Abstract
Recently, several studies have investigated the association between the rare mutation of triggering receptor expressed on myeloid cells 2 (TREM2) gene (rs75932628-T) and the risk of late-onset Alzheimer’s disease (LOAD), but they did not draw the same conclusion. Our aim was to investigate the link between the TREM2 polymorphism and LOAD in the Chinese Han population. We examined 786 patients and 803 controls in this study. The rs75932628 polymorphism was evaluated using high-resolution melting analysis and direct sequencing. rs75932628-T (predicted to cause an R47H substitution) was absent in our cohort. We did not find an association between the rs75932628 single nucleotide polymorphism of TREM2 and LOAD in this study. Thus, rs75932628 is unlikely to be related to LOAD in the Chinese Han population.
Keywords: Alzheimer’s disease, Chinese, rs75932628, triggering receptor expressed on myeloid cells 2
Introduction
Alzheimer’s disease (AD) is a genetically heterogeneous disorder characterized by the coexistence of monogenic and genetically complex forms. It is a major cause of dementia among the elderly. A twin study showed that the heritability of late-onset Alzheimer’s disease (LOAD) is ∼60–80% (Gatz et al., 2006). Increasingly more attention is being paid to the gene mutations of AD, especially to LOAD. Until 2009, apolipoprotein E (ApoE) was the only established susceptibility marker for LOAD (Gatz et al., 2006). To identify the other genes for LOAD, efforts were focused on carrying out genome-wide association studies. Since 2009, several large genome-wide association studies and meta-analyses have identified significant associations of LOAD with single-nucleotide polymorphisms, such as CLU, CR1, and PICALM (Harold et al., 2009; Lambert et al., 2009; Seshadri et al., 2010; Lambert et al., 2013).
The role of rare genetic variants in common disorders has been gaining attention in the last few years. Triggering receptor expressed on myeloid cells 2 (TREM2) has only recently been added to the list of associated LOAD genes. So far, several studies have shown that rs75932628-T (predicted to cause an R47H substitution) increases susceptibility to AD (Benitez et al., 2013; Giraldo et al., 2013; Guerreiro et al., 2013; Jonsson et al., 2013; Pottier et al., 2013; Cuyvers et al., 2014; Jin et al., 2014; Roussos et al., 2014; Ruiz et al., 2014; Slattery et al., 2014). The rare R47H variant was found in both the Icelandic population (Jonsson et al., 2013) and an international cohort of European descent (Guerreiro et al., 2013), and this association has been replicated in a cohort of French patients with an early-onset form of AD (age of onset<65 years) (Pottier et al., 2013) and in a Spanish study with a series of patients including both LOAD and early-onset AD patients (Benitez et al., 2013). Subsequently, other further studies have also reported the association of P.R47H with AD in American, Belgian, and British populations (Cuyvers et al., 2014; Jin et al., 2014; Roussos et al., 2014; Slattery et al., 2014). A meta-analysis of these studies and others reports an odds ratio of 2.7 (Lu et al., 2015), further strengthening the case for the rare p.R47H substitution as a major genetic risk factor for AD. However, recent studies in Japanese and Chinese populations showed that mutations in exon 2 of TREM2 were unlikely to play a key role in the susceptibility of LOAD (Ma et al., 2014; Miyashita et al., 2014; Yu et al., 2014). Given the conflicting roles of TREM2 in LOAD, it would be of interest to investigate the link between the TREM2 polymorphism and LOAD in our study group of Han Chinese patients.
Participants and methods
Study participants
A total of 786 LOAD patients (mean age at onset 72.3±11.1 years, 51.8% men) and 803 healthy controls (mean age at clinical assessment 71.9±10.7 years, 51.3% men) were included in the current study. From a power analysis on the basis of the study by Guerreiro et al. (2013) on Whites (risk allele frequency in the AD group=0.010, risk allele frequency in the control group=0.002), this sample set was estimated to be large enough to detect risk alleles using PASS 11 software (NCSS, Kaysville, Utah, USA) (α=0.05, power=80.8%). AD patients were recruited from the Memory Clinic, Huashan Hospital, and healthy adults were recruited from the community; they were matched for age, sex, and education with the AD patients. All the AD patients were recruited after they had completed the laboratory tests and cranial computed tomography/MRI scan and were found to have no clinically significant abnormalities in vitamin B12, folic acid, thyroid function (free triiodothyronine, free tetraiodothyronine, thyroid-stimulating hormone), rapid plasma regain, or Treponema pallidum particle agglutination.
AD was diagnosed as probable AD according to the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association (NINCDS-ADRDA/NINCDS-AIREN) criteria.
Extraction of genomic DNA
Genomic DNA was isolated from fresh peripheral blood using the QIAamp DNA Blood Kit (Qiagen, Valencia, California, USA) according to the manufacturer’s instruction.
High-resolution melting analysis
PCR cycling was performed on GeneAmp PCR System 9700 (Applied Biosystems, Foster City, California, USA) and high-resolution melting (HRM) was performed on Rotor-Gene 6000 TM (Corbett Research, Mortlake, New South Wales, Australia). The primer sequences were designed to amplify a small fragment surrounding the polymorphism and to avoid the presence of other sequence variations. The primers for the 120-bp amplicon that spanned the rs75932628 polymorphism were AAG GAC AGC AGC CAC AAG TT and reverse primers GTC TTG CCC CTA TGA CTC CA. PCR was performed in a 20 μl volume containing 10 μl Premix Taq Hot Start Version, 0.5 μmol/l of each primer, 5 μmol/l of SYTO 9, 100 ng of genomic DNA, and PCR-grade water using Premix Taq Hot Start Version (TaKaRa Biotechnology Co. Ltd, Dalian, People’s Republic of China). Each reaction was performed in triplicate. The PCR cycling conditions were as follows: one cycle of 95°C for 2 min, 40 cycles of 95°C for 30 s, 56°C for 30 s, and 72°C for 30 s, one cycle of 72°C for 5 s, followed by an HRM step of 95°C for 2 min, 60°C for 2 min, and continuous acquisition from 87° to 93°C at one acquisition per 0.1°C. Fluorescence acquisition was set as recommended by the manufacturer. The potential samples of the TREM2 rs75932628-T mutation were found by analyzing the PCR-product melting curves using the accompanying Rotor-Gene 6000 1.7 software (Corbett Research, Mortlake, New South Wales, Australia).
Sequencing
A total of 46 potential mutations identified with HRM were subjected to direct sequencing. Amplicons were gel-purified using the QIAquick gel purification kit (Qiagen). DNA sequencing analysis was carried out in an ABI PRISM 310 genetic analyzer (Applied Biosystems).
Ethical compliance
This study was approved by the ethics committee of Shanghai Huashan Hospital Fudan University. All participants or their families provided written informed consent.
Statistical analyses
Descriptive statistics are presented as mean values (SD). Comparison of means was performed using an unpaired Student’s t test. ApoE ε4 genotype and sex distribution were compared using the χ2-test. P values less than 0.05 were considered statistically significant.
Results and discussion
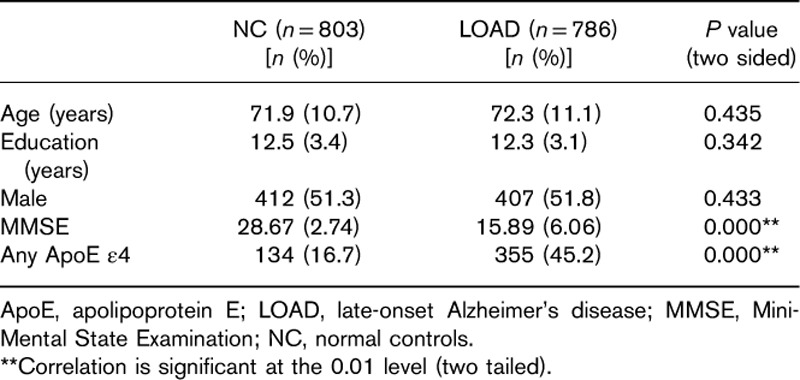
The characteristics of the study population are summarized in Table 1. No statistically significant differences were observed in age (age at onset for patients with LOAD compared with age at examination for control participants; P=0.435), education (P=0.342), and sex (P=0.433) between patients with LOAD and the control participants. As expected, the Mini-Mental State Examination scores were significantly lower in patients with LOAD than in the control participants (P=0.000). The number of patients with any ApoE ε4 in LOAD was more than that among the normal controls (P=0.000).
Table 1.
Demographic features of the participants
The rare variant rs75932628-T, reported previously in European populations, was not detected in our cohorts. All patients and controls in our study had the TREM2 rs75932628 CC genotype, and none showed CT or TT genotypes. Our results, combined with other five studies consisting of 7286 Chinese participants (Feng et al., 2014; Ma et al., 2014; Yu et al., 2014; Li et al., 2016; Tan et al., 2016), indicate that the minor allele frequency of the variant rs75932628-T in the Han Chinese population was only 0.007%. Moreover, this rare variant was not detected in AD patients, indicating that the minor allele frequency expected for rs75932628-T was lower than 0.02% in the Han Chinese AD individuals.
TREM2 is a transmembrane glycoprotein that is expressed primarily on microglia, the resident histiocytes of the central nervous system. This protein interacts directly with a type I transmembrane adapter protein, DAP12 (Colonna, 2003). With respect to the function, TREM2 has been found to suppress inflammatory responses by repression of microglia-mediated cytokine production and secretion, as well as promote phagocytosis of Aβ and damaged neurons (Colonna, 2003). Several recent studies showed that the levels of TREM2 increased in both plasma and brain in patients with AD (Giraldo et al., 2013; Guerreiro et al., 2013; Hu et al., 2014).
In the current study, we evaluated the rs75932628 polymorphism in the Chinese Han population. However, we did not detect any rs75932628-T in our cohort, indicating that the single nucleotide polymorphism of TREM2 may not be a genetic marker to assess the risk of LOAD in the Chinese Han population.
Our result was consistent with that reported previously in Asia, including Japan (Miyashita et al., 2014) and China (Ma et al., 2014; Yu et al., 2014). However, these results are not in agreement with recent data from Whites (Benitez et al., 2013; Guerreiro et al., 2013; Jonsson et al., 2013; Ruiz et al., 2014), indicating that TREM2 may contribute toward the susceptibility of LOAD only in Whites. One possible reason could be the population-specific genetic causes related to AD. Therefore, the information on ancestry will be critical to evaluate the role of TREM2 polymorphism rs75932628 in AD and other neurodegenerative disorders.
Because of the vast population in China, our sample size is insufficient to illustrate that there is a lack of association between TREM2 polymorphism rs75932628 and LOAD in the Chinese population. Further study with a large population is highly warranted.
Acknowledgements
The authors thank all the participants for their understanding and cooperation, which made this study possible.
The study was supported by the National Project of Chronic disease (2016YFC1306400), the Science and Technology Commission of Shanghai Municipality (17411950106), the National Natural Science Foundation of China (81200835, 81773513), the Shanghai Brain-Intelligence Project from STCSM (16JC1420500), and the Natural Science Foundation and Major Basic Research Program of Shanghai (16JC1420100).
Conflicts of interest
There are no conflicts of interest.
References
- Benitez BA, Cooper B, Pastor P, Jin SC, Lorenzo E, Cervantes S, Cruchaga C. (2013). TREM2 is associated with the risk of Alzheimer’s disease in Spanish population. Neurobiol Aging 34:1711.e15–1711.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M. (2003). TREMs in the immune system and beyond. Nat Rev Immunol 3:445–453. [DOI] [PubMed] [Google Scholar]
- Cuyvers E, Bettens K, Philtjens S, van Langenhove T, Gijselinck I, van der Zee J, et al. (2014). Investigating the role of rare heterozygous TREM2 variants in Alzheimer’s disease and frontotemporal dementia. Neurobiol Aging 35:e711–e729. [DOI] [PubMed] [Google Scholar]
- Feng SJ, Nie K, Gan R, Huang J, Zhang YW, Wang LM, et al. (2014). Triggering receptor expressed on myeloid cells 2 variants are rare in Parkinson’s disease in a Han Chinese cohort. Neurobiol Aging 35:1780.e11–1780.e12. [DOI] [PubMed] [Google Scholar]
- Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, et al. (2006). Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 63:168–174. [DOI] [PubMed] [Google Scholar]
- Giraldo M, Lopera F, Siniard AL, Corneveaux JJ, Schrauwen I, Carvajal J, et al. (2013). Variants in triggering receptor expressed on myeloid cells 2 are associated with both behavioral variant frontotemporal lobar degeneration and Alzheimer’s disease. Neurobiol Aging 34:2077.e11–2077.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, et al. (2013). TREM2 variants in Alzheimer’s disease. N Engl J Med 368:117–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold D, Abraham R, Hollingworth P, Sims R, Gerrish A, Hamshree ML, et al. (2009). Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat Genet 41:1088–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu N, Tan MS, Yu JT, Sun L, Tan L, Wang YL, et al. (2014). Increased expression of TREM2 in peripheral blood of Alzheimer’s disease patients. J Alzheimers Dis 38:497–501. [DOI] [PubMed] [Google Scholar]
- Jin SC, Benitez BA, Karch CM, Cooper B, Skorupa T, Carrell D, et al. (2014). Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum Mol Genet 23:5838–5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. (2013). Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med 368:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Heath S, Even G, Campion D, Sleegers K, Hiltunen M, et al. (2009). Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat Genet 41:1094–1099. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, Naj AC, Sims R, Bellenguez C, et al. (2013). Meta-analysis of 74 046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat Genet 45:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhong L, Gu L, Huang W, Shi X, Zhang X, et al. (2016). Association study of TREM2 polymorphism rs75932628 with leucoaraiosis or Parkinson’s disease in the Han Chinese population. BMJ Open 6:e009499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Liu W, Wang X. (2015). TREM2 variants and risk of Alzheimer’s disease: a meta-analysis. Neurol Sci 36:1881–1888. [DOI] [PubMed] [Google Scholar]
- Ma J, Zhou Y, Xu J, Liu X, Wang Y, Deng Y, et al. (2014). Association study of TREM2 polymorphism rs75932628 with late-onset Alzheimer’s disease in Chinese Han population. Neurol Res 36:894–896. [DOI] [PubMed] [Google Scholar]
- Miyashita A, Wen Y, Kitamura N, Matsubara E, Kawarabayashi T, Shoji M, et al. (2014). Lack of genetic association between TREM2 and late-onset Alzheimer’s disease in a Japanese population. J Alzheimers Dis 41:1031–1038. [DOI] [PubMed] [Google Scholar]
- Pottier C, Wallon D, Rousseau S, Rovelet-Lecrux A, Richard AC, Rollin-Sillaire A, et al. (2013). TREM2 R47H variant as a risk factor for early-onset Alzheimer’s disease. J Alzheimers Dis 35:45–49. [DOI] [PubMed] [Google Scholar]
- Roussos P, Katsel P, Fam P, Tan W, Purohit DP, Haroutunian V. (2014). The triggering receptor expressed on myeloid cells 2 (TREM2) is associated with enhanced inflammation, neuropathological lesions and increased risk for Alzheimer’s dementia. Alzheimers Dement 11:1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Dols-Icardo O, Bullido MJ, Pastor P, Rodríguez-Rodríguez E, López de Munain A, et al. (2014). Assessing the role of the TREM2 p.R47H variant as a risk factor for Alzheimer’s disease and frontotemporal dementia. Neurobiol Aging 35:444.e1–444.e2. [DOI] [PubMed] [Google Scholar]
- Seshadri S, Fitzpatrick AL, Ikram MA, deStefano AL, Gudnason V, Boada M, et al. (2010). Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA 303:1832–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery CF, Beck JA, Harper L, Adamson G, Abdi Z, Uphill J, et al. (2014). R47H TREM2 variant increases risk of typical early-onset Alzheimer’s disease but not of prion or frontotemporal dementia. Alzheimers Dement 10:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T, Song Z, Yuan L, Xiaong W, Deng X, Ni B, et al. (2016). Genetic analysis of TREM2 variants in Chinese Han patients with sporadic Parkinson’s disease. Neurosci Lett 612:189–192. [DOI] [PubMed] [Google Scholar]
- Yu JT, Jiang T, Wang YL, Wang HF, Zhang W, Hu N, et al. (2014). Triggering receptor expressed on myeloid cells 2 variant is rare in late-onset Alzheimer’s disease in Han Chinese individuals. Neurobiol Aging 35:937.e1–937.e3. [DOI] [PubMed] [Google Scholar]


