Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
For early-stage DLBCL, R-CHOP alone is not inferior to R-CHOP followed by RT.
Abstract
The benefit of radiotherapy (RT) after chemotherapy in limited-stage diffuse large B-cell lymphoma (DLBCL) remains controversial. We conducted a randomized trial in patients with nonbulky limited-stage DLBCL to evaluate the benefit of RT after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). Patients were stratified according to the modified International Prognostic Index, including lactate dehydrogenase, Eastern Cooperative Oncology Group performance status, age, and disease stage. The patients received 4 or 6 consecutive cycles of R-CHOP delivered once every 2 weeks, followed or not by RT at 40 Gy delivered 4 weeks after the last R-CHOP cycle. All patients were evaluated by fluorodeoxyglucose-positron emission tomography scans performed at baseline, after 4 cycles of R-CHOP, and at the end of treatment. The primary objective of the trial was event-free survival (EFS) from randomization. The trial randomly assigned 165 patients in the R-CHOP arm and 169 in the R-CHOP plus RT arm. In an intent-to-treat analysis with a median follow-up of 64 months, 5-year EFS was not statistically significantly different between the 2 arms, with 89% ± 2.9% in the R-CHOP arm vs 92% ± 2.4% in the R-CHOP plus RT arm (hazard ratio, 0.61; 95% confidence interval [CI], 0.3-1.2; P = .18). Overall survival was also not different at 92% (95% CI, 89.5%-94.5%) for patients assigned to R-CHOP alone and 96% (95% CI, 94.3%-97.7%) for those assigned to R-CHOP plus RT (P = not significant). R-CHOP alone is not inferior to R-CHOP followed by RT in patients with nonbulky limited-stage DLBCL. This trial was registered at www.clinicaltrials.gov as #NCT00841945.
Introduction
Combined modality therapy based on abbreviated chemotherapy followed by radiotherapy (RT) has until recently been considered the standard-of-care for early-stage diffuse large B-cell lymphoma (DLBCL).1,2 In the context of limited-stage DLBCL, this option has been challenged by using chemotherapy or, more recently, immunochemotherapy (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone [R-CHOP]) alone.3 In 2010, the National Comprehensive Cancer guidelines recommended 3 cycles of R-CHOP followed by involved field radiotherapy (IFRT) for early-stage, nonbulky DLBCL as well as the administration of 6 to 8 cycles of R-CHOP with or without IFRT.4 To date, no definitive conclusions can be drawn regarding the need for consolidative IFRT for patients with early-stage DLBCL.
Before the rituximab era, 4 randomized trials raised the question of whether RT should be added after chemotherapy to provide a more effective local control and an advantage in terms of disease-free survival.5-8 Over the last decade, these results have been widely commented on, which has led to conflicting conclusions, depending on the various chemotherapy options used in these trials (3, 4, or 8 courses of CHOP or high-dose doxorubicin, vindesine, cyclophosphamide, bleomycin, and prednisolone [AVCBP]), patient selection, and time of analysis.9
Whether RT should be added to R-CHOP in limited-stage DLBCL is also controversial.10-14 In a recent large retrospective study, it was shown that in the United States only 39% of patients with early-stage DLBCL were given RT.11 However, no prospective trials have been published so far in this setting in the rituximab era. It has been noted by Ng et al15 that we definitely need randomized trials using contemporary R-CHOP and positron emission tomography (PET) imaging to select patients for an optimized therapy. Moreover, response to treatment is now based on PET imaging. The British Columbia Cancer Agency has suggested that patients who reach partial response (PR) after R-CHOP defined by PET imaging could optimally benefit from RT, and thus patients in complete response (CR) after 3 or 4 cycles of R-CHOP could be spared additional RT.16,17
In 2005, the Lymphoma Study Association/Groupe Ouest-Est des Leucémies et des Autres Maladies du Sang (LYSA/GOELAMS) group initiated a randomized study comparing R-CHOP alone to R-CHOP plus RT in patients with early-stage DLBCL and no bulky disease, the results of which are reported here.
Patients and methods
Patients
Patients eligible for enrollment had to be between 18 and 75 years old, with a previously untreated DLBCL according to the 2001 World Health Organization classification.18 Patients were required to have an Ann Arbor stage I or II limited lymphoma, with nonbulky mass defined by a tumor <7 cm in diameter. In fact, even in 2017, the definition of bulky disease remains arbitrary and varies between 5 and 10 cm, depending on the studies and the cooperative groups.14,19,20 Exclusion criteria were positive serology for HIV or active chronic hepatitis B or C, transformation of a previously indolent lymphoma, primary cerebral lymphoma, previous organ transplantation, concomitant or previous cancer (except in situ cervical carcinoma), liver or kidney failure, and cardiac contraindication to doxorubicin. Patients with cutaneous, testis, ovarian, breast, or intestinal lymphoma were also excluded.
The study was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. The trial was approved by the ethics committee of Rennes University Hospital, and all patients provided written informed consent.
Pathology
Histologic diagnosis was performed by local hemopathologists. Central review was conducted by an expert LYSA/GOELAMS pathologist who was allowed to reclassify tumors according to the World Health Organization 2008 classification for the final analysis.21 Tissue microarrays were constructed by using all available samples stained for CD20, CD5, CD10, BCL2, BCL6, MYC, and MUM1 in immunohistochemistry. Identification of the cell of origin was based on the Hans algorithm.
Staging
The extent of the disease was evaluated by physical examination; computed tomography (CT) scan of the head, neck, chest, abdomen, and pelvis; cerebrospinal fluid examination; bone marrow biopsy; and other investigational procedures, depending on clinical symptoms. The lymphoma stage was defined according to the Ann Arbor staging system (stage I, involvement of a single lymph node or extranodal site; stage II, involvement of 2 or more nodal regions or of an extranodal site and 1 or more adjacent nodal regions on the same side of the diaphragm). Tumor measurements were obtained before biopsy, and bulky disease was defined as more than 7 cm in diameter measured in both transverse and coronal planes. Performance status (PS) was assessed at baseline according to the Eastern Cooperative Oncology Group scale. A stage-modified version of the International Prognostic Index (mIPI) was used that considered adverse risk factors for early stage as follows: elevated serum lactate dehydrogenase (LDH) levels, age older than 60 years, Ann Arbor stage II, and PS ≥1.3
PET assessment at inclusion was mandatory and was reviewed by 2 independent nuclear physicians in real time. Patients with stage I or II disease according to CT scans were excluded if PET assessment disclosed a more extensive lymphoma dissemination that required upstaging to stage III or IV. PET positivity was defined visually as 18F-fluorodeoxyglucose (18F-FDG) uptake above the mediastinum or surrounding background in a location incompatible with normal anatomy or physiology.22 A negative scan was defined as having no abnormally increased 18F-FDG at any site.
Treatment
Patients were randomly assigned upfront to receive or not receive RT after 4 or 6 cycles of R-CHOP (doxorubicin 50 mg/m2, cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2 [up to a maximal dose of 2 mg] on day 1, and prednisone 40 mg/m2 on days 1 through 5) combined with rituximab (375 mg/m2 on day 1) repeated at 14-day intervals. Patients without any adverse prognostic factor (normal LDH level, Ann Arbor stage I, PS 0, age younger than 60 years, mIPI 0) received 4 cycles of R-CHOP, whereas those with 1 or more adverse prognostic factors (high LDH level, Ann Arbor stage II, PS >0, age older than 60 years, mIPI ≥1) received 6 cycles of R-CHOP. IFRT began 1 month after the last cycle of R-CHOP in the group of patients initially randomly assigned to receive RT. The prescribed dose of radiation was 40 Gy in 20 fractions of 2 Gy given 5 days per week. Irradiated volumes encompassed involved nodal or extranodal sites and adjacent uninvolved nodes according to IFRT. The design of the study is depicted in supplemental Figure 1 (available on the Blood Web site). Patients who achieved CR pursued treatment according to random assignment. Patients who achieved PR after the fourth cycle of R-CHOP were recommended to receive 2 additional cycles followed by RT, no matter which treatment arm they were allocated to. Patients with progressive disease were withdrawn from the study.
Response assessment
Response was evaluated 2 weeks after the fourth course of R-CHOP, based on PET and CT scan results blinded by arm assignment and 4 weeks after the end of treatment (response criteria are provided in the supplemental Appendix).
Statistical analysis
Randomization was centralized and stratified according to mIPI. The primary objective of the trial was event-free survival (EFS) from the time of random assignment. Secondary end points were overall survival (OS) and toxicity. Based on a noninferiority analysis with an upper limit of 10% difference between the 2 arms in terms of EFS, an 80% power, and a bilateral type 1 error of 5%, 170 patients were required for each arm. Because recruitment was lower than expected, this study was extended to June 2014. An interim analysis performed in 2011 after the inclusion of 270 patients showed estimated OS and EFS at 96% and 94%, respectively, and allowed decreasing the upper limit to 8% difference. Analyzes were performed by intention to treat. Response and progression under therapy were analyzed by using Fisher’s exact test. EFS was calculated from the date of random assignment to the date of first appearance of disease progression, relapse, or death as a result of any cause. Patients alive without progression or relapse were censored at the date they were last known to be alive.
EFS and OS were estimated by using the Kaplan-Meier method, and the differences between groups were compared by using the log-rank test. Differences between groups were calculated on the basis of rounded estimates, whereas 95% confidence intervals (CIs) for these differences were calculated on the basis of exact estimates. Kaplan-Meier estimates at 5 years with 95% CIs were calculated for the probability of not having an event in the end points of EFS and OS.
Results
Patients
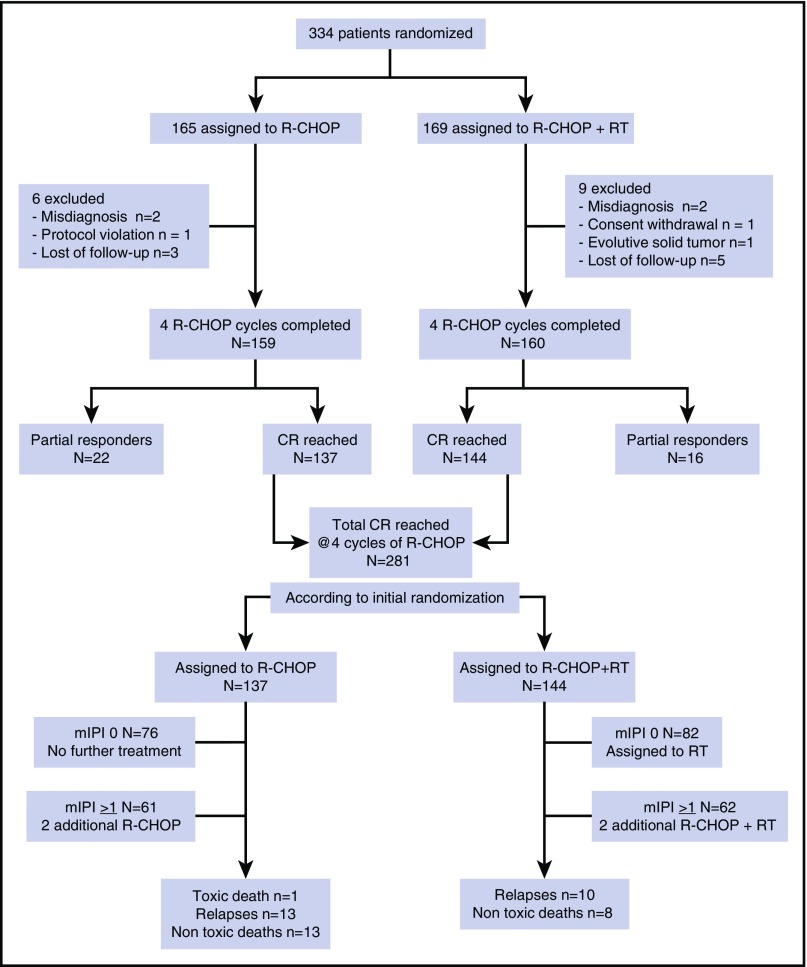
Between May 2005 and June 2014, 334 patients were enrolled at 42 participating French institutions; 165 were randomly assigned to R-CHOP alone and 169 to R-CHOP followed by RT (Figure 1). Fifteen patients were excluded for the following reasons: misdiagnosis (n = 4), protocol violation (n = 1), consent withdrawal (n = 1), lost to follow-up (n = 8), progressive solid tumor 1 month after random assignment (n = 1). The main characteristics of the patients were similar in the 2 groups (Table 1). Most of the patients (315 [94%] of 334) had a very favorable mIPI (score 0 or 1). About one-third of the whole cohort (121 [36%] of 334) were older than age 60 years and therefore allocated to receive 6 cycles of R-CHOP. Of the entire cohort, 39% had extranodal involvement. The main localizations were Waldeyer’s tonsillary ring and tonsils (n = 34), cavum and sinus (n = 23), mediastinum (n = 21), tongue (n = 11), bone (n = 10), parotid (n = 10), thyroid (n = 6), and other sites (n = 12). Sixty-two patients had a negative PET scan at baseline after complete resection of the tumor after initial surgery, but those patients were well-balanced between the 2 groups. CR patients with mIPI of 0 were allocated to receive only 4 cycles of R-CHOP (n = 187) whereas those with 1 or more adverse prognostic factors (n = 148) were allocated to receive 2 additional cycles of R-CHOP. We confirm that germinal center subtype was observed predominantly in limited-stage DLBCL compared with activated B-cell phenotype (57% vs 27%, respectively).23
Figure 1.
Consort diagram.
Table 1.
Clinical characteristics of the 327 evaluable patients
| Characteristic | R-CHOP | R-CHOP + RT | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| No. of patients randomly assigned | 163 | 164 | ||
| Ratio of males to females | 94/69 | 102/62 | ||
| Age, y | ||||
| >60 | 55 | 34 | 62 | 38 |
| <60 | 108 | 66 | 102 | 62 |
| mIPI (Miller) | ||||
| 0 | 89 | 55 | 94 | 57 |
| 1 | 65 | 40 | 59 | 36 |
| 2 | 7 | 4 | 9 | 6 |
| 3 | 2 | 1 | ||
| LDH below upper limit of normal | 131 | 80 | 138 | 84 |
| ECOG PS | ||||
| 0-1 | 157 | 161 | ||
| ≥2 | 2 | 3 | ||
| Extranodal sites | 63 | 40 | 66 | 42 |
| Anatomical site | ||||
| Cervical | 85 | 85 | ||
| Axillary | 18 | 12 | ||
| Mediastinal | 9 | 12 | ||
| Inguinal-femoral | 17 | 14 | ||
| Pathology centrally reviewed (263 [80%]) | ||||
| Misdiagnosis | 2 | 2 | ||
| Cell of origin (62% analyzed) | ||||
| Germinal center | 50 | 58 | ||
| Nongerminal center | 27 | 26 | ||
| Undetermined | 15 | 17 | ||
ECOG, Eastern Cooperative Oncology Group.
Response to treatment
An interim analysis was performed after the fourth cycle of R-CHOP on the basis of clinical examination and CT and PET scans. CR was observed in 281 patients (88%) and PR in 38 (12%) with no difference between the 2 arms. PET was omitted in 4 patients considered to be in PR, regardless of the CT scan result. R-CHOP cycles 5 and 6 were delivered to 123 and 118 patients, respectively (61 and 57 in the R-CHOP arm and 62 and 61 in the R-CHOP plus RT arm). Two patients did not receive cycles 5 and 6 by medical decision, and 5 patients did not receive cycle 6 because of toxicity (1 death as a result of toxicity during febrile neutropenia and 4 grade 4 hematologic toxicities after cycle 5). According to initial random assignment, among the 281 patients in CR after R-CHOP, 144 received RT and 137 did not receive any further treatment. Eight patients declined RT for personal reasons (Table 2).
Table 2.
Results according to treatment arm in 319 assessable patients
| Result | R-CHOP (n = 159) | R-CHOP + RT (n = 160) | ||
|---|---|---|---|---|
| No. | % | No. | % | |
| After cycle 4 | ||||
| CR | 137 | 86 | 144 | 90 |
| PR (PET-positive) | 22 | 14 | 16 | 10 |
| End of treatment | ||||
| CR | 150 | 94 | 157 | 98 |
| PR | 8 | 2 | ||
| Progression | 0 | 1 | ||
| Relapse | 13 | 8 | 10 | 6 |
| Secondary malignancy | 2 | 2 | ||
| Death | 12 | 8 | 9 | 6 |
Treatment of the 38 patients with PR after 4 cycles of R-CHOP included 2 additional cycles of R-CHOP and RT for 27 patients, 2 additional cycles of R-CHOP without RT (n = 5), high-dose chemotherapy (n = 3), and no further treatment (n = 3). CR was documented in 28 patients, and the 10 remaining patients were considered to be in PR. Two patients with positive PET scans at the end of treatment are alive and have been disease-free for more than 4 years after the end of therapy (supplemental Figure 2).
Dose delivery and toxicity
The median relative dose intensity of cytotoxic drugs (doxorubicin and cyclophosphamide) was 97% (range, 97%-98%) of that for the planned dosing schedule. Intervals between each cycle of R-CHOP were maintained from cycle 1 to cycle 4 and cycle 5 to cycle 6 with a median of 14 days. The 4-day median of delay between cycles 4 and 5 was mainly a result of the time needed for assessing response by PET. The median time between the last R-CHOP course and day 1 of RT was 36 days (range, 10-132 days).
During R-CHOP administration, hematologic toxicity was rare, with 66 episodes of febrile neutropenia occurring in 50 patients during chemotherapy. There were 4 severe infections and 1 death as a result of septic shock. Red blood cell transfusions were needed for 18 patients (6%). There was no grade 4 thrombocytopenia. After RT, 2 patients presented with grade 3 mucositis, and jaw radionecrosis was observed in 1 patient. Severe and moderate cardiac toxicity were observed in 5 and 8 patients, respectively, regardless of treatment arm. There were 4 patients with cardiomyopathy, 2 with atrial fibrillation and severe hypertension, and 1 with coronary artery disease.
Outcome
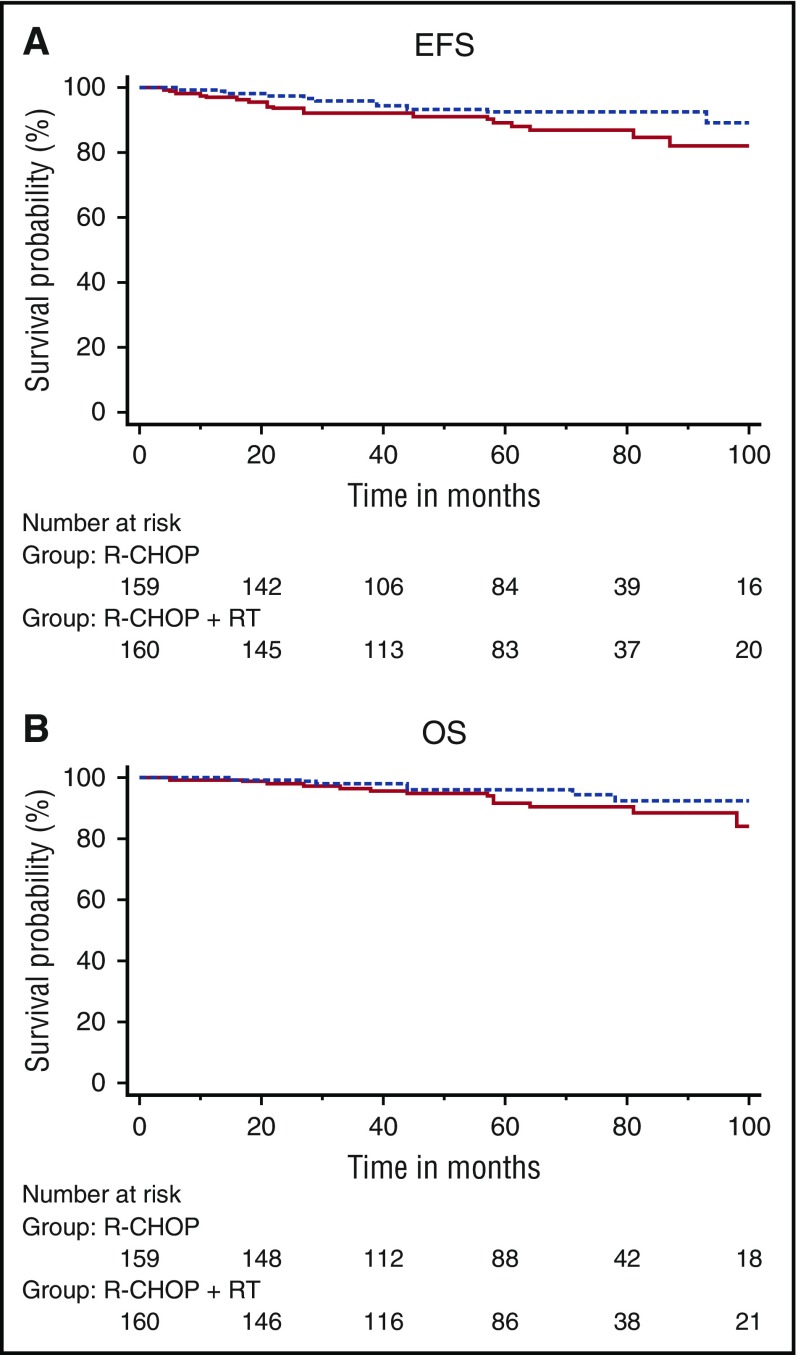
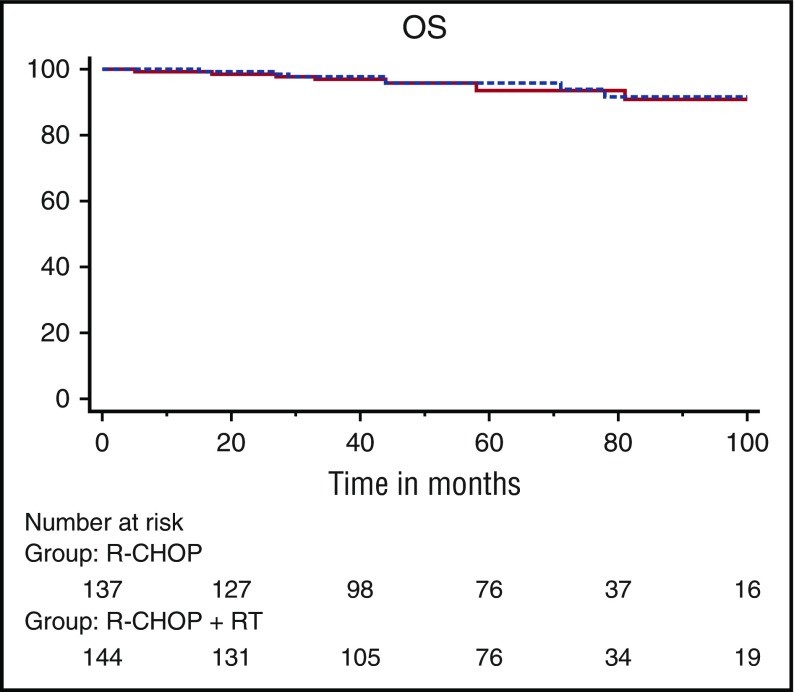
Overall, 319 patients were evaluable for response (R-CHOP arm, n = 159; R-CHOP plus RT arm, n = 160). With a median follow-up of 64 months (range, 24-132 months), in an intent-to-treat analysis, 5-year EFS was not statistically significantly different between the 2 arms: 89% ± 2.9% in the R-CHOP arm vs 92% ± 2.4% in the R-CHOP plus RT arm (hazard ratio [HR], 0.61; 95% CI, 0.3-1.2; P = .18) (Figure 2A). OS did not differ between the 2 arms, with 5-year estimates of 92% ± 2.5% for patients assigned to R-CHOP alone and 96% ± 1.7% (HR, 0.62; 95% CI, 0.3-1.5; P = .28) for those assigned to R-CHOP plus RT (Figure 2B). OS for patients in CR after the fourth cycle of R-CHOP was similar in both arms (Figure 3). After the fourth cycle of R-CHOP, OS and EFS were not statistically significantly different between patients who reached CR (n = 281) and those considered in PR (n = 38) (supplemental Figure 3A).
Figure 2.
Kaplan-Meier analysis of survival calculated from time of inclusion. (A) Event-free survival and (B) overall survival. Solid line, R-CHOP; dotted line, R-CHOP plus RT.
Figure 3.
Kaplan-Meier analysis of overall survival for patients in CR at cycle 4 according to randomization. Solid line, R-CHOP; dotted line, R-CHOP plus RT.
Pooling data from both arms, EFS was significantly affected by mIPI (HR, 2.8; 95% CI, 0.6-14.1; P = .04) comparing very good prognosis patients (mIPI 0-1) with those who had mIPI of 2 (supplemental Figure 3B). Neither the cell-of-origin pattern nor the presence or absence of extranodal sites had an impact on EFS or OS. Similarly, there was no difference in outcome between Ann Arbor stage I and II patients who had no other risk factors.
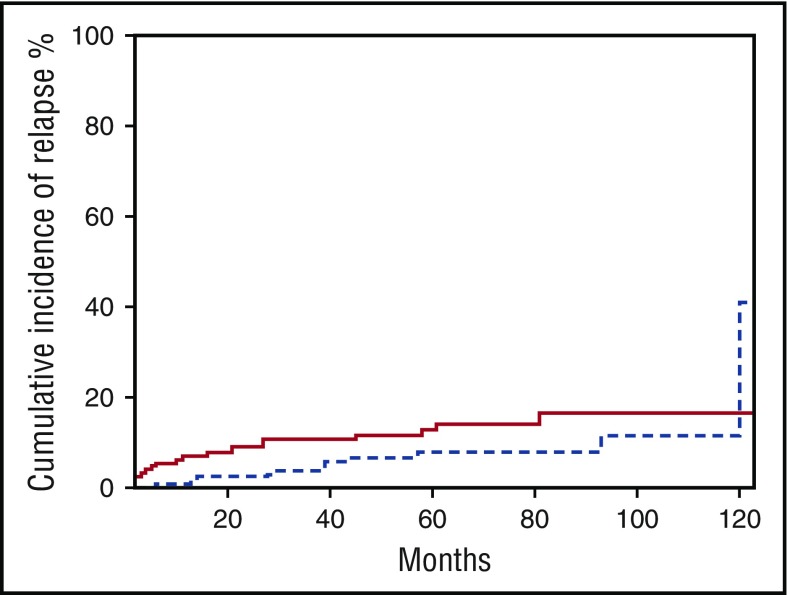
There were 21 relapses, 13 in the R-CHOP alone arm (5 at the initial site), and 10 in the R-CHOP plus RT arm (never in the irradiated field). Regarding the patients with CR after the fourth cycle of R-CHOP, 10 patients relapsed in each arm. The median time to relapse for the whole group was 21 months (range, 4-93 months) with no difference between the 2 arms (Figure 4).
Figure 4.
Incidence of relapse according to treatment arm. Solid line, R-CHOP; dotted line, R-CHOP plus RT.
There were 21 deaths, 12 in the R-CHOP alone arm, and 9 in the R-CHOP plus RT arm. Deaths were the result of progressive disease (n = 11), secondary malignancies (n = 3 [1 patient with acute myeloid leukemia, 1 with colon cancer, and 1 with pancreatic adenocarcinoma]), accident (n = 2), stroke (n = 1), late onset infection (n = 1), and unknown (n = 3).
The median relative dose intensity of cytotoxic drugs (doxorubicin and cyclophosphamide) was 97% (range, 97%-98%) of the planned schedule. Intervals between each cycle of R-CHOP were respected from cycle 1 to cycle 4 and cycle 5 to cycle 6 with a median of 14 days. The 4-day median of delay between cycle 4 and cycle 5 was mainly due to the time needed to check response assessment by PET. The median time between the last R-CHOP course and day 1 of RT was 36 days (range, 10-132 days).
During R-CHOP administration, hematologic toxicity was rare with 66 episodes of febrile neutropenia occurring in 50 patients during chemotherapy. There were 4 severe infections and 1 death as a result of septic shock. Red blood cell transfusions were needed for 18 patients (6%). There was no grade 4 thrombocytopenia. After RT, 2 patients presented with grade 3 mucositis, and 1 patient had jaw radionecrosis. Severe and moderate cardiac toxicity were observed in 5 and 8 patients, respectively, regardless of treatment arm.
Discussion
This prospective randomized trial, which enrolled 334 patients with low-tumor-burden limited-stage DLBCL, compared R-CHOP alone with R-CHOP followed by IFRT. This randomized study (the first in the rituximab era) reports definitive results showing a similar outcome with these 2 options in this specific population of patients. With more than 5 years of follow-up, EFS and OS are indeed identical between the 2 arms.
It must be stressed that only patients with nonbulky tumor (<7 cm) were selected. In fact, even in 2017, the definition of bulky disease remains arbitrary and varies between 5 and 10 cm, depending on the studies and the cooperative groups.14,19,20 Even if tumor size is not integrated into the IPI scoring system, several studies have identified it as a prognostic factor.24 Patients with a high tumor mass (usually defined as >5 cm) have a worse clinical outcome than those with low tumor burden.6,14,19,20 Whether RT should be limited to high-tumor-burden localized DLBCL in the rituximab era remains questionable. In the UNFOLDER (Unfavorable Low-Risk Patients Treated With Densification of R-Chemo Regimens) trial, patients with bulky tumor were randomly assigned to R-CHOP with or without RT, but interim analysis showed a higher incidence of relapse in patients without RT, and the R-CHOP alone arm was prematurely closed.25 The impact of tumor burden was confirmed in the MabThera International Trial (MInT) trial but not in the retrospective analysis of the RICOVER trial.20,26 Furthermore, in the LNH 03-2B Groupe d’Etude des Lymphomes de l’Adulte/LYSA (GELA/LYSA) trial, the high-dose CHOP alone regimen (ACVBP) was superior to CHOP plus RT according to IPI adjusted for younger age.6 Even if RT is supposed to induce better local tumor control, it does not prevent systemic relapse.
PET has become an essential tool for both staging and response assessment in DLBCL. More than 20% of patients with stage I or II disease evaluated by using CT scans are upstaged upon PET assessment.27 CT scan evaluation alone thus leads to underestimation of RT fields. In this trial, we included only patients with stage I or II disease based on baseline PET, a position not adopted in previously published studies.2,10,14,20 Of note, this trial was designed before the development of more accurate response criteria,28 and PET analysis relied on visual analysis. However, this did not have an impact on response assessment because nearly 90% of patients remained or became PET negative after 4 cycles of R-CHOP. The majority of patients (281 [88%] of 319) reached CR after 4 cycles of R-CHOP, which is 10% more than what is observed in patients with more advanced DLBCL treated with rituximab-combined chemotherapy.29 Whether or not these patients should receive 4 or 6 cycles of R-CHOP is currently questioned in the ongoing LNH 2009-1B trial (NCT01285765).30
Thirty-eight patients (12%) had PR after 4 cycles of R-CHOP on the basis of PET visual analysis. Persistent PET positivity may be related to disease persistence or to the inflammatory process. Biopsy was not recommended in this trial. Of these 38 patients, 28 finally achieved CR after receiving additional chemotherapy and/or RT, meaning that PET-positive signals observed after cycle 4 were mainly related to residual lymphoma. Interestingly, the outcome of these PR patients did not differ from those reaching CR after 4 cycles of R-CHOP, as previously reported.16
In this selected group of patients with limited-stage DLBCL, the relapse rate was very low, with a median time of 21 months, and indeed no patient presented relapse at the original site in the RT arm. In the literature, relapse rates after RT vary from 0% to 34%.1,5-7,14 Our study is thus the second to report the absence of local relapse after RT. This may be explained by the low tumor burden of the patients, which allowed RT to induce optimal tumor control locally, hence the low number of overall relapses. Late relapse occurred very rarely because only 4 patients had disease recurrence after 5 years. This confirms a previously reported series from our group in which 213 patients with localized DLBCL were treated with 3 cycles of high-dose CHOP and RT; only 9 patients relapsed after 5 years, with a median follow-up of 88 months.31 Of note, this was not observed in the long-term analysis of the Southwest Oncology Group S8736 (SWOG S8736) study, which compared 8 cycles of CHOP alone with 3 cycles of CHOP followed by RT.9 However, the selection of patients widely differed from that of our study: initial staging and response assessment were not based on PET imaging, patients with bulky disease were included, histology did not match with accurate classification and, importantly, treatment did not include rituximab, making the conclusions more questionable. In our trial, with an EFS higher than 90% at 5 years for CR patients, we confirm the excellent outcome of patients with low-burden DLBCL who do not present with early relapse and who achieve a survival nearly equivalent to that of the general population.32,33
This trial was designed at a time when shortening the interval between R-CHOP cycles was questioned (ie, by 2 or 3 weeks). Although we did not observe increased toxicity with a 2-week interval schedule (R-CHOP 14), a 3-week delivery (R-CHOP 21) now seems to be the best option on the basis of recent randomized studies showing similar results between these 2 regimens34,35 and could be proposed to patients who meet the inclusion criteria used in our trial.
In conclusion, this trial prompts us to recommend that patients with nonbulky limited-stage DLBCL who reach CR based on PET evaluation after 4 or 6 cycles of R-CHOP should be spared additional RT, thus avoiding radiation-related toxicity. As already proposed,16 PET could help to deliver RT to the minority of patients with residual PET-positive tumors.
Acknowledgments
The authors thank Roselyne Delepine, project medical leader, for her technical assistance; Blandine Gauthier, clinical research assistant; and all participants in the Groupe Ouest-Est des Leucémies et des Autres Maladies du Sang/French Innovative Leukemia Organization/Lymphoma Study Association groups.
This work was supported by Roche.
Footnotes
Presented in oral form at the 56th annual meeting of the American Society of Hematology, San Francisco, CA, 6-9 December 2014; and the 14th International Conference on Malignant Lymphoma, Lugano, Switzerland, 14-17 June 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: T.L., P.S., G.C., and P.C. designed the study; T.L., G.D., P.S., E.G., G.C., K.B., R.G., J.C., A.B., K.L.D., M.B., M.-P.M., S.L.G., J.F., P.G., H.M., E.D., R.H., K.L., J.P.M., O.T., B.B., A.D., J.P.V., T.F., P.C., and V.D. were responsible for patient recruitment and data collection; V.C. and V.S. performed pathological central review; J.P.V. contributed to the PET review; B.B. and M.C.B. performed statistical analysis; T.L. and M.C.B. performed the final analysis and wrote the paper; and all authors approved the final version of the manuscript and submission of the manuscript.
A complete list of the members of the LYSA Group (Lymphoma Study Association, former GOELAMS and GELA groups) appears in the online appendix.
Conflict-of-interest disclosure: T.L. received grants from Genentech. G.D. received an unrestricted research grant from Roche; G.C. served as a consultant for Celgene and Roche, and received honoraria from Bristol-Myers Squibb, Sanofi, Gilead Sciences, Roche, and Janssen Pharmaceutical. S.L.G. received grants, personal fees, and nonfinancial support from Genentech, received personal fees from Celgene, and received grants and personal fees from Janssen-Cilag outside the present work. E.D. received grants from Genentech. The remaining authors declare no competing financial interests.
The current affiliation for G.D. is Hematology Department of Basse-Normandie, CHU Caen, Microenvironment and Hematological Malignancies (MICAH), UniCaen, Caen, France.
Correspondence: Thierry Lamy, Rennes University Hospital, INSERM UMR 1236, 2 Rue du Thabor, CS 46510, 35065 Rennes, France; e-mail: thierry.lamy@univ-rennes1.fr.
References
- 1.Shenkier TN, Voss N, Fairey R, et al. Brief chemotherapy and involved-region irradiation for limited-stage diffuse large-cell lymphoma: an 18-year experience from the British Columbia Cancer Agency. J Clin Oncol. 2002;20(1):197-204. [DOI] [PubMed] [Google Scholar]
- 2.Persky DO, Unger JM, Spier CM, et al. ; Southwest Oncology Group. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26(14):2258-2263. [DOI] [PubMed] [Google Scholar]
- 3.Miller TP. The limits of limited stage lymphoma. J Clin Oncol. 2004;22(15):2982-2984. [DOI] [PubMed] [Google Scholar]
- 4.Zelenetz AD, Abramson JS, Advani RH, et al. NCCN Clinical Practice Guidelines in Oncology: non-Hodgkin’s lymphomas. J Natl Compr Canc Netw. 2010;8(3):288-334. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet C, Fillet G, Mounier N, et al. ; Groupe d’Etude des Lymphomes de l’Adulte. CHOP alone compared with CHOP plus radiotherapy for localized aggressive lymphoma in elderly patients: a study by the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2007;25(7):787-792. [DOI] [PubMed] [Google Scholar]
- 6.Reyes F, Lepage E, Ganem G, et al. ; Groupe d’Etude des Lymphomes de l’Adulte (GELA). ACVBP versus CHOP plus radiotherapy for localized aggressive lymphoma. N Engl J Med. 2005;352(12):1197-1205. [DOI] [PubMed] [Google Scholar]
- 7.Horning SJ, Weller E, Kim K, et al. Chemotherapy with or without radiotherapy in limited-stage diffuse aggressive non-Hodgkin’s lymphoma: Eastern Cooperative Oncology Group study 1484. J Clin Oncol. 2004;22(15):3032-3038. [DOI] [PubMed] [Google Scholar]
- 8.Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339(1):21-26. [DOI] [PubMed] [Google Scholar]
- 9.Stephens DM, Li H, LeBlanc ML, et al. Continued risk of relapse independent of treatment modality in limited-stage diffuse large B-cell lymphoma: Final and long-term analysis of Southwest Oncology Group Study S8736. J Clin Oncol. 2016;34(25):2997-3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yahalom J. Radiation therapy after R-CHOP for diffuse large B-cell lymphoma: the gain remains. J Clin Oncol. 2010;28(27):4105-4107. [DOI] [PubMed] [Google Scholar]
- 11.Vargo JA, Gill BS, Balasubramani GK, Beriwal S. Treatment selection and survival outcomes in early-stage diffuse large B-cell lymphoma: Do we still need consolidative radiotherapy? J Clin Oncol. 2015;33(32):3710-3717. [DOI] [PubMed] [Google Scholar]
- 12.Longo DL. Combined-modality therapy for early-stage diffuse large B-cell lymphoma: Knowing when to quit. J Clin Oncol. 2015;33(32):3684-3685. [DOI] [PubMed] [Google Scholar]
- 13.Persky DO, Miller TP. Localized large cell lymphoma: is there any need for radiation therapy? Curr Opin Oncol. 2009;21(5):401-406. [DOI] [PubMed] [Google Scholar]
- 14.Phan J, Mazloom A, Medeiros LJ, et al. Benefit of consolidative radiation therapy in patients with diffuse large B-cell lymphoma treated with R-CHOP chemotherapy. J Clin Oncol. 2010;28(27):4170-4176. [DOI] [PubMed] [Google Scholar]
- 15.Ng AK, Dabaja BS, Hoppe RT, Illidge T, Yahalom J. Re-examining the role of radiation therapy for diffuse large B-cell lymphoma in the modern era. J Clin Oncol. 2016;34(13):1443-1447. [DOI] [PubMed] [Google Scholar]
- 16.Sehn LH. Chemotherapy alone for localized diffuse large B-cell lymphoma. Cancer J. 2012;18(5):421-426. [DOI] [PubMed] [Google Scholar]
- 17.Sehn LH, Gascoyne RD. Diffuse large B-cell lymphoma: optimizing outcome in the context of clinical and biologic heterogeneity. Blood. 2015;125(1):22-32. [DOI] [PubMed] [Google Scholar]
- 18.Jaffe ES, Harris NL, Stein H, et al. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2001. [Google Scholar]
- 19.Nieder C, Licht T, Andratschke N, Peschel C, Molls M. Influence of differing radiotherapy strategies on treatment results in diffuse large-cell lymphoma: a review. Cancer Treat Rev. 2003;29(1):11-19. [DOI] [PubMed] [Google Scholar]
- 20.Pfreundschuh M, Kuhnt E, Trümper L, et al. ; MabThera International Trial (MInT) Group. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12(11):1013-1022. [DOI] [PubMed] [Google Scholar]
- 21.Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC; 2008. [Google Scholar]
- 22.Spaepen K, Stroobants S, Verhoef G, Mortelmans L. Positron emission tomography with [(18)F]FDG for therapy response monitoring in lymphoma patients. Eur J Nucl Med Mol Imaging. 2003;30(Suppl 1):S97-S105. [DOI] [PubMed] [Google Scholar]
- 23.Roberts RA, Rimsza LM, Staudt L, et al. : Gene expression differences between low and high stage diffuse large B cell lymphoma (DLBCL) [abstract]. Blood. 2006;108(11). Abstract 809.
- 24.International Non-Hodgkin’s Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329(14):987-994. [DOI] [PubMed] [Google Scholar]
- 25.German High-Grade Non-Hodgkin’s Lymphoma Study Group. Rituximab and combination chemotherapy with or without radiation therapy in treating patients with B-cell non-Hodgkin’s lymphoma. https://clinicaltrials.gov/show/NCT00278408.
- 26.Held G, Murawski N, Ziepert M, et al. Role of radiotherapy to bulky disease in elderly patients with aggressive B-cell lymphoma. J Clin Oncol. 2014;32(11):1112-1118. [DOI] [PubMed] [Google Scholar]
- 27.Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011;29(14):1844-1854. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortelazzo S, Tarella C, Gianni AM, et al. Randomized trial comparing R-CHOP versus high-dose sequential chemotherapy in high-risk patients with diffuse large B-cell lymphomas. J Clin Oncol. 2016;34(33):4015-4022. [DOI] [PubMed] [Google Scholar]
- 30.The Lymphoma Academic Research Organisation. Evaluate a Treatment Adapted to the PET Response Compared to a Standard Treatment, for Low Risk DLBCL CD 20+ Patients. http://clinicaltrials.gov/ct2/show/NCT01285765.
- 31.Bernard M, Cartron G, Rachieru P, et al. ; French GOELAMS Group. Long-term outcome of localized high-grade non-Hodgkin’s lymphoma treated with high dose CHOP regimen and involved field radiotherapy: results of a GOELAMS study. Haematologica. 2005;90(6):802-809. [PubMed] [Google Scholar]
- 32.Maurer MJ, Ghesquières H, Jais JP, et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J Clin Oncol. 2014;32(10):1066-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakobsen LH, Bøgsted M, Brown PN, et al. Minimal Loss of Lifetime for Patients With Diffuse Large B-Cell Lymphoma in Remission and Event Free 24 Months After Treatment: A Danish Population-Based Study. J Clin Oncol. 2017;35(7):778-784. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham D, Hawkes EA, Jack A, et al. Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in patients with newly diagnosed diffuse large B-cell non-Hodgkin lymphoma: a phase 3 comparison of dose intensification with 14-day versus 21-day cycles. Lancet. 2013;381(9880):1817-1826. [DOI] [PubMed] [Google Scholar]
- 35.Delarue R, Tilly H, Mounier N, et al. Dose-dense rituximab-CHOP compared with standard rituximab-CHOP in elderly patients with diffuse large B-cell lymphoma (the LNH03-6B study): a randomised phase 3 trial. Lancet Oncol. 2013;14(6):525-533. [DOI] [PubMed] [Google Scholar]