Abstract
Endovascular stent placement for decompression of an entrapped left renal vein (LRV) between the aorta and superior mesenteric artery is an alternative to surgical decompression for treating the nutcracker syndrome. However, an interventional approach may be challenging because of the unfavorable configuration of the LRV, leading to insufficient stent anchoring. We provide a case of a life-threatening stent migration from the LRV into the right ventricle 2 days after stent placement and its endovascular retrieval.
Pelvic congestion syndrome (PCS) is an increasingly recognized condition, often affecting multiparous women.1 Primary PCS is caused by incompetent venous valves in the gonadal veins, and secondary PCS is caused by impaired drainage due to compression of the left renal vein (LRV) or common iliac veins.2 Nutcracker syndrome (NCS) or renal vein entrapment syndrome is a known cause for secondary PCS, among others.3 Treatment options include endovascular stent placement and LRV transposition.4, 5, 6 Alternatives are renocaval venous bypass surgery7 and nephropexy.8 In 2011, Chen et al9 published a cohort study of 61 NCS patients treated with endovascular stent implantation. They reported excellent outcomes, with only two patients complaining of persistent symptoms after stenting. In one case, a major complication occurred that required open heart surgery to retrieve a migrated stent from the right atrium.9 Wu et al10 published a second larger follow-up study (75 patients) to call attention to the rising number of early and late stent migrations. Overall, 7% developed stent migration between 10 days and 7 months after intervention at a mean follow-up of 55 months. In 80% of the cases, the stent migrated into the inferior vena cava or the right side of the heart. Migrated stents may cause life-threatening arrhythmia, vascular or cardiac perforation, cardiac tamponade, or tricuspid valve damage. Consequently, open heart surgery with extracorporeal circulation for stent extraction or tricuspid valve repair is necessary.
We report a case of a life-threatening stent migration into the right ventricle in a young woman 2 days after LRV stent placement and its endovascular retrieval with transesophageal echocardiography (TEE) guidance and heart surgery standby. The patient provided written informed consent to the publication.
Case report
A 39-year-old woman with a history of five full-term pregnancies was referred to our department for vascular assessment of NCS. The main symptoms were pelvic pain and left-sided leg swelling. Her quality of life was reduced because of dyspareunia and pain occurring while sitting or during mild physical activities. Her recent gynecologic examination was unremarkable except for the presence of parauterine varicose veins visualized by transvaginal ultrasound. Her urine sample was free of protein or red blood cells. Duplex ultrasound and magnetic resonance venography suggested LRV entrapment and dilation of both ovarian veins (Fig 1, A). Digital selective subtraction venography with injection of contrast material into the distal LRV confirmed significant LRV stenosis by demonstrating renal drainage through the dilated left ovarian vein and not through the inferior vena cava. After bilateral sclerotherapy of the parauterine veins with 10 mL of 3% polidocanol-carbon dioxide foam, both ovarian veins were successfully embolized with 12-mm Nester coils (Cook, Bloomington, Ind) without additional foam. Subsequently, the LRV was stented with a 14- × 40-mm self-expanding S.M.A.R.T. Control stent (Cordis Europe, Roden, The Netherlands; Fig 1, B). Duplex ultrasound the day after intervention confirmed a correct position of the stent in the LRV. Anticoagulation treatment with rivaroxaban was initiated.
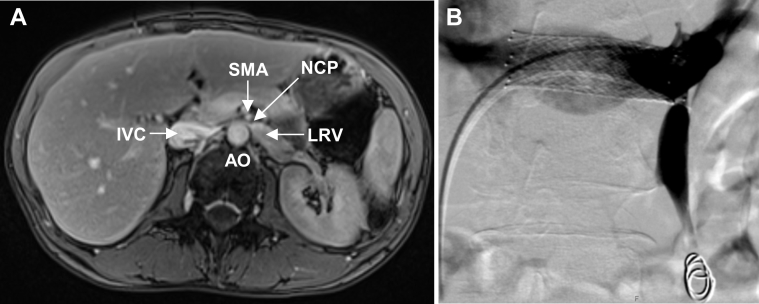
Fig 1.
Magnetic resonance venography in a 39-year old woman with nutcracker syndrome (NCS) and pelvic congestion syndrome (PCS). A, Axial image showing the compressed left renal vein (LRV) between the aorta (AO) and the superior mesenteric artery (SMA). IVC, Inferior vena cava; NCP, nutcracker point. B, Selective digital subtraction venography of LRV after successful implantation of a self-expanding S.M.A.R.T. Control stent. Both ovarian veins were embolized with polidocanol foam and 12-mm Nester coils.
Two days later, the patient was urgently referred to our hospital with severe stabbing chest pain, radiating into the left arm, and dyspnea. Her systemic blood pressure was 97/55 mm Hg, and her heart rate was 110 beats/min. Twelve-lead electrocardiography revealed a new-onset right bundle branch block. High-sensitivity cardiac troponin T level was elevated (25 ng/L). TEE revealed moderate circular pericardial effusion, tricuspid regurgitation, and the stent embedded between the tricuspid valve and the right ventricular outflow tract. A chest computed tomography scan confirmed stent migration into the right ventricle (Fig 2). The patient was prepared for endovascular stent retrieval under general anesthesia in our hybrid operating room with heart surgery standby.
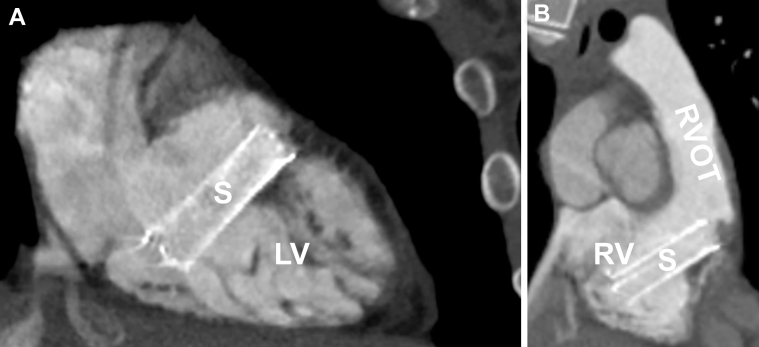
Fig 2.
A, Oblique sagittal computed tomography image showing one part of the stent (S) in proximity to the tendinous chords of the tricuspid valve. LV, Left ventricle; RA, right atrium. B, Oblique coronal computed tomography image of the stent pointing toward the right ventricle outflow tract (RVOT).
An 18F sheath (Check-Flo Performer, 40 cm; Cook Europe, Bjæverskov, Denmark) was introduced into the right common femoral vein. A 5F Judkins left diagnostic catheter (Cordis Europe) was advanced together with an exchange-length 0.035-inch Glidewire (Terumo, Leuven, Belgium) through the tricuspid valve to probe the lumen of the embedded stent in the right ventricle (Fig 3, A). After several attempts, fluoroscopy and TEE confirmed that the stent lumen was successfully probed through its ends rather than though the stent struts. The Judkins left catheter was exchanged for a 4F Glidecath (Terumo) with its tip positioned in the left pulmonary artery. The Glidewire was then exchanged for a stiff exchange-length Hi-Torque Supra Core 0.035-inch wire (Abbott Vascular, Santa Clara, Calif) to move the stent away from the tendinous chords of the tricuspid valve apparatus without additional maneuvers. TEE confirmed that the stent was now coaxial with the tricuspid valve orifice. An oversized 16- × 40-mm Atlas Gold balloon catheter (Bard Medical, Covington, Ga) was introduced over the wire into the stent with the distal balloon shoulder outside the stent to facilitate atraumatic stent extraction. The balloon was inflated at low pressure (2 atm) and then pulled back under continuous TEE observation of the tricuspid valve. The stent was retrieved into the inferior vena cava, and TEE confirmed normalized tricuspid valve function without regurgitation and no increase in pericardial effusion. A 6F sheath was now introduced into the left common femoral vein and a 6F Amplatz GooseNeck snare catheter (Medtronic, Minneapolis, Minn) advanced into the inferior vena cava. The Atlas Gold balloon catheter was retrieved once the stent was secured in the inferior vena cava, with the tip of the Hi-Torque Supra Core 0.035-inch wire snared by the Amplatz GooseNeck snare catheter (Fig 3, B). A second Amplatz GooseNeck snare catheter was introduced into the 18F sheath with the snare loop placed around the Hi-Torque Supra Core 0.035-inch wire. The snare was then partially placed around the proximal part of the stent. The stent was then successfully extracted in toto through the 18F sheath (Fig 3, C). A final venogram showed intact iliocaval veins. The patient fully recovered from the procedure except for the presence of a permanent right bundle branch block. At 3-month follow-up, she was free of pelvic congestion symptoms, and transthoracic echocardiography showed normal cardiac findings.
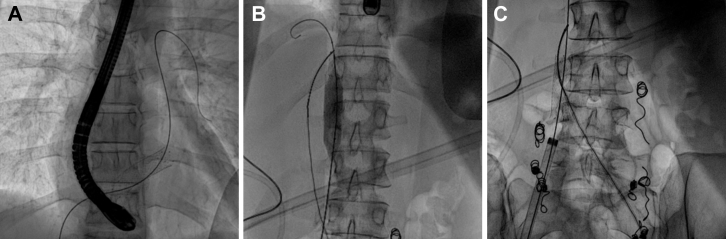
Fig 3.
Endovascular retrieval of a migrated stent from the right ventricle with transesophageal echocardiography (TEE) guidance. A, Fluoroscopic image of the stent in the right ventricle, which was successfully probed with a Glidewire. B, Fluoroscopic image showing successful retrieval of the stent from the right ventricle into the inferior vena cava. A contralaterally inserted GooseNeck snare catheter is securing the stent from migrating back into the right side of the heart. C, Fluoroscopic image showing retrieval of the captured stent with a second GooseNeck snare catheter through the 18F sheath.
Discussion
Large migrated self-expending stents are usually removed by open heart surgery. To the best of our knowledge, we report the first case of a successful endovascular extraction of a large (14-mm), laser-cut, self-expanding stent from the right ventricle in a patient with NCS. Key factors for the successful and atraumatic in toto extraction were (1) use of a large-lumen 18F sheath, (2) lifting of the stent with a stiff wire from the tricuspid valve apparatus, (3) stent extraction with an oversized balloon catheter into the inferior vena cava, and (4) use of a double snare technique to secure the stent in the inferior vena cava with the first snare from contralateral access and to extract the stent through the 18F sheath with a second snare. An alternative approach to the contralateral femoral vein access to secure migration of the captured stent is right jugular vein access.
Other extraction methods, such as direct snare maneuvers from the right ventricle, may carry a greater risk of tricuspid valve damage or cardiac tamponade. Of note, endovascular retrieval of woven stents may be easier than retrieval of laser-cut stents because woven stents can be easily collapsed by snare catheters. However, it remains unclear if this stent type has a reduced risk of stent migration in comparison to laser-cut stents. In a case report, a migrated woven 10-mm Wallstent (Boston Scientific, Marlborough, Mass) was successfully retrieved from the left pulmonary artery with the oversized balloon and snare technique.11 However, in another case of a 10-mm Wallstent that migrated into the right side of the heart, major vascular surgery was required to remove the retracted stent from the iliac vein.11
This complicated case raises two questions. First, it is controversial whether all patients with NCS and PCS require surgical or endovascular LRV decompression. In our patient without clinical signs of renal venous hypertension, such as proteinuria or hematuria, bilateral ovarian vein ablation successfully treated her pelvic venous hypertension symptoms without an LRV stent. In another case report of a patient with predominant PCS and nutcracker anatomy, ovarian vein embolization without LRV stenting was also successful.12 On the other hand, ovarian vein ablation without restoration of LRV drainage may potentially lead to flank pain, hematuria, and renal vein thrombosis.13 Although there is no evidence, measurement of the pressure gradient across the LRV may help establish the indication for LRV stent placement.
Second, our case and other cases of stent migration from the LRV raise the question of whether LRV stent placement with our currently available stents can be considered safe. Although stent sizing was based on axial imaging and venographic measurements, we cannot rule out that the stent in our case was undersized. The LRV has an unfavorable configuration leading to insufficient stent anchoring for three reasons: (1) the LRV is relatively short (4-6 cm); (2) the lumen of the LRV often tapers rapidly, with a small lumen distally and a large lumen at the entrance into the inferior vena cava; and (3) there is dynamic compression from the superior mesenteric artery anteriorly and the aorta posteriorly. The synchronous dynamic compression may play an important role in the migration of LRV stents. It remains unclear whether stent oversizing reduces the risk of migration. Unfortunately, no dedicated renal vein stents are currently available. Although open surgical repair is effective and safe, up to 30% of patients require reintervention within the first 2 years, most frequently LRV stent placement.4
Conclusions
If heart surgery standby and TEE guidance are available, an endovascular approach may be attempted to extract large venous stents migrated into the right side of the heart using the oversized balloon and snare technique.
In selected patients with the NCS and evidence of significant renal venous hypertension including hematuria or proteinuria, surgical techniques for LRV decompression should be preferred to LRV stenting. LRV stenting remains an alternative to surgery in patients who are not candidates for major vascular surgery.
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the Journal policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Beard R.W., Reginald P.W., Wadsworth J. Clinical features of women with chronic lower abdominal pain and pelvic congestion. Br J Obstet Gynaecol. 1988;95:153–161. doi: 10.1111/j.1471-0528.1988.tb06845.x. [DOI] [PubMed] [Google Scholar]
- 2.Mahmoud O., Vikatmaa P., Aho P., Halmesmaki K., Alback A., Rahkola-Soisalo P. Efficacy of endovascular treatment for pelvic congestion syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4:355–370. doi: 10.1016/j.jvsv.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Winer A.G., Chakiryan N.H., Mooney R.P., Verges D., Ghanaat M., Allaei A. Secondary pelvic congestion syndrome: description and radiographic diagnosis. Can J Urol. 2014;21:7365–7368. [PubMed] [Google Scholar]
- 4.Erben Y., Gloviczki P., Kalra M., Bjarnason H., Reed N.R., Duncan A.A. Treatment of nutcracker syndrome with open and endovascular interventions. J Vasc Surg Venous Lymphat Disord. 2015;3:389–396. doi: 10.1016/j.jvsv.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Gunka I., Navratil P., Lesko M., Jiska S., Raupach J. Laparoscopic left renal vein transposition for nutcracker syndrome. Ann Vasc Surg. 2016;31:209.e1–209.e5. doi: 10.1016/j.avsg.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Wang P., Jing T., Qin J., Xia D., Wang S. Robotic-assisted laparoscopic transposition of the left renal vein for treatment of the nutcracker syndrome. Urology. 2015;86:e27–e28. doi: 10.1016/j.urology.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y., Sun Y., Jin X. Left renocaval venous bypass with autologous great saphenous vein for nutcracker syndrome. J Vasc Surg. 2012;55:1482–1484. doi: 10.1016/j.jvs.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 8.Hmida W., Mallat F., Othmen M.B., Limayem F., Mosbah F. Modified medial nephropexy for treatment of the anterior nutcracker syndrome. Urol Ann. 2014;6:352–355. doi: 10.4103/0974-7796.141004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen S., Zhang H., Shi H., Tian L., Jin W., Li M. Endovascular stenting for treatment of Nutcracker syndrome: report of 61 cases with long-term followup. J Urol. 2011;186:570–575. doi: 10.1016/j.juro.2011.03.135. [DOI] [PubMed] [Google Scholar]
- 10.Wu Z., Zheng X., He Y., Fang X., Li D., Tian L. Stent migration after endovascular stenting in patients with nutcracker syndrome. J Vasc Surg Venous Lymphat Disord. 2016;4:193–199. doi: 10.1016/j.jvsv.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 11.El Feghaly M., Soula P., Rousseau H., Chaiban F., Otal P., Joffre F. Endovascular retrieval of two migrated venous stents by means of balloon catheters. J Vasc Surg. 1998;28:541–546. doi: 10.1016/s0741-5214(98)70142-9. [DOI] [PubMed] [Google Scholar]
- 12.Perkov D., Vrkic Kirhmajer M., Novosel L., Popic Ramac J. Transcatheter ovarian vein embolisation without renal vein stenting for pelvic venous congestion and nutcracker anatomy. Vasa. 2016;45:337–341. doi: 10.1024/0301-1526/a000547. [DOI] [PubMed] [Google Scholar]
- 13.Mallat F., Hmida W., Jaidane M., Mama N., Mosbah F. Nutcracker syndrome complicated by left renal vein thrombosis. Case Rep Urol. 2013;2013:168057. doi: 10.1155/2013/168057. [DOI] [PMC free article] [PubMed] [Google Scholar]