Abstract
OBJECTIVES
While the risk of developing colorectal cancer increases with age, there are limited prospective data regarding best treatment in the older adult population. We launched a phase III trial to evaluate difference in treatment outcome for older adults (aged ≥ 70 years) with advanced colorectal cancer. Here we review the challenges faced and reasons for poor accrual to N0949.
MATERIALS and METHODS
We describe the conceptualization, development and limited results of N0949, a randomized phase III study of fluoropyrimidine/bevacizumab with or without oxaliplatin (mFOLFOX7 or XELOX) as first line chemotherapy for metastatic colorectal cancer. Fluoropyrimidine was physician choice (e.g., 5-FU/LV or capecitabine).
RESULTS
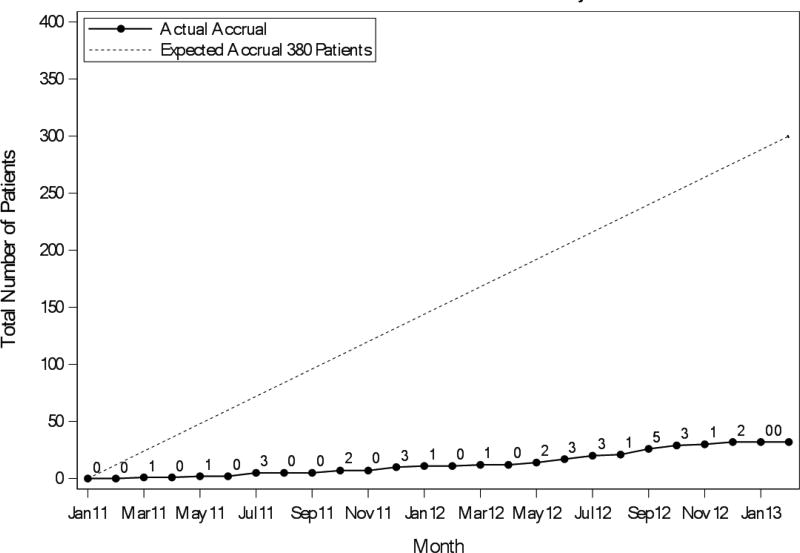
Of the projected 380 patients, only 32 patients were enrolled between the study activation in January 2011 until its closure in September 2012. Reasons for poor accrual included eligibility criteria that were too stringent, discomfort with randomizing older patients to regimens of varying intensity without considering their physical fitness, and discomfort with the use of bevacizumab in the older patient population. Several efforts were mounted to design a rationale and age-appropriate study, consider toxicities and varying study practices, and be responsive to stakeholder feedback.
CONCLUSIONS
Challenges were experienced in conducting the first prospective phase III study evaluating progression-free survival of older adults with advanced colorectal cancer receiving palliative chemotherapy with fluoropyrimidine/bevacizumab with or without oxaliplatin in the USA. Future efforts to evaluate treatment outcomes in the older adult population should reflect on lessons learned in this large national effort.
INTRODUCTION
In the United States, older patients (age ≥65) only comprise about 30% of patients enrolled in clinical trials, resulting in a lack of data on usage and tolerance of newly developed therapies in this patient population.1 There have been multiple European metastatic colorectal cancer (mCRC) clinical trials for the older adult population conducted with success,2–6 but clinical trials for this patient population in the United States are lacking.
Reasons for low enrollment are multifactorial7–10. Older adults are more prone to have other active medical conditions at the time of cancer diagnosis, leading to poorer functional status that curtails clinical trial eligibility typically requiring robust performance status. To manage concurrent medical conditions, older adults tend to have multiple medications prescribed that may compound the adverse effects of experimental therapies, making it challenging to attribute toxicity. Further, the offering of clinical trials is often a function of the physician treating the patient. This subjectivity introduces potential for physician bias in whether and how a trial is discussed with an older adult. Lastly, clinical trials are typically offered in academic treatment centers that may not be easily accessible for older adults who are often treated in community settings. For these reasons, clinical trials often do not reflect the general oncology population, comprised predominantly of older adults. Consequently, treatment decisions based on clinical trial results are not generalizable to this population. We sought to design a trial to answer a key question regarding treatment of mCRC in older adults, tailored to the needs older adults and accessible at community treatment centers.
N0949 DEVELOPMENT
Trial Hypothesis and Objectives
The specific hypothesis of North Central Cancer Treatment Group (NCCTG) N0949 was that treatment with oxaliplatin-based chemotherapy plus bevacizumab will result in superior clinical benefit compared to fluoropyrimidine-based chemotherapy plus bevacizumab, as measured by overall survival, in older adults with mCRC. The primary objective of the study was to determine whether the addition of oxaliplatin to a fluouropyrimidine and bevacizumab leads to improved overall survival (OS) among older adults with mCRC. Secondary objectives included comparison of response rates, progression-free survival (PFS), toxicity, and quality of life. If the addition of oxaliplatin did not significantly improve OS, then this patient population could be spared the toxicity of oxaliplatin and still achieve similar survival results with a fluoropyrimidine and bevacizumab alone, potentially preserving physical function for a longer period and improving quality of life.
Original Trial Design
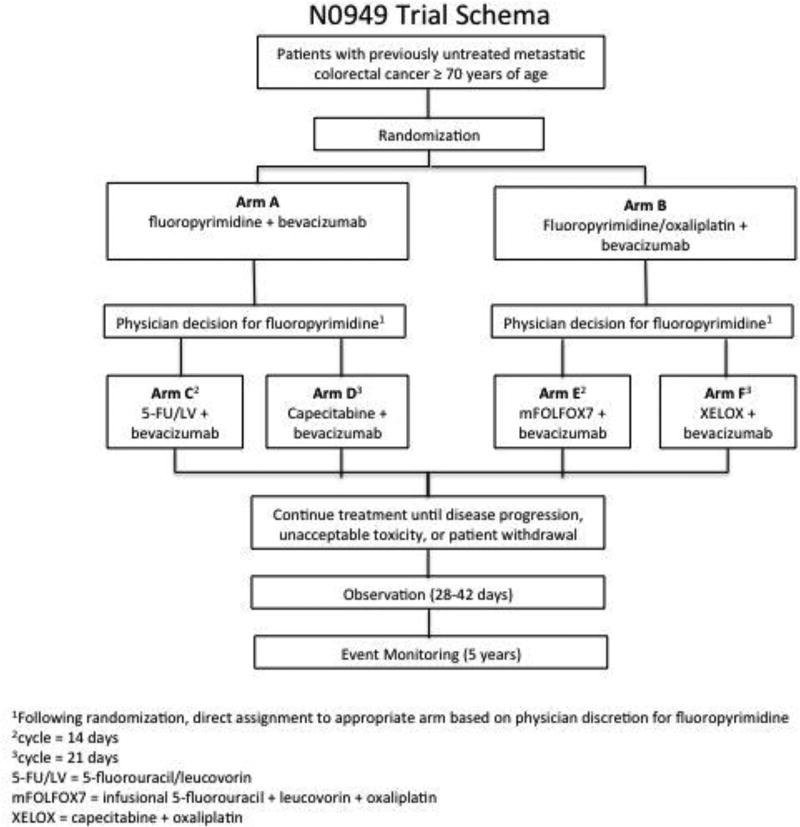
The trial was originally designed to enroll adults age ≥70 years to receive first-line treatment for mCRC. Eligible patients were randomized to either the control (Arm A) or experimental (Arm B) treatment arms. Arm A received a fluoropyrimidine of the provider’s choice (5-fluorouracil/leucovorin or capecitabine) plus bevacizumab. Arm B received modified FOLFOX7 or XELOX, plus bevacizumab. Patients were evaluated with CT imaging every 6 weeks. Patients continued their assigned treatment until progression of disease by RECIST version 1.1 criteria, unacceptable toxicity or patient withdrawal.
Eligible patients included those diagnosed with untreated mCRC age ≥ 70 years, ECOG performance status 0–2, life expectancy ≥ 3 months, able to complete questionnaire(s) by themselves or with assistance, and provide informed written consent. In addition, the following laboratory values were required <14 days prior to randomization: absolute neutrophil count ≥ 1,500/mm3, peripheral platelet count ≥ 100,000/mm3, hemoglobin > 9.0 g/dl, total bilirubin ≤1.5 × upper limit of normal (ULN), aspartate transaminase < 2.5 × ULN (<5 × ULN for patients with liver involvement), alkaline phosphatase <3 × ULN (<5 × ULN for patients with liver involvement), Creatinine <1.5 × ULN, INR <1.5 × ULN unless patients are receiving anti-coagulation therapy. Patients receiving prophylactic anti-coagulation therapy with an agent such as warfarin or heparin are allowed to participate if INR ≤ 3.0, and UPC ratio <1 or urine dipstick <2+. Those patients with co-morbid systemic illnesses or other severe concurrent disease which would make the patient inappropriate for entry, immunocompromised patients, other malignancy ≤ 3 years prior to randomization, recurrent disease ≤ 12 months of completing oxaliplatin-containing adjuvant therapy, creatinine clearance <60 mL/minute, symptomatic or untreated brain metastases, class 3+ heart failure, uncontrolled hypertension, major surgical procedure < 28 days, hemoptysis, non-healing wound, recent arterial thrombotic event, bleeding diathesis, and ≥ grade 2 peripheral neuropathy were excluded.
We estimated a median OS of 17 months in the control group11. Sample size calculations were based on obtaining enough events to achieve 80% power to detect a hazard ratio of 0.81 (an increase in median OS to 21 months in the experimental arm). We estimated an accrual rate of 12 patients per month (25 patients per month for the joint trials), an accrual period of 2.5 years and two years of minimum follow-up. We planned for two interim analyses for superiority of OS (after 33% and 66% of the planned number of events) using O’Brien-Fleming stopping boundaries. This phase III trial was to be monitored twice annually by the NCCTG Data and Safety Monitoring Board (DSMB).
Correlative Studies
Geriatric assessment was a pivotal focus of the study, introduced for the first time in a randomized trial in colorectal cancer. The cancer-specific geriatric assessment (CGA) tool was developed by Arti Hurria and colleagues within the cooperative group setting to thoroughly review and identify issues in older adults that may affect cancer treatment12–14. Details of the CGA metrics and validation studies are published elsewhere15–17. The CGA has been shown to predict chemotherapy toxicity for older adults with cancer18,19. The CGA has been validated in written format in a multicenter clinical trial15,20 and validated in a computer format in an individual center trial16. Inclusion of the CGA would have served as external validation of the tool specifically within CRC to predict moderate to severe treatment-related toxicity, hospitalization, dose delay or reduction or discontinuation of chemotherapy.
The trial included additional patient-centered correlative studies focused on patient-reported assessment of treatment-related adverse events (PRO-CTAE), neurotoxicity assessment (Neurotoxicity Symptom Experience Diary), as well as assessments of quality of life (QoL) [Fatigue/Uniscale assessment, Linear Analog Self-Assessment, and Was It Worth It questionnaire]. Pharmacokinetic and pharmacogenetic studies were added to determine if there are age-specific alterations in drug metabolism beyond the decline of basic organ function in older adults. A teleconference was held between NCCTG, CALGB and the NCI Division of Cancer Prevention representatives supporting the addition of two frailty assessments (Rockwood Canadian Study of Health and Aging Clinical Frailty Scale and NCCTG Brief Frailty Inventory), and another QoL assessment (EQ-5D).
Process of Trial Development
N0949 developed as a result of input from all relevant stakeholders. Starting in 2009, investigators from NCCTG (Grothey) and CALGB (McCleary) proposed a study to understand the additive toxicity and survival benefit of oxaliplatin in the setting of mCRC. The trial was investigator driven with input from NCCTG and the Gastrointestinal and Cancer in Elderly committees of the CALGB, active patient advocates, community oncologists and academic center leaders. Patient advocates raised concern regarding use of age as a selection factor and questioned whether the results of the trial would allow insurance payers to deny oxaliplatin to older adults. However, patient advocates were in favor of understanding factors associated with the best treatment outcomes for older adults, acknowledging that performance status alone does not adequately predict outcome. Community oncologists sought clarity on dosing and confirming provider preference for fluoropyrimidine use in the control arm. Both oncologists and patients raised concern regarding potential toxicity of oxaliplatin in older adults, somewhat reassured by the planned correlative studies to measure neurotoxicity, frailty, and quality of life directly from the patient. The proposal was revised to address these valid concerns, then submitted for approvals by the NCCTG, CALGB and National Cancer Institution (NCI).
Revised Trial Design
In May 2010, the Gastrointestinal Steering Committee (GISC) of the Cancer Therapy Evaluation Program (CTEP) of the NCI performed a consensus evaluation of the proposal, raising concerns about feasibility, statistical design and quality of life. We responded with appropriate changes in response to those concerns. Key changes to the protocol included [1] modifying the bevacizumab dose and treatment schedule in the capecitabine control arm and [2] discontinuing oxaliplatin after 8 mFOLFOX7 treatment cycles when a cumulative dose of 680 mg/m2 oxaliplatin had been administered, [3] including patients age 70–74 to stimulate trial enrollment but limiting this subset to no greater than 25% of the cohort to make sure results were also generalizable to patients greater than 75 years, [4] changing the primary endpoint to PFS, [5] making OS the secondary endpoint in a pooled analysis with JCOG, assuming the primary endpoint of PFS was achieved, [6] decreasing the total sample size from 400 to 380 and [7] including a futility analysis. The revised accrual goal of 380 patients assumed enough events to achieve 80% power to detect a hazard ratio of 0.75 (an increase in median PFS to 12 months in the experimental arm). Statistical design was modified to assume a median PFS of 9 months in the control group, still assuming an accrual rate of 25 patients/month over 2.5 years and a 1-year minimum follow-up. The previously planned two interim analyses for superiority were revised to one interim analysis for futility for the primary endpoint of PFS after 50% of the planned number of events using the rule of Wieand, Schroeder, and O’Fallon21. We added contingencies for poor accrual. If after two years from the date of study activation the accrual rate was <100 patients/year or at trial completion was less than 200 patients, the primary endpoint of PFS would become secondary and the secondary correlative patient-centered endpoints would become primary.
Final Trial Design
The trial schema is outlined in Figure 1. Each participant signed an IRB-approved, protocol-specific informed consent in accordance with federal and institutional guidelines. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson following Alliance policies.
Figure 1.
Final trial schema
Trial Timeline
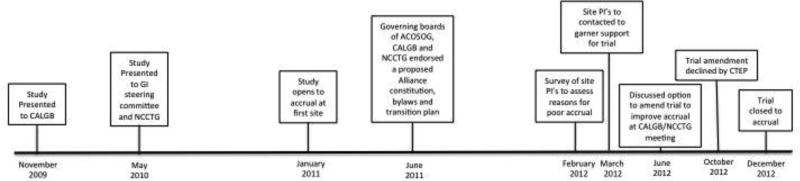
The overall timeline for the trial development and conduct is shown in Figure 2. In 2010, three adult cooperative groups, NCCTG, CALGB and ACOSOG, merged to form the Alliance for Clinical Trials in Oncology, approximately 7 months following opening accrual for N0949 on January 21, 2011. During the transition period, trials opened prior to the merger were closely monitored for compliance with accrual goals. The study was presented at each individual cooperative group and Alliance meetings. An online training module for research assistants administering the questionnaire tools was developed. We conducted a live-demonstration session for research assistants in June 2011. By December 2011, 10 patients were enrolled even though approximately 70 investigators had the study available for patients at 315 sites (6.3% academic centers). The trial accrual is shown in Figure 3.
Figure 2.
Timeline of the study (from concept to activation to closure)
Figure 3.
Cumulative Accrual (Actual vs. Expected)
Given poor accrual, we mounted a concerted four-part effort to understand barriers to accrual. First, we spoke with patient advocates, community oncologists and academic center leaders at each of the then quarterly Alliance meetings. Second, we surveyed all site principal investigators in February 2012 to assess the reasons for poor accrual. Only 34% of site principal investigators responded to the survey. Of the 24 respondents, 22 had not enrolled patients onto the study. Responses to the questionnaire are displayed in Table 1. Most respondents (83.3%) felt they did not have patients ≥70 years who were eligible for the study. Most respondents noted discomfort with randomizing frail patients to oxaliplatin or fit patients to non-oxaliplatin based regimen as barriers to enrollment. Third, we personally called each site principal investigator to learn what they perceived barriers were to enrollment at their individual sites. We learned that concerns regarding potential neurotoxicity from oxaliplatin were not the greatest concern. Investigators stated that they were uncomfortable with the use of bevacizumab in this population, despite evidence of the relative safety of bevacizumab use in the older adult population22.
Table 1.
Local Investigator Questionnaire Responses
| Response | Response % |
|
|---|---|---|
| Reasons why investigator had not enrolled a patient on the study (N=18)* | ||
| Patient declined the study | 2 | 11.1% |
| Have not had patients > 70 years eligible for the study | 15 | 83.3% |
| Not comfortable with study design | 1 | 5.6% |
| Correlative studies too burdensome | 1 | 5.6% |
| Barriers for investigator to enroll patients on the study (N=16)* | ||
| Discomfort with randomizing fit patients to a regimen without oxaliplatin | 7 | 43.8% |
| Discomfort with randomizing frail patients to a regimen with oxaliplatin | 10 | 62.5% |
| Patients are not comfortable with the randomization | 3 | 18% |
| The study involves to many correlates | 4 | 25% |
| Inclusion/exclusion criteria are too stringent | 2 | 12.5% |
More than one response allowed.
Last, we discussed the trial with the Gastrointestinal and Cancer in the Elderly committees of the Alliance, as well as the Cancer and Aging Research Group, many of whom wished to use the geriatric assessment as a risk stratification tool to assign patients to the various treatment arms. Unfortunately, this option was not feasible because it would have tripled the sample size. Another suggestion was to make bevacizumab optional, but this would have made the trial very similar to the FOCUS2 study6 and not added new information. Another suggestion was to increase advertisement for the trial, which was carried out. By September 2012, the total accrual to the trial was still below projected at 23 patients.
As a result of these cumulative efforts, we submitted an amendment to the current protocol to the Cancer Therapy Evaluation Program (CTEP) division of the NCI on October 9, 2012 proposing to (1) amend the current study to a phase II trial; (2) change the primary endpoint to PFS at 6 months thereby reducing the accrual goal to 68 patients [assuming effect size change from 0.5 (control) to 0.7 (experimental arm) with a 1-sided alpha of 0.2, 80% power] and (3) broaden inclusion criteria to include organ function more representative of the older adult population. CTEP did not consider it appropriate to re-define the trial as a randomized phase II study, citing both regimens are standard and currently given to this patient population. Despite these strategic efforts, DSMB along with Alliance leadership recommended closure of the trial, citing poor accrual and limited cooperative group resources. In December 2012, the trial was closed to accrual after 32 participants were enrolled. Follow-up was discontinued for all patients on November 1, 2014, with 5 patients still alive having a median follow-up of 33.8 months (range 3 – 56.5 months).
DISCUSSION
Multiple challenges arose during the design and conduct of this clinical trial and the study closed early due to poor accrual. Below we outline key lessons learned from this clinical trial experience that should be heeded moving forward.
Insights Gained from Failure of N0949
Lesson 1. Trials should be tailored to the population of interest to facilitate trial accrual and generalizability of trial results
Due to the heterogeneity of the physical condition of the older adult patient, randomizing older adults to regimens with substantially different activity and side effect profiles without considering their overall physical function is not feasible. This was highlighted in our principal investigator survey, which alerted us to the lack of comfort with the randomization in this trial. Clinicians did not feel comfortable randomizing fit patients to a regimen without oxaliplatin, and they did not feel comfortable randomizing frail patients to a regimen with oxaliplatin.
The FOCUS2 study was an example of a very successful clinical trial involving older adults and frail patients with mCRC6. FOCUS2 randomized older adults who were unfit to receive full dose/standard chemotherapy to treatment with either single agent fluoropyrimidine or reduced doses of combination oxaliplatin plus fluoropyrimidine (with an option to escalate doses). FOCUS2 included a more uniform population of (less fit) patients as opposed to a population based on age alone, and it offered the flexibility to increase doses at the physician discretion. These criteria were likely large contributors to its success.
Lesson 2. What happens in academic centers may differ substantially from practice in the community setting
We were surprised that a major barrier to enrollment on this trial was the lack of comfort with the use of bevacizumab in the older adult population. We heard from community providers at cooperative group meetings that bevacizumab was not the standard of care for older patients in the community, mainly out of concern for adverse events. This issue was not identified during study development despite a very careful, conscientious vetting of the study through multiple meetings and committees, which included patient advocates, community oncologists, and academic oncologists. This may highlight a disconnect between clinical trialists at academic centers and a survey conducted outside the setting of a cooperative group meeting might have increased the comfort of future site principal investigators to raise concerns about the clinical trial.
Lesson 3. Investigators must strike a delicate balance between eligibility criteria specific enough to limit toxicity exposure for suboptimal candidates but broad enough to include those factors generalizable to the target population
Specifically, inclusion criteria for trials targeting older adults should include consideration of concurrent medical conditions. N0949 used eligibility criteria that are standard for younger adults. For example, this trial had an exclusion criterion in which patients with a calculated creatinine clearance < 60 mL/min were ineligible. The equation for this calculation is as follows:
Therefore, a 78-year-old female weighing 75 kg with a serum creatinine of 1.0 would have a creatinine clearance of 55. There was an additional caveat that if the patient did not meet this eligibility requirement that a 24-hour urine could be collected to determine creatinine clearance to see if they meet the 60 mL/min criteria. It is unknown if clinicians went the extra step to do this. Other European studies referenced previously included patients with lower creatinine clearance rates (two allowed ≥ 30 mL per min, one allowed ≥ 45 mL per min, and one dose adjusted if creatinine clearance was 30–50 mL/min)3–6. Easing eligibility criteria may have made accrual more feasible in N0949.
Lesson 4. When considering trials in older adults, it is reasonable to vet the concept to ensure feasibility in a smaller trial to increase investigator and patient comfort with study design and implementation
Starting with a pilot, feasibility, or phase II study would allow demonstration of a feasible endpoint and a stronger rationale for moving forward with a larger, randomized trial. This would also prevent considerable resources going into a large trial without knowing that it is feasible to accrue well to the trial.
Lesson 5. Given the numerous challenges with conducting a clinical trial with older adults, limit additional complexity
Due to well-intentioned excitement about the conduct of a large prospective study in older adults, N0949 was laden with several and sometimes overlapping correlative studies. This trial included an 18-page booklet with 7 questionnaires. In total, patients potentially answered 92 questions for correlative studies prior to each cycle of treatment. While each correlative study had valid rationale, we received feedback that the complexity of conducting such analysis in this subpopulation was too burdensome. We propose that 1–2 correlative studies, such as comprehensive geriatric assessment and pharmacokinetic/pharmacogenetic studies, would suffice to provide detailed information regarding tolerance and mechanistic correlates on which to base treatment recommendations and further study.
Conclusions
Colorectal cancer remains largely a disease of older adults. Prospective studies have limited generalizability to this population that presents with unique challenges. We described the large effort to launch a prospective study focused on older adults diagnosed with mCRC. While the study faced significant hurdles, the lessons learned provide a path forward for targeted investigations for older adults in the future.
Continued development of educational and financial resources dedicated to the assessment and support of older adults undergoing cancer treatment will likely encourage greater enrollment of this patient population in clinical trials.
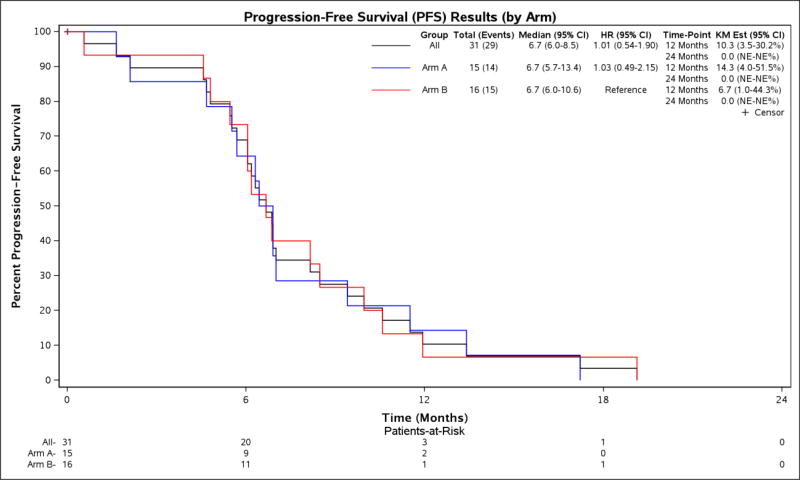
Figure 4.
PFS, by Treatment Arm
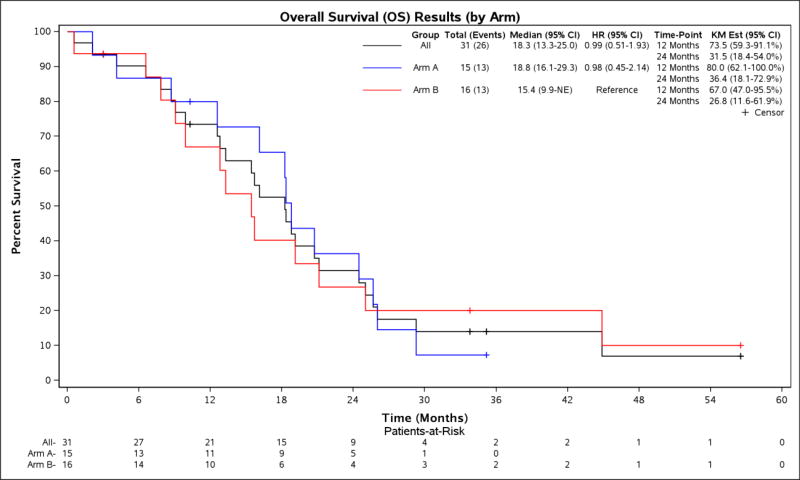
Figure 5.
OS, by Treatment Arm
Estimates calculated by the Kaplan-Meier method, with 95% Confidence Interval.
Table 2.
Baseline Patient Demographics, by Cohort
| A: Demographics, by Treatment Arm | ||||
|---|---|---|---|---|
|
| ||||
| A (N=15) |
B (N=17) |
Total (N=32) |
Fisher’s Exact p-value |
|
| Gender | 1.0000 | |||
| Female | 6 (40.0%) | 6 (35.3%) | 12 (37.5%) | |
| Male | 9 (60.0%) | 11 (64.7%) | 20 (62.5%) | |
| Race | 0.2117 | |||
| White | 13 (86.7%) | 17 (100.0%) | 30 (93.8%) | |
| Black or African American | 2 (13.3%) | 0 (0.0%) | 2 (6.3%) | |
| Ethnicity | ||||
| Not Hispanic or Latino | 15 (100.0%) | 17 (100.0%) | 32 (100.0%) | |
| Age | ||||
| N | 15 | 17 | 32 | |
| Mean (SD) | 75.0 (3.0) | 76.3 (4.0) | 75.7 (3.6) | |
| Median | 75.0 | 77.0 | 75.5 | |
| Q1, Q3 | 72.0, 78.0 | 73.0, 78.0 | 73.0, 78.0 | |
| Range | (71.0–81.0) | (71.0–86.0) | (71.0–86.0) | |
| Age Group* | 1.0000 | |||
| 70–74 years | 7 (46.7%) | 7 (41.2%) | 14 (43.8%) | |
| 75–79 years | 7 (46.7%) | 7 (41.2%) | 14 (43.8%) | |
| 80–84 years | 1 (6.7%) | 2 (11.8%) | 3 (9.4%) | |
| 85+ years | 0 (0.0%) | 1 (5.9%) | 1 (3.1%) | |
| Age Group, condensed | 1.0000 | |||
| 70–74 years | 7 (46.7%) | 7 (41.2%) | 14 (43.8%) | |
| 75+ years | 8 (53.3%) | 10 (58.8%) | 18 (56.3%) | |
| ECOG Performance Score* | 1.0000 | |||
| 0–1 | 14 (93.3%) | 15 (88.2%) | 29 (90.6%) | |
| 2 | 1 (6.7%) | 2 (11.8%) | 3 (9.4%) | |
| Number of Metastatic Sites* | 1.0000 | |||
| 1 | 5 (33.3%) | 5 (29.4%) | 10 (31.3%) | |
| >1 | 10 (66.7%) | 12 (70.6%) | 22 (68.8%) | |
| B: Demographics, by Age Group | ||||
|---|---|---|---|---|
| 70–74 years (N=14) |
75+ years (N=18) |
Total (N=32) |
p value | |
| Gender | 0.28 | |||
| Female | 7 (50.0%) | 5 (27.8%) | 12 (37.5%) | |
| Male | 7 (50.0%) | 13 (72.2%) | 20 (62.5%) | |
| Race | 1.00 | |||
| White | 13 (92.9%) | 17 (94.4%) | 30 (93.8%) | |
| Black or African American | 1 (7.1%) | 1 (5.6%) | 2 (6.3%) | |
| Ethnicity | ||||
| Not Hispanic or Latino | 14 (100.0%) | 18 (100.0%) | 32 (100.0%) | |
| ECOG Performance Score | 0.57 | |||
| 0–1 | 12 (85.7%) | 17 (94.4%) | 29 (90.6%) | |
| 2 | 2 (14.3%) | 1 (5.6%) | 3 (9.4%) | |
| Number of Metastatic Sites | 0.71 | |||
| 1 | 5 (35.7%) | 5 (27.8%) | 10 (31.3%) | |
| >1 | 9 (64.3%) | 13 (72.2%) | 22 (68.8%) | |
Stratification factors
Abbreviations: 5-FU = 5-fluorouracil, LV = leucovorin, mFOLFOX7 = modified FOLFOX (5-fluorouracil, leucovorin, oxaliplatin), XELOX = capecitabine, oxaliplatin, ECOG = Eastern Cooperative Oncology Group
Abbreviations: ECOG = Eastern Cooperative Oncology Group
Table 3.
PFS, OS, and TTP, by Age Group†
| N | Follow-up time, median (range) |
PFS, median (range) |
OS, median (range) |
TTP, median (range) |
|
|---|---|---|---|---|---|
| Age 70–74 | 14 | 6.6 mos (3–10.3) | 6.3 mos (5.7–11.9) | 18.3 mos (12.8–29.3) | 6.5 mos (6–11.9) |
| Age ≥75 | 17 | 35.2 mos (33.8–56.5) | 6.9 mos (5.5–13.4) | 18.8 mos (12.6-NE) | 6.9 mos (6.2–13.4) |
| HR (95% CI) | 1.80 (0.82–3.96) | 1.49 (0.67–3.28) | 1.61 (0.71–3.66) | ||
| P-value | 0.33 | 0.61 | 0.50 |
Abbreviations: PFS = progression-free survival, OS = overall survival, TTP = time to progression, HR = Hazard ratio, CI = confidence interval, NE = not evaluated
: Estimates calculated by the Kaplan-Meier method, with 95% Confidence Interval.
Table 4.
PFS, OS, and TTP, by Treatment Arm†
| N | Follow-up time, median (range) |
PFS, median (range) |
OS, median (range) |
TTP, median (range) |
|
|---|---|---|---|---|---|
| Overall cohort | 31 | 33.8 mos (3.0–56.5) | 6.7 mos (6.0–8.5) | 18.3 mos (13.3–25) | 6.9 mos (6.0–9.4) |
| Arm A: Fluoropyrimidine + bevacizumab | 15 | 22.7 mos (10.3–35.2) | 6.7 mos (5.7–13.4) | 18.8 mos (16.1–29.3) | 6.9 mos (5.7–17.2) |
| Arm B: Fluoropyrimidine/oxaliplatin + bevacizumab | 16 | 33.8 mos (3.0–56.5) | 6.7 mos (6.0–10.6) | 15.4 mos (9.9-NR) | 6.7 mos (6.0–11.9) |
| HR (95% CI) | 1.03 (0.49–2.17) | 0.98 (0.45–2.16) | 0.73 (0.33–1.65) | ||
| P-value | 1.00 | 1.00 | 0.75 |
Abbreviations: PFS = progression-free survival, OS = overall survival, TTP = time to progression, HR = Hazard ratio, CI = confidence interval, NR = not reached
: Estimates calculated by the Kaplan-Meier method, with 95% Confidence Interval.
Table 5.
Grade 3 and Higher Toxicity*, by Treatment Arm
| Toxicity, No. | Arm A: Fluoropyrimidine + bevacizumab (N=15) |
Arm B: Fluoropyrimidine, oxaliplatin + bevacizumab (N=16) |
|---|---|---|
| Palmar-plantar erythrodysesthesia | 5 | 1 |
| Hypertension | 2 | 4 |
| Thromboembolic events | 1 | 1 |
| Anemia | 0 | 1 |
| Neutropenia | 0 | 1 |
| Nausea | 1 | 0 |
| Vomiting | 0 | 1 |
| Mucositis | 1 | 1 |
| Fatigue | 2 | 1 |
| Peripheral sensory neuropathy | 0 | 1 |
| Fall | 1 | 0 |
| Diarrhea | 0 | 0 |
| Anorexia | 2 | 0 |
| Hyperglycemia | 0 | 1 |
| Hyponatremia | 2 | 1 |
| Dysesthesia | 0 | 1 |
| Ejection fraction decreased | 0 | 1 |
| Chronic kidney Disease | 0 | 1 |
| Back pain | 0 | 1 |
Abbreviations: No. = number
NCI CTCAE v4.0 events considered at least possibly related to treatment.
Acknowledgments
J Hubbard and JA Meyerhardt have served as consultants for Genentech. J Hubbard has received research support from Genentech.
Funding
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA025224, U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), U10CA138561 and U10CA180790. Also, supported in part by funds from the National Comprehensive Cancer Network Young Investigator Award and Dana-Farber Cancer Institute Gloria Spivak Faculty Advancement Fund (to N.J.M). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The following institutions participated in this study:
Coborn Cancer Center at Saint Cloud Hospital, Saint Cloud, MN, Dana-Farber/5
Heartland Cancer Research NCORP, Decatur, IL, James Wade, 5UG1CA189830
Mayo Clinic LAPS, Rochester, MN, Steven Alberts, 5U10CA180790
Metro Minnesota Community Oncology Research Consortium, Saint Louis Park, MN, Daniel Anderson, 5UG1CA189863
Michigan Cancer Research Consortium NCORP, Ann Arbor, MI, Philip Stella, 5UG1CA189971
Sanford NCI Community Oncology Research Program of the North Central Plains, Sioux Falls, SD, Preston Steen, 5UG1CA189825
University of Chicago Comprehensive Cancer Center LAPS, Chicago, IL, Hedy Kindler, 5U10CA180836
Washington University-Siteman Cancer Center LAPS, Saint Louis, MO, Nancy Bartlett, 5U10CA180833
Wichita NCI Community Oncology Research Program, Wichita, KS, Shaker Dakhil, 5UG1CA189808
Wisconsin NCI Community Oncology Research Program, Marshfield, WI, Anthony Jaslowski, 5UG1CA189956
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures and Conflict of Interest Statements
The authors have no other conflicts of interest to disclose.
Author Contributions
Study Concepts: NJ McCleary, J Hubbard, JA Meyerhardt, D Sargent, A Venook, A Grothey
Study Design: NJ McCleary, J Hubbard, JA Meyerhardt, D Sargent, A Venook, A Grothey
Data Acquisition: NJ McCleary, J Hubbard, MR Mahoney
Quality Control of Data and Algorithms: NJ McCleary, J Hubbard, MR Mahoney
Data Analysis and Interpretation: NJ McCleary, J Hubbard, MR Mahoney
Statistical Analysis: NJ McCleary, J Hubbard, MR Mahoney
Manuscript Preparation: NJ McCleary, J Hubbard, MR Mahoney, JA Meyerhardt, D Sargent, A Venook, A Grothey
Manuscript Editing: NJ McCleary, J Hubbard, JA Meyerhardt, D Sargent, A Venook, A Grothey
Manuscript Review: NJ McCleary, J Hubbard, JA Meyerhardt, D Sargent, A Venook, A Grothey
References
- 1.Rao AV, Cohen HJ. Preface. Clin Geriatr Med. 2016;32:xiii–xiv. doi: 10.1016/j.cger.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Aparicio T, Bouche O, Francois E, et al. PRODIGE 20: Bevacizumab + chemotherapy (BEV-CT) versus chemotherapy alone (CT) in elderly patients (pts) with untreated metastatic colorectal cancer (mCRC)--A randomized phase II trial. ASCO Meeting Abstracts. 2015;33:3541. [Google Scholar]
- 3.Aparicio T, Jouve JL, Teillet L, et al. Geriatric factors predict chemotherapy feasibility: ancillary results of FFCD 2001–02 phase III study in first-line chemotherapy for metastatic colorectal cancer in elderly patients. J Clin Oncol. 2013;31:1464–70. doi: 10.1200/JCO.2012.42.9894. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol. 2013;14:1077–85. doi: 10.1016/S1470-2045(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 5.Price TJ, Zannino D, Wilson K, et al. Bevacizumab is equally effective and no more toxic in elderly patients with advanced colorectal cancer: a subgroup analysis from the AGITG MAX trial: an international randomised controlled trial of Capecitabine, Bevacizumab and Mitomycin C. Ann Oncol. 2012;23:1531–6. doi: 10.1093/annonc/mdr488. [DOI] [PubMed] [Google Scholar]
- 6.Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet. 2011;377:1749–59. doi: 10.1016/S0140-6736(11)60399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurria A. Incorporation of geriatric principles in oncology clinical trials. J Clin Oncol. 2007;25:5350–1. doi: 10.1200/JCO.2007.13.7125. [DOI] [PubMed] [Google Scholar]
- 8.Hurria A. Clinical trials in older adults with cancer: past and future. Oncology (Williston Park) 2007;21:351–8. discussion 363-4, 367. [PubMed] [Google Scholar]
- 9.Aapro MS, Kohne CH, Cohen HJ, et al. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. Oncologist. 2005;10:198–204. doi: 10.1634/theoncologist.10-3-198. [DOI] [PubMed] [Google Scholar]
- 10.Denson AC, Mahipal A. Participation of the elderly population in clinical trials: barriers and solutions. Cancer Control. 2014;21:209–14. doi: 10.1177/107327481402100305. [DOI] [PubMed] [Google Scholar]
- 11.Kabbinavar FF, Hambleton J, Mass RD, et al. Combined analysis of efficacy: the addition of bevacizumab to fluorouracil/leucovorin improves survival for patients with metastatic colorectal cancer. J Clin Oncol. 2005;23:3706–12. doi: 10.1200/JCO.2005.00.232. [DOI] [PubMed] [Google Scholar]
- 12.Balducci L, Beghe C. The application of the principles of geriatrics to the management of the older person with cancer. Critical Reviews in Oncology-Hematology. 2000;35:147–54. doi: 10.1016/s1040-8428(00)00089-5. [DOI] [PubMed] [Google Scholar]
- 13.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. Journal of Clinical Oncology. 2007;25:1824–31. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 14.Brunello A, Sandri R, Extermann M. Multidimensional geriatric evaluation for older cancer patients as a clinical and research tool. Cancer Treatment Reviews. 2009;35:487–92. doi: 10.1016/j.ctrv.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29:1290–6. doi: 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCleary NJ, Wigler D, Berry D, et al. Feasibility of computer-based self-administered cancer-specific geriatric assessment in older patients with gastrointestinal malignancy. Oncologist. 2013;18:64–72. doi: 10.1634/theoncologist.2012-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohile SG, Velarde C, Hurria A, et al. Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J Natl Compr Canc Netw. 2015;13:1120–30. doi: 10.6004/jnccn.2015.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hurria A, Mohile S, Gajra A, et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol. 2016;34:2366–71. doi: 10.1200/JCO.2015.65.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–65. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurria A, Gupta S, Zauderer M, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104:1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 21.Wieand S, Schroeder G, O'Fallon JR. Stopping when the experimental regimen does not appear to help. Stat Med. 1994;13:1453–8. doi: 10.1002/sim.4780131321. [DOI] [PubMed] [Google Scholar]
- 22.Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol. 2010;136:737–43. doi: 10.1007/s00432-009-0712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]