TO THE EDITOR:
Histiocytic sarcoma (HS) is a rare hematopoietic neoplasm derived from non-Langerhans histiocytic cells of the monocyte/macrophage system that is diagnosed using immunohistochemistry markers, such as CD68, lysozyme, CD4, and CD163, on the tissue biopsies.1 HS can occur in isolation or in association with other hematological neoplasms like non-Hodgkin lymphoma (NHL), myelodysplasia, or acute leukemia.2 HS has variable clinical presentation and outcomes, ranging from localized disease to multiple sites within a single system, to life-threatening disseminated disease (preferentially involving the skin, soft tissue, and gastrointestinal tract). The exact incidence of HS in adults is unclear, and contemporary clinical data are mostly limited to institutional case series.2-7 In this study, we used the Surveillance, Epidemiology, and End Results (SEER) Program database (https://seer.cancer.gov/) to study the incidence, clinical presentation, and outcomes of HS.
SEER, a program of the US National Cancer Institute, collects cancer incidence and survival data from population-based cancer registries covering ∼28% of the US population. We identified HS cases (age >18 years) that were confirmed histologically using International Classification of Diseases for Oncology, Third Edition histology code 9755/3 from the SEER 18 (1973–2014) registry, and we included cases that were diagnosed after 2000. We calculated the incidence (cases per 1 000 000) and disease-specific survival (DSS) rates using the 2000–2014 SEER 18 registries and age-adjusted those to the US 2000 standard population. Patient-level data were analyzed to determine demographic findings and clinical outcome. We used SEER*Stat (version 8.3.4; https://seer.cancer.gov/seerstat/) for incidence and survival statistical calculations. The SEER*Stat Multiple Primary-SIR tool was used to calculate standard incidence ratios (SIRs) for secondary malignancies by comparing these patients’ subsequent cancer diagnoses with the number of cancers that would be expected based on incidence rates for the general US population.
A total of 159 cases with HS were reported in the SEER database between 2000 and 2014. We excluded 1 case that was reported with concomitant myeloid monocytic leukemia because that could be potentially classified as myeloid sarcoma. The median age at diagnosis was 63 years (range, 18-96 years) and was similar in both sexes. The overall incidence of HS was 0.17 per 1 000 000 individuals (95% confidence interval [CI]: 0.14-0.19]. The incidence according to racial groups was: whites, 0.18 (95% CI: 0.15-0.21), African Americans, 0.04 (95% CI: 0.01-0.11), and others (Asian/Pacific Islander/American Indian), 0.12 (95% CI: 0.06-0.21). The incidence was significantly lower among African Americans compared with whites (incidence rate ratio: 0.27; 95% CI: 0.08-0.64; P = .0009), and was higher among males (female:male incidence ratio = 0.49; P = .0001). The most common sites of the presentation were skin and connective tissue (35.8%), followed by lymph nodes (17%), respiratory system (8.2%), and nervous system (7.5%). Patient characteristics are summarized in Table 1.
Table 1.
Characteristics of HS patients
| Variable | Number |
|---|---|
| Total number of patients (2000–2014) | 159 |
| Median age, y | 63 (range, 18-86) |
| Males | 63 (18-95) |
| Females | 63.5 (18-96) |
| Sex (%) | |
| Males | 99 (62.3) |
| Females | 60 (37.7) |
| Year of diagnosis (%) | |
| 2000–2007 | 63 (39.6) |
| 2008–2014 | 96 (60.4) |
| Race (%) | |
| White | 138 (86.8) |
| African American | 5 (3.1) |
| Asian/Pacific Islander | 12 (7.5) |
| American Indian | 0 (0.0) |
| Unknown | 4 (2.5) |
| Site frequencies (%) | |
| Connective tissue and skin | 57 (35.8) |
| Nodal | 27 (17) |
| Respiratory system (including sinuses) | 13 (8.2) |
| Nervous system | 11 (6.9) |
| GI tract (including hepatobiliary and pancreas) | 12 (7.5) |
| Bone marrow and hematopoietic system | 7 (4.4) |
| Spleen and RES | 8 (5.0) |
GI, gastrointestinal; RES, reticuloendothelial system.
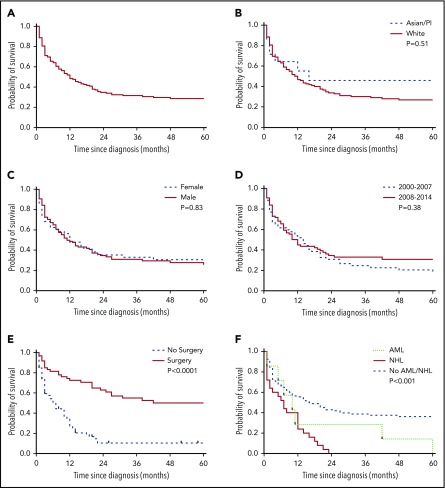
The median overall survival (OS) in the entire cohort of HS was 6 months (Figure 1A). Five-year DSS was similar in males (42.3% [95% CI: 29.8-54.2]) and females (33.6% [95% CI: 18.9-48.9]) (Figure 1B). Similarly, median OS was similar among all races (Figure 1C). Although the incidence of HS significantly increased over time (P = .006), the 5-year OS was similar between patients diagnosed from 2000 to 2007 and 2008 to 2014 (Figure 1D). The median OS did not differ significantly based on the primary site (P = .06). After censoring patients with bone marrow, spleen, and reticuloendothelial system involvement, patients who were managed surgically had a better OS compared with those who were not (hazard ratio: 0.33 [95% CI: 0.21-0.50]; P < .0001) (Figure 1E).
Figure 1.
OS analyses by Kaplan-Meier curves in patients with HS. (A) Predicted OS in patients with HS. (B) Comparison of OS by race. (C) Comparison of OS by sex. (D) Comparison of OS of patients diagnosed between 2000 and 2007 and between 2008 and 2014. (E) Comparison of OS based on surgical treatment. (F) Comparison of OS based on the presence of concomitant NHL and AML. P < .05 was considered significant. PI, Pacific Islander.
After a median follow-up of 7.5 months (range, 0-178 months), 115 patients had died. Among these 115 deaths, 66 (57.3%) were due to a malignancy, either HS alone (n = 24) or in combination with other neoplasms (n = 42). The coexisting malignancies in patients who died were as follows: NHL (n = 28), acute myeloid leukemia (AML; n = 6 patients), chronic lymphocytic leukemia (n = 3), myeloma (n = 1) mixed germ cell tumor (n = 1), splenic marginal cell lymphoma (n = 1), papillary transitional cell carcinoma of urinary bladder (n = 1), and renal cell carcinoma (n = 1). The median OS was significantly shorter in patients who had concomitant NHL (7 months) and AML (10 months) compared with that of HS alone (16 months) (P < .001) (Figure 1F). Compared with the general population, HS patients had an increased risk of developing NHL (SIR: 51.2; P < .05). HS occurred as second primary malignancy in 34 cases, which included follicular lymphoma (n = 5), precursor cell lymphoblastic leukemia (n = 4), small B-cell lymphoblastic leukemia (n = 3), breast cancer (n = 3), prostate cancer (n = 5), aleukemic leukemia (n = 1), gall bladder adenocarcinoma (n = 1), and miscellaneous cancers (n = 12). No significant difference in survival was noted whether HS occurred as a first primary malignancy or second primary malignancy (P = .38).
The major limitations of our study can be attributed to its derivation from a database, with limited information on the specific therapies received, patient performance status, and comorbidities. Additionally, in the case of patient deaths in the presence of HS and another concomitant, aggressive malignancy, we were unable to ascertain the exact cause of death. Nevertheless, our study provides important insights into disease incidence, clinical presentation, and patient outcomes, where traditional studies are limited due to the rarity of the condition.
In summary, this study is the largest report on adult HS patients in the United States. It shows that HS is a very rare malignancy that is less common among the African American population and in females. The prognosis was relatively poor for patients who had concomitant hematological neoplasms (NHL and AML). Also, there is an increased risk of subsequent development of NHL. Our study highlights that, despite the rising incidence, there has not been an improvement in survival, clearly emphasizing the unmet need for better therapies for this rare but lethal malignancy.
Authorship
Contribution: A.K., S.H.T., and G.G. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis; A.K. and S.H.T. conceptualized and designed the study and drafted the manuscript; A.K., S.H.T., and M.D. contributed to acquisition, analysis, or interpretation of data and statistical analysis; A.K., S.H.T., M.D., G.G., and R.S.G. critically revised the manuscript for important intellectual content; and G.G. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gaurav Goyal, Division of Hematology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: goyal.gaurav@mayo.edu.
References
- 1.Yoshida C, Takeuchi M. Histiocytic sarcoma: identification of its histiocytic origin using immunohistochemistry. Intern Med. 2008;47(3):165-169. [DOI] [PubMed] [Google Scholar]
- 2.Pileri SA, Grogan TM, Harris NL, et al. Tumours of histiocytes and accessory dendritic cells: an immunohistochemical approach to classification from the International Lymphoma Study Group based on 61 cases. Histopathology. 2002;41(1):1-29. [DOI] [PubMed] [Google Scholar]
- 3.Mikami M, Sadahira Y, Suetsugu Y, Wada H, Sugihara T. Monocyte/Macrophage-specific marker CD163+ histiocytic sarcoma: case report with clinical, morphologic, immunohistochemical, and molecular genetic studies. Int J Hematol. 2004;80(4):365-369. [DOI] [PubMed] [Google Scholar]
- 4.Hornick JL, Jaffe ES, Fletcher CD. Extranodal histiocytic sarcoma: clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am J Surg Pathol. 2004;28(9):1133-1144. [DOI] [PubMed] [Google Scholar]
- 5.Feldman AL, Arber DA, Pittaluga S, et al. Clonally related follicular lymphomas and histiocytic/dendritic cell sarcomas: evidence for transdifferentiation of the follicular lymphoma clone. Blood. 2008;111(12):5433-5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steussy B, Lekostaj J, Qian Q, et al. Leukemic transdifferentiation of follicular lymphoma into an acute histiocytic leukemia in a 52-year-old caucasian woman. Lab Med. 2016;47(2):155-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen W, Lau SK, Fong D, et al. High frequency of clonal immunoglobulin receptor gene rearrangements in sporadic histiocytic/dendritic cell sarcomas. Am J Surg Pathol. 2009;33(6):863-873. [DOI] [PubMed] [Google Scholar]