Abstract
This scoping review investigates if, over the last 25 years in high resource countries, midwives’ patients of low socioeconomic position (SEP) were at more or less risk of adverse infant birth outcomes compared to physicians’ patients. Reviewers identified 917 records in a search of 12 databases, grey literature, and citation lists. Thirty-one full documents were assessed and nine studies met inclusion criteria. Eight studies were assessed as moderate in quality; one study was given a weak rating. Of the moderate quality studies, the majority found no statistical difference in outcomes according to model of care for preterm birth, low or very low birth weight, or NICU admission. No study reported a statistically significant difference for small for gestational age birth (2 studies), or mean or low Apgar score (4 studies). However, one study found a reduced risk of preterm birth (AOR=0.70, p<0.01), and heavier mean infant birth weight (3325 g vs. 3282 g, p<0.01) for midwifery patients. Another study reported lower risk of low (RR=0.59, 95% CI: 0.46, 0.73) and very low birthweight (RR=0.44, 95% CI: 0.23, 0.85) for midwifery care. And, a third study reported a decrease in stays (1–3 days) in NICU (Adjusted Risk Difference=−1.8, 95% CI: −3.9, 0.2) for midwifery patients, though no overall difference in NICU admission of any duration. Other studies reported significant differences favoring midwifery care for mean birth weight (3598 g vs. 3407.3 g, p<0.05; 3233 g vs. 3089 g, p<0.05; 2 studies) and very low birth weight (OR=0.35, 95% CI:0.1, 0.9), for sub-groups within the larger study populations. This scoping review documented heterogeneity in study designs and analytical methods, inconsistent findings, moderate methodological quality, and lack of currency. There is a need for new studies to definitively establish if and how a midwifery-led model of care influences birth outcomes for women of low SEP.
Keywords: Midwifery, Socioeconomic position, Vulnerable women, Prenatal care, Infant birth outcome, Preterm birth
Highlights
-
•
Some evidence, but not consistently, of modest improvements in birth outcomes.
-
•
Limitations in study designs, methods, highlight the need for high quality studies.
-
•
Prospective cohort studies needed, with adequate power, adjusting for confounders.
-
•
Future studies are needed to establish the nature of the impact of midwifery care.
Introduction
In high resource countries there are significant disparities in prevalence of adverse birth outcomes, such as preterm birth (PTB), among infants born to women of low vs. high socioeconomic position (SEP) (Blumenshine, Egerter, Barclay, Cubbin, & Braveman, 2010). SEP demarcates social class based on material and social resources (i.e. wealth and educational credentials) and prestige (i.e. occupation, or other measures of social rank) (Krieger, 2001, p. 1). When socioeconomic barriers consistently lead to adverse health outcomes for a historically marginalized population—such as women of low SEP—health disparity mirrors social injustice (Braveman & Gruskin, 2003). Therefore, there is an ethical imperative based on the principles of fairness and the universal human right to “the highest attainable standard of health” (Constitution of the World Health Organization, 1946), to rectify health disparities (Braveman & Gruskin, 2003).
Women of low SEP are more commonly exposed to the known causal determinants of PTB and intrauterine growth restriction (IUGR) compared to women of higher SEP, including: smoking, substance use, low gestational weight gain, short stature, prolonged standing and strenuous work activity, inadequate prenatal care, bacterial vaginosis, and psychological factors such as depression, physical abuse and low social support (Kramer, Séguin, Lydon, & Goulet, 2000). At birth, PTB or IUGR infants are at greater risk of neonatal death, respiratory distress, failure to regulate temperature, and hospital readmission (Bernstein, Horbar, Badger, Ohlsson, & Golan, 2000; Martens, Derksen, & Gupta, 2004; Wang, Dorer, Fleming, & Catlin, 2004). Long-term, these infants have higher rates of delayed cognitive, emotional, and developmental growth compared to those born at full-term (Alexander, 2007), and as adults may have increased odds of cardiovascular disease, hypertension, and diabetes (Barker, 1995; Ross & Beall, 2008).
Because a number of the causal determinants of adverse infant outcomes associated with low SEP are potentially avoidable, strategies that promise even modest improvements warrant serious consideration. In a Cochrane Review (2015) examining randomized trials that compared midwifery-led continuity of care models to other care models for childbearing women, researchers found that midwifery care reduced the likelihood of preterm birth by 24% (Relative Risk 0.76, 95% CI: 0.64, 0.91) and fetal loss before 24 weeks gestation by 19% (RR 0.81, 95% CI: 0.67, 0.98) (Sandall, Soltani, Gates, Shennan, & Devane, 2015). If these findings are equally applicable for women of low SEP, whose infants are at the greatest risk of adverse outcomes, midwifery-led care may be an ideal model for vulnerable women.
Typically, physician-led care equates with the biomedical model of care. In this model the aim of prenatal care is to reduce risk of maternal fetal/infant morbidity and mortality through screening, diagnosis and treatment of complications as they arise (van Teijlingen, 2005). The biomedical model assumes a standardized approach to pregnancy and childbirth, with deviations from the norm often countered through medical intervention (Gregg, 1995). Though patient-centered care is encouraged within the biomedical model, the model is shaped by pathology and the underlying medical paradigm (Barry & Edgman-Levitan, 2012).
In contrast, midwifery practice specifically focuses on the mother׳s social, psychological, and cultural well-being, as well as the normal biological processes of pregnancy, birth and transition to parenthood (ten Hoope-Bender et al., 2014). A core element of the model, as defined in The Lancet Midwifery Series, includes capacity building to strengthen women׳s ability “to care for themselves and their families” (ten Hoope-Bender et al., 2014, p. 1227). Empowering patients as partners in health care requires mutual trust, and regard for the “woman׳s need for time, information, encouragement, validation and a supportive presence” (Kennedy, 2000, p. 10). Because of long appointment times and the model׳s relational emphasis, midwives are well positioned to understand and respond to contextual factors influencing patients’ behavior (Davis, 2010), such as personal autonomy, material and social resources, and individual abilities (Downe, Finlayson, Walsh, & Lavender, 2009). For low income women, practitioner–patient trust has been linked with clinician continuity, another hallmark of midwifery care (Phillippi & Avery, 2014), and has been associated with adherence to clinical advice (Sheppard, Zambrana, & O׳Malley, 2004). In addition, personalized continuity of care, in which a woman feels that her prenatal caregiver knows and remembers her and her health history from one visit to the next, has been shown to result in a three-fold increase in “very good” patient care ratings (Davey, Brown, & Bruinsma, 2005), which is especially important for women of low SEP who have reported lower levels of satisfaction in care compared to women of higher SEP (Haviland, Morales, Dial, & Pincus, 2005). All of these elements of care: time, trusting relationship, and individualized care, along with emotional support, and the de-medicalization of pregnancy, have been identified as key attributes of quality prenatal care by women and care providers of all types (Sword et al., 2012). In addition, it is important to note that despite their names, either model, the biomedical model or midwifery model, can and has been adopted and delivered by various types of maternity providers. The attributes of midwifery care described here are not exclusive to the midwifery profession; it is a clinician׳s philosophy of care that determines his or her model of practice.
To date there has been no review of the literature examining birth outcomes of midwifery-led care compared to physician-led care for women of low SEP. The purpose of this scoping review is to identify all available information on this topic from the last 25 years, in order to present a summary of the “extent, range and nature” of the research, determine key gaps in the literature, and provide guidance for future studies (Arksey & O׳Malley, 2005, p. 6). This review will investigate if, in countries belonging to the Organization of Economic Co-operation and Development (OECD) (Organization of Economic Co-operation and Development (OECD), 2014), midwives’ patients of low socioeconomic position were at greater or lesser risk of adverse infant birth outcomes compared to physicians’ patients.
Methods
Selection of inclusion criteria
A review team, with combined expertise from obstetrics, epidemiology, midwifery, sociology, and public health conducted this review. Methods were based on Arksey and O’Malley׳s scoping studies framework (Arksey & O׳Malley, 2005), with the exception of the quality assessment in which we used the Effective Public Health Practice Project (EPHPP) Quality Assessment Instrument (Effective Public Health Practice Project (EPHPP), 2009). After determining the research topic, five inclusion criteria were identified to guide study selection. Studies must have (1) been conducted in an OECD country; (2) compared antenatal care exclusively or predominantly delivered by midwives with physician-led care; (3) reported on one or more of the following outcomes: PTB, IUGR, small-for-gestational age (SGA) birth, Apgar score, birth weight (including mean, low and very low birth weight), and/or neonatal intensive care unit (NICU) admission; (4) included participants of low SEP (defined as low income, education or prestige); and (5) had a publication date no earlier than January 1, 1990 (see Table 1). No language restrictions were applied.
Table 1.
Keywords searched.
| Prenatal care | Prenatal* OR antenatal* OR pregnan* |
|---|---|
| Low SEP | Poor OR poverty OR “low income” OR socioeconomic OR socio-economic OR depriv* OR disadvantag* OR marginali?e* OR vulnerabl* OR “low education” OR “low prestige” OR “social class” OR “social classes” OR disparit* OR inequalit* OR discriminat* OR inequit* OR indigent OR impoverish* |
| OECD countries | Australia OR Austria OR Belgium OR Canada OR Chile OR Czech Republic OR Denmark OR Estonia OR Finland OR France OR Germany OR Greece OR Hungary OR Iceland OR Ireland OR Israel OR Italy OR Japan OR “Korea Republic” OR Luxembourg OR Mexico OR Netherlands OR “New Zealand” OR Norway OR Poland OR Portugal OR “Slovak Republic” OR Slovenia OR Spain OR Sweden OR Switzerland OR Turkey OR “United Kingdom” OR UK OR England OR Scotland OR Wales OR “United States” OR US OR USA (manually searched) |
| Infant birth outcomes | “Preterm birth” OR “preterm births” OR “pre-term birth” OR “pre-term births” OR prematur* OR “small for gestational age” OR “small-for-gestational-age” OR apgar OR “birth weight” OR “birth weights” OR birthweight* “intrauterine growth restriction” OR “intrauterine growth retardation” OR “neonatal intensive care” OR NICU OR “infant outcome” OR “infant outcomes” OR “birth outcome” OR “birth outcomes” |
| Midwifery-led care | midwif* OR midwives OR nurse-midwif* OR nurse-midwives |
| Physician-led care | physician* OR obstetrician* OR doctor* OR “family practitioner” OR “family practitioners” OR “shared care” OR “medical led” OR “medical-led” OR “medical managed” OR “medical-managed” OR “medical model” OR “medical models” OR “usual care” OR “standard care” |
Only studies conducted in OECD countries were included to ensure the results of the review are relevant to healthcare systems in high resource settings. With the exception of Mexico and Turkey, infant mortality rates for OECD countries range between 0.9 and 7.7 per 1000 live births, with a median of 3.5 (Organization of Economic Co-operation and Development, 2015). As infant mortality is a commonly accepted indicator of maternal–infant health (Reidpath & Allotey, 2003), reflecting in part the quality of national healthcare systems, membership in the OECD can be considered a proxy for similarly adequate maternal infant healthcare services across study locations.
Because standards of perinatal practices and trends in birth outcomes continually change, we restricted our search to studies published after 1990 to ensure the results would be relevant for current policy and practice.
Selection strategy
The search strategy included all relevant citations in 12 databases (see Table 2) and was conducted between June 8 and 10, 2015. When possible, email alerts were requested from databases to capture any new publications, up until August 31, 2015. Grey literature, including government reports and dissertations, was searched in six databases and a hand search was conducted of all articles published between January 1, 2010 and August 31, 2015 in four journals (see Table 2). Reference lists from studies meeting the inclusion criteria were manually searched to further identify relevant studies. Because some articles omit the national setting, referring only to the city and/or state/province, the study setting was searched manually. All citations and abstracts were imported into EndNote X7 to facilitate management and remove duplicates. To minimize bias and error in the selection of the studies, two reviewers (D.N.M. and K.S.) independently assessed titles and abstracts retrieved from the initial key search against the inclusion criteria.
Table 2.
Sources searched.
| Electronic databases | Grey literature | Hand searched |
|---|---|---|
| MEDLINE | Effective Public Health Practice Projects | American Journal of Epidemiology |
| EMBASE | New York Academy of Medicine Grey Literature Report | American Journal of Public Health |
| CINAHL | Public Health Grey Literature Sources | Journal of Midwifery and Women׳s Health |
| Ovid Healthstar | Centre for Review and Dissemination (UK) | Midwifery |
| Cochrane Library | Health Evidence | |
| ProQuest: Public Health | OIAster | |
| PubMed | Google Scholar | |
| Global Health | ||
| AMED | ||
| Web of Science Core Collection | ||
| Joanna Briggs Institute EBP Database | ||
| ProQuest Dissertations and Theses Global |
Quality assessment and data extraction
Though scoping reviews generally do not assess individual study quality, we chose to include a quality assessment to evaluate the adequacy of the research evidence. The EPHPP Quality Assessment Instrument (Effective Public Health Practice Project (EPHPP), 2009) for quantitative studies was utilized to ensure standardized quality assessment. The content/construct validity and reliability of this tool has been previously assessed (Thomas, Ciliska, Dobbins, & Micucci, 2004), and the National Collaborating Centre for Methods and Tools gave it a strong methodological rating (National Collaborating Centre for Methods and Tools, 2008).
Two raters per study (from a total of six) independently scored study quality on a scale that examined selection bias, study design, confounding, blinding, data collection, and rates of participant withdrawal/attrition. The instrument required a strong rating on at least four of the six component areas, and no weak ratings in any area, to merit a “strong” quality rating. Studies with less than four strong ratings and one weak rating were deemed “moderate” and those with two or more weak ratings were considered “weak” (Thomas et al., 2004). Discrepancies between the reviewers’ overall ratings were discussed and consensus reached for all quality ratings. Data extraction, using a standardized form, was conducted by a primary reviewer (D.N.M.) and verified by a secondary reviewer (K.S.). A narrative description of the results is reported.
Results
Selection of studies
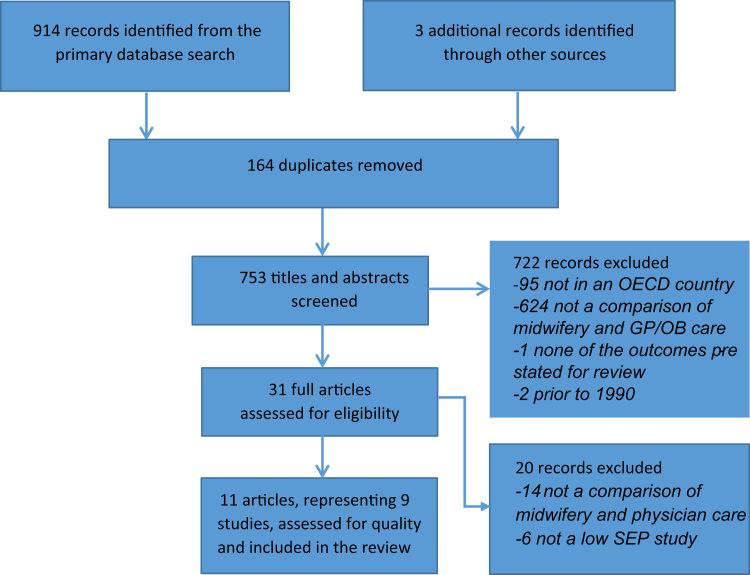
The search yielded 917 records, of which 164 were duplicates. Of the remaining 753 titles and abstracts screened using the inclusion criteria, 722 records were excluded per criteria (see Fig. 1). Thirty-one studies that either appeared to meet all of the inclusion criteria, or in which it was unclear whether or not the study met the criteria, were retained for full review. Fourteen of these studies were subsequently excluded because they did not compare midwifery-led care with physician-led care, and a further six did not specifically examine outcomes for women of low SEP. The remaining 11 articles and dissertations, representing nine studies, met all of the inclusion criteria.
Fig. 1.
Results of the study selection process.
The quality assessment determined that eight of the nine studies were of moderate methodological quality (Heins et al., 1990, McLaughlin et al., 1992, Benatar et al., 2013, Jackson et al., 2003, Visintainer et al., 2000, Fischler and Harvey, 1995, Simonet et al., 2009, Cragin, 2002); one study was given a weak rating (Blanchette, 1995); and none received a strong quality rating. Of the nine studies selected, seven were reported in peer-reviewed articles (Benatar et al., 2013; Heins et al., 1990, McLaughlin et al., 1992, Benatar et al., 2013, Jackson et al., 2003, Visintainer et al., 2000, Fischler and Harvey, 1995, Blanchette, 1995), one was described in a dissertation (Cragin, 2002), and one was documented in both a dissertation and a peer-reviewed article (Simonet et al., 2009).
Quality of included studies
Confounding due to differences in perinatal risk between groups was adequately controlled for in four studies through: (a) inclusion/exclusion criteria based on established birth centre midwifery eligibility (Benatar et al., 2013, Jackson et al., 2003); (b) a previously developed scale and risk scoring conducted by public health nurses (Heins et al., 1990); and (c) by state and national clinical guidelines (Cragin, 2002). With the exception of three studies (Blanchette, 1995; Visintainer et al., 2000, Simonet et al., 2009, Blanchette, 1995), the remainder of the studies also employed analytical methods, such as matching, to control for known perinatal risk. However, in the study by Visintainer et al. (2000) the administrative data utilized lacked information on current/prior health complications, potentially introducing major confounding as physicians’ scope of practice includes higher risk patients, more likely to experience poor birth outcomes. In the study by Simonet and colleagues (Simonet et al., 2009) there was, likewise, no adjustment for differences in current/prior health complications, due to a lack of data, but the study design may have helped to mitigate confounding. Women were classified as midwifery or physician patients according to the type of practitioner that provided the majority of care in their place of residence. This could have introduced some misclassification of provider type but may have minimized confounding, if the residents of the two communities had relatively equal prevalence of current/prior maternal health complications. In the study by Blanchette, there was no attempt to control for any type of confounding, and the comparison groups had significantly different characteristics, therefore it was given a weak quality rating.
Intent to treat analysis (ITT), in which a woman׳s birth outcomes were analyzed according to the practitioner type with whom she initiated care—regardless of subsequent cross-over—was utilized in five of the studies (Benatar et al., 2013; Heins et al., 1990; Jackson et al., 2003; McLaughlin et al., 1992; Visintainer et al., 2000). Three studies either did not use ITT, or failed to report it (Blanchette, 1995; Fischler & Harvey, 1995; Simonet et al., 2009). One study used a “modified” approach in which ITT was used for all cases, with the exception of women who transferred between provider types and received greater than 60% of their care from their second provider (n=21) (Cragin, 2002). These cases were then excluded from the analysis. Studies that failed to utilize an ITT analysis may have introduced bias, as the exclusion of women referred from midwifery-led care to physician-led care could have skewed the overall health profile and related outcomes in the midwifery cohorts.
In this review, power estimates were described for primary outcomes in four studies (Heins et al., 1990, Jackson et al., 2003, Simonet et al., 2009, Cragin, 2002), with Simonet et al. and Cragin citing rare outcomes or small samples sizes as limitations (Cragin, 2002; Simonet et al., 2009,). Without adequate power or any reported power analysis (which was also the case for all post hoc analyses) it is difficult to determine if small sample sizes prevented the detection of clinically relevant and statistically significant differences (Button et al., 2013). The two studies which found an overall statistically significant difference in PTB (Benatar et al., 2013) and low birth weight (LBW) (Visintainer et al., 2000) prevalence for midwifery patients each included more than 15 000 cases.
Though LBW (< 2500 g) is a frequently reported birth outcome in the literature, this classification often includes preterm infants and those born SGA because of IUGR (Kramer et al., 2000). In order to understand what factors influence the relationship between SEP and gestational age, and SEP and fetal growth, it is necessary to examine each outcome separately; however none of the studies reviewed examined IUGR.
Adverse birth outcomes
Six studies reported on PTB (Benatar et al., 2013; Blanchette, 1995; Fischler & Harvey, 1995; Heins et al., 1990; Jackson et al., 2003; Simonet et al., 2009)—with only Benatar et al.׳s (2013) study finding a statistically significant reduction (30%) in odds for women in the care of midwives vs. physicians (AOR 0.70, p<0.01). A sub-analysis of outcomes among African American women demonstrated similar results (AOR 0.71, p<0.01). The other five studies reported no statistically significant association.
The most frequently investigated outcome was LBW. Though LBW was examined in all nine studies, only Visintainer et al. (2000) reported a statistically significant lower risk (41%) of LBW among midwives’ patients (RR 0.59, 95% CI: 0.46, 0.73) compared to physicians’ patients. An even lower risk was reported when the analysis was restricted to Medicaid recipients (RR=0.44, 95% CI: 0.34, 0.57). Six of the remaining studies reported findings that favored midwifery care, but were not statistically significant (Benatar et al., 2013; Blanchette, 1995; Cragin, 2002; Heins et al., 1990; Jackson et al., 2003; Simonet et al., 2009).
Three studies reported on very low birth weight (VLBW) (Heins et al., 1990, Jackson et al., 2003, Visintainer et al., 2000), all indicating lower risk for midwifery compared to physician patients, but only two reported a statistically significant difference either overall, or for a sub-group of participants. Visintainer et al. reported reduced risk for VLBW (RR 0.44, 95% CI: 0.23, 0.85) for midwifery patients; the risk for VLBW babies was further reduced when the analysis was restricted to only Medicaid recipients (RR 0.32, 95% CI: 0.16, 0.63) (Visintainer et al., 2000). Heins et al. reported no statistical difference in outcomes according to practitioner type for the overall sample, but a post hoc, sub-analysis found reduced odds in VLBW babies for African American women with high risk scores for adverse outcomes cared for by midwives compared to similar women cared for by obstetricians (OR 0.35, 95% CI: 0.1, 0.9) (Heins et al., 1990).
Three studies reported mean birth weight of newborns; one indicated birth weights that overall were statistically significantly higher for women receiving midwifery care; the second study reported significantly higher newborn birth weight for patients in the care of private practice nurse-midwives, but not for women receiving care from nurse-midwives in a hospital clinic, and the third study reported significantly higher birth weight only for primiparous women. Benatar et al. reported average birth weights of 3325 g for midwives’ patients vs. 3282 g (p<0.01) for physicians’ patients (Benatar et al., 2013). Fischler et al. reported that, in a private-practice setting, nurse-midwives’ patients had a 191 g higher mean birth weight (Beta 0.13, p<0.05), but no statistically significant difference if cared for by midwives in a hospital clinic, compared to physicians’ patients (Fischler & Harvey, 1995). And McLaughlin et al. found, in a post hoc, sub-analysis, mean birth weight was significantly higher, by 144 g, for primiparous but not multiparous women in the care of midwives (Beta 0.17, p<0.05) (McLaughlin et al., 1992).
Two studies reported on NICU admission. Fischler et al. found no difference in NICU admissions for midwifery compared to physician patients (Fischler & Harvey, 1995). Jackson et al. found a significantly lower risk for NICU admission of short duration (1–3 days) for newborns of midwifery patients (Adjusted Risk Difference −1.8, 95% CI: −3.9, 0.2), but no significantly lowered risk for NICU admissions of any or longer duration (more than 3 days) (Jackson et al., 2003).
Two studies examined SGA (Jackson et al., 2003, Simonet et al., 2009), and four studies reported on Apgar scores (Benatar et al., 2013, Jackson et al., 2003, Fischler and Harvey, 1995, Blanchette, 1995), but none found significant associations between midwifery-led care and these outcomes, compared to physician-led care. None of the selected studies reported on IUGR.
Discussion
Of the eight moderate quality studies reviewed, primary care delivered by midwives—either exclusively or as part of a comprehensive prenatal intervention—was associated with similar outcomes to that of physician-led care. Significant associations favoring midwifery care were found in: one of five studies for preterm birth, one of eight studies for low birth weight, one of three studies for very low birth weight, one of three studies investigating higher mean birth weight, and one study examining NICU stays (1–3 days), though no association with NICU admission of any duration was found in this or a second study examining this outcome. Sub-analyses also found significantly better outcomes for midwifery patients in one study examining very low birth weight, and in two other studies investigating mean birth weight. However, instances of inadequate adjustment for confounding, inadequate power, and variability in design, limit the conclusiveness of the evidence.
Mean birth weight was significantly higher among midwifery patients, in every moderate quality study in which it was examined (Heins et al., 1990; McLaughlin et al., 1992; Visintainer et al., 2000). Other studies have reported a birth weight gradient associated with maternal education, a common measure of SEP (Mortensen et al., 2008; Mortensen, Diderichsen, Smith, & Andersen, 2009). In a Danish study by Mortensen et al. maternal smoking was identified as the key mediator reducing infant birth weight for women with low education (Mortensen et al., 2009). The three studies in this review that found a significant positive association between midwifery care and heavier birth weights, controlled for smoking in their analyses. However, none of the studies measured smoking reduction or cessation over the course of pregnancy by practitioner-type, a factor that could have influenced the outcomes.
This raises the question of self-selection bias, commonly suspected in midwifery/physician comparison studies, in which cohorts have systematically different health or behavioral characteristics associated with choice of caregiver. Four of the moderate quality studies demonstrated evidence of adjustment for self-selection bias. Both of the randomized controlled trials included in this review (Heins et al., 1990; McLaughlin et al., 1992) attained comparability between cohorts on all measured demographic characteristics, with the exception of marital status for primiparas in the study by McLaughlin et al., suggesting unknown confounders were likely controlled for through design. Benatar et al. utilized propensity score modeling to create a comparison group with almost identical observable characteristics to that of the midwifery cohort (Benatar et al., 2013). And, in the study by Simonet et al. there was likely little to no self-selection bias as all women were classified as midwifery or physician patients on the basis of their community of residence, regardless of the actual maternity provider involved in care (Simonet et al., 2009).
Of interest, in Fischler et al.’s study, a significant difference in average birth weights was reported between private practice midwifery patients and physician patients (191 g, p<0.05), but not among midwifery patients serviced at a hospital-based clinic compared to physician patients—despite controlling for demographic and medical risk (Fischler & Harvey, 1995). In interpreting these differing results, Fischler et al. speculate that the model of care provided by midwives in a hospital setting may bear a greater resemblance to the medical model of care than to midwifery care, thus producing outcomes similar to those of physician-led care.
Among reviewed studies that found an association between midwifery care and lower prevalence of adverse birth outcomes, three included women with more than one social or medical predictor of risk. In the study by McLaughlin et al. meaningful differences were found for average birth weight for midwives’ patients who were nulliparous and poor, compared to physicians’ patients (McLaughlin et al., 1992), but not for multiparous women who are at less risk of poor birth outcomes (Shah, 2010). Though these results should be viewed with caution because of a small sample size (n=165), they are in agreement with theory underlying other successful antenatal interventions aimed at lowering prevalence of adverse infant birth outcomes for low income women. For example, the Nurse–Family Partnership Program (Olds, Henderson, Tatelbaum, & Chamberlin, 1986) has traditionally only included first time mothers, as it is hypothesized that they are especially receptive to perinatal and lifestyle counselling (a major component of midwifery care), compared to multiparous women who may resist new advice in favor of deferring to previous personal experience (Olds, 1981).
Secondly, Benatar et al. utilized a sample population comprised of 85% African American, low-income women, finding a significant improvement in PTB rates for midwifery patients (Benatar et al., 2013). In the U.S., women of African American race/ethnicity have higher prevalence of PTB, as do women of low-income (Martin & Osterman, 2013). Lastly, in a post hoc, sub-analysis Heins et al. found midwifery care to significantly lower VLBW only for African American women who had high medical and/or social risk scores (Heins et al., 1990).
In examining why African American patients of midwifery care had lower prevalence of adverse infant birth outcomes in two of these studies, it is important to assess the significance of “race”. Nancy Krieger defines race/ethnicity as “a social, not biological, category, referring to social groups, often sharing cultural heritage and ancestry, that are forged by oppressive systems of race relations …” Krieger (2001, p. 696). Persistent discrimination, experienced across the life course, can invoke psychological distress resulting from feelings of inferiority and social exclusion (Williams & Mohammed, 2009), as well as the internalization of racialized stereotypes (Nuru-Jeter et al., 2009). Studies have found that perceived racial discrimination is a significant predictor of adverse infant outcomes for African American women, after controlling for socioeconomic and health characteristics (Collins, David, Handler, Wall, & Andes, 2004; Dominguez, Dunkel-Schetter, Glynn, Hobel, & Sandman, 2008). Racial discrimination may biologically manifest as chronic stress (Dominguez et al., 2008), which has been measured at higher levels among parturient African American women compared to non-Hispanic White women (Borders et al., 2015). Pregnant women experiencing high stress are more than twice as likely to have bacterial vaginosis, compared to women with lower stress levels (Culhane et al., 2001), increasing their odds of PTB by 60%, compared to uninfected women (Flynn, Helwig, & Meurer, 1999). Likewise, elevated cortisol levels caused by chronic stress have been associated with PTB (Giurgescu, 2009), and maternal stress has been found to increase the risk of hypertensive disorders such as preeclampsia (Leeners, Neumaier-Wagner, Kuse, Stiller, & Rath, 2007)—a leading cause of elective preterm delivery (Wadhwa, Entringer, Buss, & Lu, 2011).
Race, as a powerful marker of social risk, may have an independent effect on health status, or modify an existing relationship (Kawachi, Daniels, & Robinson, 2005), as suggested in the studies by Heins et al. and Visintainer et al. Yet, controlling for race (as was done in six studies) could obscure its effect (Kawachi et al., 2005). Just as the causes of disparity in PTB and LBW have yet to be fully elucidated (Giurgescu, McFarlin, Lomax, Craddock, & Albrecht, 2011), so the mechanisms for countering these disparities are not fully identified to date; however, studies reviewed provide evidence that midwifery care, with its emphasis on relationship, anticipatory guidance and shared-decision making, could play an important role.
Midwifery care may be a particularly effective model for all women experiencing multiple, intersecting forms of systematic marginalization. Intersectionality theory is useful in exposing how the interaction between discriminated social identities leads to unique experiences of disadvantage, often greater than what is understood by examining individual sources of discrimination singly or consecutively (Bauer 2014; Veenstra, 2011). Combined experiences of inequality due to race, class, sex, gender, ability, religion, immigrant status, etc. may modify health disparities, as was demonstrated in the study by Heins et al. in which racism and classism appear to increase the prevalence of LBW, compared to the effects of classism (low SEP) alone. In a conceptual model developed by Bogossian (2007) it is suggested that the individualized social and emotional support midwives offer effects birth outcomes by alleviating maternal stress—a by-product of oppressed social identity. Drawing on four theories of social support, Bogossian hypothesizes that midwifery care moderates stress by improving mood and emotional wellbeing, effecting positive behavior and biopsychological response; minimizing or eliminating a woman׳s “stress appraisal response”; promoting security and worth; and helping to establish a respectful clinician-patient relationship, which in turn develops maternal self-esteem (Bogossian, 2007, p. 171).
However, caution is warranted in suggesting social and emotional support as the causal mechanism promoting improved infant birth outcomes for midwifery patients. To date, numerous observational studies have examined social support interventions in relation to adverse pregnancy outcomes (Orr, 2004), with varying results, yet almost all experimental studies have found no association. In a 2010 Cochrane systematic review of seventeen trials, researchers concluded that there was no evidence of a statistically significant association between interventions enhancing social support through emotional support (i.e. counseling, or sympathetic listening), information, advice, or tangible assistance (i.e. childcare, transportation to prenatal appointments), and a reduced likelihood of LBW or PTB (Hodnett, Fredericks, & Weston, 2010). Yet, because of ambiguity in definition and measurement of “social support” it is plausible that research involving cohorts with different characteristics than those studied, or women exposed to different duration and intensity of support, type of support, support provider, or an interaction between these factors (Orr, 2004) could produce differing results.
Lastly, of the two studies that examined NICU admission rates (Fischler & Harvey, 1995; Jackson et al., 2003) a single study found a significantly lower risk difference in NICU admission for 1–3 days for midwifery patients, though no association was found for overall admission rates (Jackson et al., 2003). As some infants may be admitted to a NICU for observation for only a short period of time, admission for more than one day may be a better indicator of infant morbidity than any NICU admission.
Limitations of the review
In some instances, differences in sample populations and study designs inhibited comparability between studies. In the study by Simonet et al. (2009) the educational preparation of apprenticeship trained midwives differed from that of the Certified Nurse-Midwives in the other eight studies, therefore the results could be a measure of risk associated with model of care and/or a reflection of the practitioners’ education. Likewise, quantity of practitioner exposure was only measured in four studies (Cragin, 2002; Fischler & Harvey, 1995; Jackson et al., 2003; McLaughlin et al., 1992), thus differences in exposure between study populations may have influenced the results. And, differing measures of low SEP and varying definitions of PTB, SGA, and LBW (see Definitions following Table 3) could have impacted study outcomes, as well as hampering comparability.
Table 3.
Study characteristics.
| Author, Setting | Study design | Participant characteristics | Relevant outcomesa | Quality rating, comments |
|---|---|---|---|---|
| Benator et al.(2013) | Matched, retrospective cohort | Midwifery group (n=872); primarily low income, 21.9%<19 years old, 85% African American, African American subgroup (n=744) |
|
Moderate quality |
| Washington DC, USA 2005-2008 | Intent to treat analysis | |||
| Birth certificate data | ||||
| Clients initiating prenatal care from nurse-midwives at a free-standing birth center vs. women receiving usual care | Propensity scoring used to construct a matched comparison group | |||
| Usual care group (n=42 987); derived from propensity scoring, matched to the study population on sociodemographic, medical, and health history characteristics; AA subgroup (n=27 095) | ||||
| No reported distinction between primary and secondary outcomes | ||||
Included:
| ||||
| Simonet et al.(2009) | Retrospective cohort | Hudson Bay Inuit births (n=1529); 36.0% primiparous, 39.1% single mothers, 61.5%<11 yrs. education | Moderate quality | |
| 14 Inuit communities of Hudson Bay and Ungava Bay, Nunavik, QC, Canada 1989-2000 | Statistics Canada׳s linked live birth, infant death, and stillbirth data | Adjustment for age, educ., marital status, parity, infant, sex, plurality, community size and community-level random effects | ||
| No adjustment for preexisting health complications or maternal morbidity | ||||
| Authors acknowledged failure to reach 80% power (a=0.05) for a 30% difference in the primary outcome | ||||
| Ungava Bay Inuit births (n=1197); 29.7% primiparous, 43.1% single mothers, 64.6%<11 yrs. education | ||||
| Midwives provided majority of prenatal care and attended over 73% of deliveries in Hudson Bay vs. physicians who provided prenatal care and attended 95% of deliveries in Ungava Bay | ||||
Included:
| ||||
| Primary outcome: perinatal death, relevant secondary outcomes: PTB, SGA, LBW | ||||
| Jackson et al.(2003) | Prospective cohort study/ retrospective chart review | Collaborative care (n=1808); 22%<20 yrs. old, 54% single mothers, 86% Hispanic |
|
Moderate quality |
| Intent to treat analysis | ||||
| Medical records and a self-administered patient survey | OB-led traditional care (n=1149); 22%<20 yrs. old, 57% single mothers, 61% Hispanic | |||
| Adjusted for race/ethnicity, parity and caesarean section history, educ., age, marital status, country of origin, height, smoking during pregnancy | ||||
| Crossover between study groups, 1.9% for collaborative care vs. 1.3% for traditional care | ||||
| Power of 80% (a=.05) to detect significant risk differences of 3% to 5% for primary outcomes | ||||
| Collaborative care offered at a birth center vs. OB/OB resident care | Excluded:
|
|||
| For collaborative care, 95% of the prenatal care was delivered by CNMs (65% of participants collaboratively managed through consultation or necessary visits with an OB), 5% by OBs | ||||
| Collaborative care included case management, health education, nutrition counselling, social services | ||||
| Primary outcomes: cesarean section; major antepartum, major intrapartum, or neonatal complications; NICU admissions | ||||
| San Diego CA, USA Feb. 1, 1994-Nov. 1, 1996 | ||||
| Cragin, L.(2002) | Retrospective cohort | Nurse-midwifery care (n=801); 62% single mothers,>90% non-White, average educ. 9.6 yrs., 99% receiving Medicaid |
|
Moderate quality |
| Paper/computerized medical records | Provider type determined by clinician with whom a patient had >60% of their care | |||
| Outcomes for nurse midwifery patients vs. OB patients at 2 study sites | ||||
| OB-led care (n=372); 55% single mothers, >85% non-White, average educ. 11 yrs., 71% receiving Medicaid | ||||
| “Modified intent to treat analysis”, ITT used except for women who transferred between provider types and received >60% of care from the second provider (n=21) | ||||
| Adjustment made for maternal demographics and medical complications | ||||
| Power estimated at 80% (a=0.05) to detect ß-371 for the primary outcome | ||||
| Author acknowledged sample size was too small to find a statistically significant difference | ||||
| Primary outcome: LBW, no relevant secondary outcomes | ||||
Inclusion:
| ||||
| CA, USA April 1, 1999–March 31, 2000 | ||||
| Visintainer et al.(2000) | Retrospective cohort study | Enhanced care births (n=1474); 37% of women initiated care during the first trimester, 13% teen mothers | Moderate quality | |
| Outcomes of enhanced care, which included prenatal care administered by nurse-midwives, vs. all County births | Intent to treat analysis | |||
| County births (n=39 749); 77% of women initiated care during the first trimester, 5% teen mothers | ||||
| Westchester County, NY, USA 1992-1994 | Results stratified by 5 year age groups, race and Medicaid | |||
| Sub-analysis compared enhanced care cohort with country Medicaid births only | Inclusion:
|
|||
| No adjustment for preexisting health complications or perinatal risk | ||||
| Enhanced care included: access to counselling, individual and group instruction on childbirth, nutrition and exercise, and a Medicaid worker to assist in enrollment in federal assistance programs | ||||
| 89% of a sample of women who began the enhanced care program delivered through it | ||||
| Primary outcome: LBW | ||||
| Blanchette(1995) | Retrospective cohort | CNM patients (n=496); 15.5%<19 yrs. old, 19.6% White, 19.2% initiated prenatal care < 12 wks., 10.3% substance abuse |
|
Weak quality |
| Berkeley, CA | Clinic medical records | No adjustment for confounders | ||
| Significantly different comparison groups | ||||
| Compared outcomes for patients of a primary Care Access Clinic, the Clinic offered comprehensive care to all patients, with primary care delivered by CNMs who were supervised by 4 OBs vs. the OBs private practice patients | OB patients (n=611); 2.6%<19 yrs. old, 62.4% White, 58.8% initiated prenatal care < 12 wks., substance use unknown | |||
| Patients transferring antepartum or intrapartum from midwifery to physician care (n=12) were excluded from the analysis | ||||
Included:
| ||||
| Excluded: CNM patients who transferred care antepartum/intrapartum due to medical risk | ||||
| No reported distinction between primary and secondary outcomes | ||||
| Fischler et al.(1995) | Retrospective cohort | CNM patients in private practice (n=111); 100% receiving Medicaid, 25%<12 yrs educ., 33% primiparous, 33% smokers |
|
Moderate quality |
| A rural county in northwestern USA Jan. 1, 1989–June 30, 1990 | Medical charts | Adjustment for age, race, marital status, parity, educ., medical factors of pregnancy, smoking, adequacy of prenatal care, and setting | ||
| Compared outcomes for CNM patients in private practice to CNM patients in a hospital sponsored clinic, and to MD patients in a private practice setting | CNM patients in a hospital-sponsored clinic (n=309); 17% receiving Medicaid, 32%<12 yrs. educ., 48% primiparous, 32% smokers | |||
| MD patients in private practice (n=297); 100% Medicaid, 51%<12 yrs. educ., 39% primiparous, 47% smokers | No mention of how analysis was conducted for clients requiring transfer of care from CNMs to MDs/OBs for medical indication | |||
| No reported distinction between primary and secondary outcomes | ||||
Included:
| ||||
| McLaughlin et al.(1992) | RCT | Comprehensive care (n=217); complete perinatal data (n=170), birth weight and demographic data only (n=183) |
|
Moderate quality |
| Davidson County, TN, USA | Comprehensive care from a multi-disciplinary team including primary care from nurse-midwives vs. standard care from OB residents | Intent to treat analysis | ||
| Sub-analysis of primiparas (n=86), sub-analysis of multiparas (n=97) | ||||
| Subject loss for comprehensive group (n=34), for standard care group (n=44) | ||||
| Standard care (n=211); complete perinatal data (n=138), birth weight and demographic data only (n=167) | ||||
| Comprehensive care included care from social workers, a nutritionist, paraprofessional home visitors, and a psychologist | ||||
| Sub-analysis of primiparas (n=79), sub-analysis of multiparas (n=88) | Adjustment for age, African American race, marital status, educ., pregravid weight, male sex of infant, maternal height, pregravid medical problems, drug/alcohol use and smoking | |||
Inclusion:
| ||||
| Primary outcome: infant birth weight | ||||
| Heins et al.(1990) | RCT | Clients randomized to nurse-midwifery care (n=728); <grade 12 63.1%, 10-19 risk score 73.5%, smoking >11 cig./day 38.0% | Moderate quality | |
| South Carolina, USA July 1, 1983-Oct. 31, 1987 | Comprehensive prenatal care provided primarily by nurse-midwives and nurses under their supervision vs. standard high risk prenatal care provided by OBs | Intent to treat analysis | ||
| Midwifery subjects lost or ineligible (n=61), OB subjects lost or ineligible (n=51) | ||||
| Sub-analysis of African American women (n=348) | ||||
| Patients randomized to OB care (n=730); <grade 12 61.7%, 10-19 risk score 74.8%, smoking>11 cig./day 25.0% | ||||
| Sub-analysis of African American women (n=370) | ||||
| Power of 90% (a=0.05) to detect significant reduction in odds of LBW from 13% to 8% | ||||
| Primary outcome: LBW, secondary outcome: VLBW | ||||
Inclusion:
|
Abbreviations: PTB preterm birth; AA African American, OR odds ratio; nssd non-statistically significant difference, LBW low birthweight; CI confidence interval; SGA small for gestational age birth; OB obstetrician; ITT intent to treat analysis; CNM certified nurse-midwife; RD risk difference, VLBW very low birthweight; NICU neonatal intensive care unit; MD medical doctor; RR relative risk
Reference group is physician-led care; adjusted effect measures reported unless otherwise noted.
PTB birth at <36 wks.
LBW<2500 g.
PTB<37 completed wks. gestation.
SGA <10th percentile.
VLBW < 1500 g.
PTB<36 wks. gestation.
Undefined.
LBW<2500 g.
In five of the studies, midwifery care was part of an enhanced care intervention to improve birth outcomes which included strategies such as case management, health and nutrition education, intense follow-up of missed appointments, counselling, social services, and home visitation (Blanchette, 1995; Heins et al., 1990; Jackson et al., 2003; McLaughlin et al., 1992; Visintainer et al., 2000,). In the remaining studies, the objective was to specifically examine the effects of midwifery care as practiced in a particular setting, such as a hospital or public clinic, private practice, free-standing birth center or geographical location. The degree to which enhanced services may have influenced the results is unknown, and the effect of midwifery care cannot be considered independent of the influence of these additional services; though both positive and null associations were found for programs offering specialized care compared to those providing standard midwifery care.
In seven of the studies (Heins et al., 1990, McLaughlin et al., 1992, Jackson et al., 2003, Fischler and Harvey, 1995, Simonet et al., 2009, Cragin, 2002, Blanchette, 1995), comparison cohorts were comprised of physician (obstetrician, general practitioner, resident) patients, whereas the other two studies (Benatar et al., 2013; Visintainer et al., 2000) conducted in the U.S. compared midwifery patients’ birth outcomes to a similar population receiving “usual” perinatal care. Studies comparing outcomes of midwifery care to “usual care”, rather than physician care, may have included a small percentage of midwifery services, weakening the observed associations. But, only 7.8% of U.S. deliveries are midwifery-led (Centres for Disease Control (CDC), 2013), therefore “usual care” is primarily non-midwifery care.
Because the EPHPP Quality Assessment Instrument has only three global ratings—“weak”, “moderate” or “strong”—there is a range of quality variation within each category. Using this instrument, studies can have one weak component rating (i.e. control for confounding, a major limitation for this type of study) but still have a moderate overall rating. Of the moderate studies, some were clearly stronger than others, with some of them being of borderline, moderate quality.
Though all eligible studies conducted in OECD countries are included in this review, only one study was conducted outside of the U.S. Because of the high utilization of midwifery care in other OECD countries, there is less opportunity for observational study of midwifery care in contrast to physician-based care. It is uncertain how results from this review apply in environments with differing health care systems, rates of midwifery utilization, and/or rates of adverse birth outcomes due to divergent socioeconomic and cultural influences.
With only nine studies eligible for review, and seven of them published between 10 and 25 years ago, there is a paucity of recent research investigating this topic. Likewise, because none of the studies received a strong quality rating there is opportunity for greater rigor in design and reporting, leading to more definitive conclusions.
Recommendations
Our findings indicate that there may be benefit from evaluating different models of care when seeking solutions to improving infant outcomes among women of low SEP and socially disadvantaged contexts. RCTs would be valuable in determining the nature of this relationship, however, women in North American and other settings where midwifery care has been well established, have been unwilling to be assigned randomly to midwifery vs. other models of care (Allen, Stapleton, Tracy, & Kildea, 2013). Likewise, prospective cohort studies should be conducted, based on carefully defined comparison groups comprised of women with equivalent perinatal risk, who remain in the care of their initial primary providers throughout pregnancy. Studies need to be adequately powered, utilize intent to treat analysis, and control for confounders, including quantity of practitioner exposure. Defining and operationalizing low SEP according to theoretical principles, including the use of a composite indicator that includes measures of income/education/prestige would increase the sensitivity of SEP classification, allowing for dose–response analyses. Data collection on various risk characteristics such as perceived racial discrimination, domestic abuse, housing vulnerability, neighborhood segregation, and early childhood disadvantage would facilitate an understanding of how these factors contribute independently and modify this association. This could help to determine whether midwifery models of care benefit only women of specific demographics, or all women experiencing social marginalization; and if improvement in prevalence of poor birth outcomes is proportionate to the magnitude of a woman׳s social disadvantage. Analysis of change in health behavior over the course of pregnancy, according to practitioner-type, would also be useful in identifying mechanisms involved in improving outcomes. Future research should examine differences in practice characteristics such as duration of practitioner contact, content of care, and quality of the clinician–patient relationship, to delineate for all practitioner types, what components of care are advantageous for women of low SEP and in particular, among communities of color. Qualitative research, from the women׳s and practitioners’ perspectives, could contribute by exploring what characteristics of midwifery care they feel confer the greatest benefits and why.
Conclusion
This review provides a summary and critique of the current body of knowledge concerning the association between midwifery-led care and infant birth outcomes, compared to physician-led care, for women of low SEP. Individual studies provide evidence, in some instances, of modest improvements in birth outcomes for vulnerable women in the care of midwives. Yet overall, divergent results, heterogeneity in study designs, definitions, outcomes and analytical methods, and methodological weaknesses, highlight the need for more high quality studies to definitively establish if and how midwifery-led care influences birth outcomes for vulnerable women.
Acknowledgements
D.N.M. received financial support for this research from the University of Saskatchewan, College of Medicine and from the Department of Community Health and Epidemiology. P.A.J. is supported by a Senior Scholar award from the Child and Family Research Institute, Vancouver BC, Canada.
Contributor Information
Daphne N. McRae, Email: daphne.mcrae@usask.ca.
Nazeem Muhajarine, Email: nazeem.muhajarine@usask.ca.
Kathrin Stoll, Email: kathrin.stoll@midwifery.ubc.ca.
Maureen Mayhew, Email: maureen.mayhew@ubc.ca.
Saraswathi Vedam, Email: saraswathi.vedam@midwifery.ubc.ca.
Deborah Mpofu, Email: debbie.mpofu@saskatoonhealthregion.ca.
Patricia A. Janssen, Email: patti.janssen@ubc.ca.
References
- Alexander G.R. Prematurity at birth: Determinants, consequences, and geographic variation. In: Behrman R.E., Butler A.S., editors. Preterm birth: Causes, consequences, and prevention. National Academies Press; Washington, DC: 2007. pp. 604–643. [Google Scholar]
- Allen J., Stapleton H., Tracy S., Kildea S. Is a randomised controlled trial of a maternity care intervention for pregnant adolescents possible? An Australian feasibility study. BMC Medical Research Methodology. 2013;13:138. doi: 10.1186/1471-2288-13-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arksey H., O׳Malley L. Scoping studies: Towards a methodological framework. International Journal of Social Research Methodology. 2005;8(1):19–32. [Google Scholar]
- Barker D.J. Fetal origins of coronary heart disease. British Medical Journal. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry M.J., Edgman-Levitan S. Shared decision making—The pinnacle of patient-centered care. New England Journal of Medicine. 2012;366:780–781. doi: 10.1056/NEJMp1109283. [DOI] [PubMed] [Google Scholar]
- Bauer G.R. Incorporating intersectionality theory into population health research methodology: Challenges and the potential to advance health equity. Social Science & Medicine. 2014;110:10–17. doi: 10.1016/j.socscimed.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Benatar S., Garrett A.B., Howell E., Palmer A. Midwifery care at a freestanding birth center: a safe and effective alternative to conventional maternity care. Health Services Research. 2013;48(5):1750–1768. doi: 10.1111/1475-6773.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein I.M., Horbar J.D., Badger G.J., Ohlsson A., Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. American Journal of Obstetrics and Gynecology. 2000;182(1 Part 1):198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- Blanchette H. Comparison of obstetric outcome of a primary-care access clinic staffed by certified nurse-midwives and a private practice group of obstetricians in the same community. American Journal of Obstetrics and Gynecology. 1995;172(6):1864–1868. doi: 10.1016/0002-9378(95)91424-2. [DOI] [PubMed] [Google Scholar]
- Blumenshine P., Egerter S., Barclay C.J., Cubbin C., Braveman P.A. Socioeconomic disparities in adverse birth outcomes: A systematic review. American Journal of Preventive Medicine. 2010;39(3):263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- Bogossian F.E. Social support: Proposing a conceptual model for application to midwifery practice. Women and Birth. 2007;20(4):169–173. doi: 10.1016/j.wombi.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Borders A.E., Wolfe K., Qadir S., Kim K.-Y., Holl J., Grobman W. Racial/ethnic differences in self-reported and biologic measures of chronic stress in pregnancy. Journal of Perinatology. 2015;35(8):580–584. doi: 10.1038/jp.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P., Gruskin S. Defining equity in health. Journal of Epidemiology and Community Health. 2003;57(4):254–258. doi: 10.1136/jech.57.4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button K.S., Ioannidis J.P., Mokrysz C., Nosek B.A., Flint J., Robinson E.S. Power failure: Why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Centres for Disease Control (CDC) (2013). Births: Final data for 2012. National Vital Statistics Report (Vol. 62 (9)). 〈www.cdc.gov/nchs/data/nvsr/nvsr621nvsr62-09.pdf〉; Accessed 27.06.15. [PubMed]
- Collins J.W.J., David R.J., Handler A., Wall S., Andes S. Very low birthweight in African American infants: The role of maternal exposure to interpersonal racial discrimination. American Journal of Public Health. 2004;94(12):2132–2138. doi: 10.2105/ajph.94.12.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constitution of the World Health Organization American Journal of Public Health and the Nation׳s Health. 1946;36(11):1315–1323. doi: 10.2105/ajph.36.11.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragin L.E. University of California; Ann Arbor, San Francisco: 2002. Comparisons of care by nurse-midwives and obstetricians: Birth outcomes for moderate risk women (Ph.D. thesis) [Google Scholar]
- Culhane J.F., Rauh V., McCollum K.F., Hogan V.K., Agnew K., Wadhwa P.D. Maternal stress is associated with bacterial vaginosis in human pregnancy. Maternal and Child Health Journal. 2001;5(2):127–134. doi: 10.1023/a:1011305300690. [DOI] [PubMed] [Google Scholar]
- Davey M.A., Brown S., Bruinsma F. What is it about antenatal continuity of caregiver that matters to women? Birth. 2005;32(4):262–271. doi: 10.1111/j.0730-7659.2005.00384.x. [DOI] [PubMed] [Google Scholar]
- Davis J.A. Midwives and normalcy in childbirth: A phenomenologic concept development study. Journal of Midwifery & Women׳s Health. 2010;55(3):206–215. doi: 10.1016/j.jmwh.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Dominguez T.P., Dunkel-Schetter C., Glynn L.M., Hobel C., Sandman C.A. Racial differences in birth outcomes: The role of general, pregnancy, and racism stress. Health Psychology. 2008;27(2):194–203. doi: 10.1037/0278-6133.27.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downe S., Finlayson K., Walsh D., Lavender T. ‘Weighing up and balancing out’: A metasynthesis of barriers to antenatal care for marginalised women in high-income countries. British Journal of Gynaecology. 2009;116(4):518–529. doi: 10.1111/j.1471-0528.2008.02067.x. [DOI] [PubMed] [Google Scholar]
- Effective Public Health Practice Project (EPHPP) (2015). Quality assessment tool for quantitative studies. 〈http://www.ephpp.ca/tools.html〉; Accessed 22.06.15.
- Fischler N.R., Harvey S.M. Setting and provider of prenatal care: Association with pregnancy outcomes among low-income women. Health Care for Women International. 1995;16(4):309–321. doi: 10.1080/07399339509516184. [DOI] [PubMed] [Google Scholar]
- Flynn C.A., Helwig A.L., Meurer L.N. Bacterial vaginosis in pregnancy and the risk of prematurity: A meta-analysis. Journal of Family Practice. 1999;48(11):885–892. [PubMed] [Google Scholar]
- Giurgescu C. Are maternal cortisol levels related to preterm birth? Journal of Obstetric, Gynecologic & Neonatal Nursing. 2009;38(4):377–390. doi: 10.1111/j.1552-6909.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Giurgescu C., McFarlin B.L., Lomax J., Craddock C., Albrecht A. Racial discrimination and the black–white gap in adverse birth outcomes: A review. Journal of Midwifery and Women׳s Health. 2011;56(4):362–370. doi: 10.1111/j.1542-2011.2011.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg R. New York University Press; New York, NY: 1995. Pregnancy in a high-tech age. Paradoxes of choice. [Google Scholar]
- Haviland M.G., Morales L.S., Dial T.H., Pincus H.A. Race/ethnicity, socioeconomic status, and satisfaction with health care. American Journal of Medical Quality. 2005;20(4):195–203. doi: 10.1177/1062860605275754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heins H.C., Jr., Nance N.W., McCarthy B.J., Efird C.M. A randomized trial of nurse-midwifery prenatal care to reduce low birth weight. Obstetrics and Gynecology. 1990;75(3 Part 1):341–345. [PubMed] [Google Scholar]
- Hodnett, E.D., Fredericks, S., & Weston, J. (2010). Support during pregnancy for women at increased risk of low birthweight babies. Cochrane Database of Systematic Reviews (6), Article no. CD000198. [DOI] [PubMed]
- ten Hoope-Bender P., de Bernis L., Campbell J., Downe S., Fauveau V., Fogstad H. Improvement of maternal and newborn health through midwifery. Lancet. 2014;384(9949):1226–1235. doi: 10.1016/S0140-6736(14)60930-2. [DOI] [PubMed] [Google Scholar]
- Jackson D.J., Lang J.M., Swartz W.H., Ganiats T.G., Fullerton J., Ecker J. Outcomes, safety, and resource utilization in a collaborative care birth center program compared with traditional physician-based perinatal care. American Journal of Public Health. 2003;93(6):999–1006. doi: 10.2105/ajph.93.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I., Daniels N., Robinson D.E. Health disparities by race and class: Why both matter. Health Affairs (Millwood) 2005;24(2):343–352. doi: 10.1377/hlthaff.24.2.343. [DOI] [PubMed] [Google Scholar]
- Kennedy H.P. A model of exemplary midwifery practice: Results of a Delphi study. Journal of Midwifery and Women׳s Health. 2000;45(1):4–19. doi: 10.1016/s1526-9523(99)00018-5. [DOI] [PubMed] [Google Scholar]
- Kramer M.S., Séguin L., Lydon J., Goulet L. Socio-economic disparities in pregnancy outcome: Why do the poor fare so poorly? Paediatric and Perinatal Epidemiology. 2000;14(3):194–210. doi: 10.1046/j.1365-3016.2000.00266.x. [DOI] [PubMed] [Google Scholar]
- Krieger N. A glossary for social epidemiology. Journal of Epidemiology and Community Health. 2001;55(10):693–700. doi: 10.1136/jech.55.10.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeners B., Neumaier-Wagner P., Kuse S., Stiller R., Rath W. Emotional stress and the risk to develop hypertensive diseases in pregnancy. Hypertension in Pregnancy. 2007;26(2):211–226. doi: 10.1080/10641950701274870. [DOI] [PubMed] [Google Scholar]
- Martens P.J., Derksen S., Gupta S. Predictors of hospital readmission of Manitoba newborns within six weeks postbirth discharge: A population-based study. Pediatrics. 2004;114(3):708–713. doi: 10.1542/peds.2003-0714-L. [DOI] [PubMed] [Google Scholar]
- Martin J.A., Osterman M.J. Vol. 62. National Centre for Health Statistics, CDC; 2013. CDC health disparities and inequalities report – United States. Preterm births – United States, 2006–2010. Morbidity and mortality weekly report supplement; pp. 134–138. [Google Scholar]
- McLaughlin F.J., Altemeier W.A., Christensen M.J., Sherrod K.B., Dietrich M.S., Stern D.T. Randomized trial of comprehensive prenatal care for low-income women: Effect on infant birth weight. Pediatrics. 1992;89(1):128–132. [PubMed] [Google Scholar]
- Mortensen L.H., Diderichsen F., Arntzen A., Gissler M., Cnattingius S., Schnor O. Social inequality in fetal growth: A comparative study of Denmark, Finland, Norway and Sweden in the period 1981–2000. Journal of Epidemiology and Community Health. 2008;62(4):325–331. doi: 10.1136/jech.2007.061473. [DOI] [PubMed] [Google Scholar]
- Mortensen L.H., Diderichsen F., Smith G.D., Andersen A.M. The social gradient in birthweight at term: Quantification of the mediating role of maternal smoking and body mass index. Human Reproduction. 2009;24(10):2629–2635. doi: 10.1093/humrep/dep211. [DOI] [PubMed] [Google Scholar]
- National Collaborating Centre for Methods and Tools. Quality Assessment Tool for Quantitative Studies. 2008; http://www.nccmt.ca/registry/view/eng/14.html. Accessed 04.06.15.
- Nuru-Jeter A., Dominguez T.P., Hammond W.P., Leu J., Skaff M., Egerter S. "It׳s the skin you׳re in": African-American women talk about their experiences of racism. An exploratory study to develop measures of racism for birth outcome studies. Maternal and Child Health Journal. 2009;13(1):29–39. doi: 10.1007/s10995-008-0357-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olds D.L. Improving formal services for mothers and children. In: Garbarino J., Stocking S.H., editors. Protecting children from abuse and neglect. Jossey-Bass; San Francisco: 1981. pp. 173–197. [Google Scholar]
- Olds D.L., Henderson C.R.J., Tatelbaum R., Chamberlin R. Improving the delivery of prenatal care and outcomes of pregnancy: A randomized trial of nurse home visitation. Pediatrics. 1986;77(1):16–28. [PubMed] [Google Scholar]
- Organization of Economic Co-operation and Development (OECD) (2015). Infant mortality rates (indicator). 〈http://dx.doi.org/10.1787/83dea506-en〉. Accessed 13.08.15.
- Organization of Economic Co-operation and Development (OECD) (2014). Health: Key tables from OECD – ISSN 2075-8480. 〈http://www.oecd-ilibrary.org/social-issues-migration-health/infant-mortality_20758480-table9〉. Accessed 14.07.15.
- Orr S.T. Social support and pregnancy outcome: A review of the literature. Clinical Obstetrics and Gynecology. 2004;47(4):842–855. doi: 10.1097/01.grf.0000141451.68933.9f. [DOI] [PubMed] [Google Scholar]
- Phillippi J.C., Avery M.D. The 2012 American College of Nurse-Midwives core competencies for basic midwifery practice: History and revision. Journal of Midwifery & Women׳s Health. 2014;59(1):82–90. doi: 10.1111/jmwh.12148. [DOI] [PubMed] [Google Scholar]
- Reidpath D.D., Allotey P. Infant mortality rate as an indicator of population health. Journal of Epidemiology and Community Health. 2003;57(5):344–346. doi: 10.1136/jech.57.5.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross M.G., Beall M.H. Adult sequelae of intrauterine growth restriction. Seminars in Perinatology. 2008;32(3):213–218. doi: 10.1053/j.semperi.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandall, J., Soltani, H., Gates, S., Shennan, A., & Devane, D. (2015). Midwifery-led continuity models versus other models of care for childbearing women. Cochrane Database of Systematic Reviews (9), Article no. CD004667. [DOI] [PubMed]
- Shah P.S. Knowledge Synthesis Group on Determinants of LBW/PT births. Parity and low birth weight and preterm birth: A systematic review and meta-analyses. Acta Obstetricia et Gynecologica Scandinavica. 2010;89(7):862–875. doi: 10.3109/00016349.2010.486827. [DOI] [PubMed] [Google Scholar]
- Sheppard V.B., Zambrana R.E., O׳Malley A.S. Providing health care to low-income women: A matter of trust. Family Practice. 2004;21(5):484–491. doi: 10.1093/fampra/cmh503. [DOI] [PubMed] [Google Scholar]
- Simonet F., Wilkins R., Labranche E., Smylie J., Heaman M., Martens P. Primary birthing attendants and birth outcomes in remote Inuit communities—A natural "experiment" in Nunavik, Canada. Journal of Epidemiology and Community Health. 2009;63(7):546–551. doi: 10.1136/jech.2008.080598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sword W., Heaman M.I., Brooks S., Tough S., Janssen P.A., Young D. Women׳s and care providers׳ perspectives of quality prenatal care: A qualitative descriptive study. BMC Pregnancy and Childbirth. 2012;12(29) doi: 10.1186/1471-2393-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Teijlingen E. A critical analysis of the medical model as used in the study of pregnancy and childbirth. Social Research Online. 2005;10(2) [Google Scholar]
- Thomas B.H., Ciliska D., Dobbins M., Micucci S. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews on Evidence-Based Nursing. 2004;1(3):176–184. doi: 10.1111/j.1524-475X.2004.04006.x. [DOI] [PubMed] [Google Scholar]
- Veenstra G. Race, gender, class, and sexual orientation: Intersecting axes of inequality and self-rated health in Canada. International Journal of Equity in Health. 2011;10(3) doi: 10.1186/1475-9276-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visintainer P., Uman J., Horgan D., Ibald A., Verma U., Tejani N. Reduced risk of low weight births among indigent women receiving care from nurse-midwives. Journal of Epidemiology and Community Health. 2000;54(3):233–238. doi: 10.1136/jech.54.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa P.D., Entringer S., Buss C., Lu M.C. The contribution of maternal stress to preterm birth: Issues and considerations. Clinics in Perinatology. 2011;38(3):351–384. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M.L., Dorer D.J., Fleming M.P., Catlin E.A. Clinical outcomes of near-term infants. Pediatrics. 2004;114(2):372–376. doi: 10.1542/peds.114.2.372. [DOI] [PubMed] [Google Scholar]
- Williams D.R., Mohammed S.A. Discrimination and racial disparities in health: Evidence and needed research. Journal of Behavioral Medicine. 2009;32(1):20–47. doi: 10.1007/s10865-008-9185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]