Abstract
Isolated and spontaneous superior mesenteric artery dissection is a rare cause of acute abdominal pain. Whereas there is widespread consensus on conservative treatment of asymptomatic forms, revascularization would seem indicated in symptomatic complicated cases. A 73-year-old man presented with worsening epigastric pain. A computed tomography scan revealed an isolated and spontaneous superior mesenteric artery dissection with aneurysmal evolution of the false lumen, involving multiple side branches. The postdissection aneurysm was treated by endovascular exclusion with flow-diverting stents. The abdominal pain was completely relieved, and the patient remained asymptomatic at follow-up.
Isolated and spontaneous superior mesenteric artery dissection (ISMAD) is a rare cause of acute abdominal pain, described for the first time by Bauersfeld in 1947, with an incidence of 0.06% from postmortem studies.1, 2 ISMAD etiology is not well defined. Atherosclerosis, fibromuscular dysplasia, cystic medial necrosis, connective tissue disorders, and hemodynamic stress due to the curvature of the vessel at its origin have been suggested as possible causes, even though they have never been clearly demonstrated in the literature. Uncontrolled hypertension, reported in the literature in 30% of the cases, is considered an additional risk factor.3
Early identification of ISMAD in the acute stage is increasingly feasible nowadays because of advanced imaging technology like computed tomography (CT) scanning. In the literature, several ISMAD radiologic classifications and many different treatment algorithms have been proposed, but optimal decision-making has not yet been defined, especially in case of aneurysmal evolution of the false lumen, for which some failures are reported.4, 5, 6, 7, 8, 9 For this reason, we considered the use of a flow-diverting stent (FDS) in the treatment of a patient with ISMAD complicated by symptomatic aneurysmal evolution of the false lumen.
The patient described in this case report has consented to the publication of this article.
Clinical case
A 73-year-old man was referred to our department with worsening epigastric pain. His medical history included previous cigarette smoking and high blood pressure. Contrast-enhanced CT showed an ISMAD with aneurysmal evolution of the false lumen (maximum transverse diameter, 32 mm). The patent false lumen had connections with the true lumen at both ends. The lesion length was approximately 10.0 cm, involving multiple superior mesenteric artery (SMA) side branches (Fig 1). The ISMAD started before the origin of the inferior pancreaticoduodenal artery and ended just before the origin of the ileocolic artery. The middle colic artery, the right colic artery, and several jejunal branches (at least seven) originated from the true lumen of the dissection.
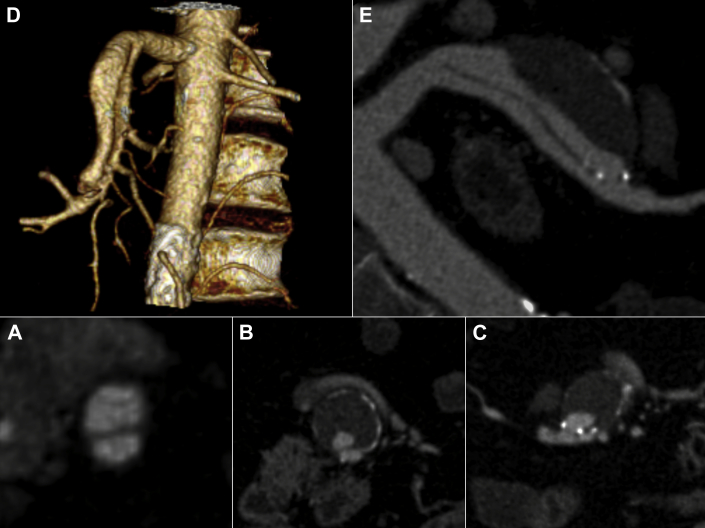
Fig 1.
Preoperative contrast-enhanced computed tomography (CT) scan (A-C) with three-dimensional reconstructions (D and E) showing the isolated and spontaneous superior mesenteric artery dissection (ISMAD) with aneurysmal evolution of the false lumen (maximum transverse diameter, 32 mm). The false lumen has connections with the true lumen at both ends. The lesion length is approximately 10 cm, involving multiple superior mesenteric artery (SMA) side branches.
The diameter of the SMA was 12 mm proximal to the entry tear of the dissection and 4 mm distal to the re-entry tear. In consideration of this significant difference between the proximal and distal diameters of the vessel, we chose to treat the lesion with two overlapping FDSs of different caliber. Under local anesthesia, two FDSs (6 × 100 mm and 12 × 80 mm; Cardiatis SA, Isnes, Belgium) were deployed through the percutaneous femoral approach, covering the entry and re-entry tears of the dissection from distal to proximal (Fig 2).
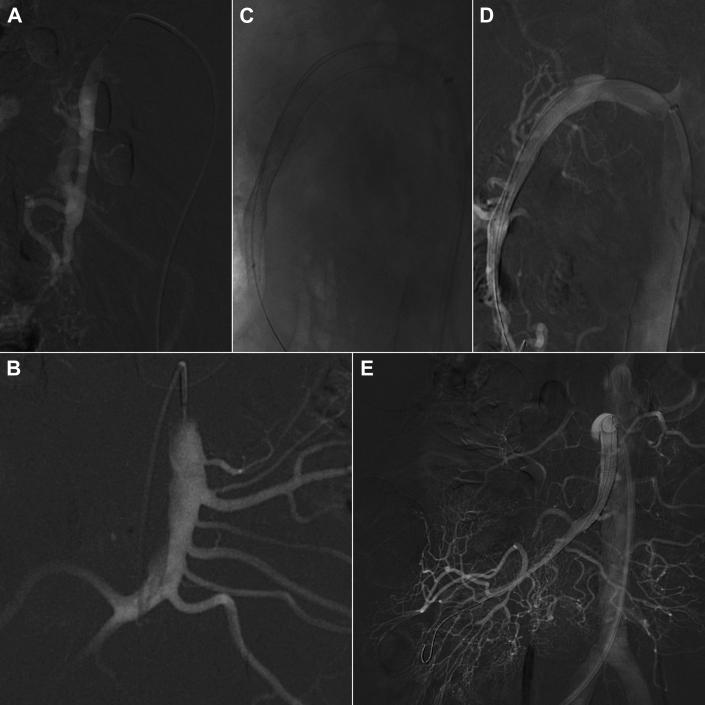
Fig 2.
Intraoperative angiography showing the isolated and spontaneous superior mesenteric artery dissection (ISMAD; A and B), the deployment of the two flow-diverting stents (FDSs; C and D), and the final result of the procedure with good expansion of the true lumen and patency of the superior mesenteric artery (SMA) and its side branches (E).
The 6-mm diameter of the distal FDS was chosen because it is the smallest diameter peripheral FDS available. The proximal stent diameter was decided on the normal internal lumen of the proximal SMA without any oversizing to reduce the caliber mismatch between the two FDSs.
The abdominal pain was completely relieved on the first postoperative day, and the patient remained asymptomatic at follow-up. The patient was instructed to take dual antiplatelet therapy with acetylsalicylic acid 100 mg and clopidogrel 75 mg for 8 weeks followed by single antiplatelet therapy (acetylsalicylic acid 100 mg) indefinitely, and he has complied with this medical regimen. At 1 month and 3 months of follow-up, duplex ultrasound scan showed patency of the stents and progressive thrombosis of the false lumen. The CT scan at 6 months revealed complete thrombosis of the false lumen, aneurysm shrinkage (reduction of the transverse diameter from 32 mm to 27 mm), and patency of the SMA and its branches (Fig 3). Eighteen months after the procedure, a duplex ultrasound examination confirmed these results, and the patient remains asymptomatic.
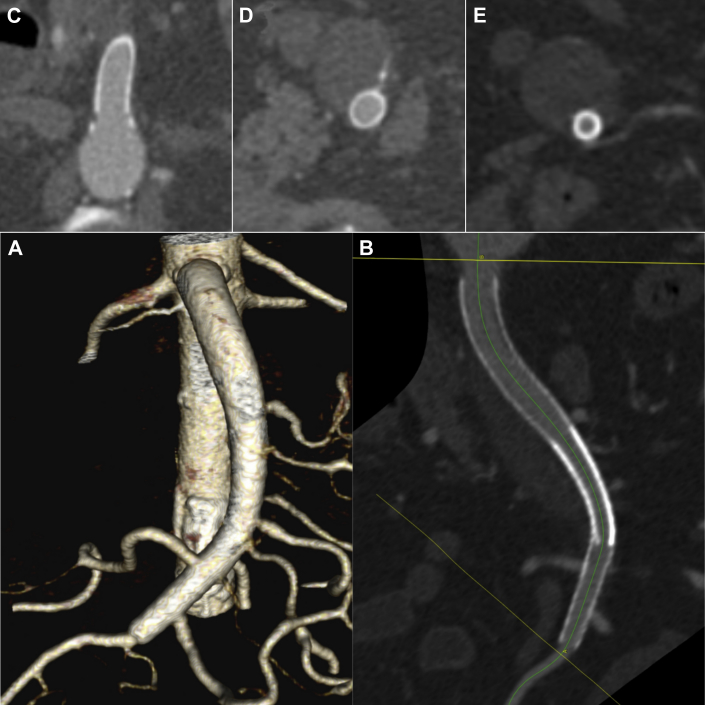
Fig 3.
The 6-month follow-up contrast-enhanced computed tomography (CT) scan showing patency of the superior mesenteric artery (SMA) and its side branches (A and B) and complete thrombosis of the false lumen of the dissection (C-E).
Discussion
Several ISMAD classifications attempting to correlate radiologic findings and clinical course are reported in the literature, but no one so far has been able to prove a clear relationship between them.4, 5, 6, 7, 8 Similarly, many different ISMAD treatment algorithms have been proposed, but an optimal decision-making protocol has not yet been defined. Whereas there is widespread consensus on conservative treatment of asymptomatic ISMAD, the same cannot be said for its symptomatic forms.4, 5, 6, 7, 8, 9
Today, open surgery remains the treatment of choice only for ISMAD cases complicated by intestinal necrosis or vessel rupture.7, 9 Symptomatic cases complicated by severe true lumen compression, angina abdominis, or aneurysmal evolution of the false lumen are instead directly referred to endovascular treatment. In all other cases, an initial attempt of conservative treatment with antiplatelet or anticoagulant therapy is suggested.7, 8
Endovascular treatment is taken into account in the event of persistent or worsening abdominal pain despite appropriate medical therapy and progression or aneurysmal evolution of the dissection at early CT follow-up.7, 8 Entry tear dissection coverage with self-expandable bare stent is currently the first choice for ISMAD endovascular treatment. However, in case of significant aneurysmal evolution of a patent ISMAD false lumen, some failures requiring microcoil embolization have been reported.4, 7, 9 The use of covered stents is limited by the need to preserve the patency of the SMA side branches, and it is feasible only in case of short lesions because a more extensive vessel coverage will most likely cause segmental bowel necrosis.
In the case reported, the abdominal pain was not only related to the ISMAD itself but more likely caused by the remarkable aneurysmal evolution of the false lumen. Believing that abdominal pain could be a threat of pending arterial rupture, we chose a procedure that, compared with a self-expandable bare stent, could immediately reduce the risk of such an event and at the same time maintain the patency of the SMA side branches, thus avoiding the risk of bowel necrosis. There was no concern about the risk of a type II endoleak during follow-up because all the collateral vessels of the SMA arose from the true lumen of the dissection.
FDSs are stentlike devices that have been initially developed to treat intracranial aneurysms, and their role in this area is evolving and expanding. Further studies with longer follow-up are needed to evaluate their role in the therapeutic armamentarium of intracranial aneurysms.10 These endovascular devices are designed to reduce flow and shear stress within the aneurysm sac and to improve laminar flow in the parent artery, resulting in a gradual aneurysm thrombosis. FDSs, however, have some limitations: the aneurysm occlusion process occurs over time; the only peripheral FDS available (Cardiatis MFM) has a wide range of choice in terms of diameter (6 to 16 mm) and length (30 to 120 mm), but there is not a tapered configuration that would be very useful in the treatment of long lesions; the costs of aneurysm exclusion with FDSs are high, and therefore they should be reserved for complex lesions otherwise untreatable.
The initial clinical experiences with the use of FDSs in the treatment of visceral aneurysms have provided satisfactory results in terms of aneurysm thrombosis (87.5%) and shrinkage (75%) as well as in terms of patency of the branch vessels (100%).11, 12, 13 These good results prompted us to perform the exclusion of the ISMAD aneurysmal evolution with such a device.
Conclusions
The use of FDSs seems an attractive and ideal solution to treat complex ISMAD and, at the same time, to maintain patency of the SMA side branches. The good results obtained in the case reported seem to confirm this hypothesis, but larger series are needed.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Bauersfeld S.R. Dissecting aneurysm of the aorta: a presentation of fifteen cases and a review of the recent literature. Ann Intern Med. 1947;26:873–879. doi: 10.7326/0003-4819-26-6-873. [DOI] [PubMed] [Google Scholar]
- 2.Foord A.G., Lewis R.D. Primary dissecting aneurysms of peripheral and pulmonary arteries: dissecting hemorrhage of media. Arch Pathol. 1959;68:553–577. [PubMed] [Google Scholar]
- 3.Park Y.J., Park C.W., Park K.B., Roh Y.N., Kim D.I., Kim Y.W. Inference from clinical and fluid dynamic studies about underlying cause of spontaneous isolated superior mesenteric artery dissection. J Vasc Surg. 2011;53:80–86. doi: 10.1016/j.jvs.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 4.Sakamoto I., Ogawa Y., Sueyoshi E., Fukui K., Murakami T., Uetani M. Imaging appearances and management of isolated spontaneous dissection of the superior mesenteric artery. Eur J Radiol. 2007;64:103–110. doi: 10.1016/j.ejrad.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 5.Yun W.S., Kim Y.W., Park K.B., Cho S.K., Do Y.S., Lee K.B. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur J Vasc Endovasc Surg. 2009;37:572–577. doi: 10.1016/j.ejvs.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Cho B.S., Lee M.S., Lee M.K., Choi Y.J., Kim C.N., Kang Y.J. Treatment guidelines for isolated dissection of the superior mesenteric artery based on follow-up CT findings. Eur J Vasc Endovasc Surg. 2011;41:780–785. doi: 10.1016/j.ejvs.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Li D., He Y., Alkalei A.M., Chen X., Jin W., Li M. Management strategy for spontaneous isolated dissection of the superior mesenteric artery based on morphologic classification. J Vasc Surg. 2014;59:165–172. doi: 10.1016/j.jvs.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 8.Xiong J., Wu Z., Guo W., Liu X., Wang L., Zhang H. The value of a new image classification system for planning treatment and prognosis of spontaneous isolated superior mesenteric artery dissection. Vascular. 2015;23:504–512. doi: 10.1177/1708538115589527. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida R.A., Yoshida W.B., Kolvenbach R., Vieira P.R., Zuppardo R.L., Lunardi O. Spontaneous isolated dissection of the superior mesenteric artery—which is the best therapeutic choice ? J Vasc Bras. 2013;12:34–39. [Google Scholar]
- 10.Alderazi Y.J., Shastri D., Kass-Hout T., Prestigiacomo C.J., Gandhi C.D. Flow diverters for intracranial aneurysms. Stroke Res Treat. 2014;2014:415653. doi: 10.1155/2014/415653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruffino M.A., Rabbia C. Endovascular treatment of visceral artery aneurysms with Cardiatis multilayer flow modulator: preliminary results at six-month follow-up. J Cardiovasc Surg. 2011;52:311–321. [PubMed] [Google Scholar]
- 12.Ruffino M.A., Rabbia C., Italian Cardiatis Registry Investigators Group Endovascular repair of peripheral and visceral aneurysms with the Cardiatis multilayer flow modulator: one-year results from the Italian Multicenter Registry. J Endovasc Ther. 2012;19:599–610. doi: 10.1583/JEVT-12-3930MR2.1. [DOI] [PubMed] [Google Scholar]
- 13.Sfyroeras G.S., Dalainas I., Giannakopoulos T.G., Antonopoulos K., Kakisis J.D., Liapis C.D. Flow-diverting stents for the treatment of arterial aneurysms. J Vasc Surg. 2012;56:839–846. doi: 10.1016/j.jvs.2012.04.020. [DOI] [PubMed] [Google Scholar]