Abstract
Objectives:
Data on cardiovascular disease risks among HIV-infected patients taking antiretroviral therapy (ART) over long periods of time are lacking in Sub-Saharan Africa.
Methods:
A cross-sectional study was conducted in Chiradzulu, Malawi from December 2015 to June 2016. HIV-infected persons on ART for more than 10 years (patients) and HIV-negative individuals (controls) from selected clinics participated. Following informed consent, a standardized questionnaire, clinical and laboratory examinations were performed. The prevalence of cardiovascular disease risk factors was calculated and stratified by age group.
Results:
Overall, 379 HIV-infected patients and 356 controls participated. Median time on ART among patients was 11.6 years (interquartile range 10.6–12.4).Within the 30–44, 45–59, and at least 60-year age groups, respectively, the prevalence of hypertension was 10.8, 20.4, and 44.7% among patients and 6.1, 25.8, and 42.9% among controls. Hypertension was previously undiagnosed in 60.3% patients and 37.0% controls with elevated blood pressure. The prevalence of diabetes within the respective age groups was 5.0, 6.4, and 13.2% among patients, and 3.4, 4.2, and 1.7% among controls. HIV-infected patients were more likely to have an glycated hemoglobin at least 6.0% (adjusted odds ratio 1.9; 95% confidence interval 1.1–3.2, P = 0.02). Prevalence of low-density lipoprotein cholesterol more than 130 mg/dl within the respective age groups was 8.0, 15.4, and 23.7% among patients and 1.8, 12.5, and 11.8% among controls.
Conclusion:
Noncommunicable diseases were a significant burden in Malawi, with high prevalence of hypercholesterolemia in all survey participants and an especially acute diabetes burden among older HIV infected. Hypertension screening and treatment services are needed among identified high-risk groups to cover unmet needs.
Keywords: cardiovascular risk factors, diabetes, HIV, hypertension, long-term antiretroviral therapy
Introduction
Antiretroviral therapy (ART) has transformed HIV infection from a death sentence to a long-term condition and, as patients live longer, noncommunicable diseases (NCDs) are becoming increasingly important illnesses among these patients. Individual care models in Europe predict that 84.0% of HIV infected individuals receiving ART will have at least one NCD comorbidity by the year 2030 [1] with comorbidities most significant among older patients [2]. Studies in high-income countries and recently published data from Sub-Saharan Africa confirm a high prevalence of noninfectious comorbidities among the HIV infected [3,4].
HIV infection itself, independent of ART, has been linked to cardiovascular disease (CVD), as well as several organ system dysfunctions [5]. Yet, research also suggests that ART can be a risk factor for NCDs like CVD [6,7] and diabetes [8,9], with HIV-infected individuals on ART twice as likely to have CVD as their HIV-negative counterparts [10]. Even in patients on treatment who achieve adequate viral suppression and high CD4+ cell counts, there is increasing evidence that low-level chronic inflammation may still play a part in morbidity and mortality over the long term [11–13].
In Malawi, previous study has established that 36% of CVD-related deaths and 45% of cardiac disease and hypertension-related deaths were among HIV-positive individuals [14]. The prevalence of risk factors for CVD and CVD-related mortality are also high in Malawi, with 33 and 14% of participants in a nationwide survey reporting hypertension and cigarette smoking, respectively [15]. And though CVD risk factors have previously been described among adult HIV-positive people on ART in some Sub-Saharan contexts [16–18], data are still lacking in of the continent among aging HIV patients who have been taking ART for long periods of time. This is further complicated by growing concerns that the multimorbidity associated with living with HIV disease will impact healthcare systems in the resource-limited countries that have yet to develop and implement a chronic care model. We measured the prevalence of elevated cholesterol, diabetes, hypertension, smoking, and elevated BMI among HIV-positive patients receiving ART years for more than 10 years.
Methodology
The cross-sectional survey of HIV-infected patients on ART for more than 10 years occurred in two Médecins Sans Frontières (MSF)-supported facilities between November 2015 and June 2016 in Chiradzulu District, in southern Malawi (population 291 000) which has an HIV prevalence of 17% [19,20]. MSF has been working in Chiradzulu district since 1997, started providing ART in 2001, and has been supporting decentralized care since 2006 [21]. Two facilities (Bilal health center and Chiradzulu district hospital) with large proportions of long-term ART patients were selected as study sites. HIV-positive individuals who were at least 30 years old and on ART for more than 10 years in Chiradzulu district (patients), and HIV-negative individuals at least 30 years living in Bilal and Chiradzulu hospital catchment areas (controls) were considered eligible.
The MSF follow-up and care of HIV infection and AIDS database (Epicentre, Paris, France) was used to generate a daily list of eligible patients who were then invited to participate in the study. Controls were matched to the patient cohort by sex. Community awareness about the study objectives and scope was conducted in meetings with key community leaders and groups. These sessions were also used to invite controls to participate in the research, encouraging them to go to the study clinics for screening and possible inclusion.
Data collection and variables
Demographic data, medical history, and physical examination findings were recorded on the day of inclusion into the study using a standard case report form. Demographic data collected included sex, age, level of education, occupation, and marital status. Physical examination findings included SBP and DBP, weight, and height. BMI was define as underweight (BMI < 18.5 kg/m2), normal (BMI 18.5 to 24.9), overweight (BMI 25.0 to 29.9), and obese (BMI ≥30). High blood pressure (BP) was defined as a SBP of at least 140 mmHg, a DBP of at least 90 mmHg, or both [22,23]. The Korotkoff technique was used to measure BP over the brachial artery using an aneroid sphygmomanometer with an adult cuff [23]. The patient sat in upright position in a quiet room. Two consecutive BP measurements were taken and the average recorded as the final result. The BP was measured on the day of enrolment into study.
Blood was collected on the day of inclusion to be tested for the following measures: glycated hemoglobin (HbA1c), lipid profile, total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), and triglycerides. Analysis was performed on the BS120 Mindray (Nanshan, People's Republic of China) at Queen Elizabeth Central Hospital Laboratory in Blantyre, Malawi. CD4+ cell counts were measured using Partec CyFlow Counter (Cyflow SL, Partec, Munster, Germany), whereas semiquantitative viral-load measurements were performed using the simple amplification-based assay semiquantitative test for HIV-1, SAMBA (Diagnostics for the Real World, Sunnyvale, California, USA). Viral load and CD4+ tests were performed at the Chiradzulu District Hospital laboratory. To confirm HIV-negative status, controls received HIV testing following the Ministry of Health Malawi guidelines [24]. A serial algorithm with Determine Rapid HIV-1/2 antibody (Abbott Laboratories, Abbott Park, Illinois, USA) followed by Unigold Rapid HIV Test (Trinity Biotech, PLC, IDA Business Park, Bray, County Wicklow, Ireland) was used.
Cutoff points considered as being risk factors for CVD were TC at least 200 mg/dl, HDL less than 40 mg/dl (men) and less than 50 mg/dl (women), LDL of at least 130 mg/dl, and triglycerides at least 150 mg/dl [25,26]. For diabetes, HbA1c of 6.0–6.4% was considered prediabetes and levels at least 6.5% were indicative of diabetes [27]. Diabetes status was also established using a self-reported history or use of antidiabetic medication.
The Framingham 10-year risk of CVD, the Framingham risk score (FRS) was calculated using the formula from the Framingham Heart Study [28,29]. Variables used in the FRS calculation were sex, age, SBP, current treatment of hypertension, current smoking status, HDL and TC, and diabetes.
Analysis
Study data were double entered and managed using Research Electronic Data Capture (REDCap, Vanderbilt University). Statistical analysis was performed in Stata 13 (College Station, Texas, USA). The demographic and cardiovascular risk factors were described separately for patients and controls and were stratified by age. The prevalence of hypocholesteremia, diabetes, hypertension, and other CVD risk factors were reported with 95% confidence intervals (CI). Means with their SDs, or medians with corresponding interquartile ranges (IQR) were calculated for continuous variables, and frequencies and proportions were established for categorical data. Univariate and multivariate logistic regressions models were used to assess factors associated with elevated LDL cholesterol (cutoff at 130 mg/dl), and elevated HbA1c (cutoff at 6.0%). Multivariate analyses were adjusted by HIV status (patient or control), age group, sex, education, and BMI.
Ethics
Ethical approval was granted in Malawi by the National Health Sciences Research Committee (ref 1359). Each participant provided written informed consent to participate in the study. The study was conducted in accordance with the Helsinki Declaration on ethical principles for medical research involving human study participants.
Results
Characteristics of participants
A total of 379 HIV-positive (patients) and 356 HIV- negative (controls) were included in the study (Fig. 1).
Fig. 1.
Inclusion flow chart.
Women made up 73.0% of patients (n = 281) and 73.3% of controls (n = 261). HIV patients were younger than controls [median age 47 years (IQR 42–52) vs. 52 years (IQR 40–63)]; Table 1), had higher educational attainment (30.8 vs. 13.0%, P < 0.001), more skilled work (29.6 vs. 14.7%, P < 0.001), and were more often divorced (18.8 vs. 10.4%, P < 0.001) or widowed (26.5 vs. 18.9%, P < 0.001).
Table 1.
Baseline characteristics of patients and controls stratified by age.
| Patients | Controls | |||||||
| 30–44 (n = 139) % (95% CI) | 45–59 (n = 202) % (95% CI) | ≥60 (n = 38) % (95% CI) | Total | 30–44 (n = 116) % (95% CI) | 45–59 (n = 121) % (95% CI) | ≥60 (n = 119) % (95% CI) | Total | |
| Sex | ||||||||
| Female | 86.3 (79.5–91.1) | 67.3 (60.6–73.5) | 55.3 (39.4–70.1) | 73.1 (68.4–77.3) | 72.4 (63.6–79.8) | 80.2 (72.1–86.4) | 67.2 (58.3–75.1) | 73.3 (68.5–77.7) |
| Male | 13.7 (8.9–20.5) | 32.7 (26.6–39.5) | 44.7 (29.9–60.6) | 26.9 (22.7–31.6) | 27.6 (20.2–36.4) | 19.8 (13.7–27.9) | 32.8 (24.9–41.7) | 26.7 (22.3–31.6) |
| Current residence | ||||||||
| In Chiradzulu district | 81.3 (73.9–87.0) | 68.8 (62.1–74.8) | 68.4 (52.2–81.1) | 73.3 (68.7–77.6) | 99.1 (94.0–99.9) | 97.5 (92.5–99.2) | 100 | 98.9 (97.0–99.6) |
| Outside Chiradzulu district | 18.7 (13.1–26.1) | 31.2 (25.2–37.9) | 31.6 (18.9–47.8) | 26.7 (22.4–31.6) | 0.9 (0.1–6.0) | 2.5 (0.8–7.5) | 0 | 1.1 (0.4–3.0) |
| Education | ||||||||
| Primary education or less | 71.7 (63.6–78.6) | 66.2 (59.3–72.4) | 76.3 (60.4–87.2) | 69.2 (64.4–73.7) | 74.8 (66.0–81.9) | 91.7 (85.3–95.5) | 94.1 (88.2–97.2) | 87.0 (83.1–90.2) |
| Secondary education or more | 28.3 (21.4–36.4) | 33.8 (27.6–40.7) | 23.7 (12.8–39.6) | 30.8 (26.3–35.6) | 25.2 (18.1–34.0) | 8.3 (4.5–14.7) | 5.9 (2.8–11.8) | 13.0 (9.8–16.9) |
| Occupation | ||||||||
| Unskilled work | 74.8 (66.9–81.4) | 63.9 (57.0–70.2) | 89.5 (75.1–96.0) | 70.5 (65.6–74.9) | 82.8 (74.8–88.6) | 81.0 (73.0–87.0) | 92.4 (86.0–96.0) | 85.4 (81.3–88.7) |
| Skilled work | 25.2 (18.7–33.1) | 36.1 (29.8–43.0) | 10.5 (4.0–24.9) | 29.6 (25.2–34.4) | 17.2 (11.4–25.2) | 19.0 (13.0–27.0) | 7.6 (4.0–14.0) | 14.7 (11.3–18.7) |
| Marital status | ||||||||
| Never married | 1.4 (0.4–5.6) | 0.5 (0.1–3.5) | 0 | 0.8 (0.3–2.4) | 2.6 (0.8–7.8) | 0 | 0 | 0.8 (0.3–2.6) |
| Married | 56.1 (47.7–64.2) | 54.7 (47.7–61.5) | 42.1 (27.6–58.1) | 54.0 (48.9–59.0) | 78.5 (70.0–85.0) | 76.0 (67.6–82.8) | 55.1 (46.0–63.8) | 69.9 (64.9–74.4) |
| Divorced | 23.7 (17.4–31.6) | 16.2 (12.3–22.8) | 10.5 (4.0–25.0) | 18.8 (15.1–23.1) | 13.8 (8.6–21.4) | 9.1 (5.1–15.7) | 8.5 (4.6–15.1) | 10.4 (7.6–14.1) |
| Widowed | 18.7 (13.0–26.1) | 27.9 (22.1–34.5) | 47.4 (32.1–63.1) | 26.5 (22.2–31.2) | 5.2 (2.3–11.1) | 14.9 (9.6–22.4) | 36.4 (28.2–45.5) | 18.9 (15.1–23.3) |
| Toilet | ||||||||
| Nonimproved facility | 64.8 (56.4–72.3) | 48.5 (41.7–55.4) | 57.9 (41.9–72.4) | 55.4 (50.3–60.4) | 84.4 (76.7–90.0) | 80.2 (72.1–86.4) | 83.2 (75.4–88.9) | 82.6 (78.3–86.2) |
| Improved facility | 35.3 (27.8–43.6) | 51.5 (44.6–58.3) | 42.1 (27.6–58.1) | 44.6 (39.6–49.7) | 15.5 (10.0–23.3) | 19.8 (13.7–27.9) | 16.8 (11.1–24.6) | 17.4 (13.8–21.7) |
CI, confidence interval.
The median time on ART for patients was 11.6 years (IQR 10.6–12.4). Initial ART regimens were all composed of two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitors (NNRTIs); mainly stavudine (88.7%), lamivudine (100%), and nevirapine (97.9%). At the time of the study, 90.5% (n = 343) of the patients were on first line, 9.2% (n = 35) on second line, and 0.3% (n = 1) on third line. Fixed dose combination based on tenofovir, lamivudine (3TC), and efavirenz was the most common first line (86.6%). Among patients, 92.7% (95% CI 90.6–94.4) had a viral load less than 1000 copies/ml and 61.3% (95% CI 56.2–66.1) had a CD4+ at least 500 cells/μl.
Prevalence of cardiovascular disease risk factors
Prevalence of CVD risk factors are presented in the Table 2. Smoking was low among patients (2.4%; 95% CI 1.2–4.5) and controls (5.1%; 95% CI 3.2–7.9), though men were more likely to smoke than women among both HIV patients (5.9 vs. 1.1%; P < 0.01) and controls (13.7 vs. 1.9%, P < 0.01). The proportion of HIV patients considered overweight or obese (BMI above or equal to 25 kg/m2) was 17.3% (95% CI 11.8–24.5), 20.8% (95% CI 15.7- 27.0), and 10.5% (95% CI 4.0–24.9) among the 30–44, 45–59, and at least 60 years age groups, respectively. Among the controls it was 21.7% (95% CI 15.1–30.2), 30.0% (95% CI 22.5–38.8), and 16.0% (95% CI 10.4–23.7). Women had significantly higher proportions with a BMI at least 25 kg/m2; among patients (22.0 vs. 8.8%, P = 0.01), and controls (27.4 vs. 9.5%, P < 0.01), as well as higher rates of abnormal waist–hip ratio among patients (52.4 vs. 12.8%, P < 0.001) and controls (44.1 vs. 3.2%, P < 0.001).
Table 2.
Prevalence of cardiovascular risk factors among patients and controls stratified by age.
| Patients | Controls | |||||||
| Risk factors | 30–44 (n = 139) % (95% CI) | 45–59 (n = 202) % (95% CI) | ≥60 (n = 38) % (95% CI) | Total | 30–44 (n = 116) % (95% CI) | 45–59 (n = 121) % (95% CI) | ≥60 (n = 119) % (95% CI) | Total |
| BMI (kg/m2): | ||||||||
| Overweight (≥25 < 30) | 15.1 (10.1–22.1) | 12.4 (8.5–17.7) | 5.3 (1.3–18.8) | 12.7 (9.7–16.4) | 14.8 (9.4–22.5) | 18.3 (12.4–26.3) | 12.6 (7.7–19.9) | 15.3 (11.9–19.4) |
| Obese (≥30) | 2.2 (0.7–6.5) | 8.4 (5.3–13.1) | 5.3 (1.3–18.8) | 5.8 (3.8–8.7) | 7.0 (3.5–13.3) | 11.7 (7.0–18.8) | 3.3 (1.3–8.6) | 7.3 (5.0–10.6) |
| Waist–hip ratio | ||||||||
| >0.95 (male) and >0.85 (female) | 41.0 (33.1–49.4) | 42.1 (35.5–49.0) | 42.1 (27.6–58.1) | 41.7 (36.8–46.7) | 30.2 (22.5–39.1) | 38.8 (30.6–47.8) | 30.3 (22.7–39.1) | 33.2 (28.4–38.2) |
| SBP (mmHg): | ||||||||
| 140–159 | 1.4 (0.4–5.6) | 9.5 (6.1–14.4) | 31.6 (18.9–47.8) | 8.7 (6.3–12.0) | 3.5 (1.3–9.0) | 17.5 (11.7–25.4) | 24.4 (17.5–32.9) | 15.3 (11.9–19.5) |
| 160–179 | 1.4 (0.4–5.6) | 4.5 (2.3–8.4) | 2.6 (0.4–16.5) | 3.2 (1.8–5.5) | 0 | 5.8 (2.8–11.8) | 9.2 (5.2–15.9) | 5.1 (3.2–8.0) |
| ≥180 | 0 | 1.0 (0.2–3.9) | 5.3 (1.3–18.8) | 1.1 (0.4–2.8) | 0 | 0 | 7.5 (4.0–13.9) | 2.5 (1.3–4.8) |
| SBP ≥140 mmHg | 2.9 (1.1–7.4) | 14.9 (10.6–20.6) | 39.5 (25.4–55.6) | 13.0 (9.9–16.8) | 3.5 (1.3–9.0) | 23.3 (16.6–31.7) | 41.2 (32.7–50.2) | 23.0 (18.9–27.6) |
| DBP (mmHg) | ||||||||
| 90–99 | 8.6 (5.0–14.6) | 11.9 (8.1–17.2) | 15.8 (7.3–31.0) | 11.1 (8.3–14.7) | 6.1 (3.0–12.3) | 13.3 (8.3–20.7) | 19.3 (13.2–27.4) | 13.0 (9.9–17.0) |
| 100–109 | 1.4 (0.4–5.6) | 4.5 (2.3–8.4) | 10.5 (4.0–24.9) | 4.0 (2.4–6.5) | 0 | 4.2 (1.7–9.6) | 5.9 (2.8–11.8) | 3.4 (1.9–5.9) |
| ≥110 | 0 | 1.5 (0.5–4.5) | 2.6 (0.4–16.5) | 1.1 (0.4–2.8) | 0 | 0 | 5.9 (2.8–11.8) | 2.0 (0.9–4.1) |
| DBP ≥90 mmHg | 10.1 (6.0–16.3) | 17.9 (13.2–23.9) | 29.0 (16.8–45.1) | 16.1 (12.8–20.2) | 6.1 (3.0–12.3) | 17.5 (11.7–25.4) | 31.1 (23.4–40.0) | 18.4 (14.7–22.8) |
| Hypertension (BP ≥140/90 mmHg) | 10.8 (6.6–17.2) | 20.4 (15.4–26.6) | 44.7 (29.9–60.6) | 19.3 (15.6–23.6) | 6.1 (3.0–12.3) | 25.8 (18.8–34.4) | 42.9 (34.3–51.9) | 25.2 (21.0–30.0) |
| Self- reported history of hypertension | 5.0 (2.4–10.2) | 14.1 (9.9–19.4) | 34.2 (21.0–50.5) | 12.8 (9.7–16.6) | 9.2 (5.0–16.2) | 32.1 (24.2–41.3) | 37.6 (29.3–46.7) | 26.6 (22.2–31.6) |
| Total cholesterol | ||||||||
| ≥200 mg/dl: | 10.1 (6.1–16.4) | 19.8 (14.9–25.9) | 26.3 (14.8–42.1) | 16.9 (13.5–21.1) | 2.6 (0.8–7.9) | 16.7 (11.0–24.5) | 17.7 (11.8–25.6) | 12.5 (9.4–16.4) |
| Median TC: mg/dl (IQR) | 140.5 (110–175) | 154 (118–187) | 166 (137–202) | 151 (115–181) | 131 (103–169) | 152 (115.5–186.5) | 148 (114–185) | 144 (111–180) |
| LDL cholesterol | ||||||||
| ≥130 mg/dl: | 8.0 (4.5–13.9) | 15.4 (11.1–21.1) | 23.7 (12.8–39.6) | 13.6 (10.5–17.4) | 1.8 (0.4–6.8) | 12.5 (7.7–19.7) | 11.8 (7.1–18.9) | 8.8 (6.2–12.2) |
| Median LDL (IQR) | 77 (59–104) | 88 (65–116) | 96.5 (66–128) | 85 (63–111) | 71.5 (55–92) | 86.5 (63.5–110) | 84 (63–109) | 80 (61–106) |
| HDL cholesterol | ||||||||
| <40 (male)/<50 (female) | 53.6 (45.3–61.8) | 49.0 (42.1–55.9) | 34.2 (21.0–50.5) | 49.2 (44.1–54.3) | 57.0 (47.8–65.8) | 52.5 (43.6–61.3) | 49.6 (40.7–58.5) | 53.0 (47.7–58.1) |
| Median HDL female (IQR) | 45 (35–60) | 47 (34–65) | 60 (43–69) | 47 (35–63) | 46 (35–54) | 46 (35.5–58) | 45.5 (33–59) | 46 (35–58) |
| Median HDL male (IQR) | 46 (35–62) | 46 (39–59) | 45 (31–62) | 46 (38–60) | 41.5 (32–54) | 46.5 (32–58.5) | 43 (32–57) | 43 (32–57) |
| Triglycerides | ||||||||
| ≥150 mg/dl | 13.0 (8.3–19.6) | 19.3 (14.4–25.4) | 10.5 (4.0–24.9) | 16.1 (12.7–20.2) | 7.8 (4.1–12.3) | 19.0 (13.0–27.0) | 12.6 (7.7–19.9) | 13.2 (10.1–17.1) |
| Median TG (IQR) | 76 (48–116) | 88.5 (63–129) | 87.5 (65–132) | 84 (60–126) | 77.5 (53–105) | 92 (63.5–132.5) | 91 (69–121) | 66 (63–121) |
| HbA1c | ||||||||
| 6.0–6.4% | 6.5 (3.4–12.0) | 7.9 (4.9–12.6) | 10.5 (4.0–24.9) | 7.7 (5.4–10.8) | 3.5 (1.3–8.8) | 5.8 (2.8–11.8) | 6.8 (3.4–13.0) | 5.4 (3.4–8.3) |
| ≥6.5% | 5.0 (2.4–10.2) | 6.4 (3.8–10.8) | 13.2 (5.6–28.0) | 6.6 (4.5–9.6) | 3.4 (1.3–8.8) | 4.2 (1.7–9.6) | 1.7 (0.4–6.5) | 3.1 (1.7–5.5) |
| Current smoker | ||||||||
| 2.2 (0.7–6.5) | 2.5 (1.0–5.8) | 2.6 (0.4–16.5) | 2.4 (1.2–4.5) | 2.6 (0.8–7.7) | 4.1 (1.7–9.6) | 8.4 (4.6–14.9) | 5.1 (3.2–7.9) | |
BP, blood pressure; CI, confidence interval; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; TC, total cholesterol; TG, triglycerides.
Among HIV patients, the prevalence of hypertension was 19.5% (95% CI 15.6–23.6), of which 60.3% (n = 44) was previously undiagnosed. Stratified by age group, 10.8% (95% CI 6.6–17.2), 20.4% (95% CI 15.4–26.6), and 44.7% (95% CI 29.9–60.6) of HIV patients’ hypertension was undiagnosed in the 30–44, 45–59, and at least 60 years age groups, respectively. Among controls, 25.8% (95% CI 21.6–30.7) and among these 37.0% (n = 30) of hypertension was previously undiagnosed. In controls, 6.1% (95% CI 3.0–12.3), 25.8% (18.8–34.4), and 42.9% (95% CI 34.3–51.9) of hypertension was undiagnosed in the 30–44, 45–59, and at least 60 years age groups, respectively.
The proportion of elevated LDL cholesterol was higher among HIV patients [13.6% (95% CI 10.5–17.4) vs. 8.9% (95% CI 6.2–12.2)]. Patients who were initiated on ART with a regimen including stavudine had higher frequencies of abnormal LDL compared with those who were initiated with zidovudine (14.7 vs. 2.5%, P = 0.03). In the multivariate analysis, an LDL of at least 130 mg/dl; was significantly associated with having a secondary education or more [adjusted odds ratio (aOR) 2.4; 95% CI 1.4–4.3] and being overweight or obese, (aOR 3.4; 95% CI 1.9–5.8; Table 3).
Table 3.
Association between LDL cholesterol, glycated hemoglobin with key risk factors: multivariate logistic models.
| OR of LDL ≥130 mg/dl | OR of HbA1c ≥6.0% | |||
| Stratification variables | Crude OR (95% CI) on univariate analysis | Adjusted OR (95% CI) on multivariable analysis | Crude OR (95% CI) on univariate analysis | Adjusted OR (95% CI) on multivariable analysis |
| Type of participant | ||||
| Control | 1 | 1 | 1 | 1 |
| Patient | 1.6 (1.0–2.6) | 1.7 (1.0–3.0) | 1.8 (1.1–2.9) | 2.1 (1.2–3.5) |
| Age group | ||||
| 30–44 | 1 | 1 | 1 | 1 |
| 45–59 | 3.0 (1.6–5.8) | 2.9 (1.5–5.7) | 1.4 (0.8–2.4) | 1.3 (0.8–2.3) |
| ≥60 | 3.1 (1.5–6.4) | 5.1 (2.3–11.1) | 1.3 (0.7–2.5) | 1.8 (0.9–3.6) |
| Sex | ||||
| Female | 1 | 1 | 1 | 1 |
| Male | 1.2 (0.7–2.0) | 1.0 (0.6–1.8) | 0.8 (0.5–1.4) | 0.8 (0.5–1.5) |
| Education | ||||
| Primary or less | 1 | 1 | 1 | 1 |
| Secondary or more | 2.7 (1.7–4.4) | 2.4 (1.4–4.3) | 1.2 (0.7–2.0) | 1.0 (0.6–1.8) |
| BMI | ||||
| Normal | 1 | 1 | 1 | 1 |
| Underweight | 0.9 (0.4–1.8) | 0.8 (0.4–1.8) | 0.8 (0.4–1.6) | 0.7 (0.3–1.4) |
| Overweight/obese | 3.4 (2.0–5.6) | 3.4 (1.9–5.8) | 1.6 (1.0–2.7) | 1.6 (0.9–2.8) |
CI, confidence interval; HbA1c, glycated hemoglobin; OR, odds ratio.
Four patients (1.1%; 95% CI 0.4–2.8) and two controls (0.6%; 95% CI 0.1–2.3) had a known diabetes diagnoses at study inclusion. The prevalence of diabetes was 6.6% (95% CI 4.5–9.6) among HIV patients and 3.1% (95% CI 1.7–5.5) in controls. There was a higher proportion of patients at least 60 years with an HbA1c at least 6.5% compared with controls in the same age group; 13.2% (95% CI 5.6–28.0) vs. 1.7% (95% CI 0.4–6.5), respectively (Table 2). In the multivariate analysis, HIV patients were more likely than controls to have an HbA1c at least 6.0% (aOR 2.1; 95% CI 1.2–3.5) Table 3.
Framingham risk score
Among patients, the prevalence of FRS more than 20% in the 30–44, 45–59, and at least 60-year age groups was 0.7% (95% CI 0.1–5.0), 2.5% (95% CI 1.1–6.0), and 23.7% (95% CI 12.8–39.6), respectively, compared with 0, 3.3 (95% CI 1.2–8.6), and 23.5% (95% CI 16.8–32.0) among controls. Among all study participants, the proportion of FRS at least 20% was significantly higher in men compared with women (10.9 vs. 1.5%, P < 0.01 in HIV patients; 23.7 vs. 3.9%, P < 0.001 in controls), and among those with at least a secondary education when compared with those with at most a primary education (9.6 vs. 1.6%, P < 0.01 in HIV patients; 13.3 vs. 8.6%, P < 0.01) (Fig. 2).
Fig. 2.
Framingham 10-year risk of CVD stratified by age and sex.
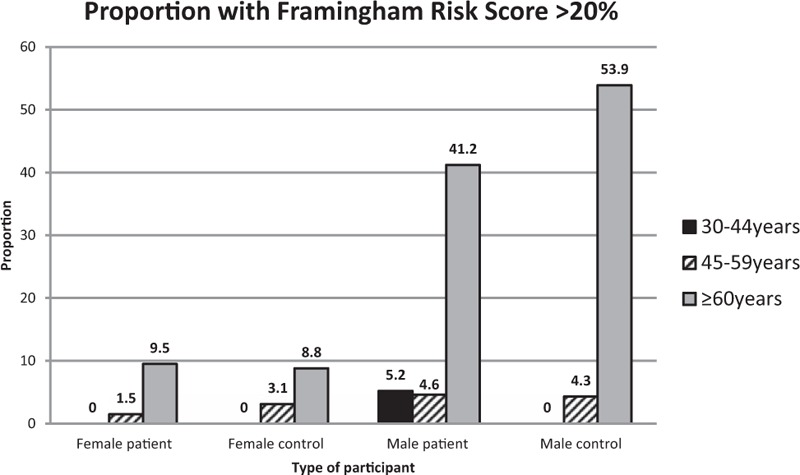
CVD, cardiovascular disease.
Discussion
Our results show an aging cohort of HIV patients who, after taking ART over more than a 10-year period, had a disproportionate burden of CVD risk factors. To our knowledge, it is the first study from Sub-Saharan Africa to describe CVD risks among the HIV-infected exposed to uninterrupted ART for such a long period of time. In Malawi, these patients had a two-fold higher prevalence of diabetes and a high prevalence of hypercholesterolemia, suggesting that long-term exposure to ART increased patients’ risk profiles, especially among older patients.
We found no significant difference in the 10-year risk of developing CVD between patients and controls, deviating from European studies where HIV patients on ART were observed to be more at risk of developing CVD [30]. Differences between our findings and European studies may be partially explained by the different ART regimens used. In Europe, regimens which include protease inhibitors are commonly used compared to Malawi were NNRTIs are used in more than 90% of cases [31,32]. The use of protease inhibitors increases the risk of some key CVD risk factors such as cholesterol when compared to NNRTIs [7]. Additionally, in Europe HIV is mainly found in key subpopulations such as MSM or people who inject drugs who also have a significant burden of other key CVD risk factors like smoking or hepatitis C [33–35]. In Malawi, the more generalized HIV epidemic more closely reflects the general population's CVD risk factors, softening differences between HIV infected and the uninfected. All the same, men in both groups had a significantly a higher prevalence of Framingham risk score more than 20% compared to women, similar to findings from Uganda [36,37]. As HIV-positive men in Malawi have poorer health-seeking behaviors overall than women [19], our findings reinforce the need to tailor programs that improve the access of men to healthcare services regardless of their HIV status. Risks associated with sex were not limited to men in our study. Female study participants exhibited significantly higher proportions of abnormal waist-to-hip ratios and abnormal BMI, perhaps related to sociocultural perceptions surrounding obesity [38–40]. A larger body size is often associated with wealth and well-being in this context without concurrent understanding of the health risks of being overweight. Further study of the interaction of obesity, HIV, and health outcomes in the Malawi context are warranted.
Men also reported higher frequencies of smoking, although smoking rates were lower in HIV patients overall, perhaps because of healthy lifestyle messages given during clinical or counselling interactions, or because of poorer economic conditions. The prevalence of smoking among all study participants was lower than rates reported in national surveys, a surprising finding in Chiradzulu as national surveys also have reported higher rural rates of smoking when compared to urban centers [41].
Our study identified a clear gap in the identification and management of chronic comorbidities especially diabetes and hypertension, in all study participants. High prevalence rates of undiagnosed diabetes were previously described in Malawi and Sub-Saharan Africa setting [42,43]. Undiagnosed hypertension has also been described in other similar contexts [41,44,45]. Yet, our study found that HIV patients’ undiagnosed hypertension was 23% higher than controls despite the fact that, during the course of their HIV management, they had at least one contact with the health system every 3–6 months. Routine HIV follow-up care provides many opportunities for screening for hypertension and other CVD and risk factors, as well as for managing hypertensive disorders and other NCDs. The proportion of hypertensive individuals significantly increased with age among all study participants, although it remained lower than the 33% observed in a Malawi national survey [15]. We observed that the largest proportion of hypertensive participants had stage 1 hypertension. As there is no evidence of reducing cardiac events and mortality through treatment of stage 1 hypertension [46], it may be prudent to focus treatment on high risk older or severely hypertensive patients and actively monitoring the rest, considering the limited human and material resources available in this context [42].
We found a high prevalence of LDL cholesterol which increased significantly with age. The prevalence of hypercholesterolemia among our study patients was higher than that observed among the general population [41]. Other studies in Sub-Saharan Africa found higher hypercholesterolemia rates among HIV patients when compared to ours [18,47–50]. Higher proportions of hypercholesterolemia were also observed to be associated with a variety of factors including the patient being ART experienced (vs. ART naive) [51,52] or having prolonged exposure to the drugs stavudine and efavirenz [53,54]. The lower prevalence of hypercholesterolemia observed in our study compared to other contexts may be partially explained by the different ART regimens used by the patients; most of our study patients had at least 3 years exposure to the less toxic tenofovir-based regimen at the time of study inclusion.
Findings from this study should be considered within the context of its limitations. Though we had a low refusal rate, reducing the risk of selection bias, we cannot rule out information bias as participants may have given socially acceptable responses, for example, on smoking status. There are other lifestyle variables we did not explore in this study which may be possible sources of confounding in the association between HIV status and CVD risk factors. For the Framingham risk score calculation, we used HbA1c as a measure of diabetes and made the assumption that none of the patients or controls had an existing cardiac problem, thus overestimates of at risk individuals may not be ruled out.
In conclusion, there are clearly unmet needs in the diagnosis and management of NCDs among both HIV patients and in uninfected community members in Chiradzulu, and CVD risk factor screening and NCD treatment should be an area of focus for HIV actors in Malawi. Sex differences found here demand that interventions target men and women differently, especially male smokers and overweight women, and that men's overall higher 10-year CVD risk and poorer HIV health-seeking behaviors be acknowledged. Our findings highlight the need to implement screening and treatment of diabetes, hypertension, and hypercholesterolemia for at risk, HIV-infected individuals on ART. However, they also serve as a reminder that CVD risk is not limited to those with HIV, and that at risk uninfected individuals, especially older community members, have NCD screening and treatment needs as well.
Acknowledgements
The authors would like to thank the District Commissioner Chiradzulu and traditional Authorities from Bilal and Chiradzulu hospital catchment areas for allowing us to engage their communities for the study. We thank the District AIDS Committee team (Chiradzulu District), Ministry of Health, District Health Management Team of Chiradzulu, the clinical and counselling staff in Chiradzulu District Hospital and Bilal Health Centre, and the Queen Elizabeth Central Hospital laboratory team for their support. We extend a special acknowledgement to the MSF France Chiradzulu project team for their commitment in implementing the study. We are particularly grateful to the patients from the Chiradzulu HIV cohort and the communities of Bilal and Chiradzulu hospital catchment areas for agreeing to participate in this study.
A.T., F.R., E.H.B-A., and D.M. designed the study protocol. S.C.M.R. and D.M. participated in data collection and cleaning. J.B-B. performed laboratory analysis. S.C.M.R. and D.M. performed statistical analysis and drafted the article. E.S. and D.M. provided technical assistance. A.T., F.R., E.H.B-A., D.M., E.S., I.A.Q., L.S., and Z.C. critically revised the abstract and article for content. All authors read and approved the final manuscript.
Funding for this study was provided by Medecins Sans Frontières (Operational Center Paris).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Smit M, Brinkman K, Geerlings S, Smit C, Thyagarajan K, Sighem A, et al. ATHENA observational cohort. Future challenges for clinical care of an ageing population infected with HIV: a modelling study. Lancet Infect Dis 2015; 15:810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, et al. Swiss HIV Cohort Study. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011; 53:1130–1139. [DOI] [PubMed] [Google Scholar]
- 3.Guaraldi G, Orlando G, Zona S, Menozzi M, Carli F, Garlassi E, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–1126. [DOI] [PubMed] [Google Scholar]
- 4.Magodoro IM, Esterhuizen TM, Chivese T. A cross-sectional, facility based study of comorbid noncommunicable diseases among adults living with HIV infection in Zimbabwe. BMC Res Notes 2016; 9:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbaro G. Cardiovascular manifestations of HIV infection. Circulation 2016; 106:1420–1425. [DOI] [PubMed] [Google Scholar]
- 6.Hemken LG, Bucher HC. HIV infection and cardiovascular disease. Eur Heart 2014; 35:1373–1381. [DOI] [PubMed] [Google Scholar]
- 7.Friis-Moller N, Weber R, Reiss P, Thiebaut R, Kirk O, d’Arminio MA, et al. DAD study group. Cardiovascular disease risk factors in HIV patients–association with antiretroviral therapy. Results from the DAD study. AIDS 2003; 17:1179–1193. [DOI] [PubMed] [Google Scholar]
- 8.Ledergerber B, Furrer H, Rickenbach M, Lehmann R, Elzi L, Hirschel B, et al. Swiss HIV Cohort Study. Factors associated with the incidence of type 2 diabetes mellitus in HIV-infected participants in the Swiss HIV Cohort Study. Clin Infect Dis 2007; 45:111–119. [DOI] [PubMed] [Google Scholar]
- 9.Smith CJ, Levy I, Sabin CA, Kaya E, Johnson MA, Lipman MC. Cardiovascular disease risk factors and antiretroviral therapy in an HIV-positive UK population. HIV Med 2004; 5:88–92. [DOI] [PubMed] [Google Scholar]
- 10.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012; 13:453–468. [DOI] [PubMed] [Google Scholar]
- 11.Samaras K, Gan SK, Peake PW, Carr A, Campbell LV. Proinflammatory markers, insulin sensitivity, and cardiometabolic risk factors in treated HIV infection. Obesity (Silver Spring) 2009; 17:53–59. [DOI] [PubMed] [Google Scholar]
- 12.Langkilde A, Petersen J, Klausen HH, Henriksen JH, Eugen-Olsen J, Andersen O. Inflammation in HIV-infected patients: impact of HIV, lifestyle, body composition, and demography: a cross sectional cohort study. PLoS One 2012; 7:e51698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tien PC, Choi AI, Zolopa AR, Benson C, Tracy R, Scherzer R, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM study cohort. J Acquir Immune Defic Syndr 2010; 55:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SanJoaquin MA, Allain TJ, Molyneux ME, Benjamin L, Everett DB, Gadabu O, et al. Surveillance Programme of IN-patients and Epidemiology (SPINE): implementation of an electronic data collection tool within a large hospital in Malawi. PLoS Med 2013; 10:e1001400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Msyamboza KP, Ngwira B, Dzowela T, Mvula C, Kathyola D, Harries AD, et al. The burden of selected chronic noncommunicable diseases and their risk factors in Malawi: nationwide STEPS survey. PLoS One 2011; 6:e20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abebe M, Kinde S, Belay G, Gebreegziabxier A, Challa F, Gebeyehu T, et al. Antiretroviral treatment associated hyperglycemia and dyslipidemia among HIV infected patients at Burayu Health Center, Addis Ababa, Ethiopia: a cross-sectional comparative study. BMC Res Notes 2014; 7:380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrahams Z, Dave JA, Maartens G, Levitt NS. Changes in blood pressure, glucose levels, insulin secretion and anthropometry after long term exposure to antiretroviral therapy in South African women. AIDS Res Ther 2015; 12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bekolo CE, Nguena MB, Ewane L, Bekoule PS, Kollo B. The lipid profile of HIV-infected patients receiving antiretroviral therapy in a rural Cameroonian population. BMC Public Health 2014; 14:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maman D, Chilima B, Masiku C, Ayouba A, Masson S, Szumilin E, et al. Closer to 90-90-90. The cascade of care after 10 years of ART scale-up in rural Malawi: a population study. J Int AIDS Soc 2016; 19:20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Statistical Office. Malawi population and housing census preliminary report. Malawi: National Statistical Office; 2008. [Google Scholar]
- 21.Ferradini L, Jeannin A, Pinoges L, Izopet J, Odhiambo D, Mankhambo L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet 2006; 367:1335–1342. [DOI] [PubMed] [Google Scholar]
- 22.Waist circumference and waist-hip ratio: report of a WHO expert consultation. 2008; Geneva: World Health Organization, 19–28. [Google Scholar]
- 23.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American heart association council on high blood pressure research. Hypertension 2005; 45:142–161. [DOI] [PubMed] [Google Scholar]
- 24.Ministry of Health Malawi. Malawi guidelines for clinical management of HIV in children. Malawi: Ministry of Health; 2014. [Google Scholar]
- 25.International Diabetes Federation. The IDF Consesus Worldwide Definition of the Metabolic Syndrome. 2006. p. 11 [Google Scholar]
- 26.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 2002; 106:3143–3421. [PubMed] [Google Scholar]
- 27.WHO Geneva. Use of glycated Hb (HbA1C) in the diagnosis of diabetes mellitus. Geneva: WHO; 2011. [Google Scholar]
- 28.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 2008; 117:743–753. [DOI] [PubMed] [Google Scholar]
- 29.Linden A. Framingham: Stata module for calculating the Framingham 10- year Coronary Vascular Disease Risk Prediction. 2015 [Google Scholar]
- 30.Bergersen BM, Sandvik L, Bruun JN, Tonstad S. Elevated Framingham risk score in HIV-positive patients on highly active antiretroviral therapy: results from a Norwegian study of 721 subjects. Eur J Clin Microbiol Infect Dis 2004; 23:625–630. [DOI] [PubMed] [Google Scholar]
- 31.Friis-Moller N, Sabin CA, Weber R, d’Arminio MA, El-Sadr WM, Reiss P, et al. Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993–2003.14627784 [Google Scholar]
- 32.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa F, Phillips AN, Lundgren JD. Update on HIV in Western Europe. Curr HIV/AIDS Rep 2014; 11:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Socio GV, Martinelli L, Morosi S, Fiorio M, Roscini AR, Stagni G, Schillaci G. Is estimated cardiovascular risk higher in HIV-infected patients than in the general population?. Scand J Infect Dis 2007; 39:805–812. [DOI] [PubMed] [Google Scholar]
- 35.Bedimo R, Abodunde O. Metabolic and cardiovascular complications in HIV/HCV-co-infected patients. Curr HIV/AIDS Rep 2016; 13:328–339. [DOI] [PubMed] [Google Scholar]
- 36.Mateen FJ, Kanters S, Kalyesubula R, Mukasa B, Kawuma E, Kengne AP, Mills EJ. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertens 2013; 31:1372–1378. [DOI] [PubMed] [Google Scholar]
- 37.Muyanja D, Muzoora C, Muyingo A, Muyindike W, Siedner MJ. High prevalence of metabolic syndrome and cardiovascular disease risk among people with HIV on stable ART in southwestern Uganda. AIDS Patient Care STDS 2016; 30:4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCormick CL, Francis AM, Iliffe K, Webb H, Douch CJ, Pakianathan M, Macallan DC. Increasing obesity in treated female HIV patients from Sub-Saharan Africa: potential causes and possible targets for intervention. Front Immunol 2014; 5:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devanathan R, Esterhuizen TM, Govender RD. Overweight and obesity amongst Black women in Durban, KwaZulu-Natal: a ’disease’ of perception in an area of high HIV prevalence. Afr J Prim Healthcare Fam Med 2013; 5:450. [Google Scholar]
- 40.Micklesfield LK, Lambert EV, Hume DJ, Chantler S, Pienaar PR, Dickie K, et al. Socio-cultural, environmental and behavioural determinants of obesity in black South African women. Cardiovasc J Afr 2013; 24:369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ministry of Health Malawi, WHO. Malawi National STEPS Survey for chronic non-communicable diseases and their risk factors. Malawi: Ministry of Health and WHO; 2010. [Google Scholar]
- 42.Divala OH, Amberbir A, Ismail Z, Beyene T, Garone D, Pfaff C, et al. The burden of hypertension, diabetes mellitus, and cardiovascular risk factors among adult Malawians in HIV care: consequences for integrated services. BMC Public Health 2016; 16:1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mbanya JC, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in sub-Saharan Africa. Lancet 2010; 375:2254–2266. [DOI] [PubMed] [Google Scholar]
- 44.Matenga JA, Allain TJ, Wilson AO, Adamchak DJ, Senzanje B, Mushangi E, Gomo Z. Hypertension management in Zimbabwe: awareness, treatment and blood pressure control. A community-based study. S Afr Med J 1997; 87:1371–1373. [PubMed] [Google Scholar]
- 45.Kavishe B, Biraro S, Baisley K, Vanobberghen F, Kapiga S, Munderi P, et al. High prevalence of hypertension and of risk factors for noncommunicable diseases (NCDs): a population based cross-sectional survey of NCDS and HIV infection in Northwestern Tanzania and Southern Uganda. BMC Med 2015; 13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database Syst Rev 2012. CD006742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Doupa D, Seck SM, Dia CA, Diallo FA, Kane MO, Kane A, et al. Dyslipidemia, obesity and other cardiovascular risk factors in the adult population in Senegal. Pan Afr Med J 2014; 19:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Feleke Y, Fekade D, Mezegebu Y. Prevalence of highly active antiretroviral therapy associated metabolic abnormalities and lipodystrophy in HIV infected patients. Ethiop Med J 2012; 50:221–230. [PubMed] [Google Scholar]
- 49.Julius H, Basu D, Ricci E, Wing J, Basu JK, Pocaterra D, Bonfanti P. The burden of metabolic diseases amongst HIV positive patients on HAART attending The Johannesburg Hospital. Curr HIV Res 2011; 9:247–252. [DOI] [PubMed] [Google Scholar]
- 50.Thiombiano LP, Mbaye A, Sarr SA, Ngaide AA, Kane A, Diao M, et al. [Prevalence of dyslipidemia in the rural population of Gueoul (Senegal)]. Ann Cardiol Angeiol (Paris) 2016; 65:77–80. [DOI] [PubMed] [Google Scholar]
- 51.Nsagha DS, Weledji EP, Assob NJ, Njunda LA, Tanue EA, Kibu OD, et al. Highly active antiretroviral therapy and dyslipidemia in people living with HIV/AIDS in Fako Division, South West Region of Cameroon. BMC Cardiovasc Disord 2015; 15:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Obirikorang C, Quaye L, Osei-Yeboah J, Odame EA, Asare I. Prevalence of metabolic syndrome among HIV-infected patients in Ghana: a cross-sectional study. Niger Med J 2016; 57:86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feeney ER, Mallon PW. HIV and HAART-associated dyslipidemia. Open Cardiovasc Med J 2011; 5:49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jones R, Sawleshwarkar S, Michailidis C, Jackson A, Mandalia S, Stebbing J, et al. Impact of antiretroviral choice on hypercholesterolaemia events: the role of the nucleoside reverse transcriptase inhibitor backbone. HIV Med 2005; 6:396–402. [DOI] [PubMed] [Google Scholar]


