac function and survival outcome by improving mitochondrial function and inflammatory responses (67). Collectively, the current published literature indicates that sepsis and endotoxin could increase aerobic metabolism and produce more lactate that may ultimately alter the function of immune cells. Understanding the mechanisms by which metabolic switching regulates the processes of immune response could be a novel research topic in sepsis.
LACTATE MODULATES THE IMMUNE RESPONSE
Previous studies have shown that high levels of lactate could downregulate the rate-limiting glycolytic enzymes hexokinase and phosphofructokinase in a variety of tissues (68) and immune cells (69). Therefore, given the importance of aerobic glycolysis in activated immune cells, the downregulation of these rate-limiting glycolytic enzymes may have important implications on cellular function. Indeed, a growing body of evidence suggests that tumor-derived lactate has clinically relevant immunosuppressive effects on a variety of cell types in the surrounding microenvironment (70). Interestingly, in cancer research, the immunologic changes of immune cells are very similar to those observed in the immunosuppressive phase of sepsis. It would be interesting to investigate the effect of increased lactate on immune cell function during sepsis.
LACTATE AND INNATE IMMUNE CELL FUNCTION
Recent and ongoing studies have explored the potential immunomodulatory effects of lactate on innate immune cells, primarily macrophages and DCs. These cells serve a fundamental role as antigen-presenting cells, and act as gatekeepers for the activation of lymphocyte B and T cells in the adaptive response. In the context of sepsis, impaired function of innate immunity not only limits the response to primary infection, but also damages important barriers to secondary nosocomial infection (17). A prominent feature of protracted sepsis is the inappropriate development of immunologic tolerance toward pathogens (9, 71). For example, DCs transition toward a progressively tolerogenic phenotype and promote immunosuppressive regulatory T-cell differentiation (72). The mechanisms underlying this transition may involve a significant metabolic dysfunction within these cells (23, 73). Indeed, recent studies have reported that the addition of exogenous lactate to growth medium containing DCs induced metabolic reprogramming and ultimately triggered innate immune cells to adapt a more tolerogenic phenotype (73, 74). These authors proposed that the unfavorable concentration gradient of lactate may prevent its diffusion-mediated export from immunogenic DCs that rely on aerobic glycolysis. It has also been reported that lactate in peritoneal dialysis solutions may inhibit LPS-induced maturation of DCs (75).
Recently, the effects of lactate on the functioning and differentiation of macrophages have been reported (56, 76, 77). In the late stage of sepsis, macrophages are often observed as having a predominantly immunosuppressive M2 phenotype configuration that may have a critical role in the pathogenesis of immune system dysfunction (56, 77). Interestingly, the M2 phenotype has also been observed in the local environments of tumor cells, where it may contribute to immune system evasion (76). Colegio et al. (76) reported that lactate may serve as the primary mediator responsible for promoting the M2 inhibitory polarization of macrophages. In subsequent in vitro experiments involving bone marrow-derived macrophages, these authors reported that lactate was consistently capable of inducing an M2-like macrophage polarization by an HIF-1α-dependent mechanism. In addition, lactate treatment also increased production of M2-associated genes (VEGF and Arg1) and markers (Fizz1, Mgl1, and Mgl2) in a dosage-dependent manner (76). Selleri et al. (78) have similarly reported that lactate induces a preferential differentiation of monocytes into M2 macrophages in a dose-dependent fashion by metabolic reprogramming. Furthermore, it has been reported that lactate decreases TNF-α secretion by human monocytes (79), potentially by reducing NF-κB activation and delaying LPS-induced signal transduction (80).
To explore the mechanisms by which lactate can induce the macrophage transition to an anti-inflammatory phenotype, Hoque et al. (81) recently proposed a novel cellular signaling pathway. This pathway involves the GPR81 receptor that recognizes lactate and has the ability to induce the transition of macrophages to the M2 phenotype. As presented in Figure 1, these authors showed that macrophages treated with LPS in the presence of lactate exhibited a significant reduction in proinflammatory cytokine production such as Pro-IL1 β, Pro-IL18, Casp1, and Nlrp3, whereas production of anti-inflammatory cytokines like IL-10 was not affected. The mechanisms by which lactate significantly affects LPS-induced production of proinflammatory cytokines involve the GPR81-dependent antagonism of the TLR4/TLR9-mediated signaling pathway, and consequently attenuation of LPS-induced NF-κB activation. It has been reported that GPR81 has an impressively high affinity for lactate with an estimated EC50 of ∼4.3 mM/L (82), suggesting that relatively low concentrations of lactate may have an effect on TLR-mediated NF-κB activation pathway (81).
Fig. 1.
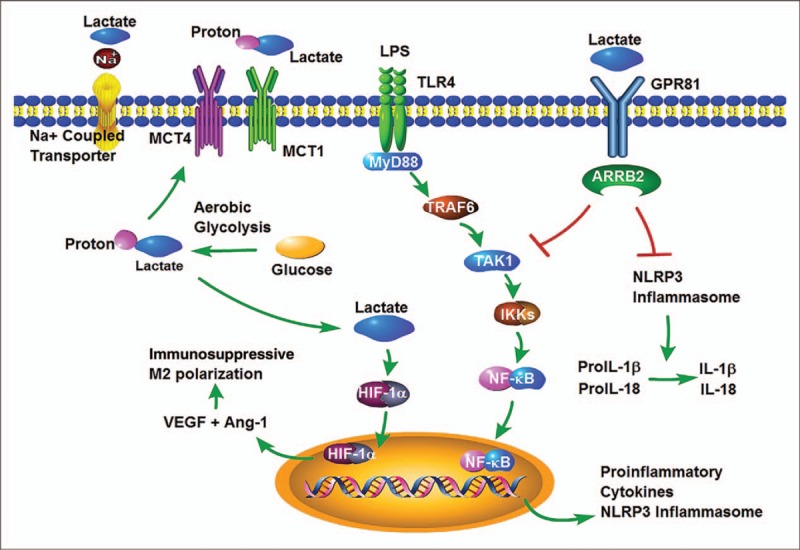
Possible mechanisms by which lactate can induce the macrophage transition to an anti-inflammatory phenotype.
Lactate has also been reported to influence the bone marrow stem cell maturation process. Husain et al. have recently shown that the addition of lactate to the growth medium before induction of bone marrow stem cell differentiation results in a significantly increased production of myeloid-derived suppressor cells (MDSCs) when compared with the control (83). MDSCs are a heterogenous group of cells that have predominantly immunosuppressive effects and play an important role in cancer development and chronic infectious diseases (84). In addition, increased MDSCs in sepsis were recently implicated in the pathogenesis of protracted immune dysfunction (20, 85). However, to date few studies have neither examined the metabolic properties of these cells nor fully described the mechanisms surrounding their origin.
LACTATE AND ADAPTIVE IMMUNE CELL FUNCTION
In addition to influencing the function of the innate immune cells, lactate has also been reported to have effects on T-cell functioning (86). Haas et al. (69) recently reported that lactate accumulation in the synovia of rheumatoid arthritis patients may play a role in the localization of T cells to the site of inflammation. In vitro studies by this group demonstrated that sodium lactate inhibited CD4+ cell motility, whereas an acidic lactate was required to inhibit the motility of CD8+ T cells. In CD4+ T cells, the effect of lactate (at physiologic pH) seemed to be dependent on the interruption of glycolysis and required expression of the Na+/lactate cotransporter, Slc5a12 (69). In contrast, the inhibitory effect of lactate on CD8+ T cells required expression of the Slc16a1 proton-coupled lactate transporter in addition to an acidic microenvironment (69). These effects were dose-dependent and estimated the EC50 of lactate to be ∼10 mM/L (69). However, this concentration is not significantly greater than what is observed in severe sepsis. Finally, the authors reported that buffered sodium lactate induced CD4+ T helper cells toward a TH17 subset, whereas unbuffered lactic acid inhibited the cytolytic function of CD8+ T cells (69). At present, there is no study to investigate the effects of lactate on Treg cells. It is possible that the proposed Treg cells are less dependent on aerobic glycolysis and primarily use oxidative phosphorylation for their energy production (61). Thus, theoretically they may not be susceptible to this type of metabolic regulation.
CONCLUSION
We have highlighted some of the current research surrounding the potential role of lactate as an immunosuppressive metabolite (see Fig. 2). Although there are clearly a number of contributing factors to the development of immune suppression in sepsis, the recent developments in other fields of research suggest that lactate could be a potential and critical contributory factor in the regulation of immune function in sepsis.
Fig. 2.
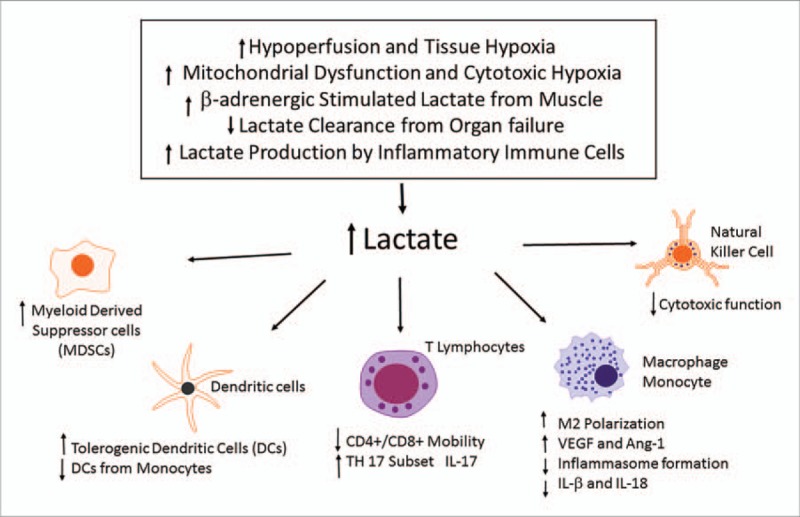
Potential role of increased lactate in the regulation of immune cell function.
Footnotes
REFERENCES
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369:840–851. [DOI] [PubMed] [Google Scholar]
- 2.Mira JC, Gentile LF, Mathias BJ, Efron PA, Brakenridge SC, Mohr AM, Moore FA, Moldawer LL. Sepsis pathophysiology, chronic critical illness, and persistent inflammation-immunosuppression and catabolism syndrome. Crit Care Med 2017; 45:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delano MJ, Ward PA. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol Rev 2016; 274:330–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kakihana Y, Ito T, Nakahara M, Yamaguchi K, Yasuda T. Sepsis-induced myocardial dysfunction: pathophysiology and management. J Intensive Care 2016; 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus DC. The search for effective therapy for sepsis: back to the drawing board? JAMA 2011; 306:2614–2615. [DOI] [PubMed] [Google Scholar]
- 6.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med 2003; 348:138–150. [DOI] [PubMed] [Google Scholar]
- 7.Boomer JS, To K, Chang KC, Takasu O, Osborne DF, Walton AH, Bricker TL, Jarman SD, Kreisel D, Krupnick AS, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 2011; 306:2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med 2009; 15:496–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol 2013; 13:862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamers L, Kox M, Pickkers P. Sepsis-induced immunoparalysis: mechanisms, markers, and treatment options. Minerva Anestesiol 2015; 81:426–439. [PubMed] [Google Scholar]
- 11.Sundar KM, Sires M. Sepsis induced immunosuppression: implications for secondary infections and complications. Indian J Crit Care Med 2013; 17:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leentjens J, Kox M, van der Hoeven JG, Netea MG, Pickkers P. Immunotherapy for the adjunctive treatment of sepsis: from immunosuppression to immunostimulation. Time for a paradigm change? Am J Respir Crit Care Med 2013; 187:1287–1293. [DOI] [PubMed] [Google Scholar]
- 13.Patil NK, Bohannon JK, Sherwood ER. Immunotherapy: a promising approach to reverse sepsis-induced immunosuppression. Pharmacol Res 2016; 111:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delano MJ, Ward PA. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J Clin Invest 2016; 126:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas SA, Lange T, Saugel B, Petzoldt M, Fuhrmann V, Metschke M, Kluge S. Severe hyperlactatemia, lactate clearance and mortality in unselected critically ill patients. Intensive Care Med 2016; 42:202–210. [DOI] [PubMed] [Google Scholar]
- 16.Hotchkiss RS, Sherwood ER. Immunology. Getting sepsis therapy right. Science 2015; 347:1201–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Monneret G, Payen D. Immunosuppression in sepsis: a novel understanding of the disorder and a new therapeutic approach. Lancet Infect Dis 2013; 13:260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Vught LA, Klein Klouwenberg PM, Spitoni C, Scicluna BP, Wiewel MA, Horn J, Schultz MJ, Nurnberg P, Bonten MJ, Cremer OL, et al. Incidence, risk factors, and attributable mortality of secondary infections in the intensive care unit after admission for sepsis. JAMA 2016; 315:1469–1479. [DOI] [PubMed] [Google Scholar]
- 19.Sweeney DA, Kalil AC. Secondary infection in patients with sepsis. JAMA 2016; 316:771–772. [DOI] [PubMed] [Google Scholar]
- 20.Mathias B, Delmas AL, Ozrazgat-Baslanti T, Vanzant EL, Szpila BE, Mohr AM, Moore FA, Brakenridge SC, Brumback BA, Moldawer LL, et al. Human myeloid-derived suppressor cells are associated with chronic immune suppression after severe sepsis/septic shock. Ann Surg 2017; 265:827–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terashima A, Okamoto K, Nakashima T, Akira S, Ikuta K, Takayanagi H. Sepsis-induced osteoblast ablation causes immunodeficiency. Immunity 2016; 44:1434–1443. [DOI] [PubMed] [Google Scholar]
- 22.McCall CE, Yoza B, Liu T, El GM. Gene-specific epigenetic regulation in serious infections with systemic inflammation. J Innate Immun 2010; 2:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng SC, Scicluna BP, Arts RJ, Gresnigt MS, Lachmandas E, Giamarellos-Bourboulis EJ, Kox M, Manjeri GR, Wagenaars JA, Cremer OL, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol 2016; 17:406–413. [DOI] [PubMed] [Google Scholar]
- 24.Hirschhaeuser F, Sattler UG, Mueller-Klieser W. Lactate: a metabolic key player in cancer. Cancer Res 2011; 71:6921–6925. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly RP, Finlay DK. Glucose, glycolysis and lymphocyte responses. Mol Immunol 2015; 68:513–519. [DOI] [PubMed] [Google Scholar]
- 26.Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol 2014; 32:609–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corcoran SE, O’Neill LA. HIF1alpha and metabolic reprogramming in inflammation. J Clin Invest 2016; 126:3699–3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mills EL, O’Neill LA. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur J Immunol 2016; 46:13–21. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen HB, Rivers EP, Knoblich BP, Jacobsen G, Muzzin A, Ressler JA, Tomlanovich MC. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med 2004; 32:1637–1642. [DOI] [PubMed] [Google Scholar]
- 30.Suetrong B, Walley KR. Lactic acidosis in sepsis: it's not all anaerobic—implications for diagnosis and management. Chest 2016; 149:252–261. [DOI] [PubMed] [Google Scholar]
- 31.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rishu AH, Khan R, Al-Dorzi HM, Tamim HM, Al-Qahtani S, Al-Ghamdi G, Arabi YM. Even mild hyperlactatemia is associated with increased mortality in critically ill patients. Crit Care 2013; 17:R197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichol AD, Egi M, Pettila V, Bellomo R, French C, Hart G, Davies A, Stachowski E, Reade MC, Bailey M, et al. Relative hyperlactatemia and hospital mortality in critically ill patients: a retrospective multi-centre study. Crit Care 2010; 14:R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puskarich MA, Trzeciak S, Shapiro NI, Albers AB, Heffner AC, Kline JA, Jones AE. Whole blood lactate kinetics in patients undergoing quantitative resuscitation for severe sepsis and septic shock. Chest 2013; 143:1548–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen HB, Kuan WS, Batech M, Shrikhande P, Mahadevan M, Li CH, Ray S, Dengel A. Outcome effectiveness of the severe sepsis resuscitation bundle with addition of lactate clearance as a bundle item: a multi-national evaluation. Crit Care 2011; 15:R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen HB, Loomba M, Yang JJ, Jacobsen G, Shah K, Otero RM, Suarez A, Parekh H, Jaehne A, Rivers EP. Early lactate clearance is associated with biomarkers of inflammation, coagulation, apoptosis, organ dysfunction and mortality in severe sepsis and septic shock. J Inflamm (Lond) 2010; 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chertoff J, Chisum M, Garcia B, Lascano J. Lactate kinetics in sepsis and septic shock: a review of the literature and rationale for further research. J Intensive Care 2015; 3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bolvardi E, Malmir J, Reihani H, Hashemian AM, Bahramian M, Khademhosseini P, Ahmadi K. The role of lactate clearance as a predictor of organ dysfunction and mortality in patients with severe sepsis. Mater Sociomed 2016; 28:57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vincent JL, Quintairos E Silva A, Couto L, Jr, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care 2016; 20:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, et al. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood 2010; 115:4742–4749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srivastava A, Mannam P. Warburg revisited: lessons for innate immunity and sepsis. Front Physiol 2015; 6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warburg O. On the origin of cancer cells. Science 1956; 123:309–314. [DOI] [PubMed] [Google Scholar]
- 43.Doughty CA, Bleiman BF, Wagner DJ, Dufort FJ, Mataraza JM, Roberts MF, Chiles TC. Antigen receptor-mediated changes in glucose metabolism in B lymphocytes: role of phosphatidylinositol 3-kinase signaling in the glycolytic control of growth. Blood 2006; 107:4458–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, et al. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med 2005; 201:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bauer DE, Harris MH, Plas DR, Lum JJ, Hammerman PS, Rathmell JC, Riley JL, Thompson CB. Cytokine stimulation of aerobic glycolysis in hematopoietic cells exceeds proliferative demand. FASEB J 2004; 18:1303–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang L, Xie M, Yang M, Yu Y, Zhu S, Hou W, Kang R, Lotze MT, Billiar TR, Wang H, et al. PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat Commun 2014; 5:4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML, Cho HC, Popescu NI, Coggeshall KM, Arditi M, et al. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell 2016; 166:624–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O’Neill LA, Xavier RJ. Trained immunity: a program of innate immune memory in health and disease. Science 2016; 352:aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mukhopadhyay R, Jia J, Arif A, Ray PS, Fox PL. The GAIT system: a gatekeeper of inflammatory gene expression. Trends Biochem Sci 2009; 34:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loftus RM, Finlay DK. Immunometabolism: cellular metabolism turns immune regulator. J Biol Chem 2016; 291:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol 2004; 5:987–995. [DOI] [PubMed] [Google Scholar]
- 52.Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol 2001; 1:135–145. [DOI] [PubMed] [Google Scholar]
- 53.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 2011; 11:738–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodriguez-Prados JC, Traves PG, Cuenca J, Rico D, Aragones J, Martin-Sanz P, Cascante M, Bosca L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J Immunol 2010; 185:605–614. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki H, Hisamatsu T, Chiba S, Mori K, Kitazume MT, Shimamura K, Nakamoto N, Matsuoka K, Ebinuma H, Naganuma M, et al. Glycolytic pathway affects differentiation of human monocytes to regulatory macrophages. Immunol Lett 2016; 176:18–27. [DOI] [PubMed] [Google Scholar]
- 56.Arts RJ, Gresnigt MS, Joosten LA, Netea MG. Cellular metabolism of myeloid cells in sepsis. J Leukoc Biol 2017; 101:151–164. [DOI] [PubMed] [Google Scholar]
- 57.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013; 496:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Caro-Maldonado A, Wang R, Nichols AG, Kuraoka M, Milasta S, Sun LD, Gavin AL, Abel ED, Kelsoe G, Green DR, et al. Metabolic reprogramming is required for antibody production that is suppressed in anergic but exaggerated in chronically BAFF-exposed B cells. J Immunol 2014; 192:3626–3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang CH, Curtis JD, Maggi LB, Jr, Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013; 153:1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fox CJ, Hammerman PS, Thompson CB. Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 2005; 5:844–852. [DOI] [PubMed] [Google Scholar]
- 61.Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 2011; 186:3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nalos M, Parnell G, Robergs R, Booth D, McLean AS, Tang BM. Transcriptional reprogramming of metabolic pathways in critically ill patients. Intensive Care Med Exp 2016; 4:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palsson-McDermott EM, Curtis AM, Goel G, Lauterbach MA, Sheedy FJ, Gleeson LE, van den Bosch MW, Quinn SR, Domingo-Fernandez R, Johnston DG, et al. Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the Warburg effect in LPS-activated macrophages. Cell Metab 2015; 21:65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie M, Yu Y, Kang R, Zhu S, Yang L, Zeng L, Sun X, Yang M, Billiar TR, Wang H, et al. PKM2-dependent glycolysis promotes NLRP3 and AIM2 inflammasome activation. Nat Commun 2016; 7:13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang A, Huen SC, Luan HH, Yu S, Zhang C, Gallezot JD, Booth CJ, Medzhitov R. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell 2016; 166:1512–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu L, Lu Y, Martinez J, Bi Y, Lian G, Wang T, Milasta S, Wang J, Yang M, Liu G, et al. Proinflammatory signal suppresses proliferation and shifts macrophage metabolism from Myc-dependent to HIF1alpha-dependent. Proc Natl Acad Sci USA 2016; 113:1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zheng Z, Ma H, Zhang X, Tu F, Wang X, Ha T, Fan M, Liu L, Xu J, Yu K, et al. Enhanced glycolytic metabolism contributes to cardiac dysfunction in polymicrobial sepsis. J Infect Dis 2017; 215:1396–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leite TC, Coelho RG, Da SD, Coelho WS, Marinho-Carvalho MM, Sola-Penna M. Lactate downregulates the glycolytic enzymes hexokinase and phosphofructokinase in diverse tissues from mice. FEBS Lett 2011; 585:92–98. [DOI] [PubMed] [Google Scholar]
- 69.Haas R, Smith J, Rocher-Ros V, Nadkarni S, Montero-Melendez T, D’Acquisto F, Bland EJ, Bombardieri M, Pitzalis C, Perretti M, et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLoS Biol 2015; 13:e1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Choi SY, Collins CC, Gout PW, Wang Y. Cancer-generated lactic acid: a regulatory, immunosuppressive metabolite? J Pathol 2013; 230:350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pena OM, Hancock DG, Lyle NH, Linder A, Russell JA, Xia J, Fjell CD, Boyd JH, Hancock RE. An endotoxin tolerance signature predicts sepsis and organ dysfunction at initial clinical presentation. EBioMedicine 2014; 1:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan X, Liu Z, Jin H, Yan J, Liang HP. Alterations of dendritic cells in sepsis: featured role in immunoparalysis. Biomed Res Int 2015; 2015:903720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sim WJ, Ahl PJ, Connolly JE. Metabolism is central to tolerogenic dendritic cell function. Mediators Inflamm 2016; 2016:2636701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dong H, Bullock TN. Metabolic influences that regulate dendritic cell function in tumors. Front Immunol 2014; 5:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Puig-Kroger A, Pello OM, Selgas R, Criado G, Bajo MA, Sanchez-Tomero JA, Alvarez V, del PG, Sanchez-Mateos P, Holmes C, et al. Peritoneal dialysis solutions inhibit the differentiation and maturation of human monocyte-derived dendritic cells: effect of lactate and glucose-degradation products. J Leukoc Biol 2003; 73:482–492. [DOI] [PubMed] [Google Scholar]
- 76.Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 2014; 513:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pena OM, Pistolic J, Raj D, Fjell CD, Hancock RE. Endotoxin tolerance represents a distinctive state of alternative polarization (M2) in human mononuclear cells. J Immunol 2011; 186:7243–7254. [DOI] [PubMed] [Google Scholar]
- 78.Selleri S, Bifsha P, Civini S, Pacelli C, Dieng MM, Lemieux W, Jin P, Bazin R, Patey N, Marincola FM, et al. Human mesenchymal stromal cell-secreted lactate induces M2-macrophage differentiation by metabolic reprogramming. Oncotarget 2016; 7:30193–30210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dietl K, Renner K, Dettmer K, Timischl B, Eberhart K, Dorn C, Hellerbrand C, Kastenberger M, Kunz-Schughart LA, Oefner PJ, et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J Immunol 2010; 184:1200–1209. [DOI] [PubMed] [Google Scholar]
- 80.Peter K, Rehli M, Singer K, Renner-Sattler K, Kreutz M. Lactic acid delays the inflammatory response of human monocytes. Biochem Biophys Res Commun 2015; 457:412–418. [DOI] [PubMed] [Google Scholar]
- 81.Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology 2014; 146:1763–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cai TQ, Ren N, Jin L, Cheng K, Kash S, Chen R, Wright SD, Taggart AK, Waters MG. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem Biophys Res Commun 2008; 377:987–991. [DOI] [PubMed] [Google Scholar]
- 83.Husain Z, Huang Y, Seth P, Sukhatme VP. Tumor-derived lactate modifies antitumor immune response: effect on myeloid-derived suppressor cells and NK cells. J Immunol 2013; 191:1486–1495. [DOI] [PubMed] [Google Scholar]
- 84.Greten TF, Manns MP, Korangy F. Myeloid derived suppressor cells in human diseases. Int Immunopharmacol 2011; 11:802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Janols H, Bergenfelz C, Allaoui R, Larsson AM, Ryden L, Bjornsson S, Janciauskiene S, Wullt M, Bredberg A, Leandersson K. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J Leukoc Biol 2014; 96:685–693. [DOI] [PubMed] [Google Scholar]
- 86.Fischer K, Hoffmann P, Voelkl S, Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G, Hoves S, et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 2007; 109:3812–3819. [DOI] [PubMed] [Google Scholar]


